Abstract
In the field of (food) toxicology, there is a strong trend of replacing animal trials with alternative methods for the assessment of adverse health effects in humans. The replacement of animal trials is not only driven by ethical concerns but also by the number of potential testing substances (food additives, packaging material, contaminants, and toxicants), which is steadily increasing. In vitro 2D cell culture applications in combination with in silico modeling might provide an applicable first response. However, those systems lack accurate predictions of metabolic actions. Thus, alternative in vivo models could fill the gap between cell culture and animal trials. In this review, we highlight relevant studies in the field and spotlight the applicability of alternative models, including C. elegans, D. rerio, Drosophila, HET-CAM and Lab-on-a-chip.
Introduction
Food and food safety are omnipresent issues in our modern society. Multiple institutions, including the World Health Organization (WHO) and the European Food Safety Agency (EFSA), have clearly articulated that unsafe food must be prohibited from being put on the market. Decisions for implementing new ingredients or products are therefore always based on risk assessment (World Health Organization Citation2009; More et al. Citation2019). Hazards regarding allergens, contaminants, nutritional data, kinetics (absorption, distribution, metabolism and excretion) and toxicological parameters (chronic toxicity, carcinogenicity, genotoxicity, immunotoxicity as well as reproductive and developmental toxicity) must be reliably identified and evaluated according to their risk potential (Boer and Bast Citation2018). It is presumed that people are exposed to approximately 100,000 synthetic chemicals, while only approximately 10% of these chemicals have been evaluated for safety (Hartung Citation2017). In addition to foodstuff, additives or packaging materials are also important for toxicological consideration.
The term food toxicology represents the study of the nature, properties, effects and the detection of toxic substances in food as well as their disease manifestation in humans (Bagchi, Swaroop, and Stohs Citation2017). Moreover, toxicokinetics is defined by the ICH Guideline S3A Citation1994 as “the generation of pharmacokinetic data, either as an integral component in the conduct of non-clinical toxicity studies or in specially designed supportive studies, in order to assess systemic exposure”. Toxicokinetics represents an integral part of the non-clinical testing program. It aims to enhance the value of the generated toxicological data as part of the assessment of risk and safety in humans.
In the field of toxicology, there is a strong trend of replacing animal trials with alternative methods for the assessment of adverse health effects in humans. The replacement of animal trials is not only driven by ethical concerns but also the low throughput and high costs of critical parameters. Currently, many industries (e.g., the chemical, agricultural, food and cosmetics industries) are facing this problem due to the large and increasing number of chemicals, toxicants and additives in products and applications. Hence, nonanimal, higher throughput testing is of great interest and importance in toxicology.
New approach methodologies (NAMs) including human cell-based in vitro methods combined with in silico bioinformatics and in chemico data are one trend for enhancing toxicological risk assessments of single compounds and their applications. Currently, most of the cell-based in vitro methods include 2D cell cultures growing in multiwell plates. A compound-induced change is usually measured by fluorescence- and luminescence readouts or by automated imaging. 2D cell culture represents a fast approach for evaluating the starting doses and acute toxicity. Furthermore, compared to more complex approaches, 2D cell culture provides a high degree of reproducibility (Zink, Chuah, and Ying Citation2020).
However, in vitro cell culture still remains a very enclosed system. The toxicokinetic effects of absorption, distribution, metabolism and excretion (ADME) are absent in cell culture applications. Even organ-specific systems (e.g., Lab-on-a-chip) typically include only a few cell types and therefore cannot cover the complexity of whole tissues or organs. This problem is partially addressed by including in silico modeling (e.g., Quantitative Structure-Activity Relationship (QSAR)) for predictions of the ADME characteristics of a compound without generating experimental data (Thiel et al. Citation2015). However, the reliability of in silico predictions for new chemicals based on the extrapolation of present data for analogous substances cannot be verified because there is little information about their applicability domains.
In addition to in silico bioinformatics and in vitro cell culture viability applications, lower surrogate in vivo model organisms are also gaining increasing attention to meet the ongoing demand for reliable higher throughput screening systems for the identification of potential adverse effects and the assessment of toxicants in foodstuff (Blaauboer et al. Citation2016).
Alternative lower animal models, such as soil nematode (Caenorhabditis elegans), zebrafish (Danio rerio) or fruit fly (Drosophila melanogaster), exhibit several advantages over mammalian animal testing: They have a very short generation time and are cheap to cultivate, favoring higher throughput applications (). In addition, the absence of ethical constraints allows for multiple concentration tests per substance, thus leading to better prediction rates regarding the toxic potential of the tested compounds. The main signaling pathways are highly conserved in these model organisms, and the gene homology between these model organisms and humans range from 65-80% (Lai et al. Citation2000; Howe et al. Citation2013; Nobrega and de Lyons Citation2020). Fully sequenced genomes of these model organisms are available, and genetic manipulation can be performed within several weeks. Because of the lucent surfaces of these model organisms, live-cell imaging is also possible. The use of a model system aims to measure the effects of oxidative stress, chronic inflammation, degenerative diseases, cell death, ageing and gene regulation (Bambino and Chu Citation2017; Gáliková and Klepsatel Citation2018; Hunt, Camacho, and Sprando Citation2020).
Figure 1. Overview of the reviewed alternative model systems for prediction of toxicity in food matter.
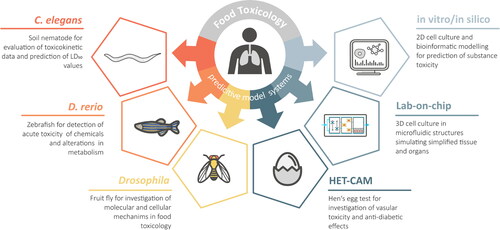
Model organisms have become irreplaceable tools for biological research. In addition to endpoint analysis, the mammals often depicted are too complex for identifying the basic biological logic. Answers, for the most part, are first discovered in lower hierarchy organisms and are then searched for in higher organisms. For future applications, human tissue cell cultures combined with in silico methods might be a promising approach for toxicity testing and drug discovery. Nevertheless, extensive research on model organisms will be necessary since many fundamental biological mechanisms still remain unsolved at present (Hunter Citation2008).
Although distinct improvements regarding those approaches have been made, currently, they are not yet approved as adequate replacements for the gold standard of animal testing (World Health Organization Citation2009). Therefore, the aim of this study is to critically review recent data on whole alternative model organisms and their possible applications in the field of food toxicology testing.
Methods
A literature search was conducted using the PubMed, ScienceDirect and Google Scholar databases and the search terms “food toxicity”, “alternative models”, “toxicokinetic”, “C. elegans”, “Drosophila”, “zebrafish”, “lab on chip”, “HET-CAM”, “in silico” and “in vitro”. Articles focusing on alternative models for predictive food toxicity testing were chosen. In this review, we focused on recent articles and reviews (2017-2021) whenever possible. The results are presented and discussed in the following sections.
Toxicokinetics in Caenorhabditis elegans
The nematode Caenorhabditis elegans (C. elegans) is a very common model organism for studying aging processes, developmental biology, and genetics (Hunt, Camacho, and Sprando Citation2020). C. elegans contains several specialized tissues, including endocrine, metabolically digestive, neuromuscular, and reproductive systems, which allow for detection of toxicological substance interactions (Wittkowski et al. Citation2019). Therefore, C. elegans serves as an attractive in vivo model for the identification of toxicokinetics. An overview is given in . The main assays used to study C. elegans comprise brood-size (number of eggs produced – total progeny), growth and reproduction, life-span and oxidative stress (Liao Citation2018; Piechulek and Mikecz Citation2018; Moyson et al. Citation2019; Spanier et al. Citation2019; Wang et al. Citation2020). Compared to rats and rabbits, in a large-scale evaluation of the developmental toxicity of chemicals in the ToxCast™ library, C. elegans was found to be very accurate for predicting developmental toxicity. For 200 phase I chemicals tested in several species, the percent active chemicals in the phase I library were 71% for C. elegans, 43% for rabbits, and 59% for rats. Balanced accuracy estimates (the average of sensitivity and specificity) for predicting rat and rabbit developmental toxicity based on C. elegans assays were 53% and 52%, respectively. C. elegans assays were the most sensitive for rabbit toxicity with 74%. For comparison, rat and rabbit studies resulted in ∼58% concordance. However, the performance was not uniform across all tested chemical classes and varied from 30-62% for rat versus C. elegans and 33-81% for rabbit versus C. elegans (Boyd et al. Citation2016). Additionally, worm development and activity tests showed promising results for identifying mammalian developmental neurotoxins. This test is currently assessed by using a panel of 20 blinded compounds with known developmental and neurotoxicity effects in mammals (Hunt et al. Citation2018). Also, the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) is highly interested in the reduction or replacement of animal use in testing, where feasible. ToxCast™ assays for identification of putative vascular disruptor compounds (pVDCs) have been tested in a variety of functional vascular development assays using zebrafish and complex in vitro cell-based models that confirmed the model to be useful in identifying environmental chemical pVDCs. The ability of a model system to respond in a similar manner compared to humans is strictly limited by how well the model and the toxicological assays are able to reproduce human exposure conditions (e.g.: stage of development, route of exposure) as well as cellular, biochemical, and molecular responses. (Boyd et al. Citation2016).
Table 1. Comparison of selected alternative models.
Wittkowski et al. Citation2019 tested triazole fungicides, which are commonly used for crop protection. However, it is assumed that development and reproduction are negatively affected by triazole fungicides in mammals. The measured half maximal inhibitory concentration (IC50) in C. elegans is correlated with the half maximal effective concentration (EC50) in rats. Based on the IC50 values, it was also shown that reproduction is a more sensitive endpoint in C. elegans than growth, while growth data are generally more robust than offspring count. Interestingly, toxicokinetic data revealed distinct differences in single substance accumulation. For example, LC-MS/MS identified 1341% prochloraz, 655% epoxiconazole, 636% tebuconazole and 144% cyproconazole accumulation in worms. Normalizing the IC50 values with these values showed a massive reduction in potency differences between the substances. Because of this normalization factor, the results of the tested substances are very comparable to EC50 in rats.
Lanzerstorfer et al. Citation2021 reported on a robust and comprehensive strategy for the assessment and prediction of the toxicological properties of selected essential oils using different alternative in vitro and in vivo approaches. The oral median lethal dose (LD50) values based on the calculated IC50 values from cell culture experiments and the lethal concentration 50% (LC50) from C. elegans data were validated by an IC50-LD50 log regression approach for estimating the starting doses for acute oral systemic toxicity testing (ICCVAM Citation2006a, Citation2006b). Importantly, the high correlation of the predicted mean LD50 values with data obtained from rats was assessed, indicating that the prediction utilizing alternative models might have sufficient potential to replace animal testing in selected applications. Nevertheless, a multiapproach system will be necessary to avoid over- or underestimation of acute toxicity in alternative model organisms. Especially, the combination of wild-type C. elegans and the bus-5 mutant, which is hypersensitive to drugs, revealed good accuracy regarding the predicted oral LD50 values from rats. Previously, it was also shown that wild-type C. elegans data were correlated with performance trials of heat-stressed broilers when Ginseng was added as a feed additive (Sandner et al. Citation2020).
