Abstract
Immune/inflammatory cells infiltrate almost all human solid tumors and affect all stages of carcinogenesis as they produce different cytokine subsets. The overproduction of TH17 cytokines marks the early stages of colorectal carcinoma (CRC) and negatively influences the prognosis of CRC patients. Studies with murine models of CRC have delineated the mechanisms by which TH17 cytokines, notably, interleukin (IL)-17A, IL-17F, IL-21, and IL-22, regulate oncogenesis and tumor progression, paving the way to the development of novel anticancer drugs. In this review article, we discuss experimental data supporting the role of TH17 cytokines in the modulation of colorectal tumorigenesis.
Introduction
Colorectal carcinoma (CRC) is one of the most common forms of malignancy and the second leading cause of cancer-related death in the western world.Citation1 The development of CRC is a multistage process characterized by a complex interaction between environmental carcinogens, genetic alterations, and the host immune system, ultimately resulting in the uncontrolled growth of transformed cells.Citation2 Like other common malignancies (e.g., hepatocellular carcinoma, prostate carcinoma, gastric cancer), chronic inflammation is an independent risk factor for the development of CRC.Citation3 Approximately 2% of CRC cases arise in patients with long-standing and extensive ulcerative colitis,Citation4 one of the 2 major forms of inflammatory bowel disease (IBD) in humans, while the majority of CRCs develops in individuals who are not affected IBD. However, even in these latter patients, the neoplastic tissue is massively infiltrated with immune/inflammatory cells, including CD4+ and CD8+ T lymphocytes, B cells, macrophages as well as natural killer (NK) cells, which can either foster or suppress the survival and growth of CRC cells, mostly through the production of cytokines.Citation5,Citation6 In this context, the production of interferon γ (IFNγ) by TH1 CD4+ cells, CD8+ cells, and NK cells has been demonstrated to limit tumor growth by activating cytotoxic immunity,Citation6 and the presence of TH1 polarization markers positively correlates with reduced tumor recurrence among CRC patients.Citation7 In contrast, cytokines produced by TH17 cells appear to exert pro-tumorigenic effects, and the presence of a TH17 immune cell infiltrate negatively influences the prognosis of CRC patients.Citation8,Citation9
In this article, we review data supporting a role for TH17 cytokines in the control of CRC.
TH17 Cells in Cancer
TH17 cells produce high amounts of interleukin (IL)-17, also termed IL-17A, and their developmental program is controlled by the transcription factor retinoic acid receptor-related orphan receptor (ROR)γt.Citation10 TH17 cells also synthesize IL-17F, IL-21, IL-22 and IL-26.Citation11,Citation12 However, not all TH17 cells secrete this cytokine cocktail, probably reflecting the heterogeneity of the TH17 cell subset. Moreover, some TH17 cytokines can be synthesized by other immune cell types, including innate lymphoid cells, NKT cells, CD8+ T lymphocytes and macrophages.Citation13-Citation16
In mice, TH17 cells differentiate from naïve T cells following activation in the presence of transforming growth factor β1 (TGFβ1) and IL-6, 2 cytokines that are sufficient to promote the expression of both RORγt and RORα, another transcription factor critical for TH17 differentiation.Citation17,Citation18 There is also evidence that the optimal expansion and maintenance of the TH17-cell response require IL-23 and the activation of the transcription factor signal transducer and activator of transcription 3 (STAT3).Citation19 The mechanism by which TH17 cells differentiate in humans is not yet fully understood, even though in vitro studies indicate that the activation of CD4+ T cells in the presence of either IL-1β and IL-6, or TGFβ1 and IL-21 induces the secretion of IL-17A.Citation20,Citation21 However, the TH17 cell lineage displays a great degree of context-dependent plasticity and can acquire functional features of TH1 cells in response to IL-12 signaling.Citation22,Citation23
Beside an important role in the host defense against extracellular microorganisms, TH17 cells participate in the pathogenesis of diverse immune-mediated diseases, including IBD, psoriasis and rheumatoid arthritis.Citation24 Compelling evidence indicates that TH17 cells are also involved in the control of tumorigenesis and TH17 cytokines have been ascribed with either a positive or a negative effect on the survival and growth of malignant cells depending on the experimental model.Citation25,Citation26 The reason why TH17 cells have a dual role in the control of tumorigenesis is unknown, even though it is conceivable that their capacity to differentiate into tumoricidal TH1-like cells under specific conditions can contribute to the antitumor effect mediated by TH17 cells in some settings.
