ABSTRACT
The angiotensin I-converting enzyme (ACE) inhibitory activity and hypocholesterolemic effect of Achatina fulica snail foot muscle protein hydrolysates (SFMPH) and its hydrolysates were studied. The SFMPHs were prepared at a temperature of 121°C for 60 min. To obtain the enzymatic hydrolysates, the SFMPHs were further hydrolysed with three proteases (papain, trypsin, or alcalase). Among all the hydrolysates, alcalase hydrolysate showed the highest degree of hydrolysis and was dominated by a small molecular size fraction (189–686 Da). The SFMPH treated by alcalase was effective in disintegrating intact cholesterol micelles. Furthermore, alcalase hydrolysate with a hydrolysis time of 60 min showed a strong ACE inhibitory activity in vitro with an IC50 of 0.024 mg/mL. Therefore, alcalase hydrolysate may be a promising ingredient for the use in functional foods.
Introduction
The development of specific food-derived protein hydrolysates is of great interest to the food industry because of their physiological effects and nutritional value.[Citation1] Many studies have focused on the functional properties of protein hydrolysates in order to understand their application in food formulation.[Citation2] Protein hydrolysates can be used as additives in food products to improve their functional properties, such as the fat adsorption capacity, foaming, and emulsifying capacity. High-temperature treatment and subsequent enzymatic hydrolysis are believed to be a better way to covert proteins into hydrolysates.[Citation3] Such hydrolysates have been reported to possess multifunctional properties, such as antioxidative, antihypertensive, and cholesterol-lowering activities, and to act as beneficial physiological modulators of metabolism in the human body.[Citation4] These facts signify that during hydrolysis processes the proteolytic system contributes to the liberation of bioactive peptides from natural protein sources. Protein hydrolysates obtained with different proteases are diverse in their functional and bioactive properties. Among these, antihypertensive and hypocholesterolemic peptides have recently received attention due to their significant effects on cardiovascular function in rodents.[Citation5] Hypercholesterolemia, the presence of a high level of total cholesterol in the bloodstream, increases the risks of heart disease and atherosclerosis. Regarding antihypertensive activity, angiotensin I-converting enzyme (ACE) plays an important role in the regulation of blood pressure. It catalyzes both the production of the vasoconstrictor angiotensin II and inactivation of the vasodilator bradykinin;[Citation6] hence, the inhibition of ACE activity is a major target in the prevention of hypertension.
The functional and bioactive properties of the hydrolysates depend on the specificity of the enzyme, amino acid composition, degree of hydrolysis, and peptide size.[Citation7] Previous studies have been conducted on animal or plant protein hydrolysates produced with various enzymes, such as gastric and pancreatic enzymes.[Citation8,Citation9] Protein hydrolysis produces different peptide sizes according the extent of hydrolysis. Thus, selective enzymatic hydrolysis under controlled conditions is the key to improving the functional and bioactive properties of a variety of plant or animal protein sources. To date, commercial proteases such as pepsin, trypsin, and alcalase have been reported to produce protein hydrolysates with various types of bioactivity.
Snails are presently utilized for food and cosmetics. In Taiwan, snails are considered a delicacy in certain seafood restaurants, where they are typically cooked in stews or sautéed. Achatina fulica Bowdich, the giant African snail, is a pest inhabiting vegetable gardens and agricultural fields.[Citation10] Our previous study has shown that the foot muscle of A. fulica is particularly rich in protein (65.6% dry weight) and thus could be a promising source of animal protein. The A. fulica foot muscle can be converted into value-added protein hydrolysates by high-temperature treatment following enzymatic hydrolysis, which can contribute to improved functional properties or bioactivities. Many studies have been conducted to produce protein hydrolysates from marine sources produced with various enzymes, with a strong focus on bioactivity.[Citation2] However, no information has been reported on protein hydrolysates from A. fulica snail foot muscle and their characterization. Therefore, the aim of this study was to prepare protein hydrolysates of A. fulica snail foot muscle using high-temperature pretreatment combined with enzymatic hydrolysis and to evaluate their functional properties, in vitro hypocholesterolemic effect, and ACE inhibitory activity.
