Abstract
The antioxidant activities of crude extract, dichloromethane, ethyl acetate, and butanolic fractions of Nasturtium officinale were investigated using the 1,1,-diphenyl-2-picryl-hydrazyl and thiobarbitaric acid reactive species methods. Total phenolics and flavonoids were determined. Rutin, chlorogenic, and caffeic acids were quantified in the crude extract by high performance liquid chromatography-photodiode array detector. The antioxidant and radical scavenging activity of the crude extract and fractions were found in the following decreasing order: butanolic fraction > ethyl acetate fraction > dichloromethane fraction > crude extract. The extent of antioxidant activities was in accordance with the amounts of phenolics and flavonoids present in the extract and fractions. Ethyl acetate and butanolic fractions caused a sharp fall in thiobarbitaric acid reactive species production, starting at the concentration of 1 μg/mL for both fractions with IC50 6.92 ± 0.19 and 12.07 ± 0.45 μg/mL, respectively. N. officinale might be a valuable antioxidant natural source and seemed to be applicable in both healthy medicine and the food industry.
Keywords:
INTRODUCTION
Fruits and vegetables contain different antioxidant substances, whose activities have been well proven in recent years. The presence of phenolic compounds, such as flavonoids, phenolic acids, and carotenoids, contribute to the beneficial effects of these foods.Citation1–3 The antioxidant effect of phenolic compounds is mainly due to their redox properties and is the result of various possible mechanisms: free-radical scavenging activity, transition-metal-chelating activity, and/or singlet-oxygen-quenching capacity.Citation4 Citation5 Emphasis has been given to the identification and purification of new compounds with antioxidant activity from natural sources, which may act alone or synergistically with other additives, as a way to prevent oxidative deterioration of food and restrict the use of synthetic antioxidants.Citation6 Citation7 In addition, studies have shown that polyphenols have a significant effect in reducing degenerative diseases, such as cancer, and epidemiological evidence showing inverse correlation between heart disease and consumption of foods rich in phenolic substances, possibly due to their antioxidant properties, are described in the literature.Citation8–12
The water watercress (Nasturtium officinale–Brassicaceae) is an herbal plant that grows in and around water. It has a distinctive smell and slightly bitter taste and is popularly used to treat children's urinary tract infections and to treat bronchitis as an expectorant.Citation[13] Phytochemical screening indicated the presence of triterpenes, steroids, flavonoids, phenylpropanoids, and saponins in water watercress growing in the conventional way.Citation14 However, several aspects of the culture can influence the chemicals found in plants. Plants grown in hydroponics, the technique of cultivation in a nutrient solution without the need of soil, have become the choice of most consumers, making it a growing market. Moreover, consumers want information about the beneficial properties and nutritional value of foods. In line with this, beyond the composition of the usual nutrients, it seems important to provide information about the presence and the levels of bioactive compounds and the antioxidant capacities of vegetables, fruits, and other important components of a diet. Considering the scarcity of information about hydroponic watercress properties, the objective of the study was to determine the levels of phenolics and flavonoids and to evaluate the antioxidant activities in plants grown in such conditions. Additionally, rutin, chlorogenic, and caffeic acids were measured by HPLC in the crude extract obtained from the plant.
MATERIALS AND METHODS
Chemicals, Apparatus, and General Procedures
All chemicals were of analytical grade. Silica Gel 60, Silica Gel 60 F254-coated plates, solvents for the extractions and analytical procedures, dichloromethane, ethyl acetate, ethanol, methanol, butanol, acetonitrile, ascorbic acid, and gallic acid were purchased from Merck (Darmstadt, Germany). Folin-Ciocalteau phenol reagent 2 N, DPPH radical (2, 2-diphenil, 1-picrylhydrazyl), rutin, caffeic, and chlorogenic acids were procured from Sigma Chemical Co. (St. Louis, MO, USA).