Role of TH17 Cytokines in CRC
IL-17A-producing CD4+ T lymphocytes and macrophages infiltrate human CRCs and the density of these cell type correlates with a poor prognosis.Citation27 CD4+ T cells co-expressing RORγt and the regulatory T cell-associated transcription factor forkhead box P3 (FOXP3) and producing IL-17A are abundant in human CRCs as well as in the intestine of polyp-ridden Apcmin/+ mice.Citation28 These cells exert potent immunosuppressive functions and are supposed to sustain oncogenesis and tumor progression.Citation28 Consistently, many studies have shown that IL-17A, IL-17F, IL-21, and IL-22 are overexpressed at both the mRNA and protein level in human CRCs as compared with the non-transformed, adjacent colonic mucosa.Citation29-Citation32 Moreover, CRC patients harboring elevated levels of IL-17A and RORγt in neoplastic tissues exhibit a drastic reduction in disease-free survival.Citation33 Studies in mouse models of CRC support the pro-tumorigenic role of TH17 cytokines. However, as discussed below, IL-17A, IL-17F, and IL-22 can also exert anti-tumorigenic effects under specific circumstances (; ).
Figure 1. Overview of the role of TH17 cytokines and downstream signaling pathways in colorectal carcinoma. Abbreviations: IL, interleukin; STAT3, signal transducer and activator of transcription 3; PGE2, prostaglandin E2; VEGF, vascular endothelial growth factor; TNFα, tumor necrosis factor α
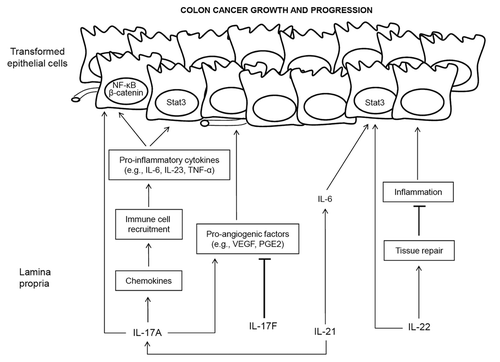
Table 1. Role of TH17 cytokines in mouse models of colorectal carcinoma
IL-17A and IL-17F
IL-17A and IL-17F can be produced by immune cells other than TH17 cells, including innate lymphoid cells, γδ T cells, NKT cells, neutrophils, and eosinophils.Citation16 Both IL-17A and IL-17F signal through the IL-17 receptor A (IL-17RA), an ubiquitously expressed type I transmembrane proteinCitation34 that triggers the mitogen activated protein kinase (MAPK) and NFκB signaling pathways, hence stimulating the production of pro-inflammatory cytokines, chemokines, and prostaglandins.Citation34
Early studies in mice subjected to the subcutaneous implantation of CRC cells showed opposite roles of IL-17A in the modulation of tumor growth in vivo. Numasaki et al. reported that IL-17A ectopically overexpressed by the murine CRC cell line MC38 enhances the growth of cancer cells in vivo and increases tumor vascularity by promoting the production of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and prostaglandin E2 (PGE2).Citation35 In contrast, Kryczek and coworkers revealed an increased growth and enhanced metastatic potential for MC38 cells implanted in IL-17A-deficient mice as compared with wild-type mice.Citation36 These effects were associated with reduced numbers of interferon γ (IFNγ)-producing NK cells and CD8+ T cells within neoplastic lesions and tumor-draining lymph nodes.Citation36 Different results were obtained by Ngiow and colleagues, who showed that MC38 cells proliferate to similar extents in IL-17A-deficient and -proficient mice.Citation37 More recent studies have investigated the role of IL-17A in genetic, chemical-driven or microbial CRC models. Bothwell’s group showed that the ablation of Il17a reduces tumor formation in mice bearing a heterozygote mutation in the adenomatous polyposis coli (Apc) gene (Apcmin/+ mice) and this effect is accompanied by a decrease of intratumoral immune cells and pro-inflammatory cytokines (e.g., IL-6, IL-23).Citation38 Wu and colleagues showed that the infection of Apcmin/+ mice with the human colonic bacterium enterotoxigenic Bacteroides fragilis (ETBF) induces colitis and causes oncogenesis in association with a marked TH17 response and activation of STAT3 in malignant cells. Blockade of IL-17A inhibits ETBF-induced colitis and tumor formation without affecting the activity or subcellular localization of STAT3, suggesting that STAT3 activation precedes the induction of IL-17A in the tumorigenic cascade of events activated in this model.Citation39