Another study measured the effects of sulfa sweeteners on the lifespan and intestinal fat deposition in C. elegans (Zhang et al. Citation2019). Recently, certain side effects of these chemicals have been identified, which has raised the question of their safety in humans. Bian et al. Citation2017 identified that saccharin (SAC) induced liver inflammation in mice by changing the microbiome, while another study in mice reported reduced behavioral activity after SAC consumption (Oishi, Higo-Yamamoto, and Yasumoto Citation2016). The results of the C. elegans study showed that 0.3 mg/mL SAC mildly impaired lifespan, while at 10 mg/mL SAC, food intake and intestinal fat deposition (IFD) were also reduced at different worm stages. Since food intake and IFD are commonly used as markers for ageing processes, SAC seems to have an impact on this metabolic action site.
Bisphenol A, propylparaben, and triclosan, which are utilized as packaging materials, antimicrobial preservatives, and additives in food matter, respectively, altered reproduction and growth in an oxidative stress-dependent manner. Most interestingly, exposure to these substances also promoted lipid accumulation in the nematode (García-Espiñeira, Tejeda-Benítez, and Olivero-Verbel Citation2018; Vingskes and Spann Citation2018). Parabens, which are used as food preservatives, are associated with significant reductions in worm length reproduction and head thrash behavior. Gene expression also revealed a significant downregulation of vitellogenin genes, vit-2 and vit-6, which are involved in lipid transporter activity and regulation of protein oxidation (García-Espiñeira, Tejeda-Benítez, and Olivero-Verbel Citation2018).
Moreover, in the growing field of mycotoxins, there is a critical need for reliable toxicity tests regarding food safety. The food-relevant mycotoxins citrinin (CIT), zearalenone-14-sulfate (ZEA-14-S) and zearalenone (ZEA) led to a significant decrease in brood size. ZEA and CIT showed reduced stress tolerance levels in C. elegans, while CIT additionally decreased the mean lifespan (Keller et al. Citation2018).
Moreover, C. elegans has become a very powerful in vivo tool for probiotic screening and studying host-probiotic interactions by monitoring aging processes and body fat storage. It was shown that Lactobacillus fermentum (L. fermentum) significantly increased the median lifespan of wild-type C. elegans, and compared to controls, the intestinal colonialization capability showed 2-4-fold higher concentrations. Furthermore, ageing processes in terms of motility or the pharyngeal pumping rate were delayed when treated with. L. fermentum (Schifano et al. Citation2019). Additionally, lipid droplet accumulation and oxidative stress were reduced in the treatment group. In the context of toxicants, the gut microbiome also plays a prevalent role. Compared to standard OP50 Escherichia coli, Lee, Kim, and Choi Citation2020 identified a significantly lower microbiome change during cadmium exposure when feeding C. elegans with soil microbial community (SMB). Additionally, gene expression revealed increased chemical stress and an increased immune response in the control group.
Nanoparticles (NPs) in food matter are also gaining increasing attraction regarding their biointeraction processes. Nano silica (E551) or titanium dioxide (E171) are commonly used as food additives and are generally considered as safe by EFSA (Younes et al. Citation2018a; Younes et al. Citation2018b). However, other studies have already revealed that NPs promoted colon microinflammation and initiated preneoplastic lesions in rats (Bettini et al. Citation2017) or reduced intestinal barrier function and proinflammatory signaling in vitro (Guo et al. Citation2017; Proquin et al. Citation2017). Moreover, foodborne NPs deriving from cooking processes play an important role in this particular context. Exposure of E551 to wild-type C. elegans showed intestinal ballooning within 24 h of exposure (Piechulek, Berwanger, and Mikecz Citation2019). Studies with E171 revealed a reduction in lifespan and increased age-associated vulval integrity defects (Ma et al. Citation2019). Foodborne NPs from roast pork fed to C. elegans (10 mg/mL NPs) showed a significantly shorter length. Moreover, the locomotive behavior in terms of body bends per minute was changed. Laser confocal microscope images of C. elegans identified the epithelium of the gastrointestinal tract as the primary target of NPs (Zhao et al. Citation2019).
Hunt et al. Citation2018 established a novel worm Development and Activity Test (wDAT) assay to simultaneously monitor neurotoxins and developmental toxins that utilizes continuous motility tracking of C. elegans to assess the timing of the larval developmental stages (L1-L4) as well as stage specific activity levels. The human neurotoxins arsenic, lead and mercury were selected for evaluation using the wDAT assay. Hyperactivity was detected at concentrations that also delayed development in the larval stages L3-L4 by 6–18%. Furthermore, hypoactivity was found for arsenic and lead. Hyperactivity at low doses of the neurotoxins has also been described in rodents and children, while higher concentrations induce hypoactivity.
In addition, only a limited number of studies is dealing with an automatized data collection and analysis of phenotypical traits regarding high throughput screenings. The approaches are aiming for the combination of multiple toxicity assays, thus resulting in an increased performance. Several parameters including the worm length and shape, motility, eccentricity, pharyngal pumping rate or developmental delay for phenotypical profiling were identified toward reliable classification of substance toxicities (Mathew, Mathew, and Ebert Citation2012; Jung et al. Citation2014; Gao et al. Citation2018).
Nevertheless, it has to be noted, that C. elegans also suffers from several limitations that have to be taken into account. For example, C. elegans lacks multiple mammalian organs such as eyes, heart, kidney, liver and lungs. While a functioning innate immune system is available, adaptive immunity is not present (Hunt Citation2017). Particular molecular pathways that are relevant in mammalian fat biology (e.g.: leptin signaling) are either entirely lacking or very rudimentary in the nematode. Additionally, C. elegans does not have dedicated adipocytes (Lemieux and Ashrafi Citation2015). Due to its tough cuticle, the nematode does not represent a good absorption model. Results from liquid or solid grown C. elegans may vary largely and cannot be directly compared with each other (Çelen, Doh, and Sabanayagam Citation2018; Lev et al. Citation2019). In addition, liquid cultures require soluble test compounds. Finally, incorrect handling of the stock cultures or small changes in temperature, nutrient or salt concentration elicit adaptive responses that can significantly alter results (e.g.: gene expression patterns), sometimes for multiple generations (Hunt Citation2017).
In summary, literature clearly shows that C. elegans is suitable for evaluating selected food toxicological issues. Combined with additional approaches, the nematode is a promising candidate for first toxicity screenings. The latest research also promises predictive toxicological studies via epigenetic modifications (histones, microRNAs), mitochondrial toxicity, immunity effects or worms carrying human genes of interest (Hunt, Camacho, and Sprando Citation2020).
Toxicity studies in Danio rerio
Zebrafish (Danio rerio) is a small freshwater fish belonging to the Cyprinidae family (Horzmann and Freeman Citation2018; Souza Anselmo et al. Citation2018). Selected characteristics make zebrafish an attractive model for studying compound toxicity and the underlying mechanisms of action (Bambino and Chu Citation2017). For instance, transparent embryos that develop ex vivo allow an easy xenobiotic exposure as well as the observation of developmental abnormalities (Bambino and Chu Citation2017). Additionally, the zebrafish genome has been fully sequenced and shows high genetic similarity to humans. Hence, 70% of human genes possess a zebrafish homologue (Cassar et al. Citation2020).
Besides several advantages, there also occur limitations in the zebrafish model. While humans ingest toxic substances through contaminated drinking water and food (oral exposure), studies with zebrafish until 5 days post fertilization (dpf) rather focus on dermal exposure. Since the routes of exposure affect absorption, tissue distribution, metabolism, and excretion, the transferability of toxicological data between fish and humans must be treated with caution (Bambino and Chu Citation2017; Souza Anselmo et al. Citation2018). Moreover, the amount of substance administered by each fish cannot be accurately controlled, thus, hindering the generation of reproducible results (Bambino and Chu Citation2017; Souza Anselmo et al. Citation2018).
In Europe, experiments with zebrafish embryos/larvae under 5 dpf are not considered animal studies because the European Commission Directive 2010/63/EU on the protection of animals used for scientific purposes (Directive 2010/63/EU 2020) does not apply to larval forms until the independent feeding stage. In fact, zebrafish larvae begin independent feeding at 5 dpf (Cassar et al. Citation2020) to 6 dpf (Souza Anselmo et al. Citation2018). Thus, an animal test authorization in the European Union is not necessary during the early developmental stages of zebrafish, and its embryos represent an alternative to in vivo toxicity studies with rodents and rabbits ().
In the U.S., the Office of Laboratory Animal Welfare (OLAW) considers fish as live, vertebrate animals once they hatch. Zebrafish larvae typically hatch at 3 dpf. Thus, zebrafish embryos are not applicable to the Public Health Service (PHS) Policy until time of hatching (Bartlett and Silk Citation2016).
The Fish Embryo Acute Toxicity (FET) test, which is described in the OECD Test Guideline (TG) 236 (OECD Citation2013), is a validated alternative assay to determine the acute toxicity of chemicals to zebrafish at different embryonic stages. In short, fertilized zebrafish eggs are exposed to different test compounds, and the indicators of lethality, including coagulation of fertilized eggs, lack of somite formation, lack of detachment of the tail-bud from the yolk sac and lack of heartbeat, are recorded every 24 h. Based on a positive outcome for any of these indicators, acute toxicity is determined after 96 h and the LC50 can be calculated.
Toxicity tests in zebrafish embryos according to OECD TG 236 have been successfully performed for several substances. Recently, herbal plants with pharmacological effects represent a major area of interest within the global health market. Therefore, evaluation of their toxicity is an important objective. For instance, embryotoxicity and teratogenicity of Curcuma longa extract were determined in selected healthy zebrafish embryos 6 hours post fertilization (hpf). At dosages above 62.50 μg/mL, toxic effects occurred, while mortality of embryos was observed at 125.0 μg/mL. Higher concentrations even led to teratogenic effects with physical body deformities (Alafiatayo et al. Citation2019). The FET Test was also applied to investigate the toxic effects of the soya isoflavones genistein and daidzein (Sarasquete, Úbeda-Manzanaro, and Ortiz-Delgado Citation2018) as well as of the Clinacanthus nutans leaf hexane fraction (Murugesu et al. Citation2019).
However, zebrafish embryos are covered by a chorionic membrane, which limits the uptake of large substances, resulting in false positives (Souza Anselmo et al. Citation2018). Therefore, alternative assays using dechorionated zebrafish embryos have been developed. Testing a training set of compounds, the chorion-off assay is more sensitive (83%) than a similar assay with chorion intact embryos (63-74%) (Panzica-Kelly, Zhang, and Augustine-Rauch Citation2015). In the study of Chousidis et al. Citation2020, the toxic effects of cannabinol on the heart physiology, morphological malformations, behavioral changes and alterations in metabolic pathways of zebrafish larvae were examined by treating dechorionated embryos with different concentrations of cannabinol. The results showed that malformations in zebrafish larvae increased significantly in a dose-dependent manner, and the LD50 value was estimated to be 1.12 mg/L.
Furthermore, adverse effects of the mycotoxin ZEA were assessed in zebrafish embryos (4 hpf) by using various endpoints, such as heart rate, oxidative stress indicators (reactive oxygen species (ROS), lipid peroxidation (LPO), nitric oxide (NO)), antioxidant responses (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase enzyme (GST) and reduced glutathione (GSH), metabolic biomarkers (lactate dehydrogenase (LDH) and NO), neurotoxicity (acetylcholinesterase (AChE)), genotoxicity (comet assay and acridine orange staining) and histological analysis. In particular, the heart rate is an important toxicological endpoint in the fish embryo toxicity test because the heart is the first functional organ in zebrafish development. Interestingly, ZEA induces oxidative stress, resulting in developmental genotoxicity and neurotoxicity in zebrafish embryos (Muthulakshmi et al. Citation2018).
