Abstract
Enterococci have the potential for resistance to virtually all clinically useful antibiotics. Their emergence as important nosocomial pathogens has coincided with increased expression of antimicrobial resistance by members of the genus. The mechanisms underlying antibiotic resistance in enterococci may be intrinsic to the species or acquired through mutation of intrinsic genes or horizontal exchange of genetic material encoding resistance determinants. This paper reviews the antibiotic resistance mechanisms in Enterococcus faecium and Enterococcus faecalis and discusses treatment options.
Keywords: :
Introduction
Enterococci are Gram-positive, facultative anaerobic organisms characterized by their ability to grow at 6.5% NaCl concentrations and at high pH and to hydrolyze bile-esculin and L-pyrrolidonyl-B-naphthylamide (PYR). Formerly considered members of Lancefield group D streptococcus, DNA homology studies suggested that they are a distinct genus. Enterococci were previously considered commensal organisms of little clinical importance, but have emerged as serious nosocomial pathogens responsible for endocarditis and infections of the urinary tract, bloodstream, meninges, wounds and the biliary tract, among others.Citation1 Recent surveillance data indicate that the enterococcus is the third most commonly isolated nosocomial pathogen (12% of all hospital infections), behind only coagulase-negative staphylococcus and Staphylococcus aureus.Citation2 The rise in prevalence of enterococcal infections in humans is influenced to some degree by the ability of enterococci to escape the action of our most commonly used antibiotics. The influence of antibiotics is most directly seen on the extent to which enterococci colonize the gastrointestinal tract. Animal data have clearly shown the relationship between exposure to parenteral antibiotics, especially extended-spectrum cephalosporins and agents with potent activity against anaerobic bacteria, and high level gastrointestinal colonization by ampicillin-resistant Enterococcus faecium.Citation3 The relationship between colonization and subsequent infection is also established. Along with increasing antimicrobial resistance, the acquisition of virulence factors and the ability of enterococcus to form biofilms have also contributed to the rise in nosocomial prevalence.Citation4
This paper reviews the mechanisms underlying antibiotic resistance in enterococci, both intrinsic (universally found within the genome of the species) and acquired (through acquisition of new genetic material or through sporadic mutations to intrinsic genes). Interspecies differences will be addressed as they arise throughout the paper. This paper will additionally provide an overview of current treatment strategies for enterococcal infections. Focus will be on Enterococcus faecalis and E. faecium, as these two species account for the overwhelming majority of human enterococcal infections ().
Table 1. Mechanisms of resistance to E. faecium and E. faecalis
Intrinsic Resistance
β-lactams and cephalosporins
Growth of most bacteria depends upon enzymatic linkage of pentapeptide precursor molecules into a peptidoglycan cell wall. The enzymes responsible for these cross-linking reactions are referred to as penicillin binding proteins (PBPs) because β-lactams (structural analogs of pentapeptide precursors) bind covalently and disrupt normal cell wall growth.Citation5 Attachment of β-lactam agents to PBPs results in impaired cell wall synthesis and, in most cases, programmed cell death via creation of reactive oxygen species.Citation6 Enterococci express low-affinity PBPs (PBP5 in E. faecium, PBP4 in E. faecalis) that bind weakly to β-lactam antibiotics. As a result, minimum inhibitory concentrations (MICs) for penicillins are typically 2–8 µg/ml for E. faecalis and 8–16 µg/ml for E. faecium,Citation7 much higher than MICs for streptococci and related Gram-positive organisms that do not contain chromosomally-encoded low-affinity PBP genes.Citation8 At the population level, enterococcal MICs have increased over time.Citation9,Citation10 Galloway-Pena et al.Citation11 demonstrated two distinct clades of E. faecium. These clades have PBP5 enzymes that vary in affinity, a result of differences in amino acid sequence and transcriptional regulation. Overproduction of non-mutated low-affinity PBPs represents a relatively rare mechanism by which enterococci express low-level resistance to penicillins.Citation7,Citation12
Early studies by Jawetz et al.Citation13 indicated that enterococci were not killed by penicillin when exposed to drug concentrations in the range of the MIC (a phenomenon known as tolerance). Tolerance in E. faecalis has been attributed to removal of reactive oxygen species by the enzyme superoxide dismutase.Citation14 In other Gram-positive species, downregulation or absence of a two-component signal transduction VncR/S autolytic system also contributes to penicillin tolerance,Citation15 but this mechanism has not been demonstrated in enterococcus. Tolerance may be induced when penicillin is administered by pulsed-dosing. As such, penicillin-naive enterococcal strains may appear susceptible in vitro but develop tolerance after exposure to the drug.Citation16,Citation17
Aminoglycosides
Both E. faecium and E. faecalis are intrinsically resistant to clinically achievable concentrations of aminoglycosides. In E. faecalis, MICs vary for the aminoglycosides, with the greatest degree of resistance seen to streptomycin (MIC up to 500 µg/ml). Intrinsic resistance in E. faecalis is attributed to an inability of the aminoglycoside to enter the cell (where they act by inhibiting ribosomal protein synthesis), as demonstrated in experiments by Moellering and colleagues in the early 1970s.Citation18,Citation19 When enterococci were exposed to radiolabeled aminoglycoside with or without penicillin, higher intracellular aminoglycoside concentrations were achieved in the presence of the cell wall synthesis inhibitor. The combination of cell wall active agents and aminoglycosides also resulted in bactericidal activity (bactericidal synergism). These studies provide physiologic context to the long-standing observations of improved clinical outcomes with aminoglycoside-penicillin combination therapy.Citation20
Some enterococci also express chromosomally-encoded enzymes that increase the MIC of aminoglycosides and prevent synergism. Ubiquitous among E. faecium, the aminoglycoside 6′ acetyltransferase [AAC(6′)-Ii] confers resistance to tobramycin with MICs as high as 1000 µg/ml and to kanamycin.Citation21 Additionally, an efmM-encoded m5C methyltransferase in E. faecium confers low-level resistance to dibekacin, tobramycin and kanamycin.Citation22 EfmM methylates the 16S rRNA resulting in a sterically-hindered ribosome target site.Citation22
Intrinsic enzyme-mediated high-level resistance to neither gentamicin nor streptomycin has been described in enterococci. As such, these drugs retain synergistic activity in enterococci and have consequently emerged as the drugs of choice to achieve synergism in severe infections caused by either E. faecium or E. faecalis.Citation23
Lincosamides and streptogramins
E. faecalis are intrinsically resistant to clindamycin (a lincosamide), quinupristin (streptogramin B class) and dalfopristin (streptogramin A class) through activity conferred by expression of the lsa gene. lsa is related structurally to ATP-binding cassette (ABC)-efflux pumps, suggesting drug efflux as a possible mechanism,Citation24 and was found in 180/180 strains of E. faecalis and 0/189 other enterococcus species, suggesting the gene is intrinsic to E. faecalis.Citation24 In general, for clinical resistance to quinupristin-dalfopristin to occur, the bacteria must be resistant to both streptogramin A and streptogramin B. E. faecium harbors a different putative ABC-efflux pump encoded by the intrinsic msrC gene.Citation25 This gene, a close relative of msrA and msrB in staphylococci, confers low-level resistance (MIC 1–2 µg/ml) to streptogramin B compounds, explaining the elevated quinupristin-dalfopristin MICs seen when E. faecium acquires a separate determinant that confers streptogramin A resistance alone.
Trimethoprim-sulfamethoxazole
Most bacteria lack the ability to absorb folate from the environment and as such require de novo folate synthesis in order to produce nucleic acids. The antibiotic combination trimethoprim-sulfamethoxazole inhibits two sequential steps in the tetrahydrofolate synthesis pathway, thereby inhibiting folate synthesis and synergistically killing a broad spectrum of bacterial species. Enterococci are unusual in that they can absorb folic acid from the environment, bypassing the effects of trimethoprim-sulfamethoxazole.Citation26 Therefore, in vitro testing of enterococcal susceptibility to trimethoprim-sulfamethoxazole in a media devoid of folate will yield a susceptible result.Citation27 Despite this apparent in vitro susceptibility, trimethoprim-sulfamethoxazole is ineffective in treating serious enterococcus infections.Citation28,Citation29
Acquired Resistance
Acquired resistance in enterococci (that which is not intrinsic to the species) can occur through sporadic mutations or through acquisition of foreign genetic material. Horizontal gene exchange among enterococci occurs through the transfer of pheromone-sensitive or broad host range plasmids, or through the movement of transposons. With few exceptions, multiple plasmids and transposons can be identified in clinical strains. These elements may interact with each other and with the bacterial chromosome to form composite mobile elements. For recent reviews of the plasmids and transposons in enterococci, refer to PalmerCitation30 and Hagstead,Citation31 respectively.
Pheromone-responsive plasmids are found predominantly in E. faecalis. Chromosomally encoded lipoprotein fragments (“pheromones”) released by recipient cells are sensed by nearby donor cells and stimulate production of aggregation substance (Asa1, PrgB and others), encoded by the plasmid.Citation32 Aggregation substance interacts with enterococcal binding substance (EBS) on the surface of the recipient cell and stimulates recipient-donor contact that promotes conjugation.Citation30 These plasmids transmit genetic information in a highly efficient manner between E. faecalis strains (10−3/donor cell during 4 h of mating), but are largely restricted to this species. pRUM plasmids in E. faecium are similar to pheromone-responsive plasmids in E. faecalis in that they transfer at a high frequency but exhibit a narrow host range.Citation31
In contrast, broad host range plasmids are capable of transferring genetic information to other gram-positive and even gram-negative species,Citation30 but at a lower frequency (10−7/donor cell during 4 h of mating) than pheromone-responsive plasmids. Transfer of these plasmids requires close contact between cells. Inc.18-type plasmids are well-known broad host range plasmids that have been implicated in the transfer of vancomycin resistance determinants to S. aureus in recent years.Citation33
Three types of transposons are responsible for most gene mobility in enterococci, Tn3 family transposons, composite transposons, and conjugative transposons.Citation31 The prototypical Tn3 family transposons are Tn917 [conferring macrolide, lincosamide and streptograminB resistance (MLSB)] and Tn1546 (conferring glycopeptide resistance), whereas the prototypical conjugative transposon is Tn916, which confers resistance to minocycline and tetracycline.Citation34 Composite transposons can readily be formed by the interaction of related IS elements that are liberally sprinkled throughout the genome of most clinical enterococcal strains. The movement of these IS elements not only confers mobility to resistance genes, but it promotes cointegration of plasmids with other plasmids and with the bacterial chromosome.
