Abstract
Dendritic cells (DCs) are highly potent initiators of adaptive immune responses and, as such, represent promising tools for immunotherapeutic applications. Despite their potential, the current efficacy of DC-based immunotherapies is poor. CD137 ligand (CD137L) signaling has been used to derive a novel type of DCs from human peripheral blood monocytes, termed CD137L-DCs. Here, we report that CD137L-DCs induce more potent cytotoxic T-cell responses than classical DCs (cDCs). Furthermore, in exploring several DC maturation factors for their ability to enhance the potency of CD137L-DCs, we found the combination of interferon γ (IFNγ) and the mixed Toll-like receptor (TLR)7/8 agonist R848, to display the highest efficacy in potentiating the T-cell co-stimulatory activity of CD137L-DCs. Of particular importance, CD137L-DCs were found to be more efficient than cDCs in activating autologous T cells targeting the cytomegalovirus (CMV)-derived protein pp65. Specifically, CD137L-DC-stimulated T cells were found to secrete higher levels of IFNγ and killed 2–3 times more HLA-matched, pp65-pulsed target cells than T cells activated by cDCs. Finally, in addition to stimulating CD8+ T cells, CD137L-DCs efficiently activated CD4+ T cells. Taken together, these findings demonstrate the superior potency of CD137L-stimulated DCs in activating CMV-specific, autologous T cells, and encourage the further development of CD137L-DCs for antitumor immunotherapy.
Introduction
Dendritic cells (DCs) are crucial initiators of adaptive immune responses. Several heterogeneous subsets of DCs have been previously characterized, mainly distinguished on the basis of cellular origin, phenotypic profile, mechanism of pathogen recognition, biological function, and tissue localization.Citation1-Citation3 These include a subset of monocyte-derived DCs that arise from peripheral blood in response to inflammatory stimuli, and support tissue-resident DCs in activating naïve T cells.Citation4 Such monocyte-derived DCs have been shown to be essential for mounting a protective immune response against Leishmania major in mice.Citation5 Similarly, monocyte-derived DCs were found to be pivotal in generating protective TH1 responses against lepromatous leprosy.Citation6
The classical protocol for generating DCs from monocyte precursors in vitro involves the step-wise differentiation of monocytes to DCs with granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4, followed by their maturation with lipopolysaccharide (LPS).Citation7 Numerous inflammatory conditions can induce monocytes to differentiate to DCs, and the resultant monocyte-derived DCs exhibit unique biological activities that at least in part depends on the differentiation stimuli.Citation8,Citation9 Classical DCs (cDCs) are being used successfully in the clinic as a form of anticancer immunotherapy.Citation10-Citation12 However, the response rate of patients to DC-based therapies remains low.Citation13 Thus, developing methods to generate potent DCs may translate into higher response rates and robust therapeutic benefits for cancer patients.
Two recent studies have established a novel method for generating human DCs with an enhanced immunogenic potential. CD137 ligand (CD137L) is expressed on the surface of antigen-presenting cells (APCs), including DCs and their precursors, and crosslinking CD137L on monocytes by exogenously applying recombinant CD137 or an agonistic anti-CD137L antibody induces their differentiation to DCs. These CD137L-derived DCs (CD137L-DCs) have been shown to robustly activate T cells, leading to increased cytokine secretion and strong T-cell proliferative responses in allogeneic mixed lymphocyte reactions (MLRs) as compared with cDCs.Citation14,Citation15
It has previously been shown that the interaction between CD137L and CD137, which is expressed on the surface of T cells, potently enhances T-cell activation.Citation16-Citation19 Concurrently, CD137L transduces a signal to APCsCitation20 that induces their differentiation to CD137L-DCs.Citation14,Citation15 Although these studies reported promising findings on new methods to generate DCs, it remains unclear whether CD137L-DCs can either evoke improved T-cell responses or have a superior potency in an autologous setting. The present study was undertaken to address these outstanding questions. In brief, using the cytomegalovirus (CMV)-derived protein pp65 as a model antigen, we demonstrated that CD137L-DCs induce an abundant secretion of interferon γ (IFNγ) and IL-13 from autologous pp65-specific T cells, endowing them with a robust cytotoxic potential toward HLA-matched, pp65-pulsed target cells.
Results
CD137L-stimulated DCs enhance the cytotoxicity of allogeneic CD8+ T cells
Allogeneic CD8+ T cells co-cultured with CD137L-DCs have previously been shown to express higher levels of perforin than cDCs exposed to LPS and IFNγ, suggesting that CD137L-DCs may be more potent effectors than mature cDCs at inducing cytotoxic T-cell functions.Citation14
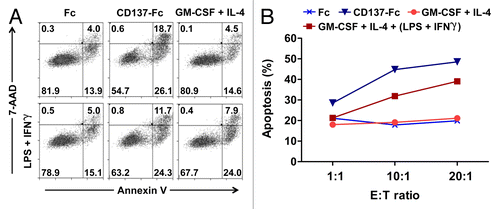
In order to assess this assumption, we compared co-cultures of allogeneic CD8+ T cells and CD137L-DCs or other APCs, including cDCs. Monocytes were pretreated for 7 d with either an immobilized variant of CD137 fused to a Fc fragment (CD137-Fc) to generate CD137L-DCs, or the Fc fragment alone, to generate control cells. For comparison, GM-CSF and IL-4 were used to generate immature cDCs, some of which were subsequently matured with LPS plus IFNγ for the final 18 h of culture. The efficacy of these differentially derived APCs was assayed by MLRs with allogeneic CD8+ T cells for additional 5 d, followed by the co-culture of T cells as effector cells (E) with carboxyfluorescein succinimidyl ester (CFSE)-labeled K562 target (T) cells (overnight). K562 cells were then stained with AnnexinV and 7-aminoactinomycin D (7-AAD) to determine the percentage of apoptotic demise.
CD137L-DCs proved to be the most potent inducers of the cytotoxic activity of CD8+ T cells. As shown in , at an E:T cell ratio of 10:1, CD137L-DC-primed CD8+ T cells induced apoptosis in nearly 45% of K562 cells (26% early-stage and 19% late-stage apoptosis). In contrast, only 32% (24% early-stage and 8% late-stage apoptosis) of K562 cells were apoptotic when the effector T cells were primed by mature cDCs (). As expected, the CD8+ T cells primed by control monocytes (exposed to the Fc only) or immature cDCs were the least cytotoxic, as only 18–19% of the corresponding K562 cells were apoptotic. A similar trend was observed at an E:T cell ratio of 20:1, although no marked difference in the percentage of apoptotic cells was observed at an E:T cell ratio of 1:1 (). Taken together, these data demonstrate that DCs arising from CD137L-stimulated monocytes can be more potent stimulators of T-cell cytotoxicity than classically-derived DC. However, it should be noted that since K562 cells are devoid of MHC class I molecules their death does not reflect the MHC/peptide complex-dependent cytolytic activity of CD8+ T cells but rather constitute a bystander effect of other cytotoxic mechanisms.
Figure 1. Dendritic cells derived in the presence of CD137L signaling enhance the cytolytic activity of CD8+ T cells. (A and B) Monocytes that had been pretreated with the differentiation (or control) stimulus indicated were co-cultured with CD8+ T cells. After 5 d T cells were harvested and co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled K562 target cells at effector to target (E:T) cell ratios of 1:1, 10:1, and 20:1. After overnight co-culture, cells were harvested and the percentage of apoptosis was determined by staining with AnnexinV and 7-aminoactinomycin D (7-AAD). Panel A reports a representative dot plot of apoptotic CFSE-cells at 10:1 E:T ratio. In panel B, the percentages of apoptotic cells at different E:T ratios are indicated. GM-CSF, granulocyte macrophage colony-stimulating factor; IFNγ, interferon γ; IL-4, interleukin-4; LPS, lipopolysaccharide.
Maturation of CD137L-DCs
Contrary to commonly used DC maturation factors (i.e., LPS and IFNγ) which give rise to CD137L-DCs that are unable to enhance CD83 expression (a DC maturation marker) and are incapable of further enhancing T-cell proliferation in allogeneic MLRs, the CD137-Fc fusion protein alone has been shown to be sufficient to generate mature and biologically potent DCs.Citation14 Here, we independently confirm the ability of CD137L-DCs to generate improved cytotoxic CD8+ T-cell responses as compared with mature cDCs ().