Plant Collection and Extractions
The watercress plants used in this study were produced in a hydroponic system by specialized producers and were purchased in supermarkets in the city of Santa Maria-RS, Brazil. The vegetables had been cleaned with tap water and the leaves and branches of the plant were dried at room temperature. The dried plants were powdered in a knife mill and the powder (270 g) was macerated with 60% ethanol (1 liter) for a week with daily shake-up. The extract was filtered and concentrated under reduced pressure in order to eliminate the ethanol. The temperature was kept at ±40°C, and this procedure furnished the hydroalcoholic extract. A portion of 100 mL of the hydroalcoholic extract was reserved and evaporated to dryness to obtain the crude extract; the remainder of the hydroalcoholic extract was partitioned with dichloromethane, ethyl acetate, and butanol, successively. The yield of the crude extract and fractions was calculated by the formula:
Determination of Total Phenolic Content
The determination of total phenolic content was performed by the Folin–Ciocalteu method with slight modifications.Citation15 The samples were read at 730 nm in a Shimadzu-UV-1201 (Shimadzu, Kyoto, Japan) spectrophotometer. The total phenolic content was expressed in milligrams equivalent of gallic acid (GAE) per gram of each fraction. The equation obtained for the calibration curve of gallic acid in the range of 0.005–0.030 mg/mL was Y = 11.969 - 0.0454 (r = 0.9984).
Determination of Total Flavonoids
The determination of flavonoids was performed as described by Woisky and Salatino.Citation16 The absorbances were determined by a spectrophotometer at 420 nm. Ethanol was used as a blank. A calibration curve with standard solutions of rutin was made (r = 0.9999). The content of flavonoids was established as rutin mg/g dry extract. The experiments were conducted in triplicate.
Radical-Scavenging Activity–DPPH Assay
The radical scavenging activity of N. officinale crude extract and fractions was quantified in the presence of DPPH• stable radical, according to a slightly modified method.Citation15 Spectrophotometric analysis was used to measure the free radical-scavenging capacity and to determine the scavenging concentration or inhibitory concentration (IC50). The DPPH quenching ability was expressed as IC50 (the extract concentration (μg/mL) required to inhibit 50% of the DPPH in the assay medium).
The crude extract and fractions were tested at six different ethanol dilutions at 7.81 to 250 μg/mL. Each sample was mixed with 1.0 mL of DPPH 0.3 mM in ethanol solution. After 30 min, the absorption was measured at 518 nm. A solution of DPPH (1 mL, 0.3 mM) in ethanol (2.5 mL) was used as a negative control and ascorbic acid in the same concentration was used for the fractions and the crude extract provided the positive control. The test was performed in triplicate and the calculation of the antioxidant activity followed the equation:
In Vitro Fe(II)-Induced Lipid Peroxidation in Rat Brains
Male Wistar rats weighing 270–320 g and with an age from 3 to 3.5 months, from our own breeding colony were kept in cages of three or four animals each, with continuous access to food and water in a room with controlled temperature (22 ± 3°C) and on a 12-h light/dark cycle with lights on at 7:00 a.m. The animals were maintained in accordance to the guidelines of the Brazilian Association for Laboratory Animal Science (COBEA). On the day of the experiments, the rats were sacrificed by decapitation under mild ether anesthesia, and the encephalic tissue was rapidly dissected and placed on ice. Tissues were immediately homogenized in cold 10 mM Tris-HCl, pH 7.5 (1/10, v/v). The homogenate centrifuged for 10 min at 4000× g to yield a pellet that was discarded and a low-speed supernatant one (S1) was used for the thiobarbitaric acid reactive species (TBARS) assay.Citation17
After centrifugation, an aliquot of 100 μl of SI was incubated for 1 h at 37°C with a pro-oxidant agent (10 μM of FeSO4) in the presence or absence of plant extracts and then used for TBARS determination. The concentration range of each tested extract was 0.1, 10, and 100 μg/mL. TBARS production was determined as described by Puntel et al.Citation17 and Ohkawa et al.Citation18
Chromatographic Conditions
High performance liquid chromatography/photodiode array detector (HPLC/DAD) was performed with the HPLC system (Shimadzu, Kyoto, Japan), Prominence Auto-sampler (SIL-20A), equipped with Shimadzu LC-20 AT reciprocating pumps connected to the degasser DGU-20A5 with integrator CBM-20A. UV-VIS detector DAD-SPD-M-20A and software LC Solution 1.22 SP1. Reversed phase chromatographic analyses were carried out in isocratic conditions using RP-C18 column (4.6 mm × 250 mm) packed with 5-μm diameter particles. The mobile phase was methanol-acetonitrile-water (40:15:45, v/v/v) containing 1.0% of acetic acid. The flow rate was 0.6 mL/min, injection volume was 40 μL, and detection was done at 365 nm for rutin and 325 nm for caffeic and chlorogenic acids. The mobile phase was filtered through a membrane filter (0.45 μm) and then degassed by an ultrasonic sound before use. The crude extract and the solutions of standards (rutin, chlorogenic acid, and caffeic acid) were prepared in the same mobile phase of HPLC.Citation19 Standard calibration curves were constructed in the concentration range of 0.0125 to 0.200 mg/mL. The chromatographic peaks were confirmed by comparing its retention time with those of reference standards and quantification was performed by peak integration using the external standard method. The calibration curve for chlorogenic acid was Y = 18763x – 145191 (r = 0.9991), the curve of caffeic acid was Y = 15972x – 708141 (r = 0.9995), and the curve of rutin was Y = 16030x – 46271 (r = 0.9998). All chromatographic operations were performed at room temperature and in triplicate.