β-lactams
Enterococci may develop increased resistance to penicillins through acquisition of β-lactamases or PBP4/5 mutations. Plasmid-mediated bla genes (encoding β-lactamases) were first described in E. faecalis in 1983.Citation35 Since that time, enterococcal β-lactamase production has been rare and described predominantly in E. faecalis. The bla genes in enterococcus are identical to those in S. aureusCitation8 and are often encoded by remnants of staphylococcal β-lactamase transposon Tn552.
High-level penicillin resistance in E. faecium is most commonly associated with accumulation of point mutations in the penicillin binding region of PBP5.Citation5 A variety of point mutations have been described in both E. faeciumCitation36,Citation37 and E. faecalis.Citation38 Although these point mutations likely originated de novo in individual bacteria under selective pressure from antibiotics, chromosome-to-chromosome transfer of low affinity pbp5 genes has been documented in vitro and likely contributes to the dissemination of high-level penicillin resistance in E. faecium.Citation39
Ceftaroline and ceftobiprole, fifth generation cephalosporins, have activity against enterococcus, but may be prone to emergence of resistance with increased clinical use. Clark et al.Citation40 subjected E. faecalis to serial passages of ceftaroline and identified two resistant isolates (one with an MIC of 8 µg/ml and the other with an MIC of 32 µg/ml). Ceftobiprole shows good in vitro activity against E. faecalis with no reports of resistance to date,Citation41,Citation42 but is ineffective against penicillin-resistant clinical strains of E. faecium.Citation43,Citation44
Aminoglycosides
While intrinsic mechanisms result in low-level aminoglycoside resistance, acquisition of mobile genetic elements typically underlies high-level aminoglycoside resistance in both E. faecium and E. faecalis. Ensuing MICs range from 2,000 µg/ml to as high as 128,000 µg/ml.Citation1 Among the genes that encode high-level resistance, the most concerning are those that result in gentamicin and streptomycin resistance because these antibiotics are used for synergistic therapy of serious enterococcal infections.
High-level gentamicin resistance most frequently occurs through acquisition of a bifunctional gene encoding APH(2′′)-Ia-AAC(6′)-Ie.Citation45 These enzymes inactivate gentamicin (and structurally related aminoglycosides) by phosphorylation at the 2′hydroxy position of gentamicin and simultaneous acetylation of the 6′hydroxy position of the other aminoglycosides.Citation46,Citation47 The modified antibiotic is no longer capable of binding to its target on the 30S ribosomal subunit and thereby loses antibacterial activity. Strains that contain aph(2′′)-Ia-aac(6′)-Ie are clinically resistant to all aminoglycosides except for streptomycin.Citation48 The aph(2′′)-Ia-aac(6′)-Ie gene is most commonly flanked by IS256 in a composite transposon designated Tn4001 in S. aureus and Tn5281 in E. faecalis.
Several other genes have been identified that confer gentamicin resistance, including aph(2′′)-Ic, aph(2′′)-Id and aph(2′′)-Ib.Citation49–Citation51 In comparison with aph(2′′)-Ia-aac(6′)-1e, these genes are minor contributors to gentamicin resistance in enterococci. Their prevalence varies by geographical region. Importantly, MICs for enterococci harboring aph(2′′)-Ic may be as low as 256 µg/ml, an MIC which would be interpreted as gentamicin-susceptible by labs that use an MIC of 500 µg/ml as a cut-off to determine high-level gentamicin resistance. Despite the lower MIC, bacteria expressing these enzymes are resistant to the synergistic activity of cell wall active agents and gentamicin.Citation48 Thus, in geographical area where aph(2′′)-Ic is present, laboratories should be alerted to lower the threshold MIC for gentamicin to enhance detection of enterococci that would be resistant to synergy.
High-level resistance to streptomycin occurs most commonly through enzymatic modification of the antibiotic or by single point mutations to the ribosome. Two well-described adenylyltransferases, Ant(6′)-Ia and Ant(3′′)-Ia, are capable of inactivating streptomycin (and structurally related aminoglycosides).Citation48,Citation52 Enterococci can also develop ribosomal mutations that result in streptomycin resistance. Whereas resistance caused by aminoglycoside-modifying enzymes (AME) will typically have MICs in the 4,000 to 16,000 µg/ml range, ribosomal mutations result in MICs of 128,000 µg/ml.Citation53
Other acquired AMEs have been identified in enterococci, including Aph(3′)-IIIa, an aminoglycoside phosphotransferase that confers resistance to kanamycinCitation54 and Ant(4′′)-Ia, a nucleotidyltransferase that confers resistance to tobramycin, amikacin, neomycin and kanamycin.Citation55 As these enzymes do not confer gentamicin or streptomycin resistance, they are of less clinical significance.
Glycopeptides
The acquisition of glycopeptide resistance by enterococci has been an epidemiological and antimicrobial dilemma for the past 25 years. First described in 1988, glycopeptide-resistant enterococci (GRE) have since emerged as a major cause of nosocomial infections. The majority of GRE infections are attributed to E. faecium, although glycopeptide resistance occurs in E. faecalis and other enterococcus species as well. Currently in the United States, an estimated 30% of clinical enterococcus isolates are resistant to glycopeptides.Citation56
Vancomycin acts by binding to the d-ala-d-ala terminus of the pentapeptide precursor, thereby inhibiting cell wall synthesis. Glycopeptide-resistant organisms modify these pentapeptide precursors, replacing the terminal d-ala with D-lac or d-ser. These modified cell wall precursors bind glycopeptides with 1,000-fold lower affinity than do normal precursors. To create the modified precursors at least seven enzymes are required. Using the VanA cassette as a model, these enzymes are VanA, H, X, Y, Z, R and S (). Initially, cellular pyruvate is converted to d-lactate by the VanH dehydrogenase. The VanA ligase then ligates d-ala to d-lac. Host enzymes ligate d-ala-d-lac to the tripeptide precursor, yielding the low affinity pentapeptide precursor. Full resistance to glycopeptides, however, requires not only construction of the altered precursor, but also elimination of normal precursors.Citation33,Citation57,Citation58 VanX hydrolyzes d-ala-d-ala to its constituent amino acids, which allows d-ala-d-lac to be the sole substrate for cell wall synthesis.Citation59 VanY hydrolyzes the terminal d-ala from any normal pentapeptide precursor, rendering it useless for normal cell wall construction.Citation60 The mechanism by which VanZ augments resistance is unknown, but when present it confers decreased susceptibility to teicoplanin.Citation61 Additional open reading frames VanW and VanV have been described on the VanB operon; their functions also are not yet known.Citation62
Figure 1. An illustration of the VanA resistance mechanism as it relates to normal cell wall synthesis. The top pathway denotes normal cell wall synthesis, and the mechanisms by which VanX and VanY disrupt this pathway. The shaded pathway denotes construction of a modified cell wall that is resistant to vancomycin. Adapted from Gold et al.Citation58
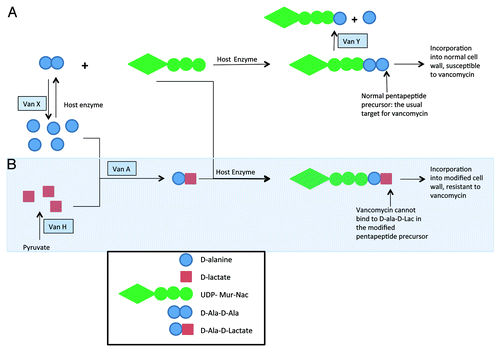
Expression of the genes for VanA, H, X, Y and Z are all regulated by VanR and VanS, a two-component sensor-transducer system that is part of the VanA operon within Tn1546. While the specific regulatory factors are not known, the presence of glycopeptides in the environment results in activation of VanS through autophosphorylation. Activated VanS then phosphorylates VanR. Phosphorylated VanR increases VanH, A, X, Y and Z transcription through interaction with specific promoter regions. VanR also interacts with its own promoter region, augmenting VanR and VanS transcription.Citation63 Clinical strains that harbor the VanA operon but contain deletions in VanR and VanS genes have been isolated and are susceptible to both vancomycin and teicoplanin. This suggests that VanR activity is required for the full expression of the VanA operon.Citation64,Citation65
VanA and VanB operons are by far the most prevalent in human GRE infections. In the VanA phenotype, the enterococcus is resistant to both vancomycin and teicoplanin. In the VanB phenotype, vancomycin but not teicoplanin induces resistance resulting in a vancomycin resistant, teicoplanin-susceptible phenotype; however, constitutive expression (which may be selected by teicoplanin exposure) results in resistance to both compounds. VanC resistance is intrinsic to E. gallinarum and E. casseliflavus. A total of nine resistance operons have been described. They may be grouped by their ligase activity. Operons that encode D-lac ligases result in high-level resistance with MICs > 256 µg/ml (VanA, VanB, VanD and VanM) while operons that encode d-ser ligases result in low-level resistance with MICs 8–16 µg/ml (VanC, VanE, VanG, VanL and VanN).Citation31,Citation66–Citation69 Of the low-level resistance phenotypes, only VanN has been shown to be transferable.
