Abstract
Regulatory T cells (Tregs) that suppress tumor-specific T cell-mediated immune responses are the subject of an intense wave of investigation. We recently reported that a subset of Tregs, namely effector/memory CD25low cells, are responsible for suppressing high avidity tumor-specific T cells in mouse mammary tumors. Here, we discuss additional findings that clarify this mechanism of Treg-mediated immunosuppression.
Introduction
Regulatory T cells (Tregs) pose a significant barrier to the success of immunotherapeutic strategies aimed at activating high-avidity tumor-specific T-cells. Initial strategies to downmodulate the effect of Tregs targeted the interleukin 2 α (IL-2α) receptor CD25 with little success.Citation1,Citation2 We have previously shown that low-dose cyclophosphamide (Cy) given one day prior to a neu-targeted, whole cell, granulocyte macrophage colony-stimulating factor (GM-CSF)-secreting vaccine (neu-vaccine) leads to the depletion of CD4+FOXP3+CD25+ Tregs.Citation3 More recently, we have demonstrated that a subpopulation of CD25low effector/memory Tregs were particularly sensitive to Cy and preferentially suppressed high- vs. low-avidity HER-2/neu-specific T cells in the HER-2/neu transgenic (neu-N) mouse model of mammary carcinoma.Citation4 Here, we present companion data showing that anti-CD25 therapy preferentially affects CD25high Tregs, leaving CD25low effector/memory Tregs unaffected and capable of suppressing high-avidity T cells. These findings provide a possible mechanism for the lack of enhanced antitumor activity when CD25-targeted approaches are used to inhibit Tregs.
Materials and Methods
All assays, adoptive transfer studies, and data analyses were performed as in Ref,Citation4 except that the anti-CD25 antibody PC61 was given in place of cyclophosphamide at a dose of 150µg/mouse one day prior to vaccine. PC61 was purified from culture supernatants of the PC61.5.3 cell line (American Type Culture Collection) grown in Protein Free Hybridoma Medium II.Citation3
Results
To elucidate differences in the activation of tumor-specific CD8+ T cells with distinct avidities, we developed high- and low-avidity CD8+ T-cell receptor transgenic mice specific for the immunodominant epitope of HER-2/neu (RNEU420–429), which is expressed by mammary tumors in the neu-N mouse model of breast carcinoma. We specifically addressed the role played by Tregs in suppressing high- and low-avidity T-cells. Using adoptive transfer studies, we showed that in non-tolerant (parental FVB/N) mice, high- and low-avidity RNEU420–429-specific Tcells given with neu-vaccine accumulate in the tumor bed, but only high-avidity T cells cause tumor rejection. In neu-N mice, high-avidity T cells failed to accumulate (and function) in tumors, unless mice were pretreated with Cy and the neu-vaccine. On the contrary, low-avidity RNEU420–429-specific T cells do not invade the tumor microenvironment even in the presence of Cy plus the neu-vaccine. High avidity RNEU420–429-specific T cells transferred with Cy and neu-vaccine upregulate integrins, which are required for trafficking to the tumor site, and secrete high levels interferon γ. Importantly, we found that Cy selectively depletes a CD4+FOXP3+CD25low subpopulation of Tregs that displays an effector/memory phenotype similar to a Treg population that has been recently described.Citation4,Citation5
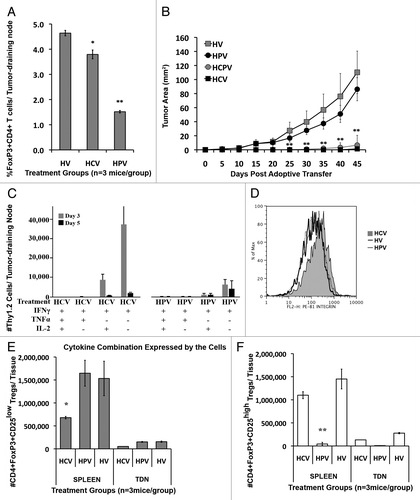
Companion data from simultaneous studies not published in ref. Citation4 evaluated the anti-CD25 antibody PC61 as another method for depleting Tregs. We previously showed that Cy given with PC61 and the neu-vaccine allowed for the systemic activation of endogenous high-avidity T cells in the neu-N model. However, in other models, PC61 given with vaccine was unable to elicit a significant anti-tumor response.Citation2,Citation6,Citation7 We therefore adoptively transferred high avidity RNEU420–429-specific T cells into tumor bearing neu-N mice receiving the neu-vaccine and either PC61, Cy, or Cy the PC61. When treated with PC61 plus the neu-vaccine, neu-N mice had significantly fewer CD4+FOXP3+ Tregs in their tumor draining lymph nodes than mice given Cy plus the neu-vaccine. However, this Treg depletion did not translate into tumor clearance as tumors in the mice treated with PC61 plus the neu-vaccine grew as quickly as tumors in mice given the vaccine and high-avidity T cells (a tumor permissive environment, ). When we compared the function of adoptively transferred high-avidity RNEU420–429-specific T cells in tumor draining nodes from mice treated with neu-vaccine plus either Cy or PC61, we found that mice given Cy plus the neu-vaccine plus high-avidity T cells had a significant population of T cells that secreted interferon γ, whereas mice given PC61 plus the neu-vaccine plus high avidity T cells had few, if any, such T cells (). In addition, high-avidity Tcells given with PC61 plus the neu-vaccine did not upregulate integrins or accumulate into the tumor bed (Fig. D). These data led us to suspect that there might be specific Treg subpopulations that are responsible for suppressing activated T cells at the tumor site that differ from those that operate in draining lymph nodes. Based on our data with PC61 depletion, we suspected that these subpopulations differed in CD25 expression. As suspected, Cy depleted mainly CD4+ FOXP3+CD25low Tregs, whereas PC61 treatment depleted only CD4+FOXP3+CD25high Tregs ().
