Abstract
The total phenolic content of methanol and ethyl-acetate extracts of pits from seven Algerian palm date (Phœnix dactylifera L.) fruit varieties was estimated by the Folin-Ciocalteau method. Their respective antioxidant activities were evaluated by the DPPH· and the Co(II)-EDTA luminol chemiluminescence tests and their phenolic profile was established by LC-DAD-MS (ESI− and ESI+). The total phenolic content of the methanolic extracts ranged from 27.2 to 38.5 mg of caffeic acid equivalents CAE/100 g fresh weight, while the ethyl acetate extracts gave contents ranging from 22.8 to 42.6 mg CAE/100 g fresh weight. The antiradical and the hydroxyl scavenging activities of the methanolic extracts were much higher than those of the ethyl acetate extracts. The different varieties that were studied did not show any major difference in their phenolic profile. Indeed, all the varieties contained mainly catechin or epi catechin derivatives, sinapic, cinnamic, and coumaric acid derivatives. However, it should be noted that, unexpectedly, a glycosylated aurone was tentatively identified in the Tazizaout variety. Further investigations are required to characterise the non-identified flavonoids.
INTRODUCTION
The fruit of the date palm (Phœnix dactylifera L.) is a key agro-product and a vital component of the population's diet living in the Algerian Sahara. This fruit holds a great importance from both nutritional and economic points of view.[Citation1–3] The industrial processing of this fruit results in the rejection of considerable quantities of waste represented mainly by the pits.Citation[4] In fact, date pits represent about 10% of the ripened fruit's total fresh weight.Citation[5] Many practices are already established to valorise this agro-industrial by-product, mainly as a precursor for the production of activated carbon.Citation[6,Citation7] Furthermore, given the content of date pits on proteins, fat, minerals, and carbohydrates, this by-product could serve as animal feed since it fulfils partially the nutritional requirements of the animals.Citation[8] Hence, it is very important to investigate the physical and chemical properties of date pits.Citation[9,Citation10]
However, date seeds contain substantial amounts of secondary metabolites, mainly phenolic compounds.Citation[11] Given the lack of studies establishing the identity of the constitutive phenolic compounds of date pits, the present study was designed to determine the phenolic content, to characterise the phenolic profile, and to evaluate the potential antioxidant activity of the date pits extracts. The outcome of this study will contribute to improve the valorisation of date pits as a new source of antioxidant compounds and as a potential tool for botanical and geographical discrimination among different date palm fruit varieties.
MATERIALS AND METHODS
Plant Materials
Seven different varieties of ripe date palm fruit (Phœnix dactylifera L.) were harvested from different oases in Ghardaia, Algeria during the year 2002. The varieties were identified by a local expert as: Akerbouche, Tazizaout, Deglet-Nour, Ougherouss, Tantbouchte, Tafiziouine, and Tazerzait. The pits were removed, cleaned, and stored at −18°C, until analysed.
Chemicals and Standards
Folin-Ciocalteau's phenol reagent, cobalt(II) chloride, hydrogen peroxide (30% v/v), ethyl acetate, and hexane were purchased from Merck (Darmstadt, Germany); methanol was obtained from Riedel-de Haën (Seelze, Germany); and DPPH• (2,2-diphenyl-1-picrylhydrazyl free radical), luminol (3-aminophthalhydrazide), boric acid, and caffeic acid were from Sigma (St. Louis, MO, USA).
Phenolic Extraction
Date pits were ground in a mortar in the presence of liquid nitrogen. Then 2 g of the ground material from each variety were rinsed twice with 35 ml of hexane. After rinsing, 50 ml of ethyl acetate were added to the samples. The resulting solutions were shaken for 3 h at room temperature. Thereafter, the solutions were centrifuged at 2500 rpm for 15 min. The supernatant layer was removed and evaporated under vacuum at 40°C. The resulting residue was re-dissolved in 3 ml of methanol and constituted the ethyl acetate extract. The precipitate from the first extraction was subjected to a second extraction by dissolving and agitating it in 50 ml of methanol overnight at room temperature in the dark. After centrifugation at 2500 rpm for 15 min, the supernatant solution was evaporated under vacuum at 40°C. The residue was re-dissolved in 3 ml of methanol and constituted the methanolic extract. The extraction was repeated in triplicate for each variety.
Phenolic Quantification
The total content of phenols in the extracts was determined according to the Folin-Ciocalteau method.Citation[12] The total phenol concentration was expressed as mg of caffeic acid equivalents (mg CAE) per 100 g fresh weight (FW) against external calibration.