Using a model of colitis-associated CRC as induced by the sequential administration of the carcinogen azoxymethane (AOM) and dextran sodium sulfate (DSS), Hyun et al. showed that IL-17A-deficient mice express reduced levels of IL-6, STAT3, IFNγ and tumor necrosis factor α (TNFα) and develop milder colitis as well as fewer and smaller tumors than wild-type mice. Moreover, IL-17A-deficient mice exhibit reduced numbers of β catenin+ cells within the intestinal crypts as well as reduction of key cell-cycle regulators (e.g., cyclin D1, cyclin-dependent kinase 2), suggesting a role for IL-17A not only in oncogenesis but also in tumor progression.Citation40 In contrast, IL-17F-deficient mice appear to develop more neoplastic lesions than wild-type mice upon the administration of AOM and DSS.Citation30 In this latter model, the lack of IL-17F is associated with the upregulation of VEGF and an increase in CD31+ cells. Altogether, these data suggest that IL-17A exerts pro-tumorigenic functions while IL-17F has a protective role in colitis-associated colon carcinogenesis. The hypothesis that IL-17F mediated antitumor effects is also supported by the observation that IL-17F-overexpressing CRC cells proliferate less vigorously than control cells when transplanted into immunodeficient mice.Citation30
Of note, Apcmin/+ mice can be genetically modified to develop tumors primarily in the distal colon (CPC-APC model).Citation41 The neoplastic lesions that develop in CPC-APC mice exhibit a marked upregulation of IL-17A and IL-17F and both tumor multiplicity and growth are reduced in mice lacking IL-17RA.Citation42 As mentioned above, both IL-17A and IL-17F signal via IL-17RA. Thus, the precise mechanism whereby IL-17A and IL-17F contribute to colorectal carcinogenesis remains unclear.
IL-21
IL-21 is a pleiotropic cytokine produced by activated CD4+ T cells, TH17 cells, NKT cells and follicular helper T cells. IL-21 signals through a receptor comprising the γ chain subunit (shared with IL-2, IL-4, IL-7, IL-9, and IL-15) and the private IL-21 receptor (IL-21R) chain.Citation15 The binding of IL-21 to its receptor complex, which is expressed by epithelial cells and monocytes, activates STAT3 and to a lesser degree STAT1 and STAT5, MAPK, and NFκB.Citation43 Pre-clinical and clinical studies have shown that IL-21 exerts potent antitumor effects owing to its ability to expand cytotoxic immune responses.Citation44 Conversely, IL-21 has been attributed a pathogenic role in many immune-mediated diseases, including IBD.Citation43,Citation45 Indeed, IL-21 is overproduced in the colonic mucosa of both ulcerative colitis and Crohn’s disease patients, where it positively regulates TH1 and TH17 responses.Citation46,Citation47 Increased IL-21 production is also found in the neoplastic tissues of patients with both sporadic and ulcerative colitis-associated CRC.Citation29 We showed that IL-21 production is increased in neoplastic lesions of AOM/DSS-treated mice while IL-21-null mice display reduced colonic inflammation and limited tumor burden compared with wild-type mice.Citation29 The analysis of the mechanisms underlying this effect revealed that IL-21 sustains the infiltration of neoplastic lesions by CD4+ T cells, the local production of IL-6 and IL-17A, as well as STAT3 activation.Citation29 In line with these observations, Jauch and coworkers reported a reduced expression of IL-17A, increased IFNγ levels, limited epithelial cell proliferation and consistent degrees of apoptosis in the intestinal tumors of IL-21-deficient mice subjected to AOM/DSS administration compared with wild-type mice.Citation48 Interestingly, the neutralization of IL-21 after the induction of colitis still reduces tumor burden, suggesting that the tumor-promoting effect of IL-21 in this model is not dependent on the inhibition of inflammation.Citation29 At present, it remains unclear whether IL-21 also regulates the development of sporadic CRC.