Zebrafish are excellent models for investigating oxidative stress, especially by using transgenic zebrafish models. Accordingly, Mourabit et al. Citation2019 produced a stable transgenic zebrafish line (3EpRE:hsp70:mCherry) to study organic-specific oxidative stress mechanisms in response to environmental conditions using electrophile response element (EpRE), otherwise known as Antioxidant Response Element (ARE), as a global marker of cellular responses to oxidative stress. However, the occurrence of oxidative stress in zebrafish can also be assessed without the use of transgenic fish lines. Hence, Lackmann et al. Citation2018 developed simple, effective and reliable procedures to monitor ROS and GSH formation in zebrafish larvae as indicators of oxidative stress. Moreover, the protective effects of dietary substances against oxidative stress can also be studied in zebrafish, as demonstrated by Tayemeh et al. Citation2020, who examined the efficacy of chitosan-nanoencapsulated quercetin against oxidative stress caused by silver nanoparticles. Additionally, Endo et al. Citation2020 used a Nrf2-mutant zebrafish line to analyze the in vivo antioxidant activity of phytochemicals derived from various spices. Curcumin, diallyl trisulfide and quercetin were found to reduce hydrogen peroxide toxicity, while cinnamaldehyde, isoeugenol and 6-(methylsulfinyl)hexyl isothiocyanate were involved in the reduction of arsenite toxicity.
Different transgenic zebrafish lines have also been used to examine the developmental toxicity of saxitoxin, a marine cyanotoxin (Chen et al. Citation2020), or psoralen, an active compound of Chinese herbs (Xia et al. Citation2018). The latter study even investigated toxic the effects of psoralen on the developing heart, liver, phagocytes, and nervous system using four specific transgenic fish lines. Furthermore, a triple-transgenic zebrafish line expressing fluorescent proteins in neurons (Cerulean), astrocytes (mCherry) and oligodendrocytes (mCitrine) even allows the investigation of the developmental neurotoxicity of chemicals during neuronal differentiation (Koiwa et al. Citation2019).
In addition to the measurement of metabolites or biomarkers, behavioral tests of both larvae and adult zebrafish are often used in toxicological assays including endpoints, such as thigmotaxis (wall hugging), scototaxis (light/dark preference), geotaxis (diving preference), exploration, habituation and stress- and anxiety-related parameters (Horzmann and Freeman Citation2018). Hence, one of the most common assays for larval behavior is the larval photomotor response (PMR), adult zebrafish can be used to study complex behaviors related to stress and anxiety. Such assays are facilitated by video tracking software, which can routinely calculate important parameters. For instance, Steele, Mole, and Brooks Citation2018 developed a behavioral profile protocol in larval fish using caffeine as a model neurostimulator and showed that zebrafish were sensitive for photomotor and locomotor endpoints. However, several factors can influence the behavior of larval fish in addition to chemical exposure. Particularly, time of day, age, well size, temperature, lighting conditions and volume of the exposure solution in each well. Another study performed the novel tank test as well as the light/dark test in adult zebrafish to confirm the anxiolytic action of Coriandrum sativum extract (Zenki et al. Citation2020).
Because of its rapid development and short life cycle, zebrafish are an interesting model to study multi- and transgenerational toxicity. Multigenerational studies observe adverse effects in generations originally exposed to the test substance, whereas transgenerational studies examine toxic consequences in subsequent generations (Horzmann and Freeman Citation2018). In a multigenerational study with zebrafish, the potential adverse effects of Δ9-tetrahydrocannabinol (THC) and cannabinol (CBD) were assessed and indicated cannabinoid-related developmental neurotoxicity (Carty et al. Citation2019).
Toxicity trials in Drosophila melanogaster
Drosophila melanogaster (Drosophila) has been widely used as a model organism since it possesses many advantages, such as a relatively short life span of 60 − 80 days (Staats and Lüersen et al. Citation2018), a well-characterized genome including over 60% homologous genes (Baenas and Wagner Citation2019) and cost-effectiveness (Nobrega and de Lyons Citation2020). In the laboratory, flies can be easily grown in glass vials and maintained in humidified and temperature-controlled incubators (Strange Citation2016). In fact, the fruit fly is one of the simplest models to study food toxicity (). Therefore, Senthilkumar et al. Citation2020 used Drosophila to investigate the developmental and behavioral toxicity of acrylamide, a by-product of the Maillard reaction in carbohydrate-rich foods. The acrylamide-induced toxic effects could be reduced by the antioxidants thymoquinone and curcumin, leading to significantly improved survival curves.
The adverse effects of different food additives have also been determined in fly-based toxicity studies. For instance, a 20-generation dietary exposure experiment in Drosophila revealed the negative impacts of the concentration of the daily human consumption of E171 on physiological, ontogenetic, genotoxic, and adaptive processes. It was found that E171 caused safety concerns, as children tend to consume high daily concentrations of E171 (Jovanović et al. Citation2018). Furthermore, Merinas-Amo, Martínez-Jurado, et al. Citation2019 advised against a high chronic intake of food colorings after in vivo testing of Riboflavin, Tartrazine, Carminic Acid, Erythrosine, Indigotine, and Brilliant Blue FCF in Drosophila. The same authors evaluated the biological effects of Czech beers and their constituents in fruit flies and human cells. Interestingly, they even found protective effects against hydrogen peroxide-induced toxicity.
Indeed, the fruit fly is an efficient model to examine the molecular and cellular mechanisms of alcohol-related behaviors. There are several assays for evaluation of alcohol sensitivity, alcohol tolerance, and preference learning. For example, the disinhibitory effects of alcohol on large fly populations can be assessed by the “inebriometer”, where flies are exposed to alcohol in a vertical tube. Subsequently, the population mean elution time (MET) can be used to estimate alcohol sensitivity (Engel et al. Citation2019).
Importantly, Drosophila is an attractive model not only for alcohol studies but also for the measurement of feed intake and nutrition research. For instance, food quantity can be determined by observation of the proboscis extension (PE) of flies, which is a sign of food uptake (Staats and Lüersen et al. 2018). Additionally, different biomarkers can be monitored in response to dietary factors, such as lifespan or locomotor activity. Usually, lifespan is determined by recording the number of dead flies in certain intervals until all flies are dead (Staats and Wagner et al. 2018). The rapid iterative negative geotaxis (RING) assay can be used to assess locomotor activity. In this assay, the climbing activity of flies in a clear vial is quantified by scoring the distance they climb. The climbing index provides information about the health status of fruit flies (Staats and Lüersen et al. 2018).
Flies are valuable models to explore dietary effects on aging and longevity (Nobrega and de Lyons Citation2020). Whereas resveratrol has no effect on life span, body composition, locomotor activity, stress response, and longevity-associated gene expression in flies (Staats and Wagner et al. 2018), curcumin supplementation increases the survival rate of heat stressed Drosophila by enhancing thermal tolerance (Chen, Liu, et al. Citation2018) and Kolaviron, a biflavonoid of garcinia kola seeds, prolongs longevity (Farombi et al. Citation2018). Similarly, ellagic acid, a component of strawberries, blackcurrants, pomegranates, walnuts and grapes, exhibited beneficial effects in a longevity assay on fruit flies. Interestingly, the mean and maximum lifespans of male flies were extended after consumption of 200 μM ellagic acid, whereas female flies laid less eggs in response to ellagic acid (Kharat et al. Citation2020). However, other studies observed prolonged lifespans in female flies compared with their male counterparts (Staats and Lüersen et al. 2018). Consequently, extreme variations of longevity between the sexes must be considered in further studies.
Another interesting field of application comprises nutrigenomics, which describes the interactions between the genome, the proteome, the epigenome, the metabolome, and the microbiome and the nutritional environment (Baenas and Wagner Citation2019). Similar to mammals, the gut of Drosophila is colonized by several microorganisms. However, compared to mammals, the number of commensal bacteria, which ranges from approximately 30 species, is limited (Staats and Lüersen et al. 2018). Thus, the simple microbiota of the fruit fly enables the analysis of microbiota-associated diseases. Microbiome studies benefit from the easy isolation, culture and engineering of the fruit fly’s gut (Baenas and Wagner Citation2019).
Additionally, Drosophila models of human diseases, especially neurodegenerative disorders, such as Parkinson’s disease (Poetini et al. Citation2018), cancer (Strange Citation2016), diabetes and obesity (Gáliková and Klepsatel Citation2018), are very popular. For example, Poetini et al. Citation2018 showed that hesperidin alleviates iron-induced oxidative damage and dopamine depletion in a Drosophila-based model of Parkinson's disease. Another study observed reduced paraquat-induced toxicity in fruit flies after coexposure with caffeic acid, a known antioxidant, cardioprotective and neuroprotective molecule (Dos Santos Nunes et al. Citation2019).
However, conventional Drosophila-based assays, including manual manipulation, cellular investigation or behavioral phenotyping techniques, are time-consuming, labor-intensive, and low in throughput. Therefore, microfluidic and Lab-on-a-chip devices have been developed to improve throughput and to facilitate performance. Accordingly, “fly-on-a-chip” tools comprise microfluidic devices for Drosophila studies at the embryonic, larval, and adulthood stages and include both in vivo assays with intact flies as well as in vitro assays with dissected flies. For instance, in vivo neuron and organ assays can be used to monitor heart activity, whereas food search behavior can be assessed using in vivo assays (Zabihihesari, Hilliker, and Rezai Citation2019).
Toxicity applications with HET-CAM
The hen’s egg – chorioallantoic membrane (HET-CAM) test is a widely used and well-established alternative test system to in vivo animal experiments. As avian embryos are not considered to be laboratory animals during the first two-thirds of embryonic development (Directive 2010/63/EU 2020), no ethics committee approval is required. Originally, the highly vascularized chorioallantoic membrane was employed as a model to study the basic mechanisms behind angiogenesis and wound healing (Ribatti Citation2016), and thus, it is frequently used to investigate the angiogenic and antiangiogenic effects of substances (Dar and Sehgal Citation2020; Saleem et al. Citation2020). Because of its versatile applicability, the in ovo system has found application in areas such as drug development, cosmetics and food toxicology and therefore represents a suitable model to investigate the irritation potential and toxicity of substances and formulations.
Tavakkoli et al. Citation2020 investigated the vascular toxicity of Dorema Ammoniacum, whose gum resin is also used in the food industry and observed vascular alterations in the CAM that probably affected embryonic development due to its consumption during the gestational period. In addition, in the emerging field of nanotechnology, which offers the use of NPs in the food industry, toxicity test systems closely mimicking the in vivo situation are required to ensure their safety. As NPs probably enter the blood stream after oral consumption of food containing NPs, their potential adverse effects on the vasculature need to be excluded. Freyre-Fonseca et al. Citation2018 showed that food-grade E171 NPs do not negatively affect the average vessel branch lengths or the microvascular density of the CAM. Similar results were obtained when testing Chitosan nanoparticles (Balan et al. Citation2020) and nanocomposite materials (Chakraborty et al. Citation2018) on the CAM.
In addition to its applicability in toxicology studies, the vascularized chorioallantoic membrane turned out to be a powerful tool for testing substances for their antidiabetic effects. Haselgrübler et al. developed a modified version of the HET-CAM test, termed Gluc-HET, to investigate the insulin-mimetic effects of phytochemicals. Extracts prepared from Bellis perennis significantly reduced blood glucose levels and did not have any adverse effects on the CAM’s vessel network (Haselgrübler and Stadlbauer et al. Citation2018; Haselgrübler and Stübl et al. Citation2018). In contrast, Jin et al. Citation2019 used the CAM model to analyze the influence of gestational diabetes on blood vessel formation in a developing embryo and observed that high glucose treatment inhibited angiogenesis and significantly reduced vascular density. Moreover, other researchers utilized the HET-CAM system to demonstrate the beneficial effect of Baicalein, which is found in the roots of Scutellaria baicalensis, on hyperglycemia-related vascular malformation in embryos, as it significantly reversed the reduced blood vessel density (Wang et al. Citation2018).
