The COP9 signalosome (CSN) is an eight-subunit protein (CSN1-CSN8) complex critical for protein degradation in all eukaryotes.Citation1 Discovered first as a regulator of constitutive photomorphogenesis (COP) in plants, it has an intrinsic deneddylation activity that removes the ubiquitin (Ub)-like protein Nedd8 from cullin-RING Ub ligases.Citation2 Through its function to coordinate ubiquitylation and proteasome degradation, CSN plays roles in cellular processes including cell cycle control, DNA damage response, apoptosis, senescence and transcription.Citation3,Citation4 CSN has recently been implicated in tumorigenesis;Citation3 however, the specific mechanisms are still emerging.
Mong-Hong Lee and colleagues have spearheaded recent efforts to understand roles for CSN in cancer. Studies focused on CSN5 and CSN6 suggest that expression of these CSN subunits may be elevated in several human cancers.Citation3 Indeed, CSN components are implicated in the negative regulation of important cancer-related genes, including p53, MDM2, P27, SMAD7, RUNX3, ID1, SKP2 and HIF1.Citation3
In studies of CSN6, Lee and colleagues have identified a role for CSN in the control of DNA damage response and cancer (). In addition to demonstrating amplification of the CSN6 gene locus at 7q22.1 in human mammary tumors,Citation5 they report that AKT phosphorylation at Ser60 stabilizes CSN6.Citation6 Interestingly, by promoting 14–3-3σ degradation through stabilization of its E3-ligase COP1, CSN6 can activate AKT to create a self-propelling positive feedback loopCitation7 (). Moreover, CSN6 promotes p53 degradation through a mechanism that stabilizes MDM2 protein by limiting its auto-ubiquitylation.Citation5 Accordingly, due to elevated p53 activity, Csn6+/− mice displayed increased apoptosis and attenuated tumorigenesis in response to irradiation.Citation5 Collectively, Lee and colleagues have linked activation of the PI3K pathway to the MDM2-p53 axis through CSN, where p53 is destabilized to promote tumorigenesis ().
Figure 1. The COP9 signalosome (CSN) is a regulator of multiple oncogenic E3-ligases. CSN6 is activated by inputs from activated PI3K kinase pathway, as observed in breast cancers with HER2 amplification. CSN6 controls the activities of the E3 ligases COP1, MDM2 and SKP2. Through COP1, CSN mediates 14–3-3σ degradation, thereby relieving negative regulation of AKT, which, in turn, forms a positive feedback to activate CSN6. By activating MDM2 and SKP2 activity toward their tumor suppressor substrates p53 and p57kip2, respectively, CSN is hypothesized to promote oncogenesis.
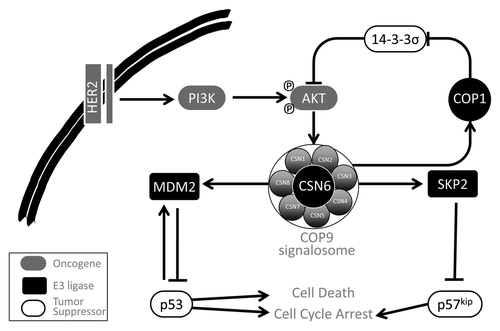
In their latest work,Citation8 this group has demonstrated that CSN6 overexpression is associated with low levels of p57Kip2, a cyclin-dependent kinase inhibitor (CKI) and putative tumor suppressor gene. p57Kip2 is known to be ubiquitylated by the Skp-Cullin-F-box (SCF) ubiquitin ligases SKP2 and FBL12.Citation9 The authors provide evidence that CSN6 interacts with SKP2 to promote p57Kip2 polyubiquitylation. By contrast, CSN6 knockdown impaired SKP2-mediated polybiquitylation of p57Kip2. Together these finding support the existence of a CSN6-p57Kip2-SKP2 complex that mediates the destruction of p57Kip2. Accordingly, the authors identified reduced levels of p57Kip2 in breast tumors with elevated CSN6 expression and an association between poor overall patient survival with either high or low expression levels of CSN6 and p57Kip2, respectively. This study highlights the importance of a CSN6-p57Kip2-SKP2 signaling axis in breast cancer and provides yet another mechanism by which CSN6 can promote cancer.
Through combinatorial effects on the MDM2-p53 signaling axis and now SKP2-mediated p57Kip2 degradation, it is hypothesized that CSN6 overexpression can effectively promote cell proliferation by simultaneously relieving cell cycle restraints, apoptosis, senescence, and presumably p53 functions in DNA damage repair and cell metabolism, among other important tumor suppressive mechanisms. The increasing importance of CNS6 in controlling different oncogenic pathways makes CSN targeting an attractive therapeutic opportunity in cancer. Indeed, CSN6 targeting is hypothesized to stabilize p53, p57Kip2 and 14–3-3σ proteins, among others (clearly, the multiple roles of CSN make this endeavor complex). Thus, therapeutic targeting the CSN awaits characterization of other substrates for this fascinating and multifunctional complex. For instance, because a majority of human tumors carry inactivated p53, it will be of great interest if inactivation of any COP9 subunit is found to be effective in tumors in a p53-independent manner. In addition, there is a need to examine the effect of COP9 inactivation on normal cells, especially since gene targeting of COP9 subunits in mice (Csn2, Csn3, Csn5, Csn6 and Csn8) results in embryonic lethality.Citation3
Overall, this study by Lee and colleagues not only opens up the attractive possibility for targeting CSN6 for cancer therapy, it also highlights the important and complex functions of the CSN, which has come a long way from its originally described repressor function of photomorphogenesis in Arabidopsis to becoming an increasingly important player in human cancer.
References
- Wei N, et al. Trends Biochem Sci 2008; 33:592 - 600; http://dx.doi.org/10.1016/j.tibs.2008.09.004; PMID: 18926707
- Hannss R, et al. FEBS Lett 2011; 585:2845 - 52; http://dx.doi.org/10.1016/j.febslet.2011.04.027; PMID: 21510940
- Lee MH, et al. Cell Cycle 2011; 10:3057 - 66; http://dx.doi.org/10.4161/cc.10.18.17320; PMID: 21876386
- Chamovitz DA. EMBO Rep 2009; 10:352 - 8; http://dx.doi.org/10.1038/embor.2009.33; PMID: 19305390
- Zhao R, et al. J Clin Invest 2011; 121:851 - 65; http://dx.doi.org/10.1172/JCI44111; PMID: 21317535
- Xue Y, et al. Cell Cycle 2012; 11:4181 - 90; http://dx.doi.org/10.4161/cc.22413; PMID: 23095642
- Choi HH, et al. Oncogene 2011; 30:4791 - 801; http://dx.doi.org/10.1038/onc.2011.192; PMID: 21625211
- Chen B, et al. Cell Cycle 2012; 11:4633 - 41; http://dx.doi.org/10.4161/cc.22887; PMID: 23187808
- Lu Z, et al. Cell Cycle 2010; 9:2342 - 52; http://dx.doi.org/10.4161/cc.9.12.11988; PMID: 20519948