Horizontal transfer of the Van genes occurs through a variety of mechanisms. VanA is mobilized on Tn3-family transposon Tn1546. Tn1546 is found on both non-conjugative and conjugative plasmids. Inc.18 plasmids are broad host range plasmids that have been implicated in the transfer of the VanA operon to methicillin-resistant S. aureus.Citation33 Vancomycin-resistant S. aureus (VRSA) has been found in clinical settings in a handful of cases. Werner et al.Citation70 demonstrated in vitro that interspecies transfer of Tn1546 is relatively uncommon compared with intraspecies transfer. It appears that while broad host-range plasmids can transfer between species, their stability within different species varies. As such, broad host range plasmids containing an intact copy of Tn1546 may transfer resistance to staphylococci more stably, since the transposon can transfer to replicons within the staphylococcal strain that are stable. Staphylococcal variants that have acquired broad host range plasmids with Tn1546 variants that have lost their ability to transfer through deletion of or insertion into the transposition genes will exhibit an unstable phenotype due to the instability of the plasmid in the staphylococcal milieu.Citation71 Additionally, in vitro studies demonstrating transfer of Tn1546 from enterococcus to S. aureus have occurred in E. faecalis.Citation72,Citation73 Sequence homology has been observed between plasmids found in VRSA isolates and GRE isolates taken from VRSA infected patients, with the most overlap occurring with E. faecalis isolates.Citation74 Compared with E. faecium, VanA-containing E. faecalis are relatively uncommon in the clinical setting. If E. faecalis is a more effective (but less common) donor than E. faecium, then this may help to explain why VanA in staphylococci is rare. VanB is most often carried on a carried on Tn5382/1549 or related conjugative transposons. VanB carrying transposons have been identified in pheromone-sensitive and conjugative plasmids.Citation30,Citation31
The complex enzymatic pathways that confer glycopeptide resistance predate the emergence of GRE in the late 1980s. E. gallinarum and E. casseliflavus exhibit innate low-level resistance through a chromosomally-encoded VanC operonCitation75 and have been implicated as a source of the genes seen in other Van phenotypes.Citation76,Citation77 Additionally, a number of soil and bowel organisms have been identified as harboring VanB genes and may have played a role in the transfer of glycopeptide-resistance genes to E. faecium.Citation78-Citation82 Enterococci are increasingly recognized as belonging to two distinct clades, one that predominates in the hospital environment and another within the community. These clades differ genetically, and may have diverged between 300,000 to a million years ago.Citation83 The nosocomial clade has acquired virulence and resistance determinants that confer a selective advantage in this setting. Acquisition of the VanA cassette in the late 1980s likely conferred further advantage that contributed to the observed increase in prevalence of infections due to E. faecium.
Streptogramins
The streptogramin B/A combination quinupristin-dalfopristin is one of two antibiotics approved by the FDA for treatment of infections caused by vancomycin-resistant E. faecium. Because E. faecalis are intrinsically resistance to streptogramins, the majority of genes that confer horizontally-transferable resistance have been isolated from E. faecium. Between 1 to 12% of E. faecium isolates are resistant to streptogramins.Citation84,Citation85 There are three mechanisms by which acquired genetic elements cause streptogramin resistance: acetylation of the antibiotic, efflux of the antibiotic, and dimethylation of the 23S rRNA target site. To date, 12 genes that cause streptogramin resistance have been described in enterococci, although additional genes have been described in staphylococci and streptococci.
The widespread use of virginamycin, a veterinary streptogramin A compound, was associated with extensive resistance among enterococci isolated from farm animals and agricultural sewage. Consequently, quinupristin-dalfopristin resistance is most common in environmental samples, although the prevalence in nosocomial infections with resistance is increasing.Citation84 Enzymatic acetylation of streptogramin A compounds was the first resistance mechanism described in the class. Virginamycin acetyltransferase genes vatD, vatE and vatH are among the streptogramin resistance genes with probable veterinary origins. vatD and vatE (formerly called satG) have been isolated from plasmids alongside erm and vgbA genes (described below) that reduce susceptibility to streptogramin B—thus providing full resistance to quinupristin-dalfopristin.Citation86–Citation89 One plasmid has been identified with both vatD and the VanA operon,Citation90 resulting in resistance to both vancomycin and quinupristin (but not dalfopristin) when expressed in recipient cells in vitro. VatH may be seen in conjunction with another streptogramin acetyltransferase, VgbA, the only known acetyltransferase with activity against streptogramin B in enterococci.Citation88 All of the above acetyltransferase genes have been isolated exclusively from E. faecium, with the exception of vatE which has been isolated from E. faecium and from E. faecalis in a veterinary setting.Citation91
The ABC-efflux channel VgaD also plays a role in acquired streptogramin resistance, independent of the intrinsic ABC-efflux channels encoded by lsa genes in E. faecalis and msrC gene in E. faecium (described above). VgaD has been described only in E. faecium. vgaD was found on a plasmid with vatH, both of which confer only streptogramin A resistance.Citation92 To date, no other acquired streptogramin efflux pumps have been described.
Perhaps the best understood mechanism of streptogramin resistance is dimethylation of the 23S rRNA.Citation25 This resistance mechanism, which confers the MLSA or MLSB phenotype occurs through acquisition of either the ermA or ermB genes on broad host range plasmids such as pAMβ1. If these plasmids also contain vatE or vatD genes, then they confer resistance to quinupristin-dalfopristin when acquired by a recipient cell.
Linezolid
Prior to FDA approval in 2000, reports of linezolid resistance in enterococci existed but were rare. The emergence of linezolid resistance occurred slowly and only in sporadic cases associated with prolonged exposure.Citation93 The industry-sponsored LEADER trial has monitored linezolid efficacy from 2004 to 2009 and has found yearly resistance rates between 0.49 and 1.83%.Citation56 In contrast, Pogue et al.Citation94 found linezolid resistance in 20% of GRE samples from the University of Pittsburgh Medical Center. Only 25% of isolates in their study were associated with prior linezolid exposure, suggesting clonal spread.
Linezolid is a first-in-class oxazolidinone, an entirely synthetic class of antibiotics that binds to the initiation complex and inhibits protein synthesis. Most bacteria, including the enterococci, have multiple copies of the genes encoding 23S rRNA. E. faecalis have four copies of the geneCitation95 and E. faecium six copies.Citation96 In theory, the presence of multiple gene copies makes resistance from sporadic mutations less likely because the unaffected gene copies would mask the effect of the mutated gene. However, recombination between susceptible and resistant copies (referred to as “gene conversion”) will yield strains with multiple mutated copies under persistent linezolid selective pressure. In clinical isolates, a mutation in one E. faecium rRNA gene conferred an MIC of 8–16 µg/ml. The same mutation in > 3 rRNA genes conferred an MIC between 64–128 µg/ml.Citation96 A variety of point mutations that confer linezolid resistance have been identified, the most common of which is G2576T. In the most recent LEADER study results (2009), the G2576T mutation was identified in all eight of the linezolid-resistant enterococci strains isolated in the United States. Four of the eight strains found in this study were isolated in Louisville, KY and appeared clonally related.Citation56,Citation97 Other sporadic point mutations have been associated with linezolid resistance, including G2505A and L4 (F101L).Citation98-Citation100
In 2006, the transferable cfr gene was identified in S. aureus as the source of resistance to linezolid, lincosamides and streptogramin A compounds, among others.Citation101 Cfr encodes an rRNA methyltransferase that modifies an adenosine in the linezolid-binding region on the 23S rRNA, preventing antibiotic binding. It is hypothesized that the cfr gene emerged from animal strains of bacteria that were exposed to natural compounds with an rRNA binding site similar to linezolid.Citation101,Citation102 In 2011, cfr was identified in an E. faecalis strain (designated EF-01) from a cattle farm in China.Citation103 In this strain, the gene was located on a plasmid (pEF-01) and flanked by IS1216, suggesting transposability. This was the first enterococcus harboring cfr to be reported in the literature, although human isolates of E. faecalis and E. faecium with cfr were reported in a 2010 abstract.Citation103 Overall, linezolid resistance remains rare in enterococci.
Daptomycin
Daptomycin is a lipoprotein with bactericidal activity against enterococci. While not FDA approved for treatment of GRE, it is often used by clinicians for this purpose.Citation104-Citation106 The epidemiology of daptomycin resistance in enterococcus (defined as MIC > 4 µg/ml) was recently reviewed by Kelesidis et al.Citation107 Rates of daptomycin resistance in this study were approximately 0.6% (111 daptomycin resistant isolates/17,084 enterococcus isolates total). In general, E. faecium is more likely than E. faecalis to express daptomycin resistance, although resistance has been reported in both species. The increased prevalence of daptomyicin resistance in E. faecium may reflect increased use of daptomycin with this species compared with E. faecalis, which is usually susceptible to penicillins. Daptomycin resistance appears to be less common in North America than in Asia or Europe.Citation107
Daptomycin incorporates itself into the cell membrane of Gram-positive organisms in the presence of physiologic calcium concentrations and promotes leakage of intracellular potassium into the extracellular space, resulting in cell death by destruction of the transcellular potassium gradient.Citation108-Citation110 Normal cell membrane polarity is required for daptomycin intercalation. In staphylococci, alteration of the cell membrane charge by virtue of modification of cell membrane lipoproteins has been associated with reduced daptomycin susceptibility.Citation111 A number of genes have been described in staphylococci that contribute to daptomycin resistance,Citation112 none of which have been identified in enterococcus to date. While the mechanism of daptomycin resistance in enterococcus remains unresolved, several reports have elucidated gene mutations associated with enterococcus daptomycin resistance.Citation113,Citation114
Palmer et al.Citation113 created three daptomycin-resistant strains by exposing E. faecalis to increasing daptomycin concentrations until stable resistance was identified. They then performed complete genome sequencing of the strains before and after emergence of daptomycin resistance and identified seven gene mutations. Of the seven mutations observed in this study, only EF1797 and EF0631 gene mutations were identified in all three resistant strains. EF1797 encodes a putative membrane protein that may be involved in phosphatidylserine and sphingolipid synthesis, but its function has yet to be determined. EF0631 encodes a putative cardiolipin sythetase (cls), a transphosphatidylase involved in the synthesis of the cell membrane protein cardiolipin. One specific mutation in this gene, R218Q, was found in two of the resistant strains and occurs in the presumed active domain of the EF0631 enzyme. Through comparison with a DNA sequence database, the authors identified one other E. faecalis strain with an EF0631 frameshift mutation, but this isolate had a daptomycin-susceptible phenotype.