In fact, the HET-CAM test is a very simple and inexpensive technique that does not require special laboratory facilities (Naik, Brahma, and Dixit Citation2018; Smeriglio et al. Citation2018) and is additionally characterized by a high degree of reproducibility (Nihad et al. Citation2018). However, since this assay is especially suitable for water-soluble and surfactant-based formulations, the HET-CAM holds possible limitations for investigating solids and insoluble compounds, as well as stains that affect the visibility of the CAM (McNamee et al. Citation2009).
Thus, the HET-CAM test serves as an important and alternative model in food toxicology and also allows the identification of candidates for functional food products with anti-diabetic properties.
Toxicological approaches with Lab-on-a-chip
In addition to the abovementioned model organisms, advances in the rapidly emerging field of microfluidics have led to the development of complex cell-based systems known as organ-on-a-chip (OOC) models (also “Lab-on-a-chip” or “Body-on-a-chip”) that have the potential to reduce animal trials in food toxicology.
In this particular context, cells or tissue cultures are usually grown in micrometer-sized chambers of polydimethylsiloxane (PDMS)-based chips containing microchannels that are continuously perfused with fluid, allowing cultivation under dynamic conditions (Wu et al. Citation2020). In contrast to traditional static monocultures and 2D-cell culture systems, the microfluidic system more accurately mimics the physiological situation in terms of shear stress, blood flow and peristalsis, as well as in terms of the morphological complexity of organs (Fois et al. Citation2019). In addition to the creation of a dynamic environment, microfluids facilitate the delivery of cells, the administration of nutrients and test substances, and the removal of waste products (Wu et al. Citation2020). To recapitulate the complex architecture of tissues and organs, surface modifications and 3D-printing of hydrogel scaffolds contribute to the controlled arrangement of cells on the microchip (Xue et al. Citation2018).
Currently, several OOC devices have been successfully established, including microchips resembling the human kidney (Yin et al. Citation2020), lung (Zhang et al. Citation2018), liver (Ma et al. Citation2018), gut (Fois et al. Citation2019), intestine (Kasendra et al. Citation2018) and placenta (Yin et al. Citation2019). Santbergen et al. Citation2020 developed a dynamic chip-based intestinal barrier model (flow-through transwell system) directly coupled to an analytical detection system (ultra-performance liquid chromatography quadrupole time-of-flight, UPLC-QTOF) to investigate the bioavailability of drug and food components. CaCo-2 and HT29-MTX cells cultivated under static and dynamic conditions were treated with ergotaminine, a natural toxin found in foods. Compared to the static situation, the permeability of ergotaminine was five times lower in the dynamic system, highlighting the influence of shear stress on transepithelial transport. Kulthong et al. Citation2018 used a very similar approach to manufacture a gut-on-a-chip model and demonstrated its applicability for bioavailability studies of food contaminants by using highly toxic dioxins that frequently accumulate in the food chain as test compounds. In addition, Yin et al. Citation2019 developed a placental barrier-on-a-chip model using BeWo and HUVEC cells to examine the potential effect of E171 nanoparticle exposure on the reproductive health of humans. Therefore, the 3D placental barrier model exposed to low (short) and high (chronic) concentrations of E171 was analyzed regarding certain placental responses (ROS production, apoptosis, permeability and inflammation). Significantly increased ROS production and higher cell death rates and interleukin (IL)-6 levels were observed after chronic exposure to E171. However, reduced expression of tight junction proteins (E- and VE-cadherin) and an increase in permeability for FITC-Dextran were already found after acute exposure, indicating disruption of the barrier, probably leading to severe dysfunctions of the human placenta.
In addition to single organ chips, microfluidics also provide the opportunity to combine several organ-on-a-chip devices or to integrate them into one “multiorgan chip” (Fois et al. Citation2019). Chen, Miller, et al. Citation2018 developed a gravity-driven fluidic gastrointestinal (GI) tract-liver model prepared from HepG2 C3A liver cells and primary human intestinal epithelial cells enabling the analysis of organ crosstalk during toxicity studies.
In summary, this innovative organ-on-a-chip technology has the potential to overcome the limitations of current in vitro methods, allowing a more accurate understanding of harmful food components to be obtained. Although a series of organ-on-a-chip models have already been developed and validated, intensive research, standardization, as well as regulatory approval, is still required for their future application in food toxicology and safety assessment. A summary of the reviewed food toxicants measured in alternative model systems is given in .
Table 2. Food related toxicants analyzed by alternative model systems.
Conclusion
To overcome the increasing demand for the evaluation of compounds in (food) toxicity testing, the application of alternative model organisms is indispensable. Furthermore, current trends are veering toward the reduction of animal experiments because of their ethical concerns, high costs and time-consuming demands. It is of particular importance to assess the toxic potential of compounds before human exposure. Therefore, regulations such as Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) encourage the use of nonanimal testing alternatives (Alberga et al. Citation2019) ().
Table 3. List of abbreviations.
The alternative models C. elegans and Drosophila can be rather easily implemented in non-specialized labs. Besides a microscope and an incubator for humidity and temperature control, no expensive equipment is needed for most of the applications. Furthermore, databases such as WormBase and FlyBase represent an excellent overview of the model organisms itself, as well as standard protocols and media recipes. Additionally, the JoVE Peer Reviewed Scientific Video Journal provides informative video training in how to perform selected experiments. However, alternative models might response sensitively to extrinsic factors including temperature, culture density and food availability. If not maintained correctly, experimental results could be negatively biased. It has to be taken into account that if exposed to unfavorable conditions, those effects could be prevalent during several generations (Ludewig et al. Citation2017; Chan, Rando, and Conine Citation2018; Gómez-Orte et al. Citation2018).
While the maintenance of C. elegans and Drosophila is manageable, zebrafish husbandry requires centralized aquaculture facilities with controlled parameters and light/dark cycles (Strange Citation2016). Besides the high acquisition costs for centralized facilities, one challenge in zebrafish research is the lack of standardized protocols for husbandry and maintenance (Cassar et al. Citation2020). Recently, recommendations for the care of laboratory zebrafish have been published that intend to set a standard for zebrafish husbandry and improve reproducibility of future research (Aleström et al. Citation2020). The HET-CAM model does not require specialized equipment apart from an incubator with constant turn-over and a candling lamp. Also, the obtainment of fertilized eggs from local breeders is rather simple. However, the proper experimental handling demands for laborious training. Regarding Lab-on-a-chip systems, the implementation of this technology is still cost-intensive, especially due to the need of special equipment such as fluidic pumps, incubators and automated dispensers. Furthermore, the operation requires highly skilled scientific personnel (Ramadan and Zourob Citation2020). Therefore, extensive user training or development of robust and automated instrumentation is necessary to achieve optimal function of the system (Bhatia and Ingber Citation2014).
The reviewed alternative models share many fundamental biological processes, such as molecular signaling pathways and cell behavior, which are common in humans. However, a detailed view clearly identifies differences including the conserved Phase I, II, and III metabolism. Nuclear hormone receptors (NHRs) such as the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR) family play a central role in the regulation of xenobiotic response in mammals. These receptors act as transcription factors and induce the expression of genes that encode for metabolic enzymes and components of multi-drug efflux pumps (Jones et al. Citation2013). In C. elegans, the abnormal DAuer Formation (DAF-12) as well as the nuclear hormone receptor family NHR-48 and NHR-8 were originally identified as PXR/CAR homologues. In Drosophila, the transcriptional response relies on the vertebrate PXR and CAR orthologue hormone receptor-like DHR96 (King-Jones et al. Citation2006).
The aryl hydrocarbon receptor (AHR) also plays an important role in xenobiotic metabolism (Pascussi et al. Citation2008). Mammalian AHR directly binds to a wide range of xenobiotics and regulates transcription of Phase I, II and III genes as well as displaying extensive cross-talk with CAR and PXR (Köhle and Bock Citation2009). AHR is conserved in both C. elegans and Drosophila and, like the vertebrate form, it is expressed in chemosensory neurons. In contrast to Drosophila, the C. elegans AHR is not associated with xenobiotic metabolism and does not bind dioxins and related chemicals. (Qin and Powell-Coffman Citation2004; Kuzin et al. Citation2014). Furthermore, the C. elegans homologue of the mammalian nuclear factor erythroid 2-related (NRF2) transcription factor, protein skinhead-1 (SKN-1), functions in the p38 MAPK pathway in parallel to the DAF-2-mediated insulin/IGF-1-like signaling pathway (IIS) in order to regulate oxidative stress responses and longevity (Harlow et al. Citation2018). In addition, differences in xenobiotic metabolizing enzymes between fish and human have to be taken into account. While many human cytochrome P450 (CYP) enzymes do have orthologues in zebrafish, this does not necessarily mean that they act identically on a specific substrate (Souza Anselmo et al. Citation2018).
Despite the known significant differences in regulatory pathways or gene functions, alternative model organisms can still be utilized as biosensors regarding several endpoints including behavioral changes, gene expression or lethality that serve as markers for cellular stress (Lindblom and Dodd Citation2006).
Lower surrogate in vivo model organisms, including C. elegans, D. rerio and Drosophila, could therefore fill the huge gap between in vitro cell culture and animal or clinical studies. The reviewed model systems are suitable as reliable indicators for toxicological assessments in foodstuff. In higher throughput applications, single substances or mixtures can be rapidly estimated regarding their potential impacts.
Furthermore, testing of multiple concentrations has led to well-defined response data and reliable IC50 values. Moreover, the understanding of the model systems and their experimental results determines the integrity of data in selected model organisms. The applications of these systems have to be clearly defined to result in a precise evaluation to depict in vivo models.
The approach of Lanzerstorfer et al. Citation2021 utilizing multiple alternative models for toxicity screening and prediction of oral LD50 values seem to be very promising. The general application of the calculation is based on the linear relationship between in vitro cytotoxicity IC50 values and rodent LD50 values. The LD50 starting dose in vivo is determined by inserting the in vitro IC50 value into a regression formula (ICCVAM Citation2006a, Citation2006b). Animal savings using this approach were found to be highest for chemicals with LD50 >5000 mg/kg, while less toxic chemicals still reduced the number of animals by up to 22% per test (Stokes et al. Citation2008).
However, it has to be mentioned that the lack of complexity of these model systems can limit their predictability in respect to toxicokinetics. There are multiple challenges in predicting effects regarding the crosstalk of toxicants by interfering with other molecules in food matrixes, different metabolic pathways (different cells, organs), modifications during metabolism (digestion, absorption, bioavailability), gender, age and health conditions. Prediction of toxicological potential regarding human risk remains a very complex topic. Interestingly, different experiments conducted by various research groups have revealed comparable effects for the same testing substance regarding its toxic potential (as shown for example with E171 in rats, C. elegans and Drosophila or ZEA in C. elegans and D. rerio). Most likely, a single model will never be able to fully replace vertebrates in toxicity testing. Nevertheless, a combination of multiple test systems based on the toxicological parameters and the strength of each individual system may be adequate for ensuring confident predictions of potential chemical toxicity toward humans.