Materials and methods
Materials
Fresh A. fulica snails were purchased from markets in Kaohsiung, Taiwan. An average of 10 snails (15 g per snail) was used for preparation of protein hydrolysates. After the shells were removed, the snail foot muscle were collected, washed, packed in polyethylene bags, and stored at –20°C until use.
Preparation of snail foot muscle protein hydrolysates
The frozen snail foot muscle was thawed to 4°C with tap water, immersed in 6% phosphoric acid with a skin-to-solution ratio of 1:4 (w/v) and hydrolysed by retorting in an autoclave (HA-300M, Hirayama, Japan) at 121°C for 60 min. After filtration with metal sieve, the pH value of the retorted solution was 1.8–2.0. The solution was then neutralized with saturated Ca (OH)2 to pH 7. To remove insoluble precipitates, the neutralized solution was filtered again through a Buchner funnel with Toyo No. 5A filter paper. The filtrate was lyophilized to obtain dry powder, which is referred to hereinafter as “SFMPH.”
Preparation of enzymatic hydrolysates
A dry SFMPH sample (1 g) was separately hydrolysed using various enzymes such as papain (pH 2.0, 37°C; P3375, Sigma, St. Louis, MO, USA), trypsin (pH 8.0, 50°C; 203-09893, Wako, Osaka, Japan), and alcalase (pH 8.0, 50°C; commercial enzyme) with a substrate-to-enzyme ratio of 50:1 (w/w) under optimum pH and temperature conditions for 0, 15, 30, and 60 min. After hydrolysis, the enzymatic mixture was heated at 100°C to inactivate the enzyme. After centrifugation at 5000g for 10 min at 4°C, the supernatant was lyophilized and stored at –20°C until use. A control sample (without added enzyme) was performed using the same procedure.
Determination of degree of hydrolysis
Following the method of Hoyle and Merritt,[Citation11] the DH was determined by measuring the nitrogen content soluble in 10% trichloroacetic acid. The SFMPH and its enzymatic hydrolysate samples were dissolved in distilled water at a concentration of 10 mg/mL, and then 10 mL of the resulting solution was mixed with 10 mL of 20% trichloroacetic acid (TCA) to obtain 10% TCA-soluble nitrogen. The mixture was centrifuged at 5000g for 15 min, and the supernatant was decanted and analyzed for nitrogen content by Kjeldahl method.[Citation12] The DH was calculated as follows:
Molecular size distribution
Molecular size distributions of the SFMPH and its enzymatic hydrolysate samples were determined by gel permeation chromatography using a Hitachi 2130 HPLC system equipped with a Superdex peptide® 10/300 column (10 × 300 mm, Amersham Pharmacia Biotech, Uppsala, Sweden). Chromatography was carried out using 0.02 M phosphate buffer (pH 7.2) as a mobile phase. An injection volume of 20 μL of each sample solution (2 mg protein/mL), a flow rate of 0.5 mL/min, and absorbance at 214 nm were used in the analysis. The molecular weight standard distributions were determined using the following standards: trypsin inhibitor (20 kDa, No. 202-09221, Wako), cytochrome C (12.5 kDa, No. C2506, Sigma), aprotinin (6511 Da, No. 52682, Serva Electrophoresis GmbH, Heidelberg, Germany), pepstatin A (686 Da, No. 616370, Calbiochem, La Jolla, CA), Gly–Gly–Gly (189 Da, No. G1377, Sigma), and Gly (75 Da, No. 17-1323-01, Amersham Pharmacia Biotech). For quantitative determinations of molecular weight distribution (%), the peak area in the HPLC chromatograms was integrated in reference to the peak area of the standard proteins, using Hitachi EZChrom Elite 3.1 chromatography data system software.