Nevertheless, the expression of the DC differentiation markers CD80, CD86, CCR7 and HLA-DR on CD137L-DCs could be further increased upon exposure to LPS and IFNγ14. This implied that the potency of CD137L-DCs can potentially be enhanced by maturation, but it was unclear whether factors other than, or in addition to, LPS and IFNγ would be required. Since our ultimate goal was to explore the utility of CD137L-stimulated DCs for use in cancer immunotherapy, we decided to prospectively evaluate candidate agents that could potentially enhance the T-cell stimulatory capacity of CD137L-DCs. To this aim, we assayed a range of well-established maturation factors in different combinations ().Citation21-Citation23 cDCs generated by GM-CSF plus IL-4 and matured in the presence IL-1β, IL-6, tumor necrosis factor α (TNFα) and prostaglandin E2 (PGE2), a combination of agents that in we referred to as cocktail C2, are currently considered the “gold standard” in immunotherapy and so were used as the benchmark for comparing the activity of various CD137L-DC-maturing candidates.Citation21
Table 1. Cytokine combinations employed for the maturation of CD137L-stimulated DCs
In brief, CD137L-DCs, generated as previously described from peripheral monocytes by a 6 d culture on immobilized CD137-Fc, were treated for 18 h with 7 different maturation cocktails. In basal conditions, the DC maturation marker CD83 was expressed on 39% of untreated (C0) CD137L-DCs, with a mean-fluorescence intensity (MFI) of 66 (arbitrary units). The expression of CD83 was significantly enhanced by treatment with cocktails C3 (64% CD83+ CD137L-DCs, MFI = 108) and C4 (58% CD83+ CD137L-DCs, MFI = 100). In contrast, C1 only moderately increased (58% CD83+ CD137L-DCs, MFI = 75) whereas cocktails C2, C5, C6, and C7 had no effect on, CD83 expression (). A similar profile was observed for the expression of the co-stimulatory molecule CD86, such that 43% of untreated CD137L-DCs expressed CD86 (MFI = 103), while exposure to cocktails C1, C3, or C4 led to a considerable upregulation of CD86 (69% CD86+ CD137L-DCs, MFI = 178; 57% CD86+ CD137L-DCs, MFI = 165; 53% CD86+ CD137L-DCs, MFI = 139, respectively). Finally, only a slight increase in CD86 expression was observed in response to C2 (55% CD86+ CD137L-DCs, MFI = 122) ().
Figure 2. Maturation of CD137L-derived dendritic cells. (A–C) Monocytes were treated with immobilized CD137-Fc for 6 d and then exposed to the indicated maturation cocktails (see also ) for 18 h, followed by a phenotypic and functional characterization. (A) Cells were immunostained for the CD83, CD86, and HLA-DR cell-surface expression and analyzed by fluorescence cytometry. Unshaded and gray histograms represent the unstained (control) and stained samples, respectively. Values associated with each histogram indicate the percentages of positive cells and mean fluorescence intensity (MFI). Bar charts on the right report the mean percentage of positive cells and mean MFI ± SD of data acquired from 2 different donors. (B) Upon 18 h of treatment with cocktails C0, C1, C3, or C4, CD137L-derived dendritic cells (CD137L-DCs) were co-cultured for additional 5 d with carboxyfluorescein succinimidyl ester (CFSE)-stained allogeneic T cells at a ratio of 1:10. T-cell proliferation was then quantified by fluorescence cytometry based on CFSE dilution. Numbers in the histograms and bar charts represent percentages of proliferating cells and CFSE MFI. (C) Supernatants from the co-cultures of mature CD137L-DCs and allogeneic T cells were harvested and the levels of interferon γ (IFNγ) were quantified by ELISA. Means ± SD of triplicate measurements are depicted. **P < 0.01 (two-tailed, unpaired Student t test). This experiment has been performed independently twice, yielding with comparable results. PHA, phytohemagglutinin; Rst, resting.
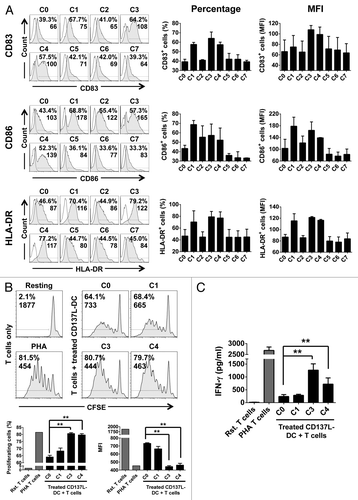
The activation of antigen-specific autologous CD4+ T cells relies on the presentation of antigenic epitopes in complex with MHC class II molecules. Thus, finding that CD137L-DCs express relatively low levels of MHC class II molecules was surprising, as these cells still possess the ability to activate CD4+ T cells.Citation14 With this in mind, we hypothesized that an increase in MHC class II expression on the surface of CD137L-DCs could potentially boost their ability to activate CD4+ T cells. We tested this hypothesis and found that the treatment of CD137L-DCs with cocktails C1, C3, or C4 markedly increased the expression of HLA-DR on the cell surface (70% HLA-DR+ CD137L-DCs, MFI = 116; 79% HLA-DR+ CD137L-DCs, MFI = 122; 77% HLA-DR+ CD137L-DCs, MFI = 117, respectively) as compared with control conditions (47% HLA-DR+ CD137L-DCs, MFI = 87) ().
Based on the expression of CD83, CD86, and HLA-DR, we deduced that cocktails C1, C3, and C4 contain factors that promote the maturation of CD137L-DCs. To verify whether these maturation cocktails not only enhance the expression of T-cell activating molecules but also enhance the biological potency of CD137L-DCs, we next assessed the ability of differentially matured CD137L-DCs to induce T-cell proliferation in allogeneic MLRs. To this aim, C1-, C3-, or C4-matured CD137L-DCs were co-cultured with CFSE-stained allogeneic T cells for 5 d and T-cell proliferation was determined by CFSE dilution. In the absence of CD137L-DCs, there was virtually no T-cell proliferation (2% proliferation, MFI = 1877). As shown in , untreated (C0) CD137L-DCs induced the proliferation of 64% of T cells (MFI = 733), while C1-treated CD137L-DCs, despite expressing comparatively higher levels of CD83, CD86, and HLA-DR, were unable to drive T-cell proliferation to further levels (68% proliferation, MFI = 665). This confirmed the previous observation that the administration of LPS and IFNγ to CD137L-DCs has no substantial effect on their ability to induce allogeneic T-cell proliferation.Citation14 Conversely, enhanced T-cell proliferation could be documented in the presence of CD137L-DCs treated with either C3 (81% proliferation, MFI = 444) or C4 (80% proliferation, MFI = 463), evincing that these maturation cocktails give rise to CD137L-DCs that are more potent T-cell activators than untreated CD137L-DCs (P < 0.01; ).
Next, as an additional measure of CD137L-DC potency and as a first indication of their ability to induce TH1 response, we determined the levels of IFNγ secreted in the supernatants of differentially matured CD137L-DCs co-cultured with T cells. The levels of IFNγ detected in supernatants of co-cultures containing C3-exposed CD137L-DCs were 5 times higher (1265 ± 243 pg/mL) than those of co-cultures involving control (C0) CD137L-DCs (241 ± 74 pg/mL). Similarly, IFNγ levels were 3 times higher (720 ± 241 pg/mL) in the supernatants of T cells exposed to C4-treated CD137L-DCs than in control co-cultures. In sharp contrast, there was no significant increase in IFNγ secretion by T cells co-cultured with C1-treated CD137L-DCs (287 ± 40 pg/mL), (). Taken together, these findings suggest that the secretion of IFNγ by T cells correlates with their proliferation rate, and that although cocktails C1, C3, and C4 enhance the expression of DC activation marker, only C3 and C4 also boost the ability of CD137L-DCs to stimulate T cells.