Evaluation of Antioxidant Activity
The antioxidant activity was estimated using two in vitro tests: the DPPH· test according to Brand-Williams et al.Citation[13] and Arnous et al.Citation[14] and the hydroxyl free radical scavenging activity test by Co (ΙΙ)/EDTA induced luminol plateau chemiluminescence (SAHFR) by Parejo et al.Citation[15,Citation16] The DPPH• test was performed by preparing three different dilutions of each sample in order to have a reduction of the DPPH• solution's absorbance, without reaching a complete discoloration. An aliquot of 50 μl of the diluted sample was added to 950 μl of the DPPH· solution (6 × 10−5 M) and vortexed. The absorbance at 515 nm was read at t = 0 and at t = 30 min. The concentration ratio: [phenols](mg/ml)/[DPPH](mg/ml) was plotted against the % of the remaining [DPPH]. The EC50 value is calculated as the concentration responsible for 50% scavenging of DPPH· and the results were expressed as antiradical efficiency AE (AE = 1/EC50).
The chemiluminescence (CL) test consisted of mixing 1 ml of a buffer solution of boric acid (50 mM, pH = 9) containing CoCl2.6H2O (0.4 mg/ml) and EDTA (2 mg/ml) with 100 μl of luminol solution (0.56 mM) in borate buffer (pH = 9). Three different dilutions of each sample were prepared in order to have a luminescence signal ranging between 0 and 100. Thereafter, 25 μl of the diluted sample were added and the mixture was vortexed for 15 s. Then, 25 μl of H2O2 aqueous solution (5.4 mM) were added and the solution was thoroughly mixed and rapidly transferred into a (10 × 10)-mm glass cuvette and the intensity of the emitted light was measured when it reached the plateau. The instantaneous reduction in the plateau CL intensity, induced by the addition of sample, was recorded as Ι, and CL intensity, induced in the absence of the sample, was recorded as Ι0. For all experiments, the ratio (Ι0/Ι) was plotted vs. concentration (mg/ml) sample. The equation was established by linear regression and the concentration of sample, which is required to decrease the CL intensity by 50%, was calculated (IC50). The hydroxyl free radical scavenging activity (SAHFR) was expressed as 1/IC50.
Phenolic Profile by LC-DAD-MS
The ethyl acetate extracts dissolved in methanol were used to establish the phenolic profile using an LC-DAD-MS system (Finnigan MAT Spectra System P4000 pump [Bremen, Germany] coupled with a UV6000LP diode array detector and a Finnigan AQA mass spectrometer [Bremen, Germany]). The separation of the phenolic compounds was performed on a 125 × 2 mm superspher 100–4 RP 18 column (Macherey-Nagel, Düren, Germany; 4 μm particle size) at a temperature of 40°C and an injection volume of 15 μl. The analysis was monitored at 290 nm and also by MS-ESI spectroscopy at a probe temperature of 300°C, probe voltage of 3.5 kV, and at 12 and 50 eV in the mass analyser. Both ESI+ and ESI− were applied in order to facilitate identification of the phenolic compounds present in the extracts. In the ESI− mode, the mobile phase was 0.1% HCOOH in water (solvent A) and methanol (solvent B). The gradient procedure at a flow rate of 0.33 ml/min was as follows: (1) 100% solvent A for 2 min; (2) a 35-min linear gradient to 100% solvent B; (3) a 5-min hold at 100% solvent B; and (4) a 3-min gradient back to 100% solvent A. For the ESI+ mode, the solvent A was replaced by 2.5% acetic acid in water. The gradient programme was the same as for the ESI−. The data were processed using Xcalibur 1.2 software (Chip PC Technologies, Essen, Germany). All the analyses were in triplicate, and processed using Excel software (2000; Microsoft, New York, USA). The statistical analysis was performed using SPSS (version 10; IBM, New York, USA) at p < 0.05.
RESULTS AND DISCUSSION
Phenolic Content
The total phenolic content ranged between 27.2 and 38.5 mg CAE/100 g FW for the methanolic extracts and between 22.8 and 42.6 mg CAE/100 g FW for the ethyl acetate extracts (). The methanolic extracts of the varieties Deglet-Nour, Ougherouss, and Tafiziouine were significantly higher than those of ethyl acetate extracts, whereas Tantbouchte's ethyl acetate extract was significantly higher than its methanolic extract. These data show that the date pit has a higher phenolic content than the fruit of the same varieties as reported by a previous study.Citation[17] However, Al-Farsi and LeeCitation[11] in their investigation on Omanian date pit showed much higher contents of total phenolics (1810–3626 mg of ferulic acid equivalents/100 g FW) using acetone and butanone as extraction solvents, respectively. The heterogeneity of these results could be attributed to several factors: (1) the origin of the plant, (2) the variety, (3) the extraction procedure, and (4) the measurement method.