IL-22
IL-22 is produced by TH17 cells, TH22 cells as well as innate lymphoid cells, and binds to a heterodimeric receptor complex composed of IL-22 receptor 1 (IL-22R1) and IL-10 receptor 2 (IL-10R2), resulting in the activation of STAT1, STAT3, and STAT5.Citation13 IL-22 can also activate multiple MAPK family members, at least in some circumstances.Citation49 While IL-10R2 is ubiquitously expressed, IL-22R1 is only found on epithelial cells, including human CRC cells.Citation50 Notably, IL-22 can be neutralized by a soluble IL-22 receptor known as IL-22 binding protein (IL-22BP), which prevents the binding of IL-22 to membrane-bound IL-22R1.Citation51-Citation53 IL-22 not only promotes the secretion of mucous and defensins but also stimulates cell proliferation and is supposed to play a major role in tissue repair and oncogenesis in the gut.Citation54 Jiang et al. showed that high levels of IL-22 promote the growth of human CRC cells (i.e., HCT-116) transplanted into immunodeficient mice, and linked this effect to STAT3 activation and the upregulation of STAT3 downstream effectors like cyclin D1.Citation31
Huber and colleagues documented accelerated AOM/DSS-driven tumorigenesis (increased number and size of lesions) in IL-22BP-deficient mice as compared with wild-type mice.Citation55 Time-course experiments in mice lacking either IL-22 or IL-22BP demonstrated that the negative effect of IL-22BP on CRC cell growth is strictly dependent on the inhibition of IL-22.Citation55 Surprisingly, IL-22-null mice developed a higher tumor load than wild-type mice.Citation55 This latter finding might reflect the fact that IL-22 deficiency delays colonic repair and increases intestinal inflammation, thereby accelerating tumor progression. A different scenario emerged in Apcmin/+ mice, as the lack of IL-22BP enhanced intestinal tumorigenesis, while IL-22-deficient Apcmin/+ mice showed a reduced tumor burden as compared with their IL-22-proficient counterparts.Citation55
A pro-tumorigenic role of IL-22 has also been reported in genetically susceptible 129SvEv.RAG-deficient mice, in which chronic colitis is caused by infection with Helicobacter hepaticus and colorectal tumorigenesis is stimulated with AOM. In these mice, the antibody-mediated neutralization of IL-22 selectively decreases STAT3 activation in epithelial cells and reduces tumor burden.Citation56 Altogether, these observations are consistent with a recent findings suggesting an association between a genetic polymorphism in IL22 and an increased risk of developing CRC.Citation57
Conclusions
Elevated levels of pro-inflammatory TH17 cytokines are found in human CRCs, and numerous studies in mice show a key role for these cytokines in facilitating the survival and growth of CRC cells Accordingly, TH17 cytokine blockers have been ascribed with robust antineoplastic effects in several CRC models. In this context, it is noteworthy that TH17 cytokines may have redundant effects on the activation of pathways that sustain colorectal carcinogenesis and tumor progression, suggesting that the inhibition of downstream effector molecules such as STAT3 could be more advantageous, from a therapeutic perspective, than the blockage of individual TH17 cytokines. This hypothesis is further supported by the observation that STAT3 is activated by other cytokines that are overexpressed by human CRCsCitation58,Citation59 and are known to exert pro-tumorigenic effects in mouse models of colon cancer, such as IL-6 and IL-11.Citation60-Citation63