Statistical Analysis
Data from the TBARS assay were analyzed statistically by one-way analysis of variance (ANOVA), followed by Duncan's multiple range tests when appropriated using the statistical software SPSS 10.0 for Windows (Statistical Product and Service Solutions Incorporations, USA). TBARS graphic was constructed using the Slide Write 4.032 Bit Edition program (Microsoft, USA). Statistical p values were calculated to quantify levels of significance for each treatment type. A significant p value (p < 0.001 when appropriate) means that there exists significant difference between the two sets of data being analyzed. Correlation coefficient (r) to determine the relationship between two variables and the standard deviations in the DPPH, flavonoids, and total phenolics assays were calculated from three separate experiments using MS Excel for Windows (Microsoft, USA). One-way ANOVA followed by a Tukey test were performed in the total phenolics and DPPH assays.
RESULTS AND DISCUSSION
Total Phenolic and Flavonoids Contents
Phenolics are aromatic secondary plant metabolites widely spread throughout the plant kingdom and associated with color, sensory qualities, and nutritional and antioxidant properties of food.Citation3 Citation4 Total phenolic and flavonoid contents of the crude extract and fractions of N. officinale are given in . The results ranged from 104.40 to 337.60 mg/g of GAE from phenolics and 71.83 to 148.12 mg/g of rutin from flavonoids contents. The butanolic fraction had the highest value, statistically different from the other fractions and the crude extract, being 23.6% higher than the ethyl acetate fraction, 50.0% higher than the dichloromethane fraction, and 69.1% higher than the crude extract, the lowest level of phenolic compounds observed. Ethyl acetate and butanolic fractions showed the highest levels of flavonoids with no significant difference among them. The crude extract showed the smallest value, differing from the other fractions, and the dichloromethane fraction presented intermediary behavior. It may be noted that the butanolic fraction showed the largest value of phenolic and flavonoids contents. Considering total phenolic and flavonoids contents, the results obtained for the crude extract of watercress in the authors' study were very close to those obtained by Yazdanparast et al.Citation20 using similar methodology.
Table 1 Yield of crude extract and fractions in percentage (%), contents of total phenols, contents of flavonoids (Flav.), and antioxidant activities (IC50/DPPH) of N. officinale grown in hydroponics system
Flavonoids are a class of secondary plant phenolics found ubiquitously in fruits and vegetables as well as food products, which act as pharmacological active compounds in many medicinal plants.Citation21 Citation22 Many of the biological actions of flavonoids have been attributed to their powerful antioxidant properties. They can act in several ways, including direct quenching of reactive oxygen species, chelating of metal ions and regeneration of membrane-bound antioxidants.
DPPH Radical Scavenging Activity
N. officinale extract and fractions demonstrated a concentration-dependent scavenging activity by quenching DPPH radicals (data not shown) when compared with ascorbic acid, the positive control. The IC50 values for DPPH scavenging ranged from 8.99 to 30.76 μg/mL (). IC50 of butanolic fraction was nearly 2-fold better than the IC50 of ascorbic acid, demonstrating the high activity of this fraction. Ethyl acetate fraction also presented better activity than ascorbic acid. In fact, many other studies have demonstrated that butanolic and ethyl acetate fractions are good sources of antioxidant compounds, evidencing the extraction capacity of these organic solvents with respect to antioxidant substances, especially belonging to the phenolic groups.Citation15 Citation23 Citation24 On the other hand, the dichloromethane fraction and the crude extract were inferior to ascorbic acid as antioxidants ().