Fat adsorption capacity
The fat adsorption capacity was measured following the method of Chalamaiah et al.[Citation13] with minor modifications. One gram of the SFMPH and its enzymatic hydrolysate samples were deposited in a 50 mL centrifuge tube, into which 10 mL sunflower oil was added. The mixture was stirred at room temperature for 30 min and centrifuged at 1500g. The volume of the supernatant was recorded and the fat adsorbed by the sample was expressed as grams of fat adsorbed per gram of dry hydrolysate.
Emulsifying capacity
The emulsifying capacity was measured according to the method of Chalamaiah et al.[Citation13] with minor modifications. One gram of the SFMPH and its enzymatic hydrolysate samples were dispersed thoroughly in 25 mL of distilled water, followed by addition of olive oil (5 mL). The mixture was homogenized at 9500 rpm for 1 min using a homogenizer (Ultra-Turrax T25-S1, IKA-Labortechnik, Staufen, Germany). The portion of emulsion was decanted into a 25 mL cylinder and the total volume was read after 30 s. The emulsifying capacity was expressed as millilitres of oil emulsified per gram of dry hydrolysate.
Foaming properties
The foam capacity (FC) and foam stability (FS) of the SFMPH and its enzymatic hydrolysate samples were measured according to the method of Chalamaiah et al.[Citation13] with minor modifications. One gram of the SFMPH sample was dispersed in 100 mL distilled water and homogenized at 8000 rpm for 1 min using a homogenizer (Ultra-Turrax T25-S1, IKA-Labortechnik, Staufen, Germany). The whipped sample was immediately transferred into a 250 mL cylinder and the total volume was read after time intervals of 15, 30, and 90 min. The FC and FS were calculated using the following equations:
where VT is total volume after whipping, V0 is the original volume before whipping, and Vt is the total volume after standing at room temperature for 15, 30, and 90 min.
Determination of disintegration of cholesterol micelles
A dietary mixture of cholesterol micelles was prepared according to the method of Raederstorff et al.[Citation14] The solution was incubated at 37°C for 24 h. Subsequently, 0.2 mL of sample solution (20 and 80 mg/mL) was added to 2.5 mL of the micellar solution, followed by gentle mixing at 37°C for 1 h and centrifugation at 1600g for 10 min. In order to separate the precipitated cholesterol from the intermicellar cholesterol, the solution was filtered through a 0.22 μm Millipore (Bedford, MA, USA). The concentration of micellar cholesterol in the filtrate was determined by the method of Searcy and Bergquist.[Citation15]
Determination of ACE inhibitory activity
ACE inhibitory activity of the SFMPH and its enzymatic hydrolysate samples was measured according to the method described by Cushman and Cheung[Citation16] with minor modifications. In the assay, 80 μL of the sample solution (10 mg/mL) was mixed with 20 μL hippuryl-His-Leu (HHL) in 100 mM sodium borate buffer containing 300 mM NaCl at pH 8.3. The above mixture was preincubated at 37°C for 5 min before 20 μL of ACE solution (0.11 U/mL) was added. The reaction was terminated by adding 150 μL of 1.0 N HCl. Released hippuric was extracted by the addition of 1.7 μL ethyl acetate with continuous vortex mixing. After centrifugation at 1600g for 10 min, 1 mL of the upper layer was transferred to a test tube and evaporated in vacuum. The resulting hippuric acid was redissolved in 1 mL distilled water, and the absorbance was measured spectrophotometrically at 280 nm. The concentration of hydrolysate caused a 50% reduction of ACE activity, expressed as the 50% inhibitory concentration (IC50).
Statistical analysis
All results were analyzed by the Duncan test using the Statistical Analysis System (SAS). An α level of 0.05 was set to determine statistical significance.