In designing therapeutic interventions targeting TH17 cytokines, one should take into consideration that, at least in mice, IL-17A, IL-17F, and IL-22 can mediate antitumor effects, at least in specific circumstances, raising the possibility that the neutralization of such cytokines may have deleterious, rather than beneficial, effects. Moreover, since TH17 cytokines are key players in the host defense against intracellular pathogens, TH17 cytokine inhibitors can enhance the risk of infection. Further work is needed to clarify these issues as well as to ascertain which TH17 cytokine is specifically upregulated in patients affected by specific types of CRC (e.g., sporadic vs. colitis-associated) and whether this occurs at specific stages of the disease (e.g., early vs. advanced disease). It would be also relevant to determine whether the excessive production of TH17 cytokines in CRC is linked to genetic polymorphisms and if the genotyping of TH17 cytokine-coding genes can be useful to identify individuals at high risk for developing CRC and/or to monitor the course of disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work received support from the “Fondazione Umberto di Mario ONLUS,” Rome, AIRC (MFAG-12108 to CS and IG-13049 to GM), and Giuliani SpA, Milan, Italy.
Citation: De Simone V, Pallone F, Monteleone G, Stolfi C. Role of TH17 cytokines in the control of colorectal cancer. OncoImmunology 2013; 2:e26617; 10.4161/onci.26617
References
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin 2009; 59:366 - 78; http://dx.doi.org/10.3322/caac.20038; PMID: 19897840
- Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 2010; 138:2101 - 14, e5; http://dx.doi.org/10.1053/j.gastro.2010.01.058; PMID: 20420949
- Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001; 91:854 - 62; http://dx.doi.org/10.1002/1097-0142(20010215)91:4<854::AID-CNCR1073>3.0.CO;2-Z; PMID: 11241255
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001; 48:526 - 35; http://dx.doi.org/10.1136/gut.48.4.526; PMID: 11247898
- Lança T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology 2012; 1:717 - 25; http://dx.doi.org/10.4161/onci.20068; PMID: 22934263
- Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci 2012; 13:11071 - 84; http://dx.doi.org/10.3390/ijms130911071; PMID: 23109839
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960 - 4; http://dx.doi.org/10.1126/science.1129139; PMID: 17008531
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298 - 306; http://dx.doi.org/10.1038/nrc3245; PMID: 22419253
- Fridman WH, Galon J, Pagès F, Tartour E, Sautès-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011; 71:5601 - 5; http://dx.doi.org/10.1158/0008-5472.CAN-11-1316; PMID: 21846822
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006; 126:1121 - 33; http://dx.doi.org/10.1016/j.cell.2006.07.035; PMID: 16990136
- Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol 2007; 19:362 - 71; http://dx.doi.org/10.1016/j.smim.2007.10.007; PMID: 18035554
- Donnelly RP, Sheikh F, Dickensheets H, Savan R, Young HA, Walter MR. Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev 2010; 21:393 - 401; http://dx.doi.org/10.1016/j.cytogfr.2010.09.001; PMID: 20947410
- Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev 2010; 21:365 - 79; http://dx.doi.org/10.1016/j.cytogfr.2010.08.002; PMID: 20870448
- Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol 2001; 167:4137 - 40; PMID: 11591732
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol 2008; 26:57 - 79; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090316; PMID: 17953510
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med 2009; 361:888 - 98; http://dx.doi.org/10.1056/NEJMra0707449; PMID: 19710487
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 2008; 28:29 - 39; http://dx.doi.org/10.1016/j.immuni.2007.11.016; PMID: 18164222
- Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol 2008; 9:1297 - 306; http://dx.doi.org/10.1038/ni.1663; PMID: 18849990