Consequently, a vast amount of data on compounds tested in different model systems is generated. However, this valuable information is scattered across numerous literature reports and nonsystematic databases. Therefore, the integration of all related data (e.g. molecular structure, absorption, distribution, metabolism, and toxicity) into a comprehensive database is indispensable and allows for a more targeted research for preliminary toxicological evaluation of food compounds. For example, the food risk component database (FRCD) is a comprehensive open-source database that provides information about more than 10.000 substances obtained from more than 150.000 literature reports and several databases (Zhang et al. Citation2020). Additionally, EFSA initialized the publicly available OpenFoodTox database on toxicological properties of substances in food and feed evaluated by EFSA (Dorne et al. Citation2021). Since the obtained data are not exclusively relevant for food risk databases, toxicology studies frequently provide important additional information for databases in other research areas. For instance, the DrugAge database, a collection of data on lifespan-extending drugs and compounds tested in different model organisms, needs to be complemented with available conflicting data from studies in this field to enable systematic compound evaluation (Barardo et al. Citation2017). Furthermore, these comprehensive databases significantly contribute to the development of the emerging field of nutri-informatics/food informatics.
Nutri-informatics represents an upcoming bioinformatics discipline for the integration of large-scale data sets from nutritional studies and omics into a stringent nutritional systems biology context (Döring and Rimbach Citation2014). Nutri-informatics aims to computationally integrate and analyze nutrition study data sets in order to unravel interactions between organisms and their nutritional environments. Thus, this novel discipline tends to focus on the analysis of food genomics and the integration of large-scale data sets derived from transcriptomics, proteomics and metabolomics in order to describe nutrient-dependent effects.
However, the lack of standardization including principles to ensure interoperability and cohesion between nutri-informatics and other biomedical resources and the technical issues present in nutri-informatics, causes serious concerns among the community. To minimize the technical concerns, a systematic approach with community-wide support and adoption will be necessary. Furthermore, incomplete coverage of nutrition-related concepts in ontologies, limited compatibility across nutrition-related databases or poor communication and accountability regarding the Findability, Accessibility, Interoperability and Reusability (FAIR) principles of scientific data management and administration among nutrition researchers, nutrition journals and nutrition research funding agencies represent multiple challenges that need to be addressed in the future.
In any case, current improvements in nutri-informatics research are very promising and give rise to novel findings and methodologies that can presumably be utilized in future research studies. Present constraints in nutri-informatics will be overcome to gain a vast impact for precision medicine and personalized health (Chan et al. Citation2021).
Hence, in addition to animal studies and clinical trials, complex in vitro cell culture systems in combination with in silico computer models may also provide mechanistic insights into toxicology in a high-throughput manner. It should be noted that insufficient data for computational modeling will lead to contradictory results across models and users, even for the same chemicals. Therefore, it is of particular importance to ensure high-quality chemical and biological data since the models are only be as reliable as the existing records. Currently, these non-testing in silico methods can only provide limited insights (Raies and Bajic Citation2018; Benfenati et al. Citation2019). However, the integration of food toxicology data obtained via cell-based in vitro and in vivo animal models as well as in silico systems have led to a mechanistic knowledge of systemic or organ-specific toxicity in humans and the identification and use of specific surrogate biomarkers in clinical settings (Gosslau Citation2016).
Overall, the achievements in the fields will lead to significant improvement of the prediction rate of drug and food safety.
Author contributions
Conceptualization, G.S. and J.W.; investigation, G.S., A.K. and M.W.; writing—original draft preparation, G.S, A.K. and M.W.; writing—review and editing, G.S. and J.W.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This work was created within a research project of the Austrian Competence Centre for Feed and Food Quality, Safety and Innovation (FFoQSI). The COMET-K1 Competence Centre FFoQSI is funded by the Austrian ministries BMVIT, BMDW and the Austrian provinces Niederoesterreich, Upper Austria and Vienna within the scope of COMET - Competence Centers for Excellent Technologies. The programme COMET is handled by the Austrian Research Promotion Agency FFG. This work was also funded by the Christian Doppler Forschungsgesellschaft (Josef Ressel Center for Phytogenic Drug Research).
Disclosure statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Alafiatayo, A. A., K.-S. Lai, A. Syahida, M. Mahmood, and N. A. Shaharuddin. 2019. Phytochemical Evaluation, Embryotoxicity, and Teratogenic Effects of Curcuma longa Extract on Zebrafish (Danio rerio). Evidence-Based Complementary and Alternative Medicine: eCAM 2019:3807207. doi: 10.1155/2019/3807207.
- Alberga, D., D. Trisciuzzi, K. Mansouri, G. F. Mangiatordi, and O. Nicolotti. 2019. Prediction of Acute Oral Systemic Toxicity Using a Multifingerprint Similarity Approach. Toxicological Sciences 167 (2):484–95. eng. doi: 10.1093/toxsci/kfy255.
- Aleström, P., L. D'Angelo, P. J. Midtlyng, D. F. Schorderet, S. Schulte-Merker, F. Sohm, and S. Warner. 2020. Zebrafish: Housing and husbandry recommendations. Lab Anim 54 (3):213–24. doi: 10.1177/0023677219869037.
- Baenas, N., and A. E. Wagner. 2019. Drosophila melanogaster as an alternative model organism in nutrigenomics. Genes & Nutrition 14:14. doi: 10.1186/s12263-019-0641-y.
- Bagchi, D., A. Swaroop, and S. J. Stohs. 2017. Food toxicology. Boca Raton, FL: CRC Press. ISBN: 1498708757.
- Balan, P., J. Indrakumar, P. Murali, and P. S. Korrapati. 2020. Bi-faceted delivery of phytochemicals through chitosan nanoparticles impregnated nanofibers for cancer therapeutics. International Journal of Biological Macromolecules 142:201–11. doi: 10.1016/j.ijbiomac.2019.09.093.
- Bambino, K., and J. Chu. 2017. Zebrafish in Toxicology and Environmental Health. Current Topics in Developmental Biology 124:331–67. doi: 10.1016/bs.ctdb.2016.10.007.
- Barardo, D., D. Thornton, H. Thoppil, M. Walsh, S. Sharifi, S. Ferreira, A. Anžič, M. Fernandes, P. Monteiro, T. Grum, et al. 2017. The DrugAge database of aging-related drugs. Aging Cell 16 (3):594–7. doi: 10.1111/acel.12585.
- Bartlett, D. H., and S. B. Silk. 2016. Office of laboratory animal welfare comments. Zebrafish 13 (6):563–4. doi: 10.1089/zeb.2016.1344.
- Benfenati, E., Q. Chaudhry, G. Gini, and J. L. Dorne. 2019. Integrating in silico models and read-across methods for predicting toxicity of chemicals: A step-wise strategy. Environment International 131:105060. doi: 10.1016/j.envint.2019.105060.
- Bettini, S., E. Boutet-Robinet, C. Cartier, C. Coméra, E. Gaultier, J. Dupuy, N. Naud, S. Taché, P. Grysan, S. Reguer, et al. 2017. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Scientific Reports 7:40373. doi: 10.1038/srep40373.
- Bhatia, S. N., and D. E. Ingber. 2014. Microfluidic organs-on-chips. Nat Biotechnol 32 (8):760–72. doi: 10.1038/nbt.2989.
- Bian, X., P. Tu, L. Chi, B. Gao, H. Ru, and K. Lu. 2017. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 107 (Pt B):530–9. doi: 10.1016/j.fct.2017.04.045.
- Blaauboer, B. J., A. R. Boobis, B. Bradford, A. Cockburn, A. Constable, M. Daneshian, G. Edwards, J. A. Garthoff, B. Jeffery, C. Krul, et al. 2016. Considering new methodologies in strategies for safety assessment of foods and food ingredients. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 91:19–35. eng. doi: 10.1016/j.fct.2016.02.019.
- Boer, A. D., and A. Bast. 2018. Demanding safe foods – Safety testing under the novel food regulation (2015/2283). Trends in Food Science & Technology 72:125–33. doi: 10.1016/j.tifs.2017.12.013.
- Boyd, W. A., M. V. Smith, C. A. Co, J. R. Pirone, J. R. Rice, K. R. Shockley, and J. H. Freedman. 2016. Developmental effects of the ToxCast™ phase I and phase II chemicals in caenorhabditis elegans and corresponding responses in zebrafish, rats, and rabbits. Environmental Health Perspectives 124 (5):586–93. eng. doi: 10.1289/ehp.1409645.
- Carty, D. R., Z. S. Miller, C. Thornton, Z. Pandelides, M. L. Kutchma, and K. L. Willett. 2019. Multigenerational consequences of early-life cannabinoid exposure in zebrafish. Toxicology and Applied Pharmacology 364:133–43. doi: 10.1016/j.taap.2018.12.021.
- Cassar, S., I. Adatto, J. L. Freeman, J. T. Gamse, I. Iturria, C. Lawrence, A. Muriana, R. T. Peterson, S. van Cruchten, and L. I. Zon. 2020. Use of zebrafish in drug discovery toxicology. Chemical Research in Toxicology 33 (1):95–118. eng. doi: 10.1021/acs.chemrestox.9b00335.
- Çelen, İ., J. H. Doh, and C. R. Sabanayagam. 2018. Effects of liquid cultivation on gene expression and phenotype of C. elegans. BMC Genomics 19 (1):562. doi: 10.1186/s12864-018-4948-7.
- Chakraborty, S., T. Ponrasu, S. Chandel, M. Dixit, and V. Muthuvijayan. 2018. Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications. Royal Society Open Science 5 (5):172017. doi: 10.1098/rsos.172017.
- Chan, I. L., O. J. Rando, and C. C. Conine. 2018. Effects of Larval Density on Gene Regulation in Caenorhabditis elegans During Routine L1 Synchronization. G3 (Bethesda, Md.) 8 (5):1787–93. doi: 10.1534/g3.118.200056.
- Chan, L., N. Vasilevsky, A. Thessen, J. McMurry, and M. Haendel. 2021. The landscape of nutri-informatics: A review of current resources and challenges for integrative nutrition research. Database. 2021:1–20. doi: 10.1093/database/baab003.
- Chen, G., Z. Jia, L. Wang, and T. Hu. 2020. Effect of acute exposure of saxitoxin on development of zebrafish embryos (Danio rerio). Environmental Research 185:109432. doi: 10.1016/j.envres.2020.109432.
- Chen, Y., X. Liu, C. Jiang, L. Liu, J. M. Ordovas, C.-Q. Lai, and L. Shen. 2018. Curcumin supplementation increases survival and lifespan in Drosophila under heat stress conditions. BioFactors (Oxford, England) 44 (6):577–87. doi: 10.1002/biof.1454.
- Chen, H. J., P. Miller, and M. L. Shuler. 2018. A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab on a Chip 18 (14):2036–46. eng. doi: 10.1039/c8lc00111a.
- Chousidis, I., T. Chatzimitakos, D. Leonardos, M. D. Filiou, C. D. Stalikas, and I. D. Leonardos. 2020. Cannabinol in the spotlight: Toxicometabolomic study and behavioral analysis of zebrafish embryos exposed to the unknown cannabinoid. Chemosphere 252:126417. doi: 10.1016/j.chemosphere.2020.126417.
- Dar, M. H., and A. Sehgal. 2020. Evaluation of antiangiogenic and antigenotoxic potential of green and black tea extracts by chicken chorioallantoic membrane assay. Journal of Entomology and Zoology Studies 8 (2):424–430.