The antioxidant activity of watercress grown in soil may be linked to the presence of phenolic acids, and also the antioxidant liposoluble betacarotene. Hassimotto et al.Citation25 determined the antioxidant capacity according to the β-carotene bleaching method for ten vegetables, the highest antioxidant capacity was found for N. officinale being 9.6 μmol BHT equiv/g. In recent years, applications of dietary plants with antioxidative properties have been the center of focus for improving the life quality of patients who suffer from severe oxidative stress.Citation9 Citation21 Citation26 Citation27 The involvement of free radical, especially their increased production, appears to be a feature of most, if not all human diseases, including cardiovascular disease and cancer. Therefore, N. officinale may be particularly important in fighting these diseases by conferring protection against free radical damage to cellular DNA, lipids and proteins.
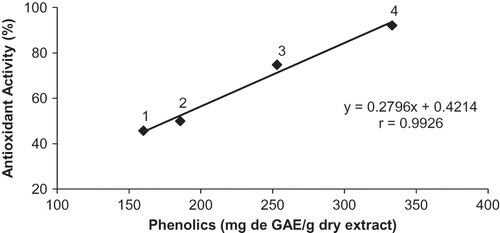
There is a high association between the content of phenols and total antioxidant activity, both for the crude extract as to the fractions of N. officinale (). The Pearson's correlation coefficient was 0.99, showing that the antioxidant activity increases with the increasing total phenolic content. Several authors have demonstrated so conclusively that there is a strong positive relationship between the content of phenolic and total antioxidant activity of fruit and vegetables.Citation15 Citation28 Citation29
In Vitro Fe(II)-Induced Lipid Peroxidation in Rat Brains
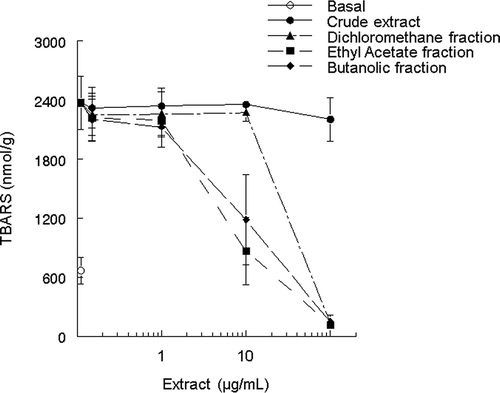
Dichloromethane, ethyl acetate and butanolic fractions of the N. officinale () significantly inhibited iron-induced TBARS production in brain preparations (for all fractions P values were 0.001). The inhibitory potency was in the following order: ethyl acetate > butanolic > dichloromethane > crude extract (, ). The calculated IC50 followed the same order, with the smallest value belonging to ethyl acetate fraction. IC50 for crude extract was not determined because it showed the smallest inhibitory potency in this study (, ). The brain is particularly susceptible to free radical damage because of its high consumption of oxygen and its relatively low concentration of antioxidants enzymes and free radicals scavengers. Several studies have focused in the use of natural therapeutic antioxidant compounds that can afford protection in a variety of in vitro and in vivo models of human pathologies, including neurotoxicity models.Citation5 Citation30 Rutin, chlorogenic, and caffeic acids are known to have antioxidative action in vitro and in vivo. The presence of these substances, especially rutin, can explain the high antioxidant effect of ethyl acetate fraction of the plant. In another study of our group, we found an IC50 = 25.8 μg/mL working with isolated rutin against the same pro-oxidant Fe(II).Citation30 Yadav and BhatnagarCitation31 working with spices used in food and medicinal plants found effects on lipid peroxidation in vitro for the species Cobra's saffron (Mesua ferrea) and Acacia (Acacia catechu), these species showed the highest antioxidant activity, probably due the highest content of phenols compared with other samples.
Table 2 Tested concentrations in the TBARS assay and IC50 (μg/mL) values for crude extract and fractions of N. officinale
Figure 2 Effects of different concentrations of crude extract, ethyl acetate dichloromethane, and butanolic fractions of N. officinale on Fe(II) (10 μM)-induced TBARS production in brain homogenates. Data show means ± SEM values average from three to four independent experiments performed in duplicate.