In a similar study, Arias et al.Citation114 compared the nucleotide sequence and cell membrane proteins of E. faecalis isolates before and after the development of daptomycin resistance in a patient with enterococcus bacteremia. Genome sequencing of the resistant strain revealed three mutated genes: cls, gdpD and liaF. A cardiolipin synthetase mutation was identified but when the mutant gene was placed in daptomycin-sensitive enterococcus strain the MIC did not change. The same cls mutation was observed in other E. faecalis and E. faecium strains resistant to daptomycin. The other two gene mutations, gdpD (glycerophosphoryl diester phosphodiesterase) and liaF (lipid II cycle-interfering antibiotic protein), did have an impact on MICs when reconstituted in the daptomycin-susceptible strain. The liaF mutation increased the MIC from 1 to 4 µg/ml. The gdpD mutation did not increase the MIC, but the combination of both proteins increased the MIC to 12 µg/ml. Mutations in both gdpD and liaF were also identified in other resistant strains of E. faecalis and E. faecium, but were not demonstrated in the Palmer study. Thus, cardiolipin synthetase, GdpD and LiaF are cell membrane proteins associated with daptomycin resistance. Given that a number of different membrane-associated proteins have been linked to reduced daptomycin susceptibility in staphylococci, it seems likely that more genes conferring enterococcal resistance to daptomycin will be identified in the future.
Tigecycline
Tigecycline, a novel glycylcycline antibiotic, gained FDA approval in 2005 for complicated intra-abdominal infections, skin and soft tissue infections, and community-acquired pneumonia. It has been used off-label to successfully treat MRSA and GRE infections.Citation115 Typical tigecycline MICs for enterococcus range from 0.125 µg/ml to 0.25 µg/ml, while MICs > 0.5 µg/ml are considered resistant. Early surveillance studies of tigecycline showed no cases of resistant enterococcus,Citation116 although two case reports of E. faecalis strains with MICs of 2 µg/ml and 6 µg/ml, respectively, have been described.Citation117,Citation118 A more recent study from Taiwan reviewed antimicrobial resistance among 219 VRE isolates and found two isolates with a tigecycline MIC of 0.5, and one isolate with an MIC of 1, with a trend toward increasing tigecycline MIC over time.Citation119 The mechanism of tigecycline resistance in enterococcus is unknown. In staphylococcus, tigecycline resistance is mediated by a novel family of efflux pumps,Citation120 but these genes have not been demonstrated in enterococcus.
Other antibiotics
Resistance occurs to other antibiotics including macrolides, tetracyclines, chloramphenicol, fosfomycin, rifampin and quinolones. These resistance mechanisms will not be described in this review, as resistance to these antimicrobial agents is so common that they are seldom involved in treatment of enterococcus infections.
Management
Because of the differences in resistance patterns between E. faecium and E. faecalis, it is imperative to differentiate the pathogen to the species level and perform susceptibility testing on strains isolated from patients with clinical infections. Treatment of enterococcal infections depends upon (1) the species, (2) the resistance patterns present in the clinical isolate and (3) the location and severity of the infection. Uncomplicated enterococcal infections may be adequately treated with monotherapy, whereas severe infections such as endocarditis benefit from a synergistic regimen.
In uncomplicated, fully susceptible E. faecalis and E. faecium infections, ampicillin remains the preferred therapy. In the uncommon presence of β-lactamase, combination with a β-lactamase inhibitor such as sulbactam may improve outcomes. When complicated infections such as endocarditis occur in susceptible enterococcal infections, an aminoglycoside should be added to a cell wall active agent for synergistic killing, as has been the standard for almost 60 years.Citation20 Among aminoglycosides, only gentamicin and streptomycin should be considered for synergistic therapy. Historically, twice-daily or three times daily aminoglycoside dosing regimens have been used. In streptococcus infections, once-daily aminoglycoside dosing was shown to be effective in humans.Citation121 For enterococcal endocarditis, though, once-daily aminoglycoside dosing has only been studied in animal models with evidence forCitation122–Citation124 and againstCitation125,Citation126 its use. Differences between these studies likely reflect the pharmacodynamic and pharmacokinetic differences between the animal models. In humans, the efficacy of once-daily aminoglycoside dosing has not been established for enterococcus infections. As such, guidelines continue to recommend three times daily dosing for gentamicin and twice-daily dosing for streptomycin.Citation127 In the presence of high-level penicillin resistance, synergy has been observed in animal models with the combination of aminoglycosides and other cell wall active antibiotics including vancomycin or daptomycin.Citation128
In the instance of complicated enterococcal infections resistant to high-levels of gentamicin and streptomycin, an alternative agent must be used for synergistic activity. Despite relative resistance to both agents, the combination of ceftriaxone and ampicillin has been shown to be efficacious in animals.Citation129 In case reports and prospective case-series, 56 patients have been treated with this combination with success rate of 71.4% (40/56);Citation130-Citation132 although these numbers may reflect publication bias. The presumed benefit of ceftriaxone-ampicillin combination therapy is attributed to full saturation of PBPs 2–4, which cannot be achieved with either agent alone. By inhibiting all PBPs, the bacteria have no alternative enzyme with which to build a cell wall. In in vitro and animal model studies, similar synergistic bactericidal activity has been shown with other cell wall active combination therapies, including ceftriaxone-fosfomycinCitation133 and ampicillin-imipenem,Citation134 but not with ampicillin-ertapenem.Citation135
Treatment of glycopeptide-resistant enterococcus
Vancomycin-resistant enterococci pose particular problems for treatment because the strains which harbor VanA and VanB resistance are also typically resistant to other classes of antibiotics. While only linezolid and quinupristin-dalfopristin have FDA approval for treatment of GRE infections, other antimicrobial agents including daptomycin, tigecycline, fosfomycin, quinolones, tetracyclines and new fifth generation cephalosporins exhibit in vitro activity and have been used with success in individual cases. In uncomplicated cases, monotherapy based upon the antibiotic susceptibility profile is appropriate. In complicated cases such as endocarditis, the ideal therapy for GRE has not been determined.
Both linezolid and quinupristin-dalfopristin have been shown to be efficacious in treatment of complicated GRE infections and are FDA approved for this indication.Citation56,Citation136 Linezolid has been used for GRE endocarditis in both E. faecalis and E. faecium, both with and without additional agents. To date, it has not been shown that combination therapy is more efficacious than monotherapy in this setting. Because linezolid is not bactericidal, treatment of GRE endocarditis with linezolid remains controversial.Citation23 Quinupristin-dalfopristin can be efficacious against E. faecium, but should not be used to treat E. faecalis due to the intrinsic presence of lsa-mediated resistance (described above). When using quinupristin-dalfopristin for treatment of severe or complicated GRE infections, combination therapy may be necessary although the optimal choice and dose of adjunct antibiotic has yet to be determined. Several studies have compared linezolid to quinupristin-dalfopristin. In a small, single-center study, Chong et al.Citation137 found increased resistance and increased number of days of bacteremia in patients treated with quinupristin-dalfopristin. Several other studies also reported more resistance to quinupristin-dalfopristin than linezolid,Citation56,Citation138 suggesting that of the two linezolid may be superior for GRE treatment.
Use of daptomycin for GRE infections, particularly endocarditis, is appealing because of its bactericidal activity against enterococci.Citation104 While monotherapy may be adequate in many GRE infections, daptomycin failure has been reported.Citation106,Citation139 Arias et al.Citation139 reported subsequent response to the combination of daptomycin/gentamicin/ampicillin after failing daptomycin monotherapy, which may be explained by a synergistic effect of the triple therapy. Daptomycin synergy has been described in vitro with ampicillin, cephalosporins, imipenem, rifampin and gentamicin.Citation140-Citation143 As with quinupristin-dalfopristin, there may be a role for adjunct antibiotics to achieve a synergistic effect, although appropriate agents and dosing regimens have not been adequately evaluated in humans.
Tigecycline has also been used off-label for treatment of GRE. Cai et al.Citation115 performed a meta-analysis of randomized trials to evaluate the use of tigecycline for GRE infections. While efficacy of treatment was no different for tigecycline monotherapy than other empirical regimens, there were significantly more adverse events and a non-significant trend toward higher mortality. As such, tigecycline monotherapy should not be considered as first line for treatment of GRE infections. Tigecycline demonstrates in vitro synergism with a number of other agents, including rifampin and daptomycin, although these combinations have not been evaluated beyond small case series in humans.Citation144
Future Directions
Over the years enterococci have demonstrated the potential to harbor and transfer resistance genes and as such have become an important clinical pathogen. A better understanding of resistance mechanisms to daptomycin and tigecycline is needed and will aid in the prediction and prevention of epidemiologic spread. Several new drugs are emerging as potential options for GRE treatment. Ceftaroline has been shown to be more efficacious than linezolid in animals and may play a larger role in the future.Citation145 Additionally, arbekacin, which is not currently available in the United States, has demonstrated synergistic killing in combination with penicillins even in the presence of high-level gentamicin and streptomycin resistance.Citation146 A number of antibiotic combinations, including those mentioned in the management section, have shown in vitro synergistic activity and are promising as potential treatment modalities for complicated GRE infections, but must first be evaluated more rigorously in humans. The novel glycopeptide oritavancin is currently under investigation and shows promise in treating GRE infections. In addition, non-antimicrobial pharmacotherapy targeted at specific virulence factors (such as anti-adhesions) may play a preventative or therapeutic role in the management of enterococcal infections. Future directions of research must focus on development of new antimicrobial agents. Finally, efforts must continue to prevent development of antibiotic resistance and spread in the enterococci through infection control and antibiotic stewardship programs.