- Directive 2010/63/EU. 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposesText with EEA relevance.
- Döring, F., and G. Rimbach. 2014. Nutri-informatics: A new kid on the block? Genes & Nutrition 9 (3):394. doi: 10.1007/s12263-014-0394-6.
- Dorne, J. L. C. M., J. Richardson, A. Livaniou, E. Carnesecchi, L. Ceriani, R. Baldin, S. Kovarich, M. Pavan, E. Saouter, F. Biganzoli, et al. 2021. EFSA's OpenFoodTox: An open source toxicological database on chemicals in food and feed and its future developments. Environ Int 146:106293. doi: 10.1016/j.envint.2020.106293.
- Dos Santos Nunes, R. G., P. S. Pereira, O. O. Elekofehinti, K. R. Fidelis, C. S. da Silva, M. Ibrahim, L. M. Barros, F. A. B. da Cunha, K. E. Lukong, I. d Menezes, et al. 2019. Possible involvement of transcriptional activation of nuclear factor erythroid 2-related factor 2 (Nrf2) in the protective effect of caffeic acid on paraquat-induced oxidative damage in Drosophila melanogaster. Pestic Biochem Physiol 157:161–8. doi: 10.1016/j.pestbp.2019.03.017.
- Endo, Y., K. Muraki, Y. Fuse, and M. Kobayashi. 2020. Evaluation of Antioxidant Activity of Spice-Derived Phytochemicals Using Zebrafish. International Journal of Molecular Sciences. 21 (3):1109. doi: 10.3390/ijms21031109.
- Engel, G. L., K. Taber, E. Vinton, and A. J. Crocker. 2019. Studying alcohol use disorder using Drosophila melanogaster in the era of 'Big Data'. Behavioral and Brain Functions : BBF 15 (1):7. doi: 10.1186/s12993-019-0159-x.
- Farombi, E. O., A. O. Abolaji, T. H. Farombi, A. S. Oropo, O. A. Owoje, and M. T. Awunah. 2018. Garcinia kola seed biflavonoid fraction (Kolaviron), increases longevity and attenuates rotenone-induced toxicity in Drosophila melanogaster. Pesticide Biochemistry and Physiology. 145:39–45. doi: 10.1016/j.pestbp.2018.01.002.
- Fois, C. A. M., T. Y. L. Le, A. Schindeler, S. Naficy, D. D. McClure, M. N. Read, P. Valtchev, A. Khademhosseini, and F. Dehghani. 2019. Models of the Gut for Analyzing the Impact of Food and Drugs. Advanced Healthcare Materials 8 (21):e1900968. doi: 10.1002/adhm.201900968.
- Freyre-Fonseca, V., E. I. Medina-Reyes, D. I. Téllez-Medina, G. L. Paniagua-Contreras, E. Monroy-Pérez, F. Vaca-Paniagua, N. L. Delgado-Buenrostro, J. O. Flores-Flores, E. O. López-Villegas, G. F. Gutiérrez-López, et al. 2018. Influence of shape and dispersion media of titanium dioxide nanostructures on microvessel network and ossification. Colloids and Surfaces B, Biointerfaces 162:193–201. doi: 10.1016/j.colsurfb.2017.11.049.
- Gáliková, M., and P. Klepsatel. 2018. Obesity and Aging in the Drosophila Model. International Journal of Molecular Sciences. 19 (7):1896. doi: 10.3390/ijms19071896.
- Gao, S., W. Chen, Y. Zeng, H. Jing, N. Zhang, M. Flavel, M. Jois, J.-D. J. Han, B. Xian, and G. Li. 2018. Classification and prediction of toxicity of chemicals using an automated phenotypic profiling of Caenorhabditis elegans. BMC Pharmacology & Toxicology 19 (1):18. doi: 10.1186/s40360-018-0208-3.
- García-Espiñeira, M. C., L. P. Tejeda-Benítez, and J. Olivero-Verbel. 2018. Toxic Effects of Bisphenol A, Propyl Paraben, and Triclosan on Caenorhabditis elegans. International Journal of Environmental Research and Public Health 15 (4):684. doi: 10.3390/ijerph15040684.
- Gómez-Orte, E., E. Cornes, A. Zheleva, B. Sáenz-Narciso, T. M de, M. Iñiguez, R. López, J.-F. San-Juan, B. Ezcurra, B. Sacristán, et al. 2018. Effect of the diet type and temperature on the C. elegans transcriptome. Oncotarget 9 (11):9556–71. doi: 10.18632/oncotarget.23563.
- Gosslau, A. 2016. Assessment of food toxicology. Food Science and Human Wellness 5 (3):103–15. doi: 10.1016/j.fshw.2016.05.003.
- Guo, Z., N. J. Martucci, F. Moreno-Olivas, E. Tako, and G. J. Mahler. 2017. Titanium Dioxide Nanoparticle Ingestion Alters Nutrient Absorption in an In Vitro Model of the Small Intestine. NanoImpact 5:70–82. doi: 10.1016/j.impact.2017.01.002.
- Harlow, P. H., S. J. Perry, A. J. Stevens, and A. J. Flemming. 2018. Comparative metabolism of xenobiotic chemicals by cytochrome P450s in the nematode Caenorhabditis elegans. Scientific Reports 8 (1):13333. doi: 10.1038/s41598-018-31215-w.
- Hartung, T. 2017. Evolution of toxicological science: The need for change. International Journal of Risk Assessment and Management 20 (1/2/3):21. doi: 10.1504/IJRAM.2017.082570.
- Haselgrübler, R., V. Stadlbauer, F. Stübl, B. Schwarzinger, I. Rudzionyte, M. Himmelsbach, M. Iken, and J. Weghuber. 2018. Insulin Mimetic Properties of Extracts Prepared from Bellis perennis. Molecules 23 (10):2605. doi: 10.3390/molecules23102605.
- Haselgrübler, R., F. Stübl, V. Stadlbauer, P. Lanzerstorfer, and J. Weghuber. 2018. An in ovo model for testing insulin-mimetic compounds. Journal of Visualized Experiments. 134:e57237. doi: 10.3791/57237.
- Horzmann, K. A., and J. L. Freeman. 2018. Making Waves: New Developments in Toxicology With the Zebrafish. Toxicological Sciences: An Official Journal of the Society of Toxicology 163 (1):5–12. doi: 10.1093/toxsci/kfy044.
- Howe, K., M. D. Clark, C. F. Torroja, J. Torrance, C. Berthelot, M. Muffato, J. E. Collins, S. Humphray, K. McLaren, L. Matthews, et al. 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496 (7446):498–503. doi: 10.1038/nature12111.
- Hunt, P. R. 2017. The C. elegans model in toxicity testing. Journal of Applied Toxicology: JAT 37 (1):50–9. doi: 10.1002/jat.3357.
- Hunt, P. R., J. A. Camacho, and R. L. Sprando. 2020. Caenorhabditis elegans for predictive toxicology. Current Opinion in Toxicology 23-24:23–8. doi: 10.1016/j.cotox.2020.02.004.
- Hunter, P. 2008. The paradox of model organisms. The use of model organisms in research will continue despite their shortcomings. EMBO Reports 9 (8):717–20. doi: 10.1038/embor.2008.142.
- Hunt, P. R., N. Olejnik, K. D. Bailey, C. A. Vaught, and R. L. Sprando. 2018. C. elegans Development and Activity Test detects mammalian developmental neurotoxins. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 121:583–92. doi: 10.1016/j.fct.2018.09.061.
- ICCVAM. 2006a. In Vitro Cytotoxicity Test Methods for Estimating Starting Doses for Acute Oral Systemic Toxicity Testing.
- ICCVAM. 2006b. In vitro ocular toxicity test methods for identifying severe irritant and corrosives.
- ICH Guideline S3A. 1994. Toxicokinetics: A Guidance for Assessing Systemic Exposure in Toxicology Studies.
- Jin, Y.-M., S.-Z. Zhao, Z.-L. Zhang, Y. Chen, X. Cheng, M. Chuai, G.-S. Liu, K. K. H. Lee, and X. Yang. 2019. High Glucose Level Induces Cardiovascular Dysplasia During Early Embryo Development. Experimental and Clinical Endocrinology & Diabetes : Official Journal, German Society of Endocrinology [and] German Diabetes Association 127 (9):590–7. doi: 10.1055/s-0043-109696.
- Jones, L. M., S. J. Rayson, A. J. Flemming, and P. E. Urwin. 2013. Adaptive and specialised transcriptional responses to xenobiotic stress in Caenorhabditis elegans are regulated by nuclear hormone receptors. PLoS ONE 8 (7):e69956. doi: 10.1371/journal.pone.0069956.
- Jovanović, B., N. Jovanović, V. J. Cvetković, S. Matić, S. Stanić, E. M. Whitley, and T. L. Mitrović. 2018. The effects of a human food additive, titanium dioxide nanoparticles E171, on Drosophila melanogaster - a 20 generation dietary exposure experiment. Scientific Reports 8 (1):17922. doi: 10.1038/s41598-018-36174-w.
- Jung, S.-K., B. Aleman-Meza, C. Riepe, and W. Zhong. 2014. QuantWorm: A comprehensive software package for Caenorhabditis elegans phenotypic assays. PLoS ONE 9 (1):e84830. doi: 10.1371/journal.pone.0084830.
- Kasendra, M., A. Tovaglieri, A. Sontheimer-Phelps, S. Jalili-Firoozinezhad, A. Bein, A. Chalkiadaki, W. Scholl, C. Zhang, H. Rickner, C. A. Richmond, et al. 2018. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Scientific Reports 8 (1):2871. doi: 10.1038/s41598-018-21201-7.
- Keller, J., A. Borzekowski, H. Haase, R. Menzel, L. Rueß, and M. Koch. 2018. Toxicity assay for citrinin, zearalenone and zearalenone-14-sulfate using the nematode caenorhabditis elegans as model organism. Toxins (Basel) 10 (7):284. doi: 10.3390/toxins10070284.
- Kharat, P., P. Sarkar, S. Mouliganesh, V. Tiwary, V. B. R. Priya, N. Y. Sree, H. V. Annapoorna, D. K. Saikia, K. Mahanta, and K. Thirumurugan. 2020. Ellagic acid prolongs the lifespan of Drosophila melanogaster. Geroscience 42 (1):271–85. doi: 10.1007/s11357-019-00135-6.
- King-Jones, K.,. M. A. Horner, G. Lam, and C. S. Thummel. 2006. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metabolism 4 (1):37–48. doi: 10.1016/j.cmet.2006.06.006.
- Köhle, C., and K. W. Bock. 2009. Coordinate regulation of human drug-metabolizing enzymes, and conjugate transporters by the Ah receptor, pregnane X receptor and constitutive androstane receptor. Biochemical Pharmacology 77 (4):689–99. doi: 10.1016/j.bcp.2008.05.020.
- Koiwa, J., T. Shiromizu, Y. Adachi, M. Ikejiri, K. Nakatani, T. Tanaka, and Y. Nishimura. 2019. Generation of a Triple-Transgenic Zebrafish Line for Assessment of Developmental Neurotoxicity during Neuronal Differentiation. Pharmaceuticals (Basel) 12 (12):14. doi: 10.3390/ph12040145.
- Kulthong, K., L. Duivenvoorde, B. Z. Mizera, D. Rijkers, G. t Dam, G. Oegema, T. Puzyn, H. Bouwmeester, and M. van der Zande. 2018. Implementation of a dynamic intestinal gut-on-a-chip barrier model for transport studies of lipophilic dioxin congeners. RSC Advances 8 (57):32440–53. doi: 10.1039/C8RA05430D.