Figure 1. Cyclophosphamide, not PC61, enhances the therapeutic effect of the neu-vaccine and high-avidity T-cell activity in neu-N mice. (A) PC61 depletes significantly more CD4+FOXP3+ T cells than cyclophosphamide (Cy) when given with neu-vaccine. The percent of CD4+FOXP3+ T cells in the tumor draining lymph nodes of neu-N mice challenged with NT2.5 tumor (5x104 cells on day 0) and treated with the neu-vaccine (3x106 cells on day 3) and high-avidity T cells (5x106 cells on day 4) (HV), with the addition of either 100 mg/kg Cy (HCV) or 150 µg/mouse PC61 (HPV) on day 2. * = p < 0.05, ** = p < 0.001. (B) Only Cy combined with high-avidity T cells and neu-vaccine treatment leads to tumor rejection in neu-N mice. Tumor growth in neu-N mice challenged with NT2.5 tumor (5x104 cells) and treated with high-avidity T cells (5x106 cells) and the neu-vaccine (3x106 cells) (HV) with the addition of either 100 mg/kg Cy (HCV), 150 µg/mouse PC61 (HPV), or Cy and PC61 combined (HCPV). ** = p < 0.001. (C) Cy plus neu-vaccine treatment leads to cytokine secretion by high-avidity T cells. High-avidity (Thy1.2) T cells from tumor draining lymph nodes on neu-N mice treated with high-avidity T cells, neu-vaccine, and Cy (HCV) or PC61 (HPV) were tested for their ability to secrete inflammatory cytokines 3 and 5 days after adoptive transfer. (D) Cy, not PC61, treatment leads to increased β1-integrin expression on high-avidity Tcells. High-avidity T cells isolated from tumor draining lymph nodes 3 d after adoptive transfer in neu-N mice treated with HCV, HPV, or HV. (E,F) Cy and PC61 preferentially deplete different subsets of CD25+ Tregs. The number of CD4+FOXP3+CD25low (E) or CD4+FOXP3+CD25high (F) Tregs in the spleen and tumor draining node (TDN) of neu-N mice treated with HCV, HPV, or HV. * = p < 0.05, ** = p < 0.001. All experiments were conducted at least 3 times (3 mice per group) with similar results.
Discussion
Here, we describe companion data to our recent publication “Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like Tregs,” in which we showed that the Treg-depleting antibody PC61 is unable to cause the same antitumor effects as the Cy-mediated depletion of Tregs. Even though PC61 treatment led to a greater decrease in CD4+FOXP3+CD25+ Tregs, high-avidity CD8+ T cells did not upregulate integrins, secrete interferon γ, or effect tumor killing after PC61-mediated Treg depletion. Though the possibility exists that PC61 might have depleted adoptively transferred T cells or activated CD4+ T cells, this is unlikely because the administration of Cy plus PC61 plus the neu-vaccine allowed for similar extents of tumor killing as the administration of Cy plus the neu-vaccine alone, and we previously reported that the combination of Cy plus PC61 plus the neu-vaccine is able to elicit endogenous high-avidity T-cell responses againt HER-2/neu.Citation7
Similar results showing the ineffectiveness of PC61 in melanoma and pancreas cancer models may be explained by the fact that PC61-like therapies fail to attack effector Tregs that traffic with regular effector CD8+ T cells into the tumor microenvironment. Recent reports have demonstrated that PC61-mediated Treg depletion effectively reduces tumor growth but only when given with lymphodepleting doses of temozolomide.Citation8 Altogether, these data highlight the complexity of Treg phenotypes, but also the fact that they can be effectively nullified to enhance tumor immunotherapy. Further elucidation of Treg subpopulations and their functions should lead to new ways to more efficiently deplete the Treg populations that de facto suppress the tumor-specific effector T cells while leaving in place populations that suppress non-specific T cell populations that are also activated by cancer immunotherapies.
| Abbreviations: | ||
| Treg | = | regulatory T cells |
| Cy | = | Cyclophosphamide |
| neu-vaccine | = | neu-targeted whole cell GM-CSF-secreting vaccine |
| neu-N | = | HER-2/neu transgenic |
Disclosure of Potential Conflicts of Interest
This work involves a GM-CSF-secreting vaccine. Through a licensing agreement with BioSante, Johns Hopkins has the potential to receive royalties in the future. None of the authors have financial interest in this work.
References
- Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res 2010; 16:5067 - 78; http://dx.doi.org/10.1158/1078-0432.CCR-10-1757; PMID: 20736326
- Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med 2008; 205:2125 - 38; http://dx.doi.org/10.1084/jem.20080099; PMID: 18725522
- Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med 2005; 201:1591 - 602; http://dx.doi.org/10.1084/jem.20042167; PMID: 15883172
- Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, et al. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS One 2012; 7:e31962; http://dx.doi.org/10.1371/journal.pone.0031962; PMID: 22359647
- Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med 2004; 199:303 - 13; http://dx.doi.org/10.1084/jem.20031562; PMID: 14757740
- Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci 2008; 1:228 - 39; http://dx.doi.org/10.1111/j.1752-8062.2008.00070.x; PMID: 20357913
- Uram JN, Black CM, Flynn E, Huang L, Armstrong TD, Jaffee EM. Nondominant CD8 T cells are active players in the vaccine-induced antitumor immune response. J Immunol 2011; 186:3847 - 57; http://dx.doi.org/10.4049/jimmunol.1000361; PMID: 21346233
- Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, et al. Monoclonal antibody blockade of IL-2 receptor α during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood 2011; 118:3003 - 12; http://dx.doi.org/10.1182/blood-2011-02-334565; PMID: 21768296