The maturation cocktails C3 and C4 differ by the presence of polyinosinic: polycytidylic acid (polyI:C), which is only included in the latter (). PolyI:C is a Toll-like receptor (TLR) 3 agonist capable of inducing the production of IL-12p70 in DCs.Citation23 Despite the presence of poly I:C, however, no IL-12p70 could be detected in the supernatants of C4-treated CD137L-DCs, nor in any of the other conditions (data not shown). Since, polyI:C apparently did not functionally contribute to CD137L-DC maturation, we surmise that the remaining components, i.e., those in common with cocktail C3, are the key mediators of this process.
A TLR7/8 agonist and IFNγ are essential and sufficient to functionally mature CD137L-DCs
We identified cocktail C3, consisting of IL-1β, TNFα, IFNγ, PGE2, and R848 (a mixed TLR7/8 agonist) as the most potent and suitable cocktail for maturing CD137L-DCs. Since reverse signaling through activated CD137L on the surface of peripheral monocytes has been shown to induce the secretion of inflammatory cytokines including TNFα and IL-1β,Citation15,Citation24 we reasoned that these potentially autocrine signaling components of C3 could be omitted without deleterious effect. Our aim was to systematically eliminate superfluous components of C3 in order to define a minimal maturation cocktail exhibiting high potency, to facilitate the future clinical use of CD137L-DCs ().
Table 2. Definition of essential factors for the maturation of CD137L-stimulated DCs
As expected, the absence of IL-1β and TNFα in the C3-derived cocktail C3A made no difference in the induction of the differentiation and activation markers CD83, CD86, and HLA-DR, as compared with the complete C3 cocktail (). Also, T cells co-cultured with CD137L-DCs matured by either C3 or C3A exhibited no apparent difference in their proliferation rates and IFNγ secretion ability ( and ). Altogether, these results suggest that the autocrine secretion of TNFα and IL-1β by CD137L-DCs is sufficient to promote their maturation as driven by IFNγ, R848 and PGE2 (maturation cocktail C3A).
Figure 3. Interferon γ and R848 are sufficient to induce the functional maturation of CD137L-derived dendritic cells. (A–C) Monocytes were treated with immobilized CD137-Fc for 6 d followed by exposure to the indicated maturation cocktails (see also ) for 18 h to obtain mature CD137L-derived dendritic cells (CD137L-DCs), which were then subjected to phenotypic and functional characterization (A) Cells were immunostained for CD83, CD86, and HLA-DR expression and analyzed by fluorescence cytometry. Unshaded and gray histograms represent the unstained (control) and stained samples, respectively. Values associated with each histogram indicate the percentages of positive cells and mean fluorescence intensity (MFI). Bar charts on the right report the mean percentage of positive cells and mean MFI ± SD of data acquired from 2 different donors. (B) Upon 18 h of treatment with cocktails C3, C3A, C3B, C3C, and C3D the CD137L-DCs were co-cultured for additional 5 d with carboxyfluorescein succinimidyl ester (CFSE)-stained allogeneic T cells at a ratio of 1:10. T-cell proliferation was then quantified by fluorescence cytometry based on CFSE dilution. Numbers in the histograms and bar charts represent percentages of proliferating cells and CFSE MFI. (C) Supernatants from the co-cultures of mature CD137L-DCs and allogeneic T cells were harvested and the levels of interferon γ (IFNγ) were quantified by ELISA. Means ± SD of triplicate measurements are depicted. *P < 0.05, **P < 0.01 (two-tailed, unpaired Student t test). This experiment has been performed independently twice, yielding with comparable results. PHA, phytohemagglutinin; Rst, resting.
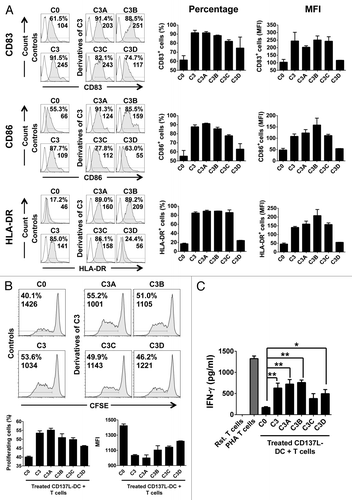
IFNγ alone (maturation cocktail C3C) was sufficient to substantially increase the expression of the phenotypic markers CD83, CD86, and HLA-DR on the surface of CD137L-DCs (). Furthermore, C3C also enhanced T-cell proliferation to levels resembling those induced by exogenous application of complete C3 (). Nevertheless, the levels of IFNγ detected in MLRs were not markedly different when untreated (C0) or C3C-treated CD137L-DCs were employed (), suggesting that C3C only partially phenocopies C3 . A salient observation was that the TLR7/8 ligand R848 alone (maturation cocktail C3D), was not able to increase the expression of the co-stimulatory molecules on the surface of CD137L-DCs, yet promoted their maturation to sufficient extent to enhance both T-cell proliferation and IFNγ secretion (). As it stands, R848 plus IFNγ (maturation cocktail C3B) were as potent as the complete C3 cocktail in its ability to drive the functional maturation of CD137L-DCs (). PGE2 was dispensable for this effect, since C3A-treated CD137L-DCs were functionally identical to their C3B-treated counterparts. This demonstrates that R848 and IFNγ are essential and sufficient for the complete functional maturation of CD137L-DCs. Additional optimization steps were undertaken to determine the minimum effective concentrations of these agents, revealing that 1 µg/mL R848 coupled to 50 ng/mL IFNγ (maturation C3B.1 in Figure S1) exert optimal effects in this setting (Fig. S1).
Phenotypic characterization of cDCs and CD137L-DCs matured in the presence of R848 and IFNγ
cDCs and CD137L-DCs were generated from autologous monocytes for 6 d and matured for a further 18 h using either TNFα, IL-1β, IL-6, and PGE2 (to produce mature cDCs) or R848 and IFNγ (in the case of CD137L-DCs). At this time point, these 2 types of DC already differed morphologically. Upon maturation, cDCs developed many small dendrites and remained non-adherent. In contrast, mature CD137L-DCs exhibited a predilection for a spindle-like shape and an increased adherence as compared with untreated CD137L-DCs, resulting in a distinctively flattened morphology (). More importantly, the expression levels of surface maturation markers were significantly different in mature cDCs and CD137L-DCs as compared with their respective immature counterparts. Upon maturation, the levels of CD14 were decreased, while the expression of CD83, CD86, and HLA-DR was augmented on both cDCs and CD137L-DCs (). However, we did not observe a complete absence of CD14 on the surface of mature DCs, a finding that is consistent with those from an independent study.Citation25 Taken together, these data confirmed that the maturation cocktails employed for each DC type were effective.
Figure 4. Generation and maturation of classical and CD137L-derived dendritic cells. (A–C) Monocytes were treated with immobilized CD137-Fc for 7 d to generate CD137L-derived dendritic cells (CD137L-DCs), or with 80 ng/mL granulocyte macrophage colony-stimulating factor (GM-CSF) plus 100 ng/mL interleukin (IL)-4 to generate classical dendritic cells (cDCs). For the last 18 h of culture, tumor necrosis factor α (TNFα) plus IL-1β plus IL-6 plus prostaglandin E2 (PGE2) were used to promote the maturations of cDCs, while 50 ng/mL interferon γ (IFNγ) plus 1 µg/mL R848 were used for the maturation of CD137L-DCs. (A) Brightfield microscopy images were taken on day 7 at a magnification of 63 × . Scale bar = 20 µm. (B) DC subsets were harvested and the expression of CD14, CD83, CD86, and HLA-DR was determined by flow cytometry upon staining with specific antibodies. These data are representative of at least 3 independent experiments yielding comparable results. (C) Viable DC numbers in each group were quantified by trypan blue exclusion assays. Bar charts represent cell count per million cells. Means ± SD of triplicate counts are depicted. These data are representative of 2–3 independent experiments yielding comparable results.