Table 1 Total phenolic content (TPC), antiradical activity (AA), and hydroxyl scavenging activity (HSA) of the ethyl acetate (EA) and the methanolic (M) extracts of the different date pit varieties.*
The Antioxidant Activity
The antiradical and the hydroxyl free radical scavenging activities of the methanolic extracts were much higher than those of the ethyl acetate extracts, except for the Tantbouchte variety (). The methanolic extracts of Tazizaout, Ougherouss, Deglet-Nour, and Akerbouche presented the highest significant antiradical activity, while the highest hydroxyl scavenging activity was manifested by the varieties Ougherouss, Deglet-Nour, and Akerbouche (). However, the results of the antiradical activity compared with the total phenolic content showed a rather weak correlation for the methanolic extracts (R = 0.34) and a strong correlation for the ethyl acetate extracts (R = 0.96). The weak correlation observed between the methanolic extracts and the antiradical activity could be due to the absence of correlation between the Folin-Ciocalteau test and the antiradical test, due to the non specificity of the method vis-à-vis structures and mechanisms,Citation[14,Citation18] as well as to the nature of the phenolic compounds. In fact, Makris and KefalasCitation[18] reported that, independently of their concentrations, the phenolic compounds show different antiradical characteristics related to their structural features. Results for antiradical activity and hydroxyl scavenging activity show strong correlation for methanolic (R = 0.993) and ethyl acetate (R = 0.990) extracts. Correlation of values for antiradical activity or hydroxyl scavenging activity for both extracts was very poor as expected, since results depend on the organic solvent used. Similar observations have been made by other workers dealing with similar samples, such as lettuce leaves.Citation[19]
Analysis of the Phenolic Profile Using LC-DAD-MS
Only the results concerning the ethyl acetate extracts are reported, since the methanolic extract seemed to contain complicated oligomeric-polymeric mixtures. Structures were elucidated by LC-MS at both ESI+ and ESI− ionisation modes. The UV and MS characteristics of the identified molecules are shown in and . Throughout the obtained results, it appeared that all the varieties contained mainly the same type of compounds with slight differences.
Table 2 LC-DAD-MS of the phenolic compounds of the different date pit fruit varieties extracts under the ESI-ionization mode
Table 3 LC-DAD-MS of the phenolic compounds of the different date pit fruit varieties extracts under the ESI+ ionization mode
Phenolic Acids
Cinnamic acids and their derivatives were the unique category of simple phenolics found in all varieties. Coumaric, sinapic, and dihydrocinamic acids were present as the main class. Two sinapic acid derivatives were identified from both the characteristic fragment 224.8 in the ESI+ and the UV-Vis spectrum and were present in almost all the varieties, except for Tazizaout and Tazerzait. Another cinnamic acid derivative was also found in Akerbouche, Deglet-Nour, Ougherouss, and Tafizioune, while the dihydrocinnamic acid derivative was found in all the varieties except the variety Tafizioune. A coumaric acid derivative (MW = 330) with its characteristic fragment 164.9, was present in all the varieties, except for Tazizaout and Tafiziouine. Tazerzait, for its part, was characterised by the presence of a caffeoyl derivative with the characteristic UV absorption spectrum with a maximum absorption at 320 nm.
The simple phenolic profiles of the different date pit fruits varieties were more or less similar to those of the fruits. Indeed, Mansouri et al.Citation[17] determined mainly p-coumaric, ferulic, and sinapic acids and some cinnamic acid derivatives in the same date fruit varieties. Al-Farsi and LeeCitation[11] found nine phenolic acids in the Omanian date pit variety, with p-hydroxybenzoic, protocatechuic, and m-coumaric acids as the main components, while Besbes et al.Citation[4] found different phenolic compounds when analyzing the pit oil of the Deglet-Nour variety. The identification of different phenolic compounds as compared to the profiles of the varieties of the present study could probably result from the different extraction conditions and the solvent used.