Results and discussion
Enzyme hydrolysis
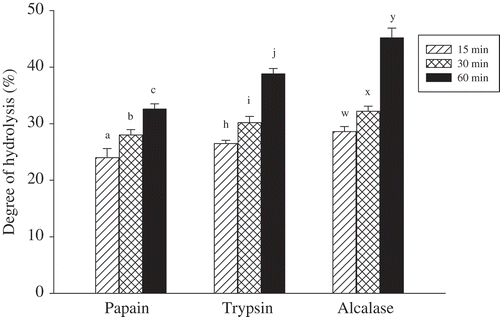
The SFMPH hydrolysed with three enzymes (papain, trypsin, or alcalase) for 15–60 min. As shown in , DH values of enzyme-hydrolysed SFMPH were in the range of 24.0–28.6% during the first 15 min. A gradual rise in DH with time was observed when all three enzymes were used. The highest DH value was found for alcalase hydrolysate (45.2%), followed by trypsin hydrolysate (38.8%) and papain hydrolysate (32.6%) as hydrolysis time was increased to 60 min. Comparing all three proteases for hydrolysis of SFMPH, alcalase showed the highest capability of hydrolysis at comparable hydrolysis times, implying the production of smaller peptides. This indicates that the proteolytic activity of alcalase toward SFMPH is higher than those of trypsin and papain. According to Zhao et al.,[Citation17] higher DH values with alkaline proteases (e.g., alcalase and trypsin) than acid or neutral proteases were found also in protein hydrolysates of rice dregs. The hydrolysis of the protein depends on the enzymes and reactions. The results of the study showed that the enzymes employed in the processing affected the SFMPH, leading to the different extents of protein hydrolysis.
Figure 1. Degree of hydrolysis of SFMPH hydrolysed with papain, trypsin, and alcalase at various times. Within the same enzymatic action, values (mean ± SD of triplicates) with different letters (a–c, h–j, w–y) are significantly different (Duncan, P < 0.05). A control test (without proteases addition) for each enzymatic treatment was performed according to normalization protocol and its DH value was in the range of 11.8–12.0%.
Molecular size distribution
The molecular weight distribution profile of SFMPH hydrolysed by various proteases is shown in . Hydrolysis of protein or hydrolysate by enzymes was applied to promote the release of low-molecular-weight peptides. The SFMPH (without proteases) was rich in the 189–686 Da fraction (approximately 41%). During the first 15 min of digestion by alcalase, trypsin, and papain, the fractions with molecular weight below 686 Da were 92.2%, 82.8%, and 76.4% of the total hydrolysates, respectively. When the hydrolysis time was increased to 60 min, the fraction with molecular weight below 189 increased, with concurrent reduction of the fraction with molecular weight in the range of 189–686 Da. Among all three enzymatic hydrolysates, the percentages of fractions with molecular weight below 189 Da in the papain-hydrolysed SFMPH were highest, indicating that papain was much more effective than the other proteases in producing dipeptides. Moreover, all enzymatic hydrolysates were mainly composed of low-molecular-weight peptides (<6511 Da). The result indicated that proteases can catalyze intra-chain breakage of peptide bonds. As predicted by DH profile, alcalase showed more extensive hydrolysis than other proteases. This phenomenon obviously suggests that the fraction of 686–6511 Da digested by alcalase could produce smaller peptides. The results are in accordance with those from You et al.,[Citation18] who demonstrated that higher DH values for protein hydrolysates indicated more small-sized peptides. The results suggested that the functional properties of the enzymatic hydrolysates were deduced from the characteristic molecular size of the peptides produced, depending on the protease specificity.
Table 1. The distribution of molecular size fractions in the SFMPH hydrolysed with papain, trypsin, and alcalase.