HPLC Analysis
The extracts of natural origin contain several chemical components in varying concentrations, so it is important to use chromatographic methods to analyze these inherently complex mixtures. The crude extract of N. officinale was analyzed by HPLC-DAD; this fraction showed, among other compounds, chlorogenic acid (tR = 7.2 min, peak 1), caffeic acid (tR = 8.6 min, peak 2), and rutin (tR = 12.4 min, peak 3). The presence of rutin (1.92%; 0.51 mg), caffeic acid (5.08%; 1.37 mg), and chlorogenic acid (1.25%; 0.33 mg) in the crude extract is related to the antioxidant activity found in this plant. Carvalho et al.Citation14observed the presence of rutin, caffeic acid, chlorogenic acid, and its derivatives in conventional growing watercress. Our results were higher than those found by Carvalho et al.,Citation14 probably due to different methodologies and different conditions of cultivation of plants used in each study.
CONCLUSION
N. officinale exhibited strong antioxidant activities in the tested in vitro assays and the extent of antioxidant activities was in accordance with the amounts of phenolics and flavonoids present in the extract and fractions (r = 0.99). The high levels of caffeic acid (5.08%) besides the presence of rutin (1.92%) and chlorogenic acid (1.25%) found in watercress could explain, at least partially, the strong antioxidant activity evidenced in this study. The results clearly indicate that the watercress and its derived phytocompounds have a great potential to prevent diseases caused by the overproduction of free radicals. Therefore, N. officinale might be a valuable antioxidant natural source and seems to be applicable in both healthy medicine and the food industry. Findings of this study support the recommendation of many national dietary guidelines that encourage eating a variety of vegetables every day, especially green leafy vegetables because they have many antioxidants.
REFERENCES
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Structure antioxidant activity relationship of flavonoids and phenolic acid . Free Radical Biology & Medicine , 20 : 933 – 956 .
- Frankel , E.N. and Meyer , A.S. 2000 . The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants . Journal of the Science of Food and Agriculture , 80 : 1925 – 1941 .
- Mandic , A.I. , Dilas , S.M. , Ćetković , G.S. , Čanadanović-Brunet , J.M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 ( 4 ) : 713 – 726 .
- Rice-Evans , C.A. , Miller , J. and Paganga , G. 1997 . Antioxidant properties of phenolic compounds . Trends in Plant Science , 2 : 152 – 159 .
- Luiz , M. , Biasutti , A and Garcia , NA. 2002 . Micellar effect on the scavenging of singlet molecular oxygen by hydroxybenzenes . Redox Report , 7 : 23 – 28 .
- Shahidi , F , Alasalvar , C. and Liyana-Pathirana , C.M. 2007 . Antioxidant phytochemicals in hazelnut kernel . Corylus avellana L.) and hazelnut byproducts. Journal of Agricultural and Food Chemistry , 55 : 1212 – 1220 .
- Asharani , V.T. , Jayadeep , A. and Malleshi , N.G. 2010 . Natural antioxidants in edible flours of selected small millets . International Journal of Food Properties , 13 ( 1 ) : 41 – 50 .
- Karakaya , S. 2004 . Bioavailability of phenolic compounds . Critical Reviews in Food Science and Nutrition , 44 : 453 – 464 .
- Ninfali , P. , Mea , G. , Giorgini , S. , Rocchi , M. and Bacchiocca , M. 2005 . Antioxidant capacity of vegetables, spices and dressings relevant to nutrition . British Journal of Nutrition , 93 : 257 – 266 .
- Szabo , M.R. , Radu , D. , Gavrilas , S. , Chambre , D. and Iditoiu , C. 2010 . Antioxidant and antimicrobial properties of selected spice extracts . International Journal of Food Properties , 13 ( 3 ) : 535 – 545 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus Alba L.) fruits . International Journal of Food Properties , 11 ( 1 ) : 44 – 52 .
- Amico , V. , Chillemi , R. , Mangiafico , S. , Spatafora , C. and Tringali , C. 2008 . Polyphenol enriched fractions from Sicilian grape pomace. HPLC-DAD analysis and antioxidant activity . Bioresource Technology , 99 : 5960 – 5966 .
- Cruz , R.M.S. , Vieira , M.C. and Silva , C.L.M. 2006 . Effect of heat and thermosonication treatments on peroxidase inactivation kinetics in watercress (Nasturtium officinale) . Journal of Food Engineering , 72 : 8 – 15 .
- Carvalho , J.L.S. , Cunico , M.M. , Dias , J.F.G. , Miguel , M.D. and Miguel , O.G. 2009 . Termoestabilidade de processos extrativos de Nasturtium officinale R. Br., Brassicaceae por sistema Soxhlet modificado . Química Nova , 32 : 1031 – 1035 .