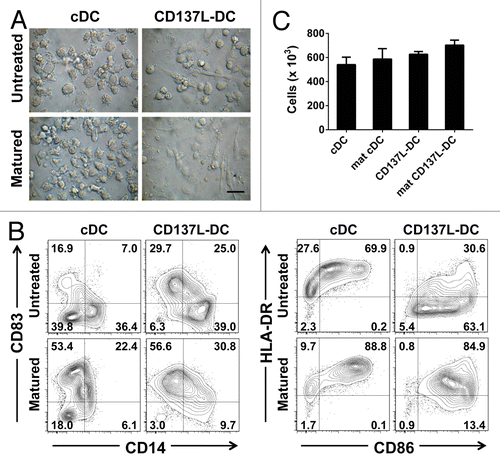
Aside from the biological potency of DCs, another important consideration involves the amounts of DCs that can be generated with a given differentiation and maturation protocol. No significant difference could be detected between the absolute numbers of mature cDCs and mature CD137L-DCs produced as described above. In each case, between 55–70% of the monocytes differentiated into mature DCs (). Hence, CD137L signaling in monocytes can promote the generation of DCs while maintaining a viability that is comparable to that ensured by classical differentiation protocols.
Mature CD137L-DCs potently activate autologous, antigen-specific T cells
So far, all the assessments involving CD137L-DCs had been performed in allogeneic systems. Therefore, we next investigated the capability of CD137L-DCs, as compared with cDCs, to initiate an antigen-specific response in an autologous setting. To this aim, we utilized a pool of peptides derived from the cytomegalovirus (CMV)-encoded protein pp65 as antigenic stimulus, and generated a pp65-specific T-cell line from a pp65+/HLA-A2+ donor. After 9–10 d, peripheral blood mononuclear cell (PBMCs) treated with pp65-derived peptides were predominantly T cells, as determined by CD3 expression (98% purity). Within this population, 79% of T cells were CD8+ while 19% were CD4+ T cells (data not shown).
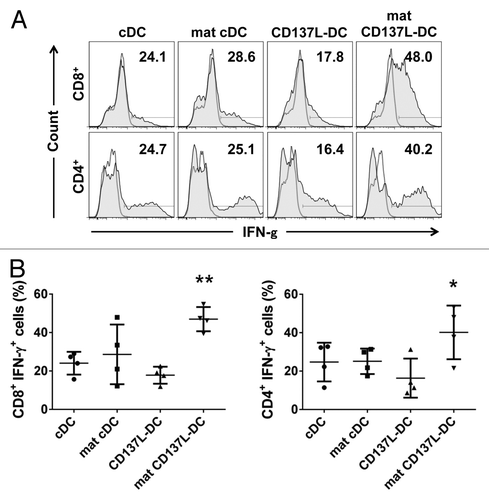
To comparatively assess the antigen-presentation capacity of the cDCs and CD137L-DCs, we first pulsed these cells with pp65-derived peptides, and then co-cultured them with autologous pp65-specific T cells. We next determined IFNγ expression among T cells as a measure of their activation status after 18h. No T-cell activation occurred in the absence of pp65-derived peptides (data not shown). Conversely, when CD8+ T cells were exposed to mature CD137L-DCs pulsed with pp65-derived peptides, 48% of them were found to express IFNγ. All other DC preparations were less active than mature CD137L-DCs as only 24.1%, 28.6%, or 17.8% of the CD8+ T cells expressed IFNγ in response to priming by immature cDCs, mature cDCs or untreated CD137L-DCs, respectively (, top panels). A similar pattern was observed with regard to the priming of CD4+ T cells by these distinct DC subsets (, bottom panels). Collated data from 4 independent experiments confirmed that mature CD137L-DCs are indeed significantly more potent activators of CD8+ and CD4+ T cells than the other types of DCs tested in this setting ().
Figure 5. Mature CD137L-derived DCs potently stimulate autologous CD8+ and CD4+ T cells. (A and B) Dendritic cells (DCs) were pulsed with a pool of pp65-derived peptides or left unpulsed (control conditions). On day 9/10, pp65-specific T cells were co-cultured with peptide-pulsed or unpulsed DCs at a ratio of 10:1 in the presence of 2 µg/mL brefeldin A for 18 h. T cells were then immunostained for cell surface expression of CD8 or CD4 as well as for the intracellular expression of interferon γ (IFNγ) prior to cytofluorometric analysis. (A) Representative histograms from a single experiment. Unshaded and gray histograms represent the unstained (control) and stained samples, respectively. The number associated with each histogram reports the percentage of IFNγ+ cells. (B) Scatter plot depicting collated data of pp65-specific, IFNγ-secreting CD8+ or CD4+ T cells from 4 independent experiments. Means ± SD are depicted. *P < 0.05, **P < 0.01 (ANOVA).
The same conclusion was reached by measuring IFNγ secretion after 5 days of co-culture. Although pp65-pulsed immature and mature cDCs were able to induce considerable IFNγ secretion by T cells (5.6 ± 0.2 ng/mL and 7.2 ± 0.2 ng/mL, respectively), untreated CD137L-DCs were significantly more effective, inducing the release of more than twice as much IFNγ (14.6 ± 0.6 ng/mL). Mature CD137L-DCs were comparatively the most efficient, stimulating T cells to secrete the highest amount of IFNγ (25.7 ± 1 ng/mL) ().
Figure 6. T cells stimulated by CD137L-derived dendritic cells stimulate the release of high levels of TH1 and TH2 cytokines. (A and B) Dendritic cells (DCs) were pulsed with a pool of pp65-derived peptides or left unpulsed (control conditions). pp65-specific T cells were subsequently co-cultured with peptide-pulsed or unpulsed DCs at a ratio of 10:1. Interleukin (IL)-2, IL-7, and IL-15 were added on day 3 of re-stimulation. The levels of interferon (IFNγ) (A) and IL-13 (B) in culture supernatants were determined by ELISA 5 d later. Means ± SD of triplicate measurements are depicted. **P < 0.01 (two-tailed, unpaired Student t test). These data are representative of at least 2 independent experiments yielding comparable results.
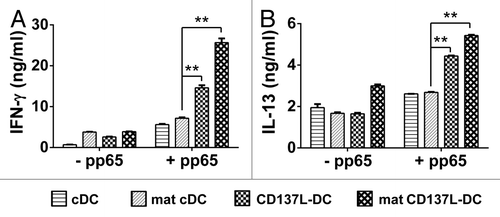
Often the levels of TH1 cytokines inversely correlate with those of TH2 cytokines. Therefore, it was surprising to detect a profile of IL-13 secretion in response to the various DC subsets that was similar to that observed for IFNγ. In general, the concentrations IL-13 were below 2000 pg/mL in the co-cultures involving unpulsed DC subsets, with the exception of those containing unpulsed mature CD137L-DCs, in which we found 2997 pg/mL of IL-13 (). Upon exposure to immature or mature cDCs pulsed with pp65-derived peptides, T cells released 2612 ± 3 pg/mL and 2684 ± 24 pg/mL IL-13, respectively. The levels of IL-13 were even higher in the supernatants of T cells co-cultured with peptide-pulsed, untreated CD137L-DCs (4438 ± 24 pg/mL), and peptide-pulsed, mature CD137L-DCs (5428 ± 35 pg/mL) ().
CD137L-DCs promote a superior killing activity in antigen-specific autologous T cells
In order to assess the cytotoxicity of T cells re-stimulated by distinct DC subsets, we next sought to determine their lytic activity against peptide-pulsed target cells. Donor monocytes and a target lymphoblastoid B-cell line (clone CM371) were confirmed to be HLA-matched, as they both expressed HLA-A2 ().
Figure 7. pp65-specific T cells re-stimulated by CD137L-derived dendritic cells are more cytotoxic than T cells re-stimulated by classical dendritic cells. (A) The lymphoblastic cell line CM371 and monocytes isolated from a HLA-A2+ donor were immunostained for HLA-A2 and CD20 expression or HLA-A2 expression, respectively, and analyzed by fluorescence cytometry. (B) CM371 cells were loaded with the DELFIA® BATDA reagent, pulsed or not with pp65-derived peptides and used as target cells. T cells re-stimulated by exposure to the indicated type of dendritic cells (DC) for 5 d were added at the depicted effector to target (E:T) cell ratios, and were incubated for 3 h. The percentages of target cell lysis are reported as means ± SD from triplicate measurements. **P < 0.01 (two-tailed, unpaired Student t test). These data are representative of at least 2 independent experiments yielding comparable results.