- Kuzin, B. A., E. A. Nikitina, R. O. Cherezov, J. E. Vorontsova, M. S. Slezinger, O. G. Zatsepina, O. B. Simonova, G. N. Enikolopov, and E. V. Savvateeva-Popova. 2014. Combination of hypomorphic mutations of the Drosophila homologues of aryl hydrocarbon receptor and nucleosome assembly protein family genes disrupts morphogenesis, memory and detoxification. PLoS ONE 9 (4):e94975. doi: 10.1371/journal.pone.0094975.
- Lackmann, C., M. M. Santos, S. Rainieri, A. Barranco, H. Hollert, P. Spirhanzlova, M. Velki, and T.-B. Seiler. 2018. Novel procedures for whole organism detection and quantification of fluorescence as a measurement for oxidative stress in zebrafish (Danio rerio) larvae. Chemosphere 197:200–9. doi: 10.1016/j.chemosphere.2018.01.045.
- Lai, C. H., C. Y. Chou, L. Y. Ch'ang, C. S. Liu, and W. Lin. 2000. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Research 10 (5):703–13. doi: 10.1101/gr.10.5.703.
- Lanzerstorfer, P., G. Sandner, J. Pitsch, B. Mascher, T. Aumiller, and J. Weghuber. 2021. Acute, reproductive, and developmental toxicity of essential oils assessed with alternative in vitro and in vivo systems. Archives of Toxicology 95 (2):673–19. . doi: 10.1007/s00204-020-02945-6.
- Lee, S., Y. Kim, and J. Choi. 2020. Effect of soil microbial feeding on gut microbiome and cadmium toxicity in Caenorhabditis elegans. Ecotoxicology and Environmental Safety 187:109777. doi: 10.1016/j.ecoenv.2019.109777.
- Lemieux, G. A., and K. Ashrafi. 2015. Insights and challenges in using C. elegans for investigation of fat metabolism. Critical Reviews in Biochemistry and Molecular Biology 50 (1):69–84. Epub 2014 Sep 17. eng. doi: 10.3109/10409238.2014.959890.
- Lev, I., R. Bril, Y. Liu, L. I. Ceré, and O. Rechavi. 2019. Inter-generational consequences for growing Caenorhabditis elegans in liquid. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 374 (1770):20180125. doi: 10.1098/rstb.2018.0125.
- Liao, V. H.-C. 2018. Use of Caenorhabditis elegans To Study the Potential Bioactivity of Natural Compounds. Journal of Agricultural and Food Chemistry 66 (8):1737–42. doi: 10.1021/acs.jafc.7b05700.
- Lindblom, T. H., and A. K. Dodd. 2006. Xenobiotic detoxification in the nematode Caenorhabditis elegans. Journal of Experimental Zoology. Part A, Comparative Experimental Biology 305 (9):720–30. doi: 10.1002/jez.a.324.
- Ludewig, A. H., C. Gimond, J. C. Judkins, S. Thornton, D. C. Pulido, R. J. Micikas, F. Döring, A. Antebi, C. Braendle, and F. C. Schroeder. 2017. Larval crowding accelerates C. elegans development and reduces lifespan. PLoS Genetics 13 (4):e1006717. doi: 10.1371/journal.pgen.1006717.
- Ma, H., K. A. Lenz, X. Gao, S. Li, and L. K. Wallis. 2019. Comparative toxicity of a food additive TiO2, a bulk TiO2, and a nano-sized P25 to a model organism the nematode C. elegans. Environmental Science and Pollution Research International 26 (4):3556–68. doi: 10.1007/s11356-018-3810-4.
- Mathew, M. D., N. D. Mathew, and P. R. Ebert. 2012. WormScan: A technique for high-throughput phenotypic analysis of Caenorhabditis elegans. PLoS ONE 7 (3):e33483. doi: 10.1371/journal.pone.0033483.
- Ma, L.-D., Y.-T. Wang, J.-R. Wang, J.-L. Wu, X.-S. Meng, P. Hu, X. Mu, Q.-L. Liang, and G.-A. Luo. 2018. Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab on a Chip 18 (17):2547–62. doi: 10.1039/c8lc00333e.
- McNamee, P., J. Hibatallah, M. Costabel-Farkas, C. Goebel, D. Araki, E. Dufour, N. J. Hewitt, P. Jones, A. Kirst, B. Le Varlet, et al. 2009. A tiered approach to the use of alternatives to animal testing for the safety assessment of cosmetics: Eye irritation. Regulatory Toxicology and Pharmacology: RTP 54 (2):197–209. doi: 10.1016/j.yrtph.2009.04.004.
- Merinas-Amo, R., M. Martínez-Jurado, S. Jurado-Güeto, Á. Alonso-Moraga, and T. Merinas-Amo. 2019. Biological Effects of Food Coloring in In Vivo and In Vitro Model Systems. Foods 8 (5):176. doi: 10.3390/foods8050176.
- Merinas-Amo, T., R. Merinas-Amo, V. García-Zorrilla, A. Velasco-Ruiz, L. Chladek, V. Plachy, M. Del Río-Celestino, R. Font, L. Kokoska, and Á. Alonso-Moraga. 2019. Toxicological Studies of Czech Beers and Their Constituents. Foods 8 (8):328. doi: 10.3390/foods8080328.
- More, S. J., V. Bampidis, D. Benford, C. Bragard, T. I. Halldorsson, A. F. Hernández‐Jerez, S. Hougaard Bennekou, K. P. Koutsoumanis, K. Machera, H. Naegeli, et al. 2019. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 17 (6):e05708. doi: 10.2903/j.efsa.2019.5708.
- Mourabit, S., J. A. Fitzgerald, R. P. Ellis, A. Takesono, C. S. Porteus, M. Trznadel, J. Metz, M. J. Winter, T. Kudoh, and C. R. Tyler. 2019. New insights into organ-specific oxidative stress mechanisms using a novel biosensor zebrafish. Environment International 133 (Pt A):105138. doi: 10.1016/j.envint.2019.105138.
- Moyson, S., R. M. Town, K. Vissenberg, and R. Blust. 2019. The effect of metal mixture composition on toxicity to C. elegans at individual and population levels. PLoS One 14 (6):e0218929. doi: 10.1371/journal.pone.0218929.
- Murugesu, S., A. Khatib, Q. U. Ahmed, Z. Ibrahim, B. F. Uzir, K. Benchoula, N. I. N. Yusoff, V. Perumal, M. F. Alajmi, S. Salamah, et al. 2019. Toxicity study on Clinacanthus nutans leaf hexane fraction using Danio rerio embryos. Toxicology Reports 6:1148–54. doi: 10.1016/j.toxrep.2019.10.020.
- Muthulakshmi, S., K. Maharajan, H. R. Habibi, K. Kadirvelu, and M. Venkataramana. 2018. Zearalenone induced embryo and neurotoxicity in zebrafish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 198:111–21. doi: 10.1016/j.chemosphere.2018.01.141.
- Naik, M., P. Brahma, and M. Dixit. 2018. A cost-effective and efficient chick ex-ovo CAM assay protocol to assess angiogenesis. Methods Protoc 1 (2):19. doi: 10.3390/mps1020019.
- Nihad, A. S. M., R. Deshpande, V. P. Kale, R. R. Bhonde, and S. P. Datar. 2018. Establishment of an in ovo chick embryo yolk sac membrane (YSM) assay for pilot screening of potential angiogenic and anti-angiogenic agents. Cell Biology International 42 (11):1474–83. doi: 10.1002/cbin.11051.
- Nobrega, A. K., and L. C. de Lyons. 2020. Aging and the clock: Perspective from flies to humans. The European Journal of Neuroscience 51 (1):454–81. doi: 10.1111/ejn.14176.
- OECD. 2013. OECD Guideline for the testing of chemicals - Fish Embryo Toxicity Test.
- Oishi, K., S. Higo-Yamamoto, and Y. Yasumoto. 2016. Moderately high doses of the artificial sweetener saccharin potentially induce sleep disorders in mice. Nutrition (Burbank, Los Angeles County, Calif.) 32 (10):1159–61. eng. doi: 10.1016/j.nut.2016.03.013.
- Panzica-Kelly, J. M., C. X. Zhang, and K. A. Augustine-Rauch. 2015. Optimization and performance assessment of the chorion-off [dechorinated] zebrafish developmental toxicity assay. Toxicological Sciences : An Official Journal of the Society of Toxicology 146 (1):127–34. doi: 10.1093/toxsci/kfv076.
- Pascussi, J.-M., S. Gerbal-Chaloin, C. Duret, M. Daujat-Chavanieu, M.-J. Vilarem, and P. Maurel. 2008. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: Crosstalk and consequences. Annual Review of Pharmacology and Toxicology 48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349.
- Piechulek, A., L. C. Berwanger, and A. v Mikecz. 2019. Silica nanoparticles disrupt OPT-2/PEP-2-dependent trafficking of nutrient peptides in the intestinal epithelium. Nanotoxicology 13 (8):1133–48. doi: 10.1080/17435390.2019.1643048.
- Piechulek, A., and A. v Mikecz. 2018. Life span-resolved nanotoxicology enables identification of age-associated neuromuscular vulnerabilities in the nematode Caenorhabditis elegans. Environmental Pollution (Barking, Essex: 1987) 233:1095–103. doi: 10.1016/j.envpol.2017.10.012.
- Poetini, M. R., S. M. Araujo, M. Trindade de Paula, V. C. Bortolotto, L. B. Meichtry, F. Polet de Almeida, C. R. Jesse, S. N. Kunz, and M. Prigol. 2018. Hesperidin attenuates iron-induced oxidative damage and dopamine depletion in Drosophila melanogaster model of Parkinson's disease. Chemico-Biological Interactions 279:177–86. doi: 10.1016/j.cbi.2017.11.018.
- Proquin, H., C. Rodríguez-Ibarra, C. G. J. Moonen, I. M. Urrutia Ortega, J. J. Briedé, T. M. de Kok, H. van Loveren, and Y. I. Chirino. 2017. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 32 (1):139–49. doi: 10.1093/mutage/gew051.
- Qin, H., and J. A. Powell-Coffman. 2004. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Developmental Biology 270 (1):64–75. doi: 10.1016/j.ydbio.2004.02.004.
- Raies, A. B., and V. B. Bajic. 2018. In silico toxicology: Comprehensive benchmarking of multi-label classification methods applied to chemical toxicity data. Wiley Interdisciplinary Reviews. Computational Molecular Science 8 (3):e1352. doi: 10.1002/wcms.1352.
- Ramadan, Q., and M. Zourob. 2020. Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 14 (4):041501. doi: 10.1063/5.0011583.
- Ribatti, D. 2016. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mechanisms of Development 141:70–7. doi: 10.1016/j.mod.2016.05.003.
- Saleem, M., M. Asif, A. Parveen, H. S. Yaseen, M. Saadullah, A. Bashir, J. Asif, M. Arif, I. U. Khan, and R. U. Khan. 2020. Investigation of in vivo anti-inflammatory and anti-angiogenic attributes of coumarin-rich ethanolic extract of Melilotus indicus. Inflammopharmacology. 29 (1):281–293. doi: 10.1007/s10787-020-00703-9.