- Boligon , A.A. , Pereiram , R.P. , Feltrin , A.C. , Machado , M.M. , Janovik , V. , Rocha , J.B.T. and Athayde , M.L. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutia buxifolia Reiss . Bioresource Technology 2009 , 100 6592 – 6598 .
- Woisky , R.G. and Salatino , A. 1998 . Analysis of própolis: Some parameters and procedures for chemical quality control . Journal of Apicultural Research , 37 : 99 – 105 .
- Puntel , R.L. , Roos , D.H. , Grotto , D. , Garcia , S.C. , Nogueira , C.W. and Rocha , J.B.T. 2007 . Antioxidant properties of Krebs cycle intermediates against malonate pro-oxidant activity in vitro . A comparative study using the colorimetric method and HPLC analysis to determine malondialdehyde in rat brain homogenates. Life Sciences , 81 : 51 – 62 .
- Ohkawa , H. , Ohishi , H. and Yagi , K. 1979 . Assay for lipid peroxide in animal tissues thiobarbituric acid reaction . Analytical Biochemistry , 95 : 351 – 358 .
- Yuangang , Z. , Chunying , L. , Yujie , F. and Chunjian , Z. 2006 . Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD . Journal of Pharmaceutical and Biomedical Analysis , 41 : 714 – 719 .
- Yazdanparast , R. , Bahramikia , S. and Ardestani , A. 2008 . Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats . Chemico-Biological Interactions , 172 : 176 – 184 .
- Heber , D. 2004 . Vegetables, fruits and phytoestrogens in the prevention of diseases . Journal of Postgraduate Medicine , 50 : 145 – 149 .
- Rababah , T.M. , Ereifej , K.I. , Al-Mahasneh , M.A. , Ismaeal , K. , Hidar , A. and Yang , W. 2008 . Total phenolics, antioxidant activities, and anthocyanins of different grape seed cultivars grown in Jordan . International Journal of Food Properties , 11 ( 2 ) : 472 – 479 .
- Schubert , A. , Pereira , D.F. , Zanin , F.F. , Alves , S.H. , Beck , R.C.R. and Athayde , M.L. 2007 . Comparison of antioxidant activities and total polyphenolic and methylxanthine contents between the unripe fruit and leaves of Ilex paraguariensis . A. St. Hil. Die Pharmazie , 62 : 876 – 880 .
- Tung , Y.T. , Wu , J.H. , Huang , C.Y. , Kuo , Y.H. and Chang , S.T. 2009 . Antioxidant activities and phytochemical characteristics of extracts from Acacia confusa bark . Bioresource Technology , 100 : 509 – 514 .
- Hassimotto , N.M.A. , Genovese , M.I. and Lajolo , F.M. 2009 . Antioxidant capacity of Brazilian fruit, vegetables and commercially-frozen fruit pulps . Journal of Food Composition and Analysis , 22 : 394 – 396 .
- Taniyama , Y. and Griendling , K.K. 2003 . Reactive oxygen species in the vasculature: Molecular and cellular mechanisms . Hypertension , 42 : 1075 – 1081 .
- Shan , B. , Cai , Y.Z. , Sun , M. and Corke , H. 2005 . Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituent . Journal of Agricultural and Food Chemistry , 53 : 7749 – 7759 .
- Abdille , M.D.H. , Singh , R.P. , Jayaprakasha , G.K. and Jena , B.S. 2005 . Antioxidant activity of the extracts from Dillenia indica fruits . Food Chemistry , 90 : 891 – 896 .
- Kaur , C. and Kapoor , H.C. 2002 . Antioxidant activity and total phenolic content of some Asian vegetables . International Journal of Food Science & Technology , 37 : 153 – 161 .
- Pereira , R.P. , Fachinetto , R. , Prestes , A.L. , Puntel , R.L. , Silva , G.N.S. , Heinzmann , B.M. , Boschetti , T.K. , Athayde , M.L. , Burger , M.E. , Morel , A.F. , Morsch , V.M. and Rocha , J.B.T. 2009 . Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus . Neurochemical Research , 34 : 973 – 983 .
- Yadav , A.S. and Bhatnagar , D. 2010 . Inhibition of iron induced lipid peroxidation and antioxidant activity of Indian spices and Acacia in vitro . Plant Foods for Human Nutrition , 65 : 18 – 24 .