References
- Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev 1990; 3:46 - 65; PMID: 2404568
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al, National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 2008; 29:996 - 1011; http://dx.doi.org/10.1086/591861; PMID: 18947320
- Rice LB, Lakticová V, Helfand MS, Hutton-Thomas R. In vitro antienterococcal activity explains associations between exposures to antimicrobial agents and risk of colonization by multiresistant enterococci. J Infect Dis 2004; 190:2162 - 6; http://dx.doi.org/10.1086/425580; PMID: 15551215
- Sava IG, Heikens E, Huebner J. Pathogenesis and immunity in enterococcal infections. Clin Microbiol Infect 2010; 16:533 - 40; http://dx.doi.org/10.1111/j.1469-0691.2010.03213.x; PMID: 20569264
- Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev 2008; 32:361 - 85; http://dx.doi.org/10.1111/j.1574-6976.2007.00095.x; PMID: 18248419
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007; 130:797 - 810; http://dx.doi.org/10.1016/j.cell.2007.06.049; PMID: 17803904
- Sifaoui F, Arthur M, Rice L, Gutmann L. Role of penicillin-binding protein 5 in expression of ampicillin resistance and peptidoglycan structure in Enterococcus faecium. Antimicrob Agents Chemother 2001; 45:2594 - 7; http://dx.doi.org/10.1128/AAC.45.9.2594-2597.2001; PMID: 11502534
- Murray BE. Beta-lactamase-producing enterococci. Antimicrob Agents Chemother 1992; 36:2355 - 9; PMID: 1489177
- Grayson ML, Eliopoulos GM, Wennersten CB, Ruoff KL, De Girolami PC, Ferraro MJ, et al. Increasing resistance to beta-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob Agents Chemother 1991; 35:2180 - 4; PMID: 1803989
- Galloway-Peña JR, Nallapareddy SR, Arias CA, Eliopoulos GM, Murray BE. Analysis of clonality and antibiotic resistance among early clinical isolates of Enterococcus faecium in the United States. J Infect Dis 2009; 200:1566 - 73; http://dx.doi.org/10.1086/644790; PMID: 19821720
- Galloway-Peña JR, Rice LB, Murray BE. Analysis of PBP5 of early U.S. isolates of Enterococcus faecium: sequence variation alone does not explain increasing ampicillin resistance over time. Antimicrob Agents Chemother 2011; 55:3272 - 7; http://dx.doi.org/10.1128/AAC.00099-11; PMID: 21576454
- Fontana R, Aldegheri M, Ligozzi M, Lopez H, Sucari A, Satta G. Overproduction of a low-affinity penicillin-binding protein and high-level ampicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother 1994; 38:1980 - 3; PMID: 7811006
- Jawetz E, Sonne M. Penicillin-streptomycin treatment of enterococcal endocarditis. A re-evaluation. N Engl J Med 1966; 274:710 - 5; http://dx.doi.org/10.1056/NEJM196603312741304; PMID: 5908873
- Bizzini A, Zhao C, Auffray Y, Hartke A. The Enterococcus faecalis superoxide dismutase is essential for its tolerance to vancomycin and penicillin. J Antimicrob Chemother 2009; 64:1196 - 202; http://dx.doi.org/10.1093/jac/dkp369; PMID: 19828491
- Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 2010; 8:423 - 35; http://dx.doi.org/10.1038/nrmicro2333; PMID: 20440275
- Krogstad DJ, Pargwette AR. Defective killing of enterococci: a common property of antimicrobial agents acting on the cell wall. Antimicrob Agents Chemother 1980; 17:965 - 8; PMID: 6902640
- Hodges TL, Zighelboim-Daum S, Eliopoulos GM, Wennersten C, Moellering RC Jr.. Antimicrobial susceptibility changes in Enterococcus faecalis following various penicillin exposure regimens. Antimicrob Agents Chemother 1992; 36:121 - 5; PMID: 1590676
- Zimmermann RA, Moellering RC Jr., Weinberg AN. Mechanism of resistance to antibiotic synergism in enterococci. J Bacteriol 1971; 105:873 - 9; PMID: 4994038
- Moellering RC Jr., Weinberg AN. Studies on antibiotic syngerism against enterococci. II. Effect of various antibiotics on the uptake of 14 C-labeled streptomycin by enterococci. J Clin Invest 1971; 50:2580 - 4; http://dx.doi.org/10.1172/JCI106758; PMID: 5001959
- Geraci JE, Martin WJ. Antibiotic therapy of bacterial endocarditis. VI. Subacute enterococcal endocarditis; clinical, pathologic and therapeutic consideration of 33 cases. Circulation 1954; 10:173 - 94; http://dx.doi.org/10.1161/01.CIR.10.2.173; PMID: 13182750
- Costa Y, Galimand M, Leclercq R, Duval J, Courvalin P. Characterization of the chromosomal aac(6′)-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother 1993; 37:1896 - 903; PMID: 8239603
- Galimand M, Schmitt E, Panvert M, Desmolaize B, Douthwaite S, Mechulam Y, et al. Intrinsic resistance to aminoglycosides in Enterococcus faecium is conferred by the 16S rRNA m5C1404-specific methyltransferase EfmM. RNA 2011; 17:251 - 62; http://dx.doi.org/10.1261/rna.2233511; PMID: 21159796
- Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect 2010; 16:555 - 62; http://dx.doi.org/10.1111/j.1469-0691.2010.03214.x; PMID: 20569266
- Singh KV, Weinstock GM, Murray BE. An Enterococcus faecalis ABC homologue (Lsa) is required for the resistance of this species to clindamycin and quinupristin-dalfopristin. Antimicrob Agents Chemother 2002; 46:1845 - 50; http://dx.doi.org/10.1128/AAC.46.6.1845-1850.2002; PMID: 12019099
- Portillo A, Ruiz-Larrea F, Zarazaga M, Alonso A, Martinez JL, Torres C. Macrolide resistance genes in Enterococcus spp. Antimicrob Agents Chemother 2000; 44:967 - 71; http://dx.doi.org/10.1128/AAC.44.4.967-971.2000; PMID: 10722498
- Bushby SR, Hitchings GH. Trimethoprim, a sulphonamide potentiator. Br J Pharmacol Chemother 1968; 33:72 - 90; PMID: 5301731
- Zervos MJ, Schaberg DR. Reversal of the in vitro susceptibility of enterococci to trimethoprim-sulfamethoxazole by folinic acid. Antimicrob Agents Chemother 1985; 28:446 - 8; PMID: 3935044
- Grayson ML, Thauvin-Eliopoulos C, Eliopoulos GM, Yao JD, DeAngelis DV, Walton L, et al. Failure of trimethoprim-sulfamethoxazole therapy in experimental enterococcal endocarditis. Antimicrob Agents Chemother 1990; 34:1792 - 4; PMID: 2126691
- Chenoweth CE, Robinson KA, Schaberg DR. Efficacy of ampicillin versus trimethoprim-sulfamethoxazole in a mouse model of lethal enterococcal peritonitis. Antimicrob Agents Chemother 1990; 34:1800 - 2; PMID: 2126692
- Palmer KL, Kos VN, Gilmore MS. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr Opin Microbiol 2010; 13:632 - 9; http://dx.doi.org/10.1016/j.mib.2010.08.004; PMID: 20837397
- Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjord A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin microbiol infect 2010; 16:541 - 54; http://dx.doi.org/10.1111/j.1469-0691.2010.03226.x; PMID: 20569265
- Wardal E, Sadowy E, Hryniewicz W. Complex nature of enterococcal pheromone-responsive plasmids. Polish J Microbiol 2010; 59:79 87
- Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009; 53:4580 - 7; http://dx.doi.org/10.1128/AAC.00346-09; PMID: 19506057
- Rice LB. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob Agents Chemother 1998; 42:1871 - 7; PMID: 9687377
- Murray BE, Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest 1983; 72:1168 - 71; http://dx.doi.org/10.1172/JCI111042; PMID: 6411768
- Fontana R, Ligozzi M, Pittaluga F, Satta G. Intrinsic penicillin resistance in enterococci. Microb Drug Resist 1996; 2:209 - 13; http://dx.doi.org/10.1089/mdr.1996.2.209; PMID: 9158761
- Rice LB, Bellais S, Carias LL, Hutton-Thomas R, Bonomo RA, Caspers P, et al. Impact of specific pbp5 mutations on expression of beta-lactam resistance in Enterococcus faecium. Antimicrob Agents Chemother 2004; 48:3028 - 32; http://dx.doi.org/10.1128/AAC.48.8.3028-3032.2004; PMID: 15273117
- Ono S, Muratani T, Matsumoto T. Mechanisms of resistance to imipenem and ampicillin in Enterococcus faecalis. Antimicrob Agents Chemother 2005; 49:2954 - 8; http://dx.doi.org/10.1128/AAC.49.7.2954-2958.2005; PMID: 15980374
- Rice LB, Carias LL, Rudin S, Lakticová V, Wood A, Hutton-Thomas R. Enterococcus faecium low-affinity pbp5 is a transferable determinant. Antimicrob Agents Chemother 2005; 49:5007 - 12; http://dx.doi.org/10.1128/AAC.49.12.5007-5012.2005; PMID: 16304165
- Clark C, McGhee P, Appelbaum PC, Kosowska-Shick K. Multistep resistance development studies of ceftaroline in gram-positive and -negative bacteria. Antimicrob Agents Chemother 2011; 55:2344 - 51; http://dx.doi.org/10.1128/AAC.01602-10; PMID: 21343467
- Arias CA, Singh KV, Panesso D, Murray BE. Evaluation of ceftobiprole medocaril against Enterococcus faecalis in a mouse peritonitis model. J Antimicrob Chemother 2007; 60:594 - 8; http://dx.doi.org/10.1093/jac/dkm237; PMID: 17606481
- Arias CA, Singh KV, Panesso D, Murray BE. Time-kill and synergism studies of ceftobiprole against Enterococcus faecalis, including beta-lactamase-producing and vancomycin-resistant isolates. Antimicrob Agents Chemother 2007; 51:2043 - 7; http://dx.doi.org/10.1128/AAC.00131-07; PMID: 17438057