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; 8:967 - 74; http://dx.doi.org/10.1038/ni1488; PMID: 17581537
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 2008; 454:350 - 2; http://dx.doi.org/10.1038/nature07021; PMID: 18469800
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007; 8:942 - 9; http://dx.doi.org/10.1038/ni1496; PMID: 17676045
- Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol 2010; 11:674 - 80; http://dx.doi.org/10.1038/ni.1899; PMID: 20644573
- Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol 2009; 21:274 - 80; http://dx.doi.org/10.1016/j.coi.2009.05.021; PMID: 19524429
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev 2008; 223:87 - 113; http://dx.doi.org/10.1111/j.1600-065X.2008.00628.x; PMID: 18613831
- Greten TF, Zhao F, Gamrekelashvili J, Korangy F. Human Th17 cells in patients with cancer: Friends or foe?. Oncoimmunology 2012; 1:1438 - 9; http://dx.doi.org/10.4161/onci.21245; PMID: 23243621
- Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance?. Carcinogenesis 2011; 32:643 - 9; http://dx.doi.org/10.1093/carcin/bgr019; PMID: 21304053
- Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun 2011; 407:348 - 54; http://dx.doi.org/10.1016/j.bbrc.2011.03.021; PMID: 21396350
- Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med 2012; 4:ra159; http://dx.doi.org/10.1126/scitranslmed.3004566; PMID: 23241743
- Stolfi C, Rizzo A, Franzè E, Rotondi A, Fantini MC, Sarra M, Caruso R, Monteleone I, Sileri P, Franceschilli L, et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med 2011; 208:2279 - 90; http://dx.doi.org/10.1084/jem.20111106; PMID: 21987656
- Tong Z, Yang XO, Yan H, Liu W, Niu X, Shi Y, Fang W, Xiong B, Wan Y, Dong C. A protective role by interleukin-17F in colon tumorigenesis. PLoS One 2012; 7:e34959; http://dx.doi.org/10.1371/journal.pone.0034959; PMID: 22509371
- Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, Gao Y, Yao A, Wang X, Yu L, et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 2013; 13:59; http://dx.doi.org/10.1186/1471-2407-13-59; PMID: 23379788
- Cui G, Yuan A, Goll R, Florholmen J. IL-17A in the tumor microenvironment of the human colorectal adenoma-carcinoma sequence. Scand J Gastroenterol 2012; 47:1304 - 12; http://dx.doi.org/10.3109/00365521.2012.725089; PMID: 22989213
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263 - 71; http://dx.doi.org/10.1158/0008-5472.CAN-10-2907; PMID: 21303976
- Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 2003; 14:155 - 74; http://dx.doi.org/10.1016/S1359-6101(03)00002-9; PMID: 12651226
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003; 101:2620 - 7; http://dx.doi.org/10.1182/blood-2002-05-1461; PMID: 12411307
- Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 2009; 114:357 - 9; http://dx.doi.org/10.1182/blood-2008-09-177360; PMID: 19289853
- Ngiow SF, Smyth MJ, Teng MW. Does IL-17 suppress tumor growth?. Blood 2010; 115:2554 - 5, author reply 2556-7; http://dx.doi.org/10.1182/blood-2009-11-254607; PMID: 20339108
- Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A 2010; 107:5540 - 4; http://dx.doi.org/10.1073/pnas.0912675107; PMID: 20212110
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009; 15:1016 - 22; http://dx.doi.org/10.1038/nm.2015; PMID: 19701202
- Hyun YS, Han DS, Lee AR, Eun CS, Youn J, Kim HY. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis 2012; 33:931 - 6; http://dx.doi.org/10.1093/carcin/bgs106; PMID: 22354874
- Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res 2007; 67:9721 - 30; http://dx.doi.org/10.1158/0008-5472.CAN-07-2735; PMID: 17942902
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012; 491:254 - 8; PMID: 23034650