- Sandner, G., A. S. Mueller, X. Zhou, V. Stadlbauer, B. Schwarzinger, C. Schwarzinger, U. Wenzel, K. Maenner, J. D. van der Klis, S. Hirtenlehner, et al. 2020. Ginseng extract ameliorates the negative physiological effects of heat stress by supporting heat shock response and improving intestinal barrier integrity: evidence from studies with heat-stressed caco-2 Cells, C. elegans and growing broilers. Molecules 25 (4):835. doi: 10.3390/molecules25040835.
- Santbergen, M. J. C., M. van der Zande, A. Gerssen, H. Bouwmeester, and M. W. F. Nielen. 2020. Dynamic in vitro intestinal barrier model coupled to chip-based liquid chromatography mass spectrometry for oral bioavailability studies. Analytical and Bioanalytical Chemistry 412 (5):1111–22. doi: 10.1007/s00216-019-02336-6.
- Sarasquete, C., M. Úbeda-Manzanaro, and J. B. Ortiz-Delgado. 2018. Toxicity and non-harmful effects of the soya isoflavones, genistein and daidzein, in embryos of the zebrafish, Danio rerio. Comparative Biochemistry and Physiology. Toxicology & Pharmacology: CBP 211:57–67. doi: 10.1016/j.cbpc.2018.05.012.
- Schifano, E., P. Zinno, B. Guantario, M. Roselli, S. Marcoccia, C. Devirgiliis, and D. Uccelletti. 2019. The foodborne strain lactobacillus fermentum MBC2 triggers pept-1-dependent pro-longevity effects in caenorhabditis elegans. Microorganisms 7 (2):45. doi: 10.3390/microorganisms7020045.
- Senthilkumar, S., R. Raveendran, S. Madhusoodanan, M. Sundar, S. S. Shankar, S. Sharma, V. Sundararajan, P. Dan, and S. Sheik Mohideen. 2020. Developmental and behavioural toxicity induced by acrylamide exposure and amelioration using phytochemicals in Drosophila melanogaster. Journal of Hazardous Materials 394:122533. doi: 10.1016/j.jhazmat.2020.122533.
- Smeriglio, A., M. Denaro, D. Barreca, V. D'Angelo, M. P. Germanò, and D. Trombetta. 2018. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.). Fitoterapia 124:49–57. doi: 10.1016/j.fitote.2017.10.006.
- Souza Anselmo, C., de, Sardela, V. F. Sousa, V. P. de, Pereira. and H. M. G. 2018. Zebrafish (Danio rerio): A valuable tool for predicting the metabolism of xenobiotics in humans? Comparative Biochemistry and Physiology. Toxicology & Pharmacology: CBP 212:34–46. doi: 10.1016/j.cbpc.2018.06.005.
- Spanier, B., R. Lang, D. Weber, A. Lechner, T. Thoma, M. Rothner, K. Petzold, T. Lang, A. Beusch, M. Bösl, et al. 2019. Bioavailability and biological effects of 2- O-β-d-glucopyranosyl-carboxyatractyligenin from green coffee in caenorhabditis elegans. Journal of Agricultural and Food Chemistry 67 (17):4774–81. doi: 10.1021/acs.jafc.8b06785.
- Staats, S., K. Lüersen, A. E. Wagner, and G. Rimbach. 2018. Drosophila melanogaster as a versatile model organism in food and nutrition research. Journal of Agricultural and Food Chemistry 66 (15):3737–53. doi: 10.1021/acs.jafc.7b05900.
- Staats, S., A. E. Wagner, B. Kowalewski, F. T. Rieck, S. T. Soukup, S. E. Kulling, and G. Rimbach. 2018. Dietary resveratrol does not affect life span, body composition, stress response, and longevity-related gene expression in drosophila melanogaster. International Journal of Molecular Sciences 19 (1):223. doi: 10.3390/ijms19010223.
- Steele, W. B., R. A. Mole, and B. W. Brooks. 2018. Experimental protocol for examining behavioral response profiles in larval fish: application to the neuro-stimulant caffeine. Journal of Visualized Experiments. 137:57938. doi: 10.3791/57938.
- Stokes, W. S., S. Casati, J. Strickland, and M. Paris. 2008. Neutral red uptake cytotoxicity tests for estimating starting doses for acute oral toxicity tests. Current Protocols in Toxicology Chapter 20:Unit 20.4. eng. doi: 10.1002/0471140856.tx2004s36.
- Strange, K. 2016. Drug Discovery in Fish, Flies, and Worms. ILAR Journal 57 (2):133–43. doi: 10.1093/ilar/ilw034.
- Tavakkoli, H., A. Derakhshanfari, J. Moayedi, and A. Poostforoosh Fard. 2020. Utilization of a chicken embryo membrane model for evaluation of embryonic vascular toxicity of Dorema ammoniacum. Journal of Phynomedicine 10 (2):152–160.
- Tayemeh, M. B., M. R. Kalbassi, H. Paknejad, and H. S. Joo. 2020. Dietary nanoencapsulated quercetin homeostated transcription of redox-status orchestrating genes in zebrafish (Danio rerio) exposed to silver nanoparticles. Environmental Research 185:109477eng. doi: 10.1016/j.envres.2020.109477.
- Thiel, C., S. Schneckener, M. Krauss, A. Ghallab, U. Hofmann, T. Kanacher, S. Zellmer, R. Gebhardt, J. G. Hengstler, and L. Kuepfer. 2015. A systematic evaluation of the use of physiologically based pharmacokinetic modeling for cross-species extrapolation. Journal of Pharmaceutical Sciences 104 (1):191–206. eng. doi: 10.1002/jps.24214.
- Vingskes, A. K., and N. Spann. 2018. The toxicity of a mixture of two antiseptics, triclosan and triclocarban, on reproduction and growth of the nematode Caenorhabditis elegans. Ecotoxicology (London, England) 27 (4):420–9. eng. doi: 10.1007/s10646-018-1905-9.
- Wang, J., N. Deng, H. Wang, T. Li, L. Chen, B. Zheng, and R. H. Liu. 2020. Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis elegans. Molecules 25 (2):351. eng. doi: 10.3390/molecules25020351.
- Wang, G., J. Liang, L.-R. Gao, Z.-P. Si, X.-T. Zhang, G. Liang, Y. Yan, K. Li, X. Cheng, Y. Bao, et al. 2018. Baicalin administration attenuates hyperglycemia-induced malformation of cardiovascular system. Cell Death & Disease 9 (2):234. eng. doi: 10.1038/s41419-018-0318-2.
- Wittkowski, P., P. Marx-Stoelting, N. Violet, V. Fetz, F. Schwarz, M. Oelgeschläger, G. Schönfelder, and S. Vogl. 2019. Caenorhabditis elegans As a Promising Alternative Model for Environmental Chemical Mixture Effect Assessment-A Comparative Study. Environmental Science & Technology 53 (21):12725–33. eng. doi: 10.1021/acs.est.9b03266.
- World Health Organization. 2009. Principles and Methods for the Risk Assessment of Chemicals in Food, 788. Geneva: World Health Organization. (Environmental Health Criteria). ISBN: 9789241572408. http://gbv.eblib.com/patron/FullRecord.aspx?p=760420.
- Wu, Q., J. Liu, X. Wang, L. Feng, J. Wu, X. Zhu, W. Wen, and X. Gong. 2020. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomedical Engineering Online 19 (1):9. doi: 10.1186/s12938-020-0752-0.
- Xia, Q., L. Wei, Y. Zhang, H. Kong, Y. Shi, X. Wang, X. Chen, L. Han, and K. Liu. 2018. Psoralen Induces Developmental Toxicity in Zebrafish Embryos/Larvae Through Oxidative Stress, Apoptosis, and Energy Metabolism Disorder. Frontiers in Pharmacology 9:1457. doi: 10.3389/fphar.2018.01457.
- Xue, D., Y. Wang, J. Zhang, D. Mei, Y. Wang, and S. Chen. 2018. Projection-Based 3D Printing of Cell Patterning Scaffolds with Multiscale Channels. ACS Applied Materials & Interfaces 10 (23):19428–35. doi: 10.1021/acsami.8b03867.
- Yin, L., G. Du, B. Zhang, H. Zhang, R. Yin, W. Zhang, and S.-M. Yang. 2020. Efficient Drug Screening and Nephrotoxicity Assessment on Co-culture Microfluidic Kidney Chip. Scientific Reports 10 (1):6568. doi: 10.1038/s41598-020-63096-3.
- Yin, F., Y. Zhu, M. Zhang, H. Yu, W. Chen, and J. Qin. 2019. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicology in Vitro: An International Journal Published in Association with BIBRA 54:105–13. doi: 10.1016/j.tiv.2018.08.014.
- Younes, M., P. Aggett, F. Aguilar, R. Crebelli, B. Dusemund, M. Filipič, M. J. Frutos, P. Galtier, D. Gott, U. Gundert‐Remy, et al. 2018a. Evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E 171. EFSA Journal 16 (7):16. doi: 10.2903/j.efsa.2018.5366.
- Younes, M., P. Aggett, F. Aguilar, R. Crebelli, B. Dusemund, M. Filipič, M. J. Frutos, P. Galtier, D. Gott, U. Gundert‐Remy, et al. 2018b. Re‐evaluation of silicon dioxide (E 551) as a food additive. EFSA Journal 16 (1):5088. doi: 10.2903/j.efsa.2018.5088.
- Zabihihesari, A., A. J. Hilliker, and P. Rezai. 2019. Fly-on-a-Chip: Microfluidics for Drosophila melanogaster Studies. IntIntegrative Biology : Quantitative Biosciences from Nano to Macro 11 (12):425–43. eng. doi: 10.1093/intbio/zyz037.
- Zenki, K. C., L. d Souza, A. M. Góis, B. D. S. Lima, AAdS. Araújo, J. S. Vieira, E. A. Camargo, E. Kalinine, D. d Oliveira, and C. I. B. Walker. 2020. Coriandrum sativum extract prevents alarm substance-induced fear- and anxiety-like responses in adult zebrafish. Zebrafish 17 (2):120–30. eng. doi: 10.1089/zeb.2019.1805.
- Zhang, D., L. Gong, S. Ding, Y. Tian, C. Jia, D. Liu, M. Han, X. Cheng, D. Sun, P. Cai, et al. 2020. FRCD: A comprehensive food risk component database with molecular scaffold, chemical diversity, toxicity, and biodegradability analysis. Food Chemistry 318:126470. Epub 2020 Feb 24. eng. doi: 10.1016/j.foodchem.2020.126470.
- Zhang, M., C. Xu, L. Jiang, and J. Qin. 2018. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicology Research 7 (6):1048–60. doi: 10.1039/c8tx00156a.
- Zhang, M., X. Yang, W. Xu, X. Cai, M. Wang, Y. Xu, P. Yu, J. Zhang, Y. Zheng, J. Chen, et al. 2019. Evaluation of the effects of three sulfa sweeteners on the lifespan and intestinal fat deposition in C. elegans. Food Research International (Ottawa, Ont.) 122:66–76. doi: 10.1016/j.foodres.2019.03.028.
- Zhao, X., S. Shan, J. Li, L. Cao, J. Lv, and M. Tan. 2019. Assessment of potential toxicity of foodborne fluorescent nanoparticles from roasted pork. Nanotoxicology 13 (10):1310–23. doi: 10.1080/17435390.2019.1652943.
- Zink, D., J. K. C. Chuah, and J. Y. Ying. 2020. Assessing Toxicity with Human Cell-Based In Vitro Methods. Trends in Molecular Medicine 26 (6):570–82. doi: 10.1016/j.molmed.2020.01.008.