- Lascols C, Legrand P, Mérens A, Leclercq R, Muller-Serieys C, Drugeon HB, et al. In vitro antibacterial activity of ceftobiprole against clinical isolates from French teaching hospitals: proposition of zone diameter breakpoints. Int J Antimicrob Agents 2011; 37:235 - 9; http://dx.doi.org/10.1016/j.ijantimicag.2010.11.035; PMID: 21295447
- Henry X, Amoroso A, Coyette J, Joris B. Interaction of ceftobiprole with the low-affinity PBP 5 of Enterococcus faecium. Antimicrob Agents Chemother 2010; 54:953 - 5; http://dx.doi.org/10.1128/AAC.00983-09; PMID: 19917749
- Mederski-Samoraj BD, Murray BE. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis 1983; 147:751 - 7; http://dx.doi.org/10.1093/infdis/147.4.751; PMID: 6404994
- Ferretti JJ, Gilmore KS, Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6′-aminoglycoside acetyltransferase 2″-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol 1986; 167:631 - 8; PMID: 3015884
- Courvalin P, Carlier C, Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol 1980; 143:541 - 51; PMID: 6259117
- Chow JW. Aminoglycoside resistance in enterococci. Clin Infect Dis 2000; 31:586 - 9; http://dx.doi.org/10.1086/313949; PMID: 10987725
- Chow JW, Zervos MJ, Lerner SA, Thal LA, Donabedian SM, Jaworski DD, et al. A novel gentamicin resistance gene in Enterococcus. Antimicrob Agents Chemother 1997; 41:511 - 4; PMID: 9055984
- Mahbub Alam M, Kobayashi N, Ishino M, Sumi A, Kobayashi K, Uehara N, et al. Detection of a novel aph(2″) allele (aph[2″]-Ie) conferring high-level gentamicin resistance and a spectinomycin resistance gene ant(9)-Ia (aad 9) in clinical isolates of enterococci. Microb Drug Resist 2005; 11:239 - 47; http://dx.doi.org/10.1089/mdr.2005.11.239; PMID: 16201926
- Tsai SF, Zervos MJ, Clewell DB, Donabedian SM, Sahm DF, Chow JW. A new high-level gentamicin resistance gene, aph(2″)-Id, in Enterococcus spp. Antimicrob Agents Chemother 1998; 42:1229 - 32; PMID: 9593155
- Hollingshead S, Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid 1985; 13:17 - 30; http://dx.doi.org/10.1016/0147-619X(85)90052-6; PMID: 2986186
- Eliopoulos GM, Farber BF, Murray BE, Wennersten C, Moellering RC Jr.. Ribosomal resistance of clinical enterococcal to streptomycin isolates. Antimicrob Agents Chemother 1984; 25:398 - 9; PMID: 6326668
- Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5″-aminoglycoside phosphotransferase type III. Gene 1983; 23:331 - 41; http://dx.doi.org/10.1016/0378-1119(83)90022-7; PMID: 6313476
- Carlier C, Courvalin P. Emergence of 4′,4″-aminoglycoside nucleotidyltransferase in enterococci. Antimicrob Agents Chemother 1990; 34:1565 - 9; PMID: 2171424
- Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob Agents Chemother 2011; 55:3684 - 90; http://dx.doi.org/10.1128/AAC.01729-10; PMID: 21670176
- Arthur M, Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother 1993; 37:1563 - 71; PMID: 8215264
- Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001; 33:210 - 9; http://dx.doi.org/10.1086/321815; PMID: 11418881
- Reynolds PE, Depardieu F, Dutka-Malen S, Arthur M, Courvalin P. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of D-alanyl-D-alanine. Mol Microbiol 1994; 13:1065 - 70; http://dx.doi.org/10.1111/j.1365-2958.1994.tb00497.x; PMID: 7854121
- Reynolds PE, Arias CA, Courvalin P. Gene vanXYC encodes D,D -dipeptidase (VanX) and D,D-carboxypeptidase (VanY) activities in vancomycin-resistant Enterococcus gallinarum BM4174. Mol Microbiol 1999; 34:341 - 9; http://dx.doi.org/10.1046/j.1365-2958.1999.01604.x; PMID: 10564477
- Arthur M, Depardieu F, Molinas C, Reynolds P, Courvalin P. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 1995; 154:87 - 92; http://dx.doi.org/10.1016/0378-1119(94)00851-I; PMID: 7867956
- Ribeiro T, Santos S, Marques MI, Gilmore M, de Fátima Silva Lopes M. Identification of a new gene, vanV, in vanB operons of Enterococcus faecalis. Int J Antimicrob Agents 2011; 37:554 - 7; http://dx.doi.org/10.1016/j.ijantimicag.2011.01.024; PMID: 21482081
- Arthur M, Molinas C, Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol 1992; 174:2582 - 91; PMID: 1556077
- Gagnon S, Lévesque S, Lefebvre B, Bourgault AM, Labbé AC, Roger M. vanA-containing Enterococcus faecium susceptible to vancomycin and teicoplanin because of major nucleotide deletions in Tn1546. J Antimicrob Chemother 2011; 66:2758 - 62; http://dx.doi.org/10.1093/jac/dkr379; PMID: 21926081
- Choi HJ, Nam D, Peck KR, Song JH, Shin D, Ko KS. Loss of vancomycin resistance not completely dependent on the Tn1546 element in Enterococcus faecium isolates. Diagn Microbiol Infect Dis 2011; 69:105 - 10; http://dx.doi.org/10.1016/j.diagmicrobio.2010.08.030; PMID: 21146722
- Fines M, Perichon B, Reynolds P, Sahm DF, Courvalin P. VanE, a new type of acquired glycopeptide resistance in Enterococcus faecalis BM4405. Antimicrob Agents Chemother 1999; 43:2161 - 4; PMID: 10471558
- Lebreton F, Depardieu F, Bourdon N, Fines-Guyon M, Berger P, Camiade S, et al. D-Ala-d-Ser VanN-type transferable vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother 2011; 55:4606 - 12; http://dx.doi.org/10.1128/AAC.00714-11; PMID: 21807981
- McKessar SJ, Berry AM, Bell JM, Turnidge JD, Paton JC. Genetic characterization of vanG, a novel vancomycin resistance locus of Enterococcus faecalis. Antimicrob Agents Chemother 2000; 44:3224 - 8; http://dx.doi.org/10.1128/AAC.44.11.3224-3228.2000; PMID: 11036060
- Xu X, Lin D, Yan G, Ye X, Wu S, Guo Y, et al. vanM, a new glycopeptide resistance gene cluster found in Enterococcus faecium. Antimicrob Agents Chemother 2010; 54:4643 - 7; http://dx.doi.org/10.1128/AAC.01710-09; PMID: 20733041
- Werner G, Freitas AR, Coque TM, Sollid JE, Lester C, Hammerum AM, et al. Host range of enterococcal vanA plasmids among Gram-positive intestinal bacteria. J Antimicrob Chemother 2011; 66:273 - 82; http://dx.doi.org/10.1093/jac/dkq455; PMID: 21131318
- Foucault ML, Courvalin P, Grillot-Courvalin C. Fitness cost of VanA-type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009; 53:2354 - 9; http://dx.doi.org/10.1128/AAC.01702-08; PMID: 19332680
- Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003; 302:1569 - 71; http://dx.doi.org/10.1126/science.1090956; PMID: 14645850
- de Niederhäusern S, Bondi M, Messi P, Iseppi R, Sabia C, Manicardi G, et al. Vancomycin-resistance transferability from VanA enterococci to Staphylococcus aureus. Curr Microbiol 2011; 62:1363 - 7; http://dx.doi.org/10.1007/s00284-011-9868-6; PMID: 21234755
- Zhu W, Murray PR, Huskins WC, Jernigan JA, McDonald LC, Clark NC, et al. Dissemination of an Enterococcus Inc18-Like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2010; 54:4314 - 20; http://dx.doi.org/10.1128/AAC.00185-10; PMID: 20660665
- Leclercq R, Dutka-Malen S, Duval J, Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother 1992; 36:2005 - 8; PMID: 1416893
- Navarro F, Courvalin P. Analysis of genes encoding D-alanine-D-alanine ligase-related enzymes in Enterococcus casseliflavus and Enterococcus flavescens. Antimicrob Agents Chemother 1994; 38:1788 - 93; PMID: 7986009
- Arias CA, Courvalin P, Reynolds PE. vanC cluster of vancomycin-resistant Enterococcus gallinarum BM4174. Antimicrob Agents Chemother 2000; 44:1660 - 6; http://dx.doi.org/10.1128/AAC.44.6.1660-1666.2000; PMID: 10817725
- Guardabassi L, Christensen H, Hasman H, Dalsgaard A. Members of the genera Paenibacillus and Rhodococcus harbor genes homologous to enterococcal glycopeptide resistance genes vanA and vanB. Antimicrob Agents Chemother 2004; 48:4915 - 8; http://dx.doi.org/10.1128/AAC.48.12.4915-4918.2004; PMID: 15561881
- Guardabassi L, Agersø Y. Genes homologous to glycopeptide resistance vanA are widespread in soil microbial communities. FEMS Microbiol Lett 2006; 259:221 - 5; http://dx.doi.org/10.1111/j.1574-6968.2006.00270.x; PMID: 16734783
- Patel R, Piper K, Cockerill FR 3rd, Steckelberg JM, Yousten AA. The biopesticide Paenibacillus popilliae has a vancomycin resistance gene cluster homologous to the enterococcal VanA vancomycin resistance gene cluster. Antimicrob Agents Chemother 2000; 44:705 - 9; http://dx.doi.org/10.1128/AAC.44.3.705-709.2000; PMID: 10681342
- Stinear TP, Olden DC, Johnson PD, Davies JK, Grayson ML. Enterococcal vanB resistance locus in anaerobic bacteria in human faeces. Lancet 2001; 357:855 - 6; http://dx.doi.org/10.1016/S0140-6736(00)04206-9; PMID: 11265957
- Tsvetkova K, Marvaud JC, Lambert T. Analysis of the mobilization functions of the vancomycin resistance transposon Tn1549, a member of a new family of conjugative elements. J Bacteriol 2010; 192:702 - 13; http://dx.doi.org/10.1128/JB.00680-09; PMID: 19966009