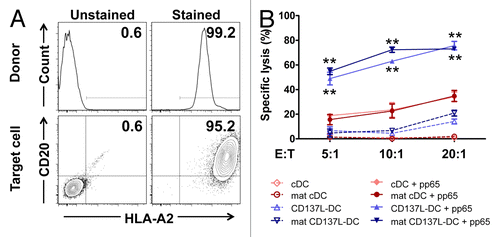
A dose-dependent killing of target cells was observed at increasing effector/target (E:T) cell ratios in all conditions (). Surprisingly, the maturation of cDCs and CD137L-DCs had no or little effect, respectively, on their ability to trigger the cytotoxic functions of T cells (). In the absence of pp65-derived peptides, T cells re-stimulated by either immature or mature cDCs had no cytotoxic activity, whereas T cells re-stimulated by CD137L-DCs, be them mature or not, killed 4–20% of target cells. Furthermore, regardless of the nature of re-stimulating DCs, pulsing target cells with pp65-derived peptides enhanced their capacity to induce T-cell killing.
Of particular importance, the T cells re-stimulated by CD137L-DCs were at least twice as effective as T cells re-stimulated by cDCs, as measured by the specific lysis of target cells at various E:T cell ratios. T cells re-stimulated by untreated CD137L-DCs killed 49%, 63%, and 75%, of target cells at E:T cell ratios of 5:1, 10:1, and 20:1, respectively, while 16%, 23%, and 35% of target cells were killed by T cells primed by mature cDCs. The efficacy of mature CD137L-DCs was quite similar to that of untreated CD137L-DCs (). These data demonstrate that autologous, antigen-specific T cells that have been re-stimulated by CD137L-DCs, regardless of their maturation status, are functionally superior to T cells re-stimulated by cDCs.
Discussion
DCs are the key orchestrators of antigen-specific T-cell responses and thus DC-based vaccines are attractive tools for the implementation of anticancer immunotherapy. However, DC vaccination has been ineffective to date, calling for technical improvements in the protocols whereby DCs are prepared, loaded with antigens and endowed with immunostimulatory properties.
It has previously been shown that DCs differentiated upon CD137L engagement are more effective as compared with classically-derived DCs in stimulating the proliferation of allogeneic T cells.Citation14,Citation15 Moreover, the high expression levels of perforin and IFNγ exhibited by CD8+ T cells exposed to CD137L-DCs suggest that these DCs may be more potent than cDCs in the induction of cytotoxic T-cell activity.Citation14 These promising activities of CD137L-DCs prompted us to investigate whether these APCs may also be functionally superior in a more challenging and clinically-relevant, antigen-specific autologous setting.
Indeed, our findings reveal that CD137L-DC-stimulated T cells exert a superior cytotoxicity toward target cells. In contrast to what is obsevered for cDCs, the maturation of CD137L-DCs upon the exogenous application of LPS and IFNγ did not further enhance their ability to promote T-cell cytotoxicity. This suggests that either CD137L-DCs are already fully mature, or that a different cytokine cocktail may be required to enhance their biological activity. Of note, low levels of MHC class II molecules are expressed on the surface of CD137L-DCs, suggesting that they have not yet reached their full potential as APCs, especially relative to the capacity to activate CD4+ T cells. The treatment of CD137L-DCs with R848 and IFNγ was sufficient to promote the maturation of these cells, as evidenced by the increased expression of typical DC maturation markers such as CD83, CD86, and HLA-DR, coupled and the reduction of CD14. Importantly, T cells activated by CD137L-DCs exposed to R848 and IFNγ underwent a greater proliferative response than T cells stimulated by untreated CD137L-DCs.
In spite of the fact that CD137L-DCs exposed to LPS plus IFNγ and R848 plus IFNγ expressed similar levels of maturation markers, the latter treatment induced a comparatively more intense T-cell stimulation. Such an enhanced co-stimulatory activity of CD137L-DCs treated with R848 and IFNγ is presumably due to the TLR7/8 agonist. Chamberlain et al. reported that the ligation of TLR7 by single-stranded RNA leads to TNFα secretion by monocytes upon the induction of the NF-kB and phosphoinositide-3-kinase (PI3K) pathways.Citation26 Although the engagement of CD137L on the surface of monocytes also induces basal TNFα production, the combination of CD137, R848, and IFNγ may lead to synergistic effects on TNFα secretion. Extracellular TNFα can interact with CD137L-DCs in an autocrine or paracrine manner, thereby favoring their maturation. An alternative mechanism underlying the synergism between R848 and IFNγ might relate to the fact that IFNγ increases TLR8 expression in human cDCs,Citation27,Citation28 Considering that R848 is a TLR7/8 agonist, the upregulation of TLR8 by IFNγ would naturally increase the sensitivity of CD137L-DCs to R848.
In order to further evaluate the immunotherapeutic potential of CD137L-DCs, we characterized their immunostimulatory function in an autologous system based on the CMV-derived antigen pp65. In this setting, mature CD137L-DCs robustly stimulated the activation of pp65-specific CD8+ and CD4+ T cells (as measured in terms of IFNγ production) even within a brief period (18 h) of re-stimulation. In contrast, the other types of DC tested in this study were not able to induce comparable levels of IFNγ, and there was no difference in IFNγ production by T cells exposed to cDCs and untreated CD137L-DCs. The re-stimulation of T cells for additional 5 d, however, allowed untreated CD137L-DCs to activate them more robustly than both immature and mature cDCs, but less robustly than mature CD137L-DCs. This observation suggests that untreated CD137L-DCs are sufficient to stimulate T cells more potently than cDCs, and that upon maturation CD137L-DCs acquire the ability to activate T cells very quickly.Citation14 Furthermore, in line with what we observed in an allogeneic setting, antigen-specific T cells primed by autologous CD137L-DCs, be them untreated or mature, had superior cytotoxic ability as compared with T cells co-cultured with cDCs. Indeed, T cells primed by autologous CD137L-DCs were 2–3 times more potent than T cells primed by mature cDCs in inducing the lysis of antigen-pulsed target cells.
It should be noted that different protocols for the generation of cDCs exist.Citation29 Here, as a basis for comparison, we used the method described by Jonuleit et al. in 1997 which is considered as a ‘gold standard’ for cDC maturation.Citation21 A recent, alternative method by Mailliard et al. reportedly results in the generation of DCs that secrete abundant levels of IL-12p70 and hence prime robust CD8+ T-cell cytotoxic responses.Citation30 This said, a third, independent study found little difference in the efficacy of cDCs generated by either of these protocols.Citation22
A potential explanation for the enhanced killing activity of CD137L-DCs may be found in the fact that CD137L-DCs stimulate not only CD8+ but also CD4+ T cells, as evinced by the increase in IFNγ expression detected in both T-cell populations. CD4+ T cells are essential to sustain CD8+ T-cell activity. Indeed, the interactions between CD4+ and CD8+ T cells via CD40 and its ligand (CD40L)Citation31 results in the downregulation of programmed cell death 1 (PDCD1, an immunosuppressive receptor best known as PD-1)Citation32 and tumor necrosis factor (ligand) superfamily, member 10 (TNFSF10, an inducer of apoptosis best known as TRAIL)Citation33 on the surface of CD8+ T cells. These phenotypic changes dramatically enhances the cytotoxicity of CD8+ T cells and limit their susceptibility to TRAIL-induced apoptosis. Soluble factors such as IL-2 that are secreted by CD4+ T cells further act onto CD8+ T cells, thus improving recall responses such as those that occur in the course of lymphocytic choriomeningitis virus (LCMV) infections.Citation34
More recently, the discovery of cytotoxic CD4+ T cells has cast doubt on the dogma that CD4+ T cells merely operate as helper for CD8+ cytotoxic T lymphocytes. Cytotoxic CD4+ T cells were observed in response to infection or vaccination against viruses such as herpes virus, West-Nile virus, and dengue virus.Citation35-Citation37 Likewise, melanoma-reactive CD4+ T cells have been shown to exert a cytotoxic activity, leading to tumor rejection via MHC class II-restricted interactions.Citation38 These cytotoxic CD4+ T cells appear to exhibit a TH1 phenotype, and the upregulation of the TH1 cytokine IFNγ by CD137L-DC-primed CD4+ T cells is suggestive of such a cytotoxic activity. It is possible that untreated and mature CD137L-DCs are equipotent in their ability to stimulate CD8+ and CD4+ T cells, and thereby induce an optimal, combined cytotoxic potential in the overall T-cell population. However, the phenotypic status of the CD4+ T cells elicited by CD137L-DCs remains to be investigated in detail, especially considering that IL-13, a prototypic TH2 cytokine, was found to be secreted by T cells primed by CD137L-DCs.