Flavonoids
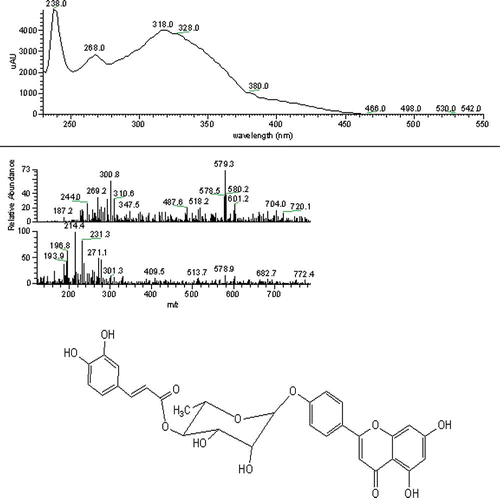
Different classes of flavonoids were identified in the varieties. Flavanols were detected in all the varieties. Flavones, flavanones, and proanthocyanins were also present as well in some of the varieties, but in low concentrations. The flavones were absent in almost all the varieties except for Tazizaout, where an apigenin derivative was detected and identified as glycoside esterified apigenin, with a molecular weight of 578.3 and the characteristic fragment of 271.1 using ESI+. The structure of a caffeoyl rhamnoside of apigenin was proposed ().
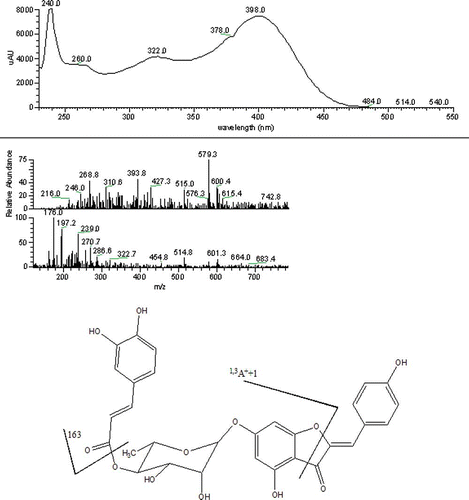
Figure 1 UV-Vis absorption spectrum, mass spectrum, and suggestive structure of an esterified apigenin glycoside. (Color figure available online.)
Flavanones were detected in three varieties only. An unknown flavanone was found in Tazizaout, with a molecular weight of 379.1 and fragments of 263.7 and 349.1 in the ESI−; the characterization was based on the UV absorption spectrum. A second compound was detected in the Tafiziouine variety as a flavanone derivative with a molecular weight of 700 based on the UV-Vis spectrum. The only identified flavanone was naringenin, detected in Tantbouchte with a molecular weight of 336.8 and UV maximal absorption at 286 nm. The main fragment 335.8 was attributed to the naringenin aglycon, associated with formic acid and a molecule of water, under negative ionization [Aglycon (272) + HCOOH (47) + H2O (18) – (1) = 335].
Flavanols
Even though the flavanols were not so numerous, they were detected in all the varieties. The main flavanol was a catechin or epi-catechin derivative identified by its characteristic UV-Vis spectrum with maximum absorption picks at 240 and 278 nm.Citation[16] The second was identified as an oligomer of catechin or epi-catechin units, and it was detected in all the varieties with its specific fragment 273.Citation[20] Three proanthocyanidins were found in Tazizaout; the first one, eluted at 13.96 min, had a molecular ion of 403.6 with a specific fragment 175, the second had a molecular ion of 358.2, and the last one had a molecular ion of 352.5. It should be emphasised that these proanthocyanidins had a characteristic UV-Vis spectrum with maxima at 240 and 274–278 nm.
Tentative Suggestion of an Aurone Structure
Aurones are natural yellow pigments of plants related to flavonoids that have a limited occurrence. Analogy with flavonoids suggests that aurones could have interesting biological properties.Citation[21] The occurrence of an aurone in date pit or fruit has not been mentioned before, to our knowledge. A glycosilated aurone was tentatively identified in Tazizaout, with a molecular weight of 578.3 and the characteristic UV-Vis spectrum with absorptions at 240, 322, and 398 nm (), typical for aurones and a molecular ion of 579 (M + 1). The suggested structure was that of a caffeoyl Rhamnoside of 4,4′,6-trihydroxyaurone. This structural assumption was further supported by fragment (1,3A+ + 1 = 427) and by the characteristic fragment for the caffeoyl moiety (163) as shown in .