- Galloway-Peña J, Roh JH, Latorre M, Qin X, Murray BE. Genomic and SNP analyses demonstrate a distant separation of the hospital and community-associated clades of Enterococcus faecium. PLoS One 2012; 7:e30187; http://dx.doi.org/10.1371/journal.pone.0030187; PMID: 22291916
- McDonald LC, Rossiter S, Mackinson C, Wang YY, Johnson S, Sullivan M, et al. Quinupristin-dalfopristin-resistant Enterococcus faecium on chicken and in human stool specimens. N Engl J Med 2001; 345:1155 - 60; http://dx.doi.org/10.1056/NEJMoa010805; PMID: 11642231
- Raad II, Hanna HA, Hachem RY, Dvorak T, Arbuckle RB, Chaiban G, et al. Clinical-use-associated decrease in susceptibility of vancomycin-resistant Enterococcus faecium to linezolid: a comparison with quinupristin-dalfopristin. Antimicrob Agents Chemother 2004; 48:3583 - 5; http://dx.doi.org/10.1128/AAC.48.9.3583-3585.2004; PMID: 15328133
- Hammerum AM, Flannagan SE, Clewell DB, Jensen LB. Indication of transposition of a mobile DNA element containing the vat(D) and erm(B) genes in Enterococcus faecium. Antimicrob Agents Chemother 2001; 45:3223 - 5; http://dx.doi.org/10.1128/AAC.45.11.3223-3225.2001; PMID: 11600385
- Jensen LB, Hammerum AM, Aarestrup FM. Linkage of vat(E) and erm(B) in streptogamin-resistant Enterococcus faecium isolates from Europe. Antimicrob Agents Chemother 2000; 44:2231 - 2; http://dx.doi.org/10.1128/AAC.44.8.2231-2232.2000; PMID: 11023445
- Jensen LB, Hammerum AM, Aerestrup FM, van den Bogaard AE, Stobberingh EE. Occurrence of satA and vgb genes in streptogramin-resistant Enterococcus faecium isolates of animal and human origins in the Netherlands. Antimicrob Agents Chemother 1998; 42:3330 - 1; PMID: 10049241
- Bozdogan B, Leclercq R. Effects of genes encoding resistance to streptogramins A and B on the activity of quinupristin-dalfopristin against Enterococcus faecium. Antimicrob Agents Chemother 1999; 43:2720 - 5; PMID: 10543753
- Bozdogan B, Leclercq R, Lozniewski A, Weber M. Plasmid-mediated coresistance to streptogramins and vancomycin in Enterococcus faecium HM1032. Antimicrob Agents Chemother 1999; 43:2097 - 8; PMID: 10484759
- Hershberger E, Donabedian S, Konstantinou K, Zervos MJ. Quinupristin-dalfopristin resistance in gram-positive bacteria: mechanism of resistance and epidemiology. Clin Infect Dis 2004; 38:92 - 8; http://dx.doi.org/10.1086/380125; PMID: 14679454
- Jung YH, Shin ES, Kim O, Yoo JS, Lee KM, Yoo JI, et al. Characterization of two newly identified genes, vgaD and vatH, [corrected] conferring resistance to streptogramin A in Enterococcus faecium. Antimicrob Agents Chemother 2010; 54:4744 - 9; http://dx.doi.org/10.1128/AAC.00798-09; PMID: 20713681
- Meka VG, Gold HS. Antimicrobial resistance to linezolid. Clin Infect Dis 2004; 39:1010 - 5; http://dx.doi.org/10.1086/423841; PMID: 15472854
- Pogue JM, Paterson DL, Pasculle AW, Potoski BA. Determination of risk factors associated with isolation of linezolid-resistant strains of vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2007; 28:1382 - 8; http://dx.doi.org/10.1086/523276; PMID: 17994519
- Lee ZM, Bussema C 3rd, Schmidt TM. rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucleic Acids Res 2009; 37:Database issue D489 - 93; http://dx.doi.org/10.1093/nar/gkn689; PMID: 18948294
- Marshall SH, Donskey CJ, Hutton-Thomas R, Salata RA, Rice LB. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother 2002; 46:3334 - 6; http://dx.doi.org/10.1128/AAC.46.10.3334-3336.2002; PMID: 12234875
- Mendes RE, Deshpande LM, Castanheira M, DiPersio J, Saubolle MA, Jones RN. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Chemother 2008; 52:2244 - 6; http://dx.doi.org/10.1128/AAC.00231-08; PMID: 18391032
- Bourgeois-Nicolaos N, Massias L, Couson B, Butel MJ, Andremont A, Doucet-Populaire F. Dose dependence of emergence of resistance to linezolid in Enterococcus faecalis in vivo. J Infect Dis 2007; 195:1480 - 8; http://dx.doi.org/10.1086/513876; PMID: 17436228
- Biedenbach DJ, Farrell DJ, Mendes RE, Ross JE, Jones RN. Stability of linezolid activity in an era of mobile oxazolidinone resistance determinants: results from the 2009 Zyvox® Annual Appraisal of Potency and Spectrum program. Diagn Microbiol Infect Dis 2010; 68:459 - 67; http://dx.doi.org/10.1016/j.diagmicrobio.2010.09.018; PMID: 21094428
- Prystowsky J, Siddiqui F, Chosay J, Shinabarger DL, Millichap J, Peterson LR, et al. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob Agents Chemother 2001; 45:2154 - 6; http://dx.doi.org/10.1128/AAC.45.7.2154-2156.2001; PMID: 11408243
- Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. The Cfr rRNA methyltransferase confers resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A antibiotics. Antimicrob Agents Chemother 2006; 50:2500 - 5; http://dx.doi.org/10.1128/AAC.00131-06; PMID: 16801432
- Toh SM, Xiong L, Arias CA, Villegas MV, Lolans K, Quinn J, et al. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol 2007; 64:1506 - 14; http://dx.doi.org/10.1111/j.1365-2958.2007.05744.x; PMID: 17555436
- Liu Y, Wang Y, Wu C, Shen Z, Schwarz S, Du X, et al. First Report of the Multidrug Resistance gene cfr in Enterococcus faecalis of Animal origin. Antimicrob Agents Chemother 2012; 56:1650 - 4; http://dx.doi.org/10.1128/AAC.06091-11; PMID: 22203597
- Cantón R, Ruiz-Garbajosa P, Chaves RL, Johnson AP. A potential role for daptomycin in enterococcal infections: what is the evidence?. J Antimicrob Chemother 2010; 65:1126 - 36; http://dx.doi.org/10.1093/jac/dkq087; PMID: 20363805
- Crank CW, Scheetz MH, Brielmaier B, Rose WE, Patel GP, Ritchie DJ, et al. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: A retrospective, multicenter, cohort study. Clin Ther 2010; 32:1713 - 9; http://dx.doi.org/10.1016/j.clinthera.2010.09.008; PMID: 21194593
- Poutsiaka DD, Skiffington S, Miller KB, Hadley S, Snydman DR. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. J Infect 2007; 54:567 - 71; http://dx.doi.org/10.1016/j.jinf.2006.11.007; PMID: 17188750
- Kelesidis T, Humphries R, Uslan DZ, Pegues DA. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin Infect Dis 2011; 52:228 - 34; http://dx.doi.org/10.1093/cid/ciq113; PMID: 21288849
- Lakey JH, Ptak M. Fluorescence indicates a calcium-dependent interaction between the lipopeptide antibiotic LY146032 and phospholipid membranes. Biochemistry 1988; 27:4639 - 45; http://dx.doi.org/10.1021/bi00413a009; PMID: 2844233
- Alborn WE Jr., Allen NE, Preston DA. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob Agents Chemother 1991; 35:2282 - 7; PMID: 1666494
- Silverman JA, Perlmutter NG, Shapiro HM. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47:2538 - 44; http://dx.doi.org/10.1128/AAC.47.8.2538-2544.2003; PMID: 12878516
- Silverman JA, Oliver N, Andrew T, Li T. Resistance studies with daptomycin. Antimicrob Agents Chemother 2001; 45:1799 - 802; http://dx.doi.org/10.1128/AAC.45.6.1799-1802.2001; PMID: 11353628
- Friedman L, Alder JD, Silverman JA. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother 2006; 50:2137 - 45; http://dx.doi.org/10.1128/AAC.00039-06; PMID: 16723576
- Palmer KL, Daniel A, Hardy C, Silverman J, Gilmore MS. Genetic basis for daptomycin resistance in enterococci. Antimicrob Agents Chemother 2011; 55:3345 - 56; http://dx.doi.org/10.1128/AAC.00207-11; PMID: 21502617
- Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, et al. Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med 2011; 365:892 - 900; http://dx.doi.org/10.1056/NEJMoa1011138; PMID: 21899450
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162 - 72; http://dx.doi.org/10.1128/AAC.01402-10; PMID: 21173186
- Kresken M, Leitner E, Seifert H, Peters G, von Eiff C. Susceptibility of clinical isolates of frequently encountered bacterial species to tigecycline one year after the introduction of this new class of antibiotics: results of the second multicentre surveillance trial in Germany (G-TEST II, 2007). Eur J Clin Microbiol Infect Dis 2009; 28:1007 - 11; http://dx.doi.org/10.1007/s10096-009-0725-5; PMID: 19296137
- Werner G, Gfrörer S, Fleige C, Witte W, Klare I. Tigecycline-resistant Enterococcus faecalis strain isolated from a German intensive care unit patient. J Antimicrob Chemother 2008; 61:1182 - 3; http://dx.doi.org/10.1093/jac/dkn065; PMID: 18285315
- Cordina C, Hill R, Deshpande A, Hood J, Inkster T. Tigecycline-resistant Enterococcus faecalis associated with omeprazole use in a surgical patient. J Antimicrob Chemother 2012; 67:1806 - 7; http://dx.doi.org/10.1093/jac/dks122; PMID: 22454491
- Tsai HY, Liao CH, Chen YH, Lu PL, Huang CH, Lu CT, et al. Trends in susceptibility of vancomycin-resistant Enterococcus faecium to tigecycline, daptomycin, and linezolid and molecular epidemiology of the isolates: results from the Tigecycline In Vitro Surveillance in Taiwan (TIST) study, 2006 to 2010. Antimicrob Agents Chemother 2012; 56:3402 - 5; http://dx.doi.org/10.1128/AAC.00533-12; PMID: 22491684