Another potential explanation for the enhanced potency of CD137L-DCs relates to the peculiarities of CD137L signaling. CD137L not only associates with TLR4 and possibly other TLRs, but also is essential for the long-term release of TNFα from murine macrophages exposed to LPS.Citation39 CD137L signaling in human monocytes has also been shown to promote the secretion of TNFα,Citation24 and it may therefore be speculated that TLR4 and CD137L synergize in these cells to promote TNFα release. TNFα is a potent immunomodulatory cytokine and may function in both an autocrine or paracrine manner to stimulate the maturation of DCs. CD137L also associates with TNF receptor 1 (TNFR1), and upon binding to CD137 utilizes TNFR1 to transduce a reverse signal to CD137L+ cells.Citation40 Therefore, CD137L, TNFR1, and TLR4 (and perhaps other TLRs) might form a large multimeric signaling complex that is activated by recombinant CD137 or anti-CD137L antibodies to deliver multiple signals to monocytes, hence synergistically promoting the differentiation and maturation of DCs.
During the first 24 h of differentiation to CD137L-DCs, monocytes activated by CD137 promote the apoptotic demise of T cells. This activity of CD137L-DCs appears to mimic the T-cell attrition that occurs in the early phase of infections. In this setting, a massive apoptotic response among T cells allows antigen-specific T cells to expand.Citation41 Thus, triggering the selective demise of T cells may be another mechanisms by which CD137L-DCs ameliorate T-cell responses.
In the present study, we employed a viral model antigen. The induction of T-cell responses against tumor-associated antigens, which are self antigens, is more challenging. Along similar lines, priming T cells in a cancer patient, and hence previously exposed to an immunosuppressive environment, will be even more technically challenging.
Our findings are in line with a large body of evidence that identifies CD137L as a general myelopoietic growth and differentiation factor. Reverse CD137L signaling is known to induce the activation, survival, proliferation, and migration of monocytes.Citation24,Citation42-Citation46 Not only does CD137L signaling activate mature myeloid cells but it also stimulates the proliferation and colony-forming potential of hematopoietic progenitor cells as well as their differentiation to macrophages.Citation47-Citation50 Through these diverse activities, CD137L pleiotropically contributes to the increase in the abundance of myeloid cells that characterize immune responses to infections, generating cells which are available to assist with pathogen clearance.Citation51 That these myelopoietic activities of CD137L signaling may be of therapeutic significance has been previously suggested by the demonstration that CD137L promotes the maturation of cDCs.Citation52-Citation54 It is worthwhile to point out that CD137L-DCs are not only more potent than their classical counterparts but also simpler to produce since fewer recombinant proteins are required for both their differentiation and maturation.
In conclusion, this study confirms and extends promising data from earlier studies by demonstrating the superior immunological potency of CD137L-DCs in an antigen-specific, autologous human system based on a viral model antigen. A further advance and a logical next step to develop CD137L-DCs for clinical use would be testing whether CD137L-DCs can also induce potent immune responses against human tumors.
Materials and Methods
Recombinant proteins, peptides, and antibodies
The human CD137-Fc chimera was purchased from R&D Systems. The human IgG Fc was purchased from Millipore. GM-CSF, IL-4, TNFα, IFNγ, IL-1β, IL-6, IL-7, and IL-15 were purchased from Peprotech while IL-2 was from R&D Systems. PGE2 and LPS were purchased from Sigma. R848, polyI:C and recombinant flagellin were purchased from Invitrogen. The CMV pp65 PepTivator peptide pool was purchased from Miltenyi Biotec. Peptide pools were composed of 15-mers with 11 amino acid overlaps, covering the complete sequence of the pp65 protein encoded by the CMV strain AD169 (Swiss-Prot Acc. no. P06725). Antibodies against human HLA-DR (clone L243), CD14 (clone 61D3), CD20 (clone 2H7), CD83 (clone HB15e), CD86 (clone IT2.2), CD3 (clone OKT3), CD4 (clone OKT4), and IFNγ (clone 4S.B3), were purchased from eBioscience. Antibodies against human CD8 (clone RPA-T8) and HLA-A2 (clone BB7.2) were purchased from BD PharMingen.
Cells and cell culture
Human PBMCs were prepared by Ficoll-Paque (GE Healthcare) density gradient centrifugation. Monocytes were isolated from PBMCs by negative selection using the Monocyte Isolation Kit II (Miltenyi Biotec) and MACS magnetic separation, according to manufacturer’s instructions. The isolation of total T cells was performed via positive selection with the MACS system and anti-CD3 microbeads (Miltenyi Biotec). To acquire the CD8+ T-cell fraction, anti-CD8 microbeads and MACS (Miltenyi Biotec) were used for positive selection. Isolated monocytes and T cells exhibited a purity > 95% based on antigenic phenotyping by CD14, CD3 and CD8 staining, respectively. To generate CD137L-DCs, monocytes were seeded onto polystyrene dishes (Becton Dickinson) pre-coated with 10 µg/mL CD137-Fc, in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 50 µg/mL streptomycin and 50 IU/mL penicillin for 7 d (R10 PS medium). Maturation stimuli were administered during the last 18 h of incubation. Classical DCs were generated by culturing monocytes in R10 PS medium in the presence of 80 ng/mL GM-CSF and 100 ng/mL IL-4. Fresh media was added on day 3. For the last 18 h, cells were matured with either 1 µg/mL LPS + 50 ng/mL IFNγ or with 10 ng/mL TNFα, 10 ng/mL IL-1β, 10 ng/mL IL-6, and 1 µg/mL PGE2.Citation21 HLA-A2+ Epstein-barr virus (EBV)-transformed B lymphoblasts (clone CM 371) were a gift from Dr Laura Rivino (National University of Singapore) and were maintained in R10 PS media. K562 cells were obtained from ATCC and were maintained in Iscove’s modified dulbecco’s medium (IMDM) supplemented with 10% FBS.
Maturation of CD137L-DCs and cDCs
Monocytes were seeded onto CD137-Fc-coated plates and cultured until day 6, when various cytokine cocktails () were added to the culture for 18 h to induce maturation. Cells were then analyzed for the expression of CD14, CD83, CD86, and HLA-DR and their ability to induce T-cell activation, as measured by proliferation of allogeneic T cells and IFNγ production. For autologous experiments, CD137L-DCs were generated using 1 µg/mL R848 plus 50 ng/mL IFNγ, while cDCs were produced with 10 ng/mL TNFα, 10 ng/mL IL-1β, 10 ng/mL IL-6, and 1 µg/mL PGE2 for 18 h.Citation21
Allogeneic MLRs
Allogeneic T cells were isolated and stained with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Invitrogen) followed by co-culture with CD137L-DCs or cDCs at a ratio of 10:1. Cultures were maintained in R10 PS media for 5 d and cell proliferation was determined by CFSE dilution on fluorescence cytometry. Supernatants from co-cultures were collected and IFNγ production was determined by ELISA.
Cell quantification by trypan blue staining
DCs were harvested upon trypsinization, washed and resuspended in PBS. Cells were mixed at 1:1 ratio with 0.4% trypan blue solution (Sigma T8154) and counted on a hemocytometer using brightfield microscopy.
Generation of a pp65-specific T-cell line
PBMCs were suspended at 106 cells/mL in AIM V medium (Invitrogen) supplemented with 2% human AB serum (Sigma-Aldrich), 50 µg/mL streptomycin sulfate and 10 µg/mL gentamicin sulfate (AIM V 2Ab medium). PBMCs were then stimulated with the PepTivator peptide pool (0.1 µg/mL per peptide; Miltenyi Biotec) for 9–10 d in the presence of 10 U/mL IL-2 added after 24 h. On day 9–10, > 95% of cells were CD3 positive.