- Monteleone G, Pallone F, Macdonald TT. Interleukin-21 as a new therapeutic target for immune-mediated diseases. Trends Pharmacol Sci 2009; 30:441 - 7; http://dx.doi.org/10.1016/j.tips.2009.05.006; PMID: 19616319
- Søndergaard H, Skak K. IL-21: roles in immunopathology and cancer therapy. Tissue Antigens 2009; 74:467 - 79; http://dx.doi.org/10.1111/j.1399-0039.2009.01382.x; PMID: 19845910
- Stolfi C, Pallone F, Macdonald TT, Monteleone G. Interleukin-21 in cancer immunotherapy: Friend or foe?. Oncoimmunology 2012; 1:351 - 4; http://dx.doi.org/10.4161/onci.19122; PMID: 22737612
- Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, Tersigni R, Alessandroni L, Biancone L, Naccari GC, et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology 2005; 128:687 - 94; http://dx.doi.org/10.1053/j.gastro.2004.12.042; PMID: 15765404
- Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 2008; 134:1038 - 48; http://dx.doi.org/10.1053/j.gastro.2008.01.041; PMID: 18395085
- Jauch D, Martin M, Schiechl G, Kesselring R, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Interleukin 21 controls tumour growth and tumour immunosurveillance in colitis-associated tumorigenesis in mice. Gut 2011; 60:1678 - 86; http://dx.doi.org/10.1136/gutjnl-2011-300612; PMID: 21948944
- Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 2002; 277:33676 - 82; http://dx.doi.org/10.1074/jbc.M204204200; PMID: 12087100
- Ziesché E, Bachmann M, Kleinert H, Pfeilschifter J, Mühl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem 2007; 282:16006 - 15; http://dx.doi.org/10.1074/jbc.M611040200; PMID: 17438334
- Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol 2001; 166:7096 - 103; PMID: 11390454
- Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol 2001; 166:7090 - 5; PMID: 11390453
- Wei CC, Ho TW, Liang WG, Chen GY, Chang MS. Cloning and characterization of mouse IL-22 binding protein. Genes Immun 2003; 4:204 - 11; http://dx.doi.org/10.1038/sj.gene.6363947; PMID: 12700595
- Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis 2012; 18:1777 - 84; http://dx.doi.org/10.1002/ibd.22929; PMID: 22359410
- Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W Jr., Murphy AJ, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012; 491:259 - 63; http://dx.doi.org/10.1038/nature11535; PMID: 23075849
- Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 2013; 210:917 - 31; http://dx.doi.org/10.1084/jem.20122308; PMID: 23589566
- Thompson CL, Plummer SJ, Tucker TC, Casey G, Li L. Interleukin-22 genetic polymorphisms and risk of colon cancer. Cancer Causes Control 2010; 21:1165 - 70; http://dx.doi.org/10.1007/s10552-010-9542-5; PMID: 20339910
- Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci 2012; 8:1248 - 53; http://dx.doi.org/10.7150/ijbs.4614; PMID: 23136553
- Yoshizaki A, Nakayama T, Yamazumi K, Yakata Y, Taba M, Sekine I. Expression of interleukin (IL)-11 and IL-11 receptor in human colorectal adenocarcinoma: IL-11 up-regulation of the invasive and proliferative activity of human colorectal carcinoma cells. Int J Oncol 2006; 29:869 - 76; PMID: 16964382
- Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 2008; 294:R393 - 401; http://dx.doi.org/10.1152/ajpregu.00716.2007; PMID: 18056981
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009; 15:103 - 13; http://dx.doi.org/10.1016/j.ccr.2009.01.001; PMID: 19185845
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 2004; 21:491 - 501; http://dx.doi.org/10.1016/j.immuni.2004.07.020; PMID: 15485627
- Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, Nguyen PM, Preaudet A, Farid R, Edwards KM, et al. Interleukin-11 Is the Dominant IL-6 Family Cytokine during Gastrointestinal Tumorigenesis and Can Be Targeted Therapeutically. Cancer Cell 2013; 24:257 - 71; http://dx.doi.org/10.1016/j.ccr.2013.06.017; PMID: 23948300