- McAleese F, Petersen P, Ruzin A, Dunman PM, Murphy E, Projan SJ, et al. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob Agents Chemother 2005; 49:1865 - 71; http://dx.doi.org/10.1128/AAC.49.5.1865-1871.2005; PMID: 15855508
- Francioli P, Ruch W, Stamboulian D. Treatment of streptococcal endocarditis with a single daily dose of ceftriaxone and netilmicin for 14 days: a prospective multicenter study. Clin Infect Dis 1995; 21:1406 - 10; http://dx.doi.org/10.1093/clinids/21.6.1406; PMID: 8749624
- Gavaldà J, Cardona PJ, Almirante B, Capdevila JA, Laguarda M, Pou L, et al. Treatment of experimental endocarditis due to Enterococcus faecalis using once-daily dosing regimen of gentamicin plus simulated profiles of ampicillin in human serum. Antimicrob Agents Chemother 1996; 40:173 - 8; PMID: 8787901
- Houlihan HH, Stokes DP, Rybak MJ. Pharmacodynamics of vancomycin and ampicillin alone and in combination with gentamicin once daily or thrice daily against Enterococcus faecalis in an in vitro infection model. J Antimicrob Chemother 2000; 46:79 - 86; http://dx.doi.org/10.1093/jac/46.1.79; PMID: 10882693
- López P, Gavaldà J, Martin MT, Almirante B, Gomis X, Azuaje C, et al. Efficacy of teicoplanin-gentamicin given once a day on the basis of pharmacokinetics in humans for treatment of enterococcal experimental endocarditis. Antimicrob Agents Chemother 2001; 45:1387 - 93; http://dx.doi.org/10.1128/AAC.45.5.1387-1393.2001; PMID: 11302800
- Fantin B, Carbon C. Importance of the aminoglycoside dosing regimen in the penicillin-netilmicin combination for treatment of Enterococcus faecalis-induced experimental endocarditis. Antimicrob Agents Chemother 1990; 34:2387 - 91; PMID: 2128443
- Marangos MN, Nicolau DP, Quintiliani R, Nightingale CH. Influence of gentamicin dosing interval on the efficacy of penicillin-containing regimens in experimental Enterococcus faecalis endocarditis. J Antimicrob Chemother 1997; 39:519 - 22; http://dx.doi.org/10.1093/jac/39.4.519; PMID: 9145826
- Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr., Bolger AF, Levison ME, et al, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association, Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394 - 434; http://dx.doi.org/10.1161/CIRCULATIONAHA.105.165564; PMID: 15956145
- Furustrand Tafin U, Majic I, Zalila Belkhodja C, Betrisey B, Corvec S, Zimmerli W, et al. Gentamicin improves the activities of daptomycin and vancomycin against Enterococcus faecalis in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 2011; 55:4821 - 7; http://dx.doi.org/10.1128/AAC.00141-11; PMID: 21807979
- Gavaldá J, Onrubia PL, Gómez MT, Gomis X, Ramírez JL, Len O, et al. Efficacy of ampicillin combined with ceftriaxone and gentamicin in the treatment of experimental endocarditis due to Enterococcus faecalis with no high-level resistance to aminoglycosides. J Antimicrob Chemother 2003; 52:514 - 7; http://dx.doi.org/10.1093/jac/dkg360; PMID: 12917251
- Miro JM, Cervera C, Garcia-de-la-Maria C, Del Rio A, Armero Y, Mestres CA, et al. Success of ampicillin plus ceftriaxone rescue therapy for a relapse of Enterococcus faecalis native-valve endocarditis and in vitro data on double beta-lactam activity. Scand J Infect Dis 2008; 40:968 - 72; http://dx.doi.org/10.1080/00365540802398945; PMID: 18767002
- Euba G, Lora-Tamayo J, Murillo O, Pedrero S, Cabo J, Verdaguer R, et al. Pilot study of ampicillin-ceftriaxone combination for treatment of orthopedic infections due to Enterococcus faecalis. Antimicrob Agents Chemother 2009; 53:4305 - 10; http://dx.doi.org/10.1128/AAC.00444-09; PMID: 19667290
- Tascini C, Doria R, Leonildi A, Martinelli C, Menichetti F. Efficacy of the combination ampicillin plus ceftriaxone in the treatment of a case of enterococcal endocarditis due to Enterococcus faecalis highly resistant to gentamicin: efficacy of the “ex vivo” synergism method. J Chemother 2004; 16:400 - 3; PMID: 15332717
- Farina C, Russello G, Chinello P, Pasticci MB, Raglio A, Ravasio V, et al, Italian Infective Endocarditis Study Group (SEI). In vitro activity effects of twelve antibiotics alone and in association against twenty-seven Enterococcus faecalis strains isolated from Italian patients with infective endocarditis: high in vitro synergistic effect of the association ceftriaxone-fosfomycin. Chemotherapy 2011; 57:426 - 33; http://dx.doi.org/10.1159/000330458; PMID: 22122863
- Brandt CM, Rouse MS, Laue NW, Stratton CW, Wilson WR, Steckelberg JM. Effective treatment of multidrug-resistant enterococcal experimental endocarditis with combinations of cell wall-active agents. J Infect Dis 1996; 173:909 - 13; http://dx.doi.org/10.1093/infdis/173.4.909; PMID: 8603970
- Pasticci MB, Mencacci A, Moretti A, Palladino N, Maria Lapalorcia L, Bistoni F, et al. In vitro Antimicrobial Activity of Ampicillin-Ceftriaxone and Ampicillin-Ertapenem Combinations Against Clinical Isolates of Enterococcus faecalis with High Levels of Aminoglycoside Resistance. Open Microbiol J 2008; 2:79 - 84; http://dx.doi.org/10.2174/1874285800802010079; PMID: 19088915
- Moellering RC, Linden PK, Reinhardt J, Blumberg EA, Bompart F, Talbot GH, Synercid Emergency-Use Study Group. The efficacy and safety of quinupristin/dalfopristin for the treatment of infections caused by vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother 1999; 44:251 - 61; http://dx.doi.org/10.1093/jac/44.2.251; PMID: 10473233
- Chong YP, Lee SO, Song EH, Lee EJ, Jang EY, Kim SH, et al. Quinupristin-dalfopristin versus linezolid for the treatment of vancomycin-resistant Enterococcus faecium bacteraemia: efficacy and development of resistance. Scand J Infect Dis 2010; 42:491 - 9; http://dx.doi.org/10.3109/00365541003699623; PMID: 20524781
- Bérenger R, Bourdon N, Auzou M, Leclercq R, Cattoir V. In vitro activity of new antimicrobial agents against glycopeptide-resistant Enterococcus faecium clinical isolates from France between 2006 and 2008. Med Mal Infect 2011; 41:405 - 9; http://dx.doi.org/10.1016/j.medmal.2010.12.013; PMID: 21550192
- Arias CA, Torres HA, Singh KV, Panesso D, Moore J, Wanger A, et al. Failure of daptomycin monotherapy for endocarditis caused by an Enterococcus faecium strain with vancomycin-resistant and vancomycin-susceptible subpopulations and evidence of in vivo loss of the vanA gene cluster. Clin Infect Dis 2007; 45:1343 - 6; http://dx.doi.org/10.1086/522656; PMID: 17968832
- Baltch AL, Ritz WJ, Bopp LH, Michelsen PB, Smith RP. Antimicrobial activities of daptomycin, vancomycin, and oxacillin in human monocytes and of daptomycin in combination with gentamicin and/or rifampin in human monocytes and in broth against Staphylococcus aureus. Antimicrob Agents Chemother 2007; 51:1559 - 62; http://dx.doi.org/10.1128/AAC.00973-06; PMID: 17283190
- Cilli F, Aydemir S, Tunger A. In vitro activity of daptomycin alone and in combination with various antimicrobials against Gram-positive cocci. J Chemother 2006; 18:27 - 32; PMID: 16572890
- Snydman DR, McDermott LA, Jacobus NV. Evaluation of in vitro interaction of daptomycin with gentamicin or beta-lactam antibiotics against Staphylococcus aureus and Enterococci by FIC index and timed-kill curves. J Chemother 2005; 17:614 - 21; PMID: 16433191
- Pankey G, Ashcraft D, Patel N. In vitro synergy of daptomycin plus rifampin against Enterococcus faecium resistant to both linezolid and vancomycin. Antimicrob Agents Chemother 2005; 49:5166 - 8; http://dx.doi.org/10.1128/AAC.49.12.5166-5168.2005; PMID: 16304195
- Entenza JM, Moreillon P. Tigecycline in combination with other antimicrobials: a review of in vitro, animal and case report studies. Int J Antimicrob Agents 2009; 34:8 - , e1-9; http://dx.doi.org/10.1016/j.ijantimicag.2008.11.006; PMID: 19162449
- Jacqueline C, Caillon J, Le Mabecque V, Miègeville AF, Ge Y, Biek D, et al. In vivo activity of a novel anti-methicillin-resistant Staphylococcus aureus cephalosporin, ceftaroline, against vancomycin-susceptible and -resistant Enterococcus faecalis strains in a rabbit endocarditis model: a comparative study with linezolid and vancomycin. Antimicrob Agents Chemother 2009; 53:5300 - 2; http://dx.doi.org/10.1128/AAC.00984-09; PMID: 19752276
- Kak V, You I, Zervos MJ, Kariyama R, Kumon H, Chow JW. In-vitro synergistic activity of the combination of ampicillin and arbekacin against vancomycin-and high-level gentamicin-resistant Enterococcus faecium with the aph(2″)-Id gene. Diagn Microbiol Infect Dis 2000; 37:297 - 9; http://dx.doi.org/10.1016/S0732-8893(00)00155-3; PMID: 10974585