Fat adsorption capacity
shows the effects of SFMPH and its enzymatic hydrolysate samples (60 min hydrolysis) on fat adsorption capacity. Fat adsorption capacity was found to be higher with SFMPH than with other hydrolysates prepared by proteases. Comparing all three of the enzymatic hydrolysates, the highest fat adsorption capacity was observed in SFMPH hydrolysed by papain (1.68 g oil/g hydrolysate), followed by trypsin hydrolysate (1.48 g oil/g) and alcalase hydrolysate (1.35 g oil/g). The decrease in fat adsorption capacity with increasing DH can be attributed to the disruption of the protein network. The result showed that papain hydrolysate adsorbed much more oil than did other hydrolysates, possibly due to the presence of non-polar groups that easily bind oil molecules. In addition, the differences in the molecular sizes of peptides released among the hydrolysates may explain the difference in the fat adsorption capacity. It has been reported that hydrolysates with higher molecular weight prepared from grass carp skin exhibited a greater fat adsorption capacity.[Citation19] Therefore, SFMPH could potentially be used as a functional ingredient in the meat industry.
Table 2. Effect of SFMPH after treatment with papain, trypsin, and alcalase for 60 min on functional propertiesa.
Foaming properties
shows the effects of SFMPH and its enzymatic hydrolysate samples (60 min hydrolysis) on foaming properties. The foam capacity of SFMPH (57.3%) was much higher than that of SFMPH hydrolysed by proteases (32.3–54.8%). Among the three enzyme-treated hydrolysates, at the same hydrolysis time, alcalase hydrolysate had the poorest foaming properties. As the smaller molecular size in the hydrolysates increased, the foaming capacity and foaming stability of alcalase hydrolysate decreased. The results imply that hydrolysates with smaller peptides could not enhance the formation of a stable film around the gas bubbles. It is apparent that the molecular size of protein hydrolysates is related to the foaming properties. According to Liu et al.[Citation20] peptides with low molecular weights probably have a reduced amphiphilicity and thus do not produce good foaming stability. The foam capacity after whipping for 15, 30, and 60 min was monitored to indicate the foam stability of these hydrolysates. The foam stability of all the hydrolysates gradually decreased with increasing incubation time. Moreover, Jamdar et al.[Citation21] have reported that treating proteins with enzymes releases the peptides in which polarity or hydrophobicity have been altered, which in turn affects the foam capacity or foam stability.
Emulsifying properties
shows the effects of SFMPH and its enzymatic hydrolysate samples (60 min hydrolysis) on the emulsifying properties. The emulsion capacities of all three enzymatic hydrolysates were lower than those of SFMPH. Of the three proteases for hydrolysis of SFMPH, the alcalase hydrolysate exhibited the lowest emulsion capacity. The higher emulsion capacity of papain hydrolysates may have been due to the presence of higher-molecular-sized peptides, which were clearly observed in the molecular size distribution profile. It appears that larger-molecular-sized peptides are more effective emulsifiers than smaller peptides are. This may be mainly attributable to the weak interfacial films around the emulsion droplets. The trend in the emulsion properties of hydrolysates in this study corresponds to the foaming properties. Owing to the excessive hydrolysis, enzymatic hydrolysates reduced the emulsifying properties,[Citation22] and the emulsion properties of protein hydrolysates from surimi processing by-products decreased with increasing DH.[Citation23] Mutilangi et al.[Citation24] deduced that hydrolysates with a large molecular size may be amphiphilic, which would contribute to emulsion stability.