CONCLUSION
This study revealed that date pits contain a variety of phenolic compounds at considerable amounts. Though the main objective was not to look for a new source of polyphenols because of the effectiveness of recovery and difficulties of extraction due to the nature of date pits, this study constitutes a contribution to further highlight the richness of this agro-industrial by-product and its potential nutritional importance. Moreover, the outcome of this study could serve as a tool to discriminate among date fruit varieties. Finally, it should be noted that the tentative identification of an aurone in the Tazizaout variety might be of utmost interest. This variety is of particular interest because it contains two markers from the flavonoids family and their glycosides. Therefore, further investigations are required to isolate these particular compounds.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Sofia Loupasaki and Miss Panagiota Goutsiou for their technical and scientific help.
REFERENCES
- Al-Farsi , M.A. and Lee , C.Y. 2008 . Nutritional and functional properties of dates: A review . Critical Reviews in Food Science , 48 : 877 – 887 .
- Al-Shahib , W. and Marshall , R.J. 2003 . The fruit of the date palm Phoenix dactylifera L. Possible use as the best food of the future . International Journal of Food Science and Nutrition , 54 : 247 – 259 .
- Benchelah , A.C. and Maka , M. 2006 . Les dattes, de la préhistoire à nos jours . Phytothérapie , 4 : 43 – 47 .
- Besbes , S. , Blecker , C. , Deroanne , C. , Drira , N.E. and Attia , H. 2004 . Date seeds: Chemical composition and characteristic profiles of the lipid fraction . Food Chemistry , 84 : 577 – 584 .
- Almana , H.A. and Mahmoud , R.M. 1994 . Palm date seeds as an alternative source of dietary fibre in Saudi bread . Ecology of Food and Nutrition , 32 : 261 – 270 .
- Banat , F. , Al-Asheh , S. and Al-Makhameh , L. 2003 . Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters . Process Biochemistry , 39 : 193 – 202 .
- Girgis , B.S. and El-Hendawy , A.N.A. 2002 . Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid . Microporous and Mesoporous Materials , 52 : 105 – 117 .
- Barreveld , W.H. 2002 . Date palm products. Bulletin No. 010., FAO, Viale delle Terme di Caracalla Rome , , Italy
- Thabet , I.B. , Besbes , S. , Attia , H. , Deroanne , C. , Francis , F. , Drira , N.-E. and Blecker , C. 2009 . Physicochemical characteristics of date sap “Lagmi” from deglet nour palm (Phoenix Dactylfera L.) . International Journal of Food Properties , 12 : 659 – 670 .
- Rahman , M.S. , Kasapis , S. , Al-Kharusi , N.S.Z. , Al-Marhubi , I.M. and Khan , A.J. 2007 . Composition characterisation and thermal transition of date pits powders . Journal of Food Engineering , 80 : 1 – 10 .
- Al-Farsi , M.A. and Lee , C.Y. 2008 . Optimization of phenolics and dietary fibre extraction from date seeds . Food Chemistry , 108 : 977 – 985 .
- Waterman , P.G. and Mole , S. 1994 . Analysis of Phenolics Plant Metabolites , 238 Blackwell Scientific Publication : Oxford .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . LWT–Food Science and Technology , 28 : 25 – 30 .
- Arnous , A. , Makris , D.P. and Kefalas , P. 2001 . Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines . Journal of Agricultural and Food Chemistry , 49 : 5736 – 5742 .
- Parejo , I. , Petrakis , C. and Kefalas , P. 2000 . A transition metal enhanced luminol chemiluminescence in the presence of a chelator . Journal of Pharmacological and Toxicological Methods , 43 : 183 – 190 .
- Parejo , I. , Codina , C. , Petrakis , C. and Kefalas , P. 2000 . Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH· (2,2-diphenyl-1-picrylhydrazyl) free radical assay . Journal of Pharmacological and Toxicological Methods , 44 : 507 – 512 .
- Mansouri , A. , Guendez , E. , Kokkalou , E. and Kefalas , P. 2005 . Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) . Food Chemistry , 89 : 411 – 420 .
- Makris , D.P. and Kefalas , P. 2005 . Association between in vitro antiradical activity and ferric reducing power in aged red wines: A mechanistic approach . Food Science and Technology International , 11 : 11 – 18 .
- Cano , A. and Arnao , M.B. 2005 . Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties . International Journal of Food Properties , 8 : 521 – 528 .
- Atoui , A.K. , Mansouri , A. , Boskou , G. and Kefalas , P. 2005 . Tea and herbal infusions: Their antioxidant activity and phenolic profile . Food Chemistry , 89 : 27 – 36 .
- Villemin , D. , Martin , B. and Bar , N. 1998 . Application of microwave in organic synthesis. Dry synthesis of 2-Arylmethylene-3(2)-naphthofuranones . Molecules , 3 : 88 – 93 .