Re-stimulation of pp65-specific T cells
CD137L-DCs or cDCs were harvested and co-cultured with autologous pp65-specific T cells at a ratio of 1:10 in the presence of pp65-derived peptides (at either 1 µg/mL per peptide, for 18 h, or 0.1 µg/mL per peptide, for 5 d). As a negative control, no peptides were added to the culture.
Cytotoxicity assays
CD8+ T cells were pre-activated in an allogeneic MLR with pre-treated monocytes or DCs at a ratio of 10:1. After 5 d of co-culture, these CD8+ T cells were used as effector cells against K562 target cells. CD8+ T cells were thus harvested and co-cultured with K562 cells labeled with CFSE at E:T cell ratios of 1:1, 10:1, and 20:1. Upon overnight co-culture, total cells were harvested and the Annexin V Apoptosis Detection Kit (BD Biosciences) was used to determine the percentage of apoptotic cells among the CFSE-positive population by flow cytometry. For pp65-specific cytotoxicity assays, pp65-specific T cells were re-stimulated with DCs pulsed with pp65-derived peptides (0.1 µg/mL per peptide) at a ratio of 10:1 for 5 d. Cells were then harvested and stained for CD3 expression, and found to be > 95% positive for this T-cell marker. EBV-transformed B lymphoblasts were loaded with the DELFIA® BATDA Reagent (Perkin Elmer) and cytotoxicity assays were performed according to manufacturer’s instruction. In brief, DELFIA® BATDA-loaded target cells were pulsed with pp65-derived peptides (1 µg/mL per peptide) for 1 h at 37° C. Unpulsed target cells were employed as a control. Cells were washed and resuspended in AIM V medium supplemented with 2% human serum, 50 µg/mL streptomycin sulfate, 10 µg/mL gentamicin sulfate, 2 mM probenecid and 50 µM β-mercaptoethanol and then seeded into ‘V’-bottom 96-well plates at 104 cells per well. pp65-specific T cells were then co-cultured with target cells at E:T ratios of 5:1, 10:1, and 20:1 for 3 h at 37° C. Europium solution was added to the harvested supernatants for 15 min and fluorescence was measured using a time-resolved fluorometer.
Fluorescence cytometry
In order to determine the expression of surface markers, cells were blocked with FcR blocking reagent (Miltenyi Biotec) and were then stained with specific antibodies in PBS containing 0.5% bovine serum albumin (BSA) and 0.1% sodium azide (staining buffer) for 45 min at 4° C in the dark. Cells were washed twice and resuspended in staining buffer. Unstained samples were used as a control. For intracellular cytokine staining, cells were cultured in the presence of 2 µg/mL brefeldin A (eBioscience) overnight and stained with anti-CD8 or anti-CD4 antibody followed by cell fixation using BD Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences). Intracellular staining of IFNγ was then performed following the manufacturer’s instruction. Fluorescence cytometry was performed on a CyAn ADP Analyzer (Dako) or BD LSR Fortessa cell analyzer (BD Bioscience). Data were anyalzed using Summit or FlowJo data acquisition and analysis software.
ELISA
The concentrations of IFNγ and IL-13 in cell supernatants were determined by the Human IFNγ and Human IL-13 ELISA Duoset (R&D Systems), respectively, according to manufacturer’s instructions. All measurements were performed in triplicates.
Photographs
Cell morphology was photographed using a Zeiss Axiovert 40 inverted microscope (Zeiss) and a Canon PowerShot G6 digital camera.
Statistical analyses
Statistical significance was determined by two-tailed, unpaired Student t tests unless otherwise specified.
| Abbreviations: | ||
| 7-AAD | = | 7-aminoactinomycin D |
| APC | = | antigen-presenting cell |
| CD137L | = | CD137 ligand |
| cDC | = | classical DC |
| CFSE | = | carboxyfluorescein succinimidyl ester |
| CMV | = | cytomegalovirus |
| DC | = | dendritic cell |
| GM-CSF | = | granulocyte macrophage colony-stimulating factor |
| IFNγ | = | interferon γ |
| IL | = | interleukin |
| LPS | = | lipopolysaccharide |
| MLR | = | mixed lymphocyte reaction |
| PBMC | = | peripheral blood mononuclear cell |
| polyI:C | = | polyinosinic:polycytidylic acid |
| R848 | = | Resiquimod |
| TLR | = | Toll-like receptor |
| TNFα | = | tumor necrosis factor α |
Additional material
Download Zip (117.2 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by grant (T1–2012 Oct-14) from the Academic Research Foundation, Singapore.
Supplemental Materials
Supplemental materials may be found here: http://www.landesbioscience.com/journals/oncoimmunology/article/26859/
Citation: Harfuddin Z, Kwajah S, Chong Nyi Sim A, MacAry P, Schwarz H. CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses. OncoImmunology 2013; 2:e26859; 10.4161/onci.26859
References
- Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003; 51:59 - 60; http://dx.doi.org/10.1016/S0369-8114(03)00096-8; PMID: 12801800
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O’Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 2007; 8:1217 - 26; http://dx.doi.org/10.1038/ni1522; PMID: 17922015
- Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol 2005; 175:1373 - 81; PMID: 16034072
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006; 211:609 - 18; http://dx.doi.org/10.1016/j.imbio.2006.05.025; PMID: 16920499
- León B, López-Bravo M, Ardavín C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007; 26:519 - 31; http://dx.doi.org/10.1016/j.immuni.2007.01.017; PMID: 17412618
- Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med 2005; 11:653 - 60; http://dx.doi.org/10.1038/nm1246; PMID: 15880118
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179:1109 - 18; http://dx.doi.org/10.1084/jem.179.4.1109; PMID: 8145033
- Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med 1998; 188:2175 - 80; http://dx.doi.org/10.1084/jem.188.11.2175; PMID: 9841930
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009; 27:669 - 92; http://dx.doi.org/10.1146/annurev.immunol.021908.132557; PMID: 19132917
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10:475 - 80; http://dx.doi.org/10.1038/nm1039; PMID: 15122249
- Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol 2006; 25:377 - 413; http://dx.doi.org/10.1080/08830180600992456; PMID: 17169781
- Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K, Thielemans K, Bonehill A. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother 2007; 56:1513 - 37; http://dx.doi.org/10.1007/s00262-007-0334-z; PMID: 17503040
- Thomas-Kaskel AK, Veelken H. [Active immunotherapy of prostate cancer with a focus on dendritic cells]. Actas Urol Esp 2007; 31:668 - 79; http://dx.doi.org/10.1016/S0210-4806(07)73704-X; PMID: 17896564
- Kwajah M M S, Schwarz H. CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur J Immunol 2010; 40:1938 - 49; http://dx.doi.org/10.1002/eji.200940105; PMID: 20432236
- Ju S, Ju S, Ge Y, Qiu H, Lu B, Qiu Y, Fu J, Liu G, Wang Q, Hu Y, et al. A novel approach to induce human DCs from monocytes by triggering 4-1BBL reverse signaling. Int Immunol 2009; 21:1135 - 44; http://dx.doi.org/10.1093/intimm/dxp077; PMID: 19684160
- Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 2009; 229:192 - 215; http://dx.doi.org/10.1111/j.1600-065X.2009.00765.x; PMID: 19426223
- Thum E, Shao Z, Schwarz H. CD137, implications in immunity and potential for therapy. Front Biosci (Landmark Ed) 2009; 14:4173 - 88; http://dx.doi.org/10.2741/3521; PMID: 19273343