Disintegration of intact cholesterol micelles
The effects of SFMPH and its enzymatic hydrolysates at concentrations of 10 and 20 mg/mL on the disintegration of cholesterol micelles are shown in . The cholesterol in mixed micelles can be absorbed efficiently by the intestinal epithelium. Micelles can accommodate a limited amount of sterols and other lipids. The cholesterol concentration in micelles can be lowered in the presence of other sterols. There is a limit to the quantity of cholesterol in micelles; hence, the cholesterol removed from the disintegrated micelles would escape the absorption process and be excreted in faeces.[Citation14] Compared with the control (without hydrolysate addition), SFMPH at a concentration of 10 mg/mL reduced the percentage of intact cholesterol micelles by 20.4%. Further hydrolysis with papain, trypsin, or alcalase affected the stability of the cholesterol micelles and significantly (P < 0.05) reduced the amount of intact cholesterol micelles by 23.0%, 28.3%, and 31.5%, respectively. When the concentration of enzymatic hydrolysates was increased to 20 mg/mL, the amount of intact cholesterol micelles was significantly (P < 0.05) decreased by 22.6–33.3%. Among the enzymatic hydrolysates, the SFMPH hydrolysed by alcalase was found to have the most potent ability to disintegrate intact cholesterol micelles. Other studies have reported that the hypocholesterolemic activity of trypsin casein hydrolysate can be attributed to the interaction of lysine and arginine residues with cholesterol, leading to a reduction of cholesterol absorption in the intestine.[Citation25] Zhong et al.[Citation26] reported that soy protein alcalsase hydrolysate showed the highest inhibition of cholesterol micellar solubility. This high inhibition was attributed to reduced solubility of cholesterol in micelles, which is related to the DH. Similarly, Zhang et al.[Citation27] evaluated in vitro tests of hypocholesterolemic properties and reported that rice bran hydrolysed by alcalase had the highest micellar cholesterol inhibition ability, relative to other proteases, due to the highest DH and the highest exposition of hydrophobic residues. This could be explained by the presence of alcalase, which can release peptides rich in hydrophobic amino acid residues that can bind bile acids. Therefore, it was speculated that the hydrophobic residues contained in the alcalase-treated SFMPH with a high DH may affect the stability of cholesterol micelles, and thus the residues may have the potential to inhibit the reabsorption of bile acid in the ileum and lower the blood cholesterol concentration.
ACE inhibitory activity
Bioactive peptides can be released by enzymatic proteolysis of food proteins and may act as potential physiological modulators. Released bioactive peptides contain 2–20 amino acid residues per molecule. The low molecular size allows easy passage through the intestinal barrier and subsequent biological activity.[Citation28] As the SFMPH hydrolysed by alcalase exhibited the highest DH and lowest-molecular-size peptides among the three proteases tested, it is likely that alcalase would be the most suitable enzyme for investigating the ACE inhibitory activity of hydrolysate. Captopril is a common orally administered ACE inhibitor used to treat hypertension. The measured IC50 was 0.0019 μg/mL, which was used as the positive control in this study. As shown in , the ACE inhibitory activities of alcalase hydrolysate obtained with different hydrolysis times were higher than that of the SFMPH. In particular, the ACE inhibitory activity of alcalase hydrolysate at a hydrolysis time of 60 min (IC50 = 0.024 mg/mL) was significantly (P < 0.05) higher than that of other times (IC50 = 0.073 and 0.031 mg/mL for hydrolysis of 15 min and 30 min, respectively). This finding is similar to that of Vastag et al.,[Citation29] who reported that the ACE inhibitory activity in alcalase hydrolysate of pumpkin oil cake increased as DH increased. Furthermore, the hydrolysis time affects the release of ACE inhibitory peptides in hydrolysate, resulting in a different molecular size. It is apparent that the ACE inhibitory activity of smaller peptides is stronger than that of larger ones. The IC50 value for SFMPH treated by alcalase in this study was lower than that of alcalase hydrolysate from mud snails.[Citation30] The results indicate that the antihypertensive activity of SFMPH could be enhanced by enzymatic hydrolysis of SFMPH. It is possible that SFMPH treated with alcalase had the highest ACE inhibitory activity at 60 min of hydrolysis because it contained more short peptides.
Table 3. Effect of SFMPH after treatment with papain, trypsin, and alcalase for 60 min on disintegration of cholesterol micellesa.
Table 4. ACE inhibitory activity (IC50) of SFMPH hydrolysed with alcalase at various hydrolysis times.
Conclusions
The results indicated that the functional properties and biological activities of SFMPH were determined by the DH and by the enzyme type employed. Among all the enzymatic hydrolysates tested, alcalase hydrolysate had the highest DH and smallest molecular size. The functional properties (i.e., fat adsorption capacity, foaming properties, and emulsifying properties) of SFMPH treated with papain and trypsin are better than those of alcalase hydrolysate. However, hydrolysis of SFMPH by alcalase can produce too many small-sized peptides, which can decrease the amount of the intact cholesterol micelles and exhibit high in vitro ACE inhibitory activity. Therefore, alcalase-hydrolysed SFMPH is a potential bioactive food ingredient for developing functional foods with a beneficial impact on cardiovascular health. Further investigations are necessary to characterize the peptide responsible for hypocholesterolemic and ACE inhibitory activities in alcalase-treated SFMPH.
Funding
This study was carried out with financial support from the Ministry of Science and Technology of the Republic of China (NSC 98-2313-B022-001).
Additional information
Funding
References
- Tavano, O.L. Protein Hydrolysis using Proteases: An Important Tool for Food Biotechnology. Journal of Molecular Catalysis B-Enzymatic 2013, 90, 1–11.
- He, S.; Franco, C.; Zhang, W. Functions, Applications and Production of Protein Hydrolysates from Fish Processing Co-Products (FPCP). Food Research International 2013, 50, 289–297.
- Yang, J.L.; Ho, H.Y.; Chu, Y.J.; Chow, C.J. Characteristic and Antioxidant Activity of Retorted Gelatin Hydrolysates from Cobia (Rachycentron canadum) Skin. Food Chemistry 2008, 110, 128–136.
- Saadi, S.; Saari, N.; Anwar, F.; Hamid, A.A.; Ghazali, H.M. Recent Advances in Food Biopeptides: Production, Biological Functionalities and Therapeutic Applications. Biotechnology Advances 2015, 33, 80–116.
- Liu, X.; Zhang, M.; Zhang, C.; Liu, C. Angiotensin Converting Enzyme (ACE) Inhibitory, Antihypertensive and Antihyperlipidaemic Activities of Protein Hydrolysates from Rhopilema esculentum. Food Chemistry 2012, 134, 2134–2140.
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. Journal of Food Science 2012, 77, R11–R24.
- Xie, N.; Huang, J.; Li, B.; Cheng, J.; Wang, Z.; Yin, J.; Yan, X. Affinity Purification and Characterisation of Zinc Chelating Peptides from Rapeseed Protein Hydrolysates: Possible Contribution of Characteristic Amino Acid Residues. Food Chemistry 2015, 173, 210–217.
- Valdez-Ortiz, A.; Fuentes-Gutierrez, C.I.; German-Baez, L.J.; Gutierrez-Dorado, R.; Medina-Godoy, S. Protein Hydrolysates Obtained from Azufrado (sulphur yellow) Beans (Phaseolus vulgaris): Nutritional, ACE-inhibitory and Antioxidative Characterization. LWT-Food Science and Technology 2012, 46, 91–96.
- Uluko, H.; Zhang, S.W.; Liu, L.; Tsakama, M.; Lu, J.; Lv, J.P. Effects of Thermal, Microwave, and Ultrasound Pretreatments on Antioxidative Capacity of Enzymatic Milk Protein Concentrate Hydrolysates. Journal of Functional Foods 2015, 18, 1138–1146.
- Thiengo, S.C.; Faraco, F.A.; Salgado, N.C.; Cowie, R.H.; Fernandez, M.A. Rapid Spread of an Invasive Snail in South America: The giant African snail, Achatina fulica, in Brasil. Biological Invasions 2007, 9, 693–702.
- Hoyle, N.T.; Merritt, J.H. Quality of Fish-Protein Hydrolysates from Herring (Clupea harengus). Journal of Food Science 1994, 59, 76–79.
- AOAC. Official Methods of Analysis; 16th Ed. Association of Official Analytical Chemists: Washington, DC, 1995.
- Chalamaiah, M.; Rao, G.N.; Rao, D.G.; Jyothirmayi, T. Protein Hydrolysates from Meriga (Cirrhinus mrigala) Egg and Evaluation of their Functional Properties. Food Chemistry 2010, 120, 652–657.