- Lee SW, Croft M. 4-1BB as a therapeutic target for human disease. Adv Exp Med Biol 2009; 647:120 - 9; http://dx.doi.org/10.1007/978-0-387-89520-8_8; PMID: 19760070
- Wang S, Lv J, Wang P, Yin X, Tan A, Chen Y. Recombinant human CD137L for cancer immunotherapy: effects of different fusions and linkers on its activity. Cancer Immunol Immunother 2012; 61:489 - 95; http://dx.doi.org/10.1007/s00262-011-1097-0; PMID: 21968735
- Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol 2011; 89:21 - 9; http://dx.doi.org/10.1189/jlb.0510315; PMID: 20643812
- Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 1997; 27:3135 - 42; http://dx.doi.org/10.1002/eji.1830271209; PMID: 9464798
- Zobywalski A, Javorovic M, Frankenberger B, Pohla H, Kremmer E, Bigalke I, Schendel DJ. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med 2007; 5:18; http://dx.doi.org/10.1186/1479-5876-5-18; PMID: 17430585
- Boullart AC, Aarntzen EH, Verdijk P, Jacobs JF, Schuurhuis DH, Benitez-Ribas D, Schreibelt G, van de Rakt MW, Scharenborg NM, de Boer A, et al. Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer Immunol Immunother 2008; 57:1589 - 97; http://dx.doi.org/10.1007/s00262-008-0489-2; PMID: 18322684
- Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol 1998; 160:2488 - 94; PMID: 9498794
- Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A 1996; 93:2588 - 92; http://dx.doi.org/10.1073/pnas.93.6.2588; PMID: 8637918
- Chamberlain ND, Kim SJ, Vila OM, Volin MV, Volkov S, Pope RM, Arami S, Mandelin AM 2nd, Shahrara S. Ligation of TLR7 by rheumatoid arthritis synovial fluid single strand RNA induces transcription of TNFα in monocytes. Ann Rheum Dis 2013; 72:418 - 26; http://dx.doi.org/10.1136/annrheumdis-2011-201203; PMID: 22730373
- Zannetti C, Bonnay F, Takeshita F, Parroche P, Ménétrier-Caux C, Tommasino M, Hasan UA. C/EBPdelta and STAT-1 are required for TLR8 transcriptional activity. J Biol Chem 2010; 285:34773 - 80; http://dx.doi.org/10.1074/jbc.M110.133884; PMID: 20829351
- Xu S, Koldovsky U, Xu M, Wang D, Fitzpatrick E, Son G, Koski G, Czerniecki BJ. High-avidity antitumor T-cell generation by toll receptor 8-primed, myeloid- derived dendritic cells is mediated by IL-12 production. Surgery 2006; 140:170 - 8; http://dx.doi.org/10.1016/j.surg.2006.03.006; PMID: 16904966
- Skalova K, Mollova K, Michalek J. Human myeloid dendritic cells for cancer therapy: does maturation matter?. Vaccine 2010;; 28:5153 - 60; http://dx.doi.org/10.1016/j.vaccine.2010.05.042; PMID: 20665974
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res 2004; 64:5934 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-04-1261; PMID: 15342370
- Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 2002; 297:2060 - 3; http://dx.doi.org/10.1126/science.1072615; PMID: 12242444
- Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H, Usherwood EJ. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol 2009; 182:4244 - 54; http://dx.doi.org/10.4049/jimmunol.0802041; PMID: 19299723
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 2005; 434:88 - 93; http://dx.doi.org/10.1038/nature03337; PMID: 15744305
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006; 441:890 - 3; http://dx.doi.org/10.1038/nature04790; PMID: 16778891
- Stuller KA, Cush SS, Flaño E. Persistent gamma-herpesvirus infection induces a CD4 T cell response containing functionally distinct effector populations. J Immunol 2010; 184:3850 - 6; http://dx.doi.org/10.4049/jimmunol.0902935; PMID: 20208003
- Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol 2008; 181:8568 - 75; PMID: 19050276
- Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 2010; 185:5405 - 16; http://dx.doi.org/10.4049/jimmunol.1001709; PMID: 20870934
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637 - 50; http://dx.doi.org/10.1084/jem.20091918; PMID: 20156971
- Kang YJ, Kim SO, Shimada S, Otsuka M, Seit-Nebi A, Kwon BS, Watts TH, Han J. Cell surface 4-1BBL mediates sequential signaling pathways ‘downstream’ of TLR and is required for sustained TNF production in macrophages. Nat Immunol 2007; 8:601 - 9; http://dx.doi.org/10.1038/ni1471; PMID: 17496895
- Moh MC, Lorenzini PA, Gullo C, Schwarz H. Tumor necrosis factor receptor 1 associates with CD137 ligand and mediates its reverse signaling. FASEB J 2013; 27:2957 - 66; http://dx.doi.org/10.1096/fj.12-225250; PMID: 23620528
- Kwajah M M S, Mustafa N, Holme AL, Pervaiz S, Schwarz H. Biphasic activity of CD137 ligand-stimulated monocytes on T cell apoptosis and proliferation. J Leukoc Biol 2011; 89:707 - 20; http://dx.doi.org/10.1189/jlb.1010569; PMID: 21330351
- Langstein J, Becke FM, Söllner L, Krause G, Brockhoff G, Kreutz M, Andreesen R, Schwarz H. Comparative analysis of CD137 and LPS effects on monocyte activation, survival, and proliferation. Biochem Biophys Res Commun 2000; 273:117 - 22; http://dx.doi.org/10.1006/bbrc.2000.2889; PMID: 10873573
- Langstein J, Michel J, Schwarz H. CD137 induces proliferation and endomitosis in monocytes. Blood 1999; 94:3161 - 8; PMID: 10556203
- Langstein J, Schwarz H. Identification of CD137 as a potent monocyte survival factor. J Leukoc Biol 1999; 65:829 - 33; PMID: 10380906
- Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE, Lim YC, Schwarz H. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB J 2007; 21:456 - 63; http://dx.doi.org/10.1096/fj.05-4739com; PMID: 17167064
- Quek BZ, Lim YC, Lin JH, Tan TE, Chan J, Biswas A, Schwarz H. CD137 enhances monocyte-ICAM-1 interactions in an E-selectin-dependent manner under flow conditions. Mol Immunol 2010; 47:1839 - 47; http://dx.doi.org/10.1016/j.molimm.2009.11.010; PMID: 20347151
- Jiang D, Yue PS, Drenkard D, Schwarz H. Induction of proliferation and monocytic differentiation of human CD34+ cells by CD137 ligand signaling. Stem Cells 2008; 26:2372 - 81; http://dx.doi.org/10.1634/stemcells.2008-0158; PMID: 18566330
- Jiang D, Chen Y, Schwarz H. CD137 induces proliferation of murine hematopoietic progenitor cells and differentiation to macrophages. J Immunol 2008; 181:3923 - 32; PMID: 18768847
- Jiang D, Schwarz H. Regulation of granulocyte and macrophage populations of murine bone marrow cells by G-CSF and CD137 protein. PLoS One 2010; 5:e15565; http://dx.doi.org/10.1371/journal.pone.0015565; PMID: 21179444
- Jiang D, Tang Q, Schwarz H. Involvement of the cytokine receptor CD137 in murine hematopoiesis. Adv Exp Med Biol 2011; 691:375 - 82; http://dx.doi.org/10.1007/978-1-4419-6612-4_38; PMID: 21153341
- Tang Q, Jiang D, Alonso S, Pant A, Martínez Gómez JM, Kemeny DM, Chen L, Schwarz H. CD137 ligand signaling enhances myelopoiesis during infections. Eur J Immunol 2013; 43:1555 - 67; http://dx.doi.org/10.1002/eji.201243071; PMID: 23519951
- Laderach D, Wesa A, Galy A. 4-1BB-ligand is regulated on human dendritic cells and induces the production of IL-12. Cell Immunol 2003; 226:37 - 44; http://dx.doi.org/10.1016/j.cellimm.2003.11.003; PMID: 14746806
- Kim YJ, Li G, Broxmeyer HE. 4-1BB ligand stimulation enhances myeloid dendritic cell maturation from human umbilical cord blood CD34+ progenitor cells. J Hematother Stem Cell Res 2002; 11:895 - 903; http://dx.doi.org/10.1089/152581602321080556; PMID: 12590704
- Lippert U, Zachmann K, Ferrari DM, Schwarz H, Brunner E, Mahbub-Ul Latif AH, Neumann C, Soruri A. CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response. Eur J Immunol 2008; 38:1024 - 32; http://dx.doi.org/10.1002/eji.200737800; PMID: 18395851