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on Lipid Absorption and Plasma Lipid Levels in Rats. Journal of Nutritional Biochemistry 2003, 14, 326–332.
- Searcy, R.L.; Bergquist, L.M. A New Color Reaction for the Quantitation of Serum Cholesterol. Clinica Chimica Acta 1960, 5, 192–199.
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochemistry Pharmacology 1971, 20, 1637–1648.
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.; Wang, S; Sun, W.; Zhou, Q. Enzymatic Hydrolysis of Rice Dreg Protein: Effects of Enzyme Type on the Functional Properties and Antioxidant Activities of Recovered Proteins. Food Chemistry 2012, 134, 1360–1367.
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Changes in the Antioxidant Activity of Loach (Misgurnus anguillicaudatus) Protein Hydrolysates During a Simulated Gastrointestinal Digestion. Food Chemistry 2010, 120, 810–816.
- Wasswa, J.; Tang, J.; Gu, X.H.; Yuan, X.Q. Influence of the Extent of Enzymatic Hydrolysis on the Functional Properties of Protein Hydrolysate from Grass Carp (Ctenopharyngodon idella) Skin. Food Chemistry 2007, 104, 1698–1704.
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant Activity and Functional Properties of Porcine Plasma Protein Hydrolysate as Influenced by the Degree of Hydrolysis. Food Chemistry 2010, 118, 403–410.
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of Degree of Hydrolysis on Functional Properties, Antioxidant Activity and ACE Inhibitory Activity of Peanut Protein Hydrolysate. Food Chemistry 2010, 121, 178–184.
- Kotlar, C.E.; Ponce, A.G.; Roura, S.I. Improvement of Functional and Antimicrobial Properties of Brewery Byproduct Hydrolysed Enzymatically. LWT-Food Science and Technology 2013, 50, 378–385.
- Liu, Y.; Li, X.; Chen, Z.; Yu, J.; Wang, F.; Wang, J. Characterization of Structural and Functional Properties of Fish Protein Hydrolysates from Surimi Processing By-Products. Food Chemistry 2014, 151, 459–465.
- Mutilangi, W.A.M.; Panyam, D.; Kilara, A. Functional Properties of Hydrolysates from Proteolysis of Heat-Denatured Whey Protein Isolate. Journal of Food Science 1996, 61, 270–275.
- Alhaj, O.A.; Kanekanian, A.D.; Peters, A.C.; Tatham, A.S. Hypocholesterolaemic Effect of Bifidobacterium animalis subsp Lactis (Bb12) and Trypsin Casein Hydrolysate. Food Chemistry 2010, 123, 430–435.
- Zhong, F.; Liu, J.M.; Ma, J.G.; Shoemaker, C.F. Preparation of Hypocholesterol Peptides from soy Protein and their Hypocholesterolemic Effect in Mice. Food Research International 2007, 40, 661–667.
- Zhang, H.J.; Yokoyama, W.H.; Zhang, H. Concentration-Dependent Displacement of Cholesterol in Micelles by Hydrophobic Rice Bran Protein Hydrolysates. Journal of the Science of Food and Agriculture 2012, 92, 1395–1401.
- Pihlanto-Leppala, A. Bioactive Peptides Derived from Bovine Whey Proteins: Opioid and Ace-Inhibitory Peptides. Trends in Food Science & Technology 2000, 11, 347–356.
- Vastag, Z.; Popovic, L.; Popovic, S.; Krimer, V.; Pericin, D. Production of Enzymatic Hydrolysates with Antioxidant and Angiotensin-I Converting Enzyme Inhibitory Activity from Pumpkin Oil Cake Protein Isolate. Food Chemistry 2011, 124, 1316–1321.
- Xia, S.H.; Wang, Z.; Xu, S.Y. Characteristics of Bellamya Purificata Snail Foot Protein and Enzymatic Hydrolysates. Food Chemistry 2007, 101, 1188–1196.
