?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study evaluated the effect of energy-gathered ultrasound treatment on the antioxidant and physicochemical properties of soluble dietary fiber (SDF) from garlic straw. Results showed ultrasonic power and time play an important role in the antioxidant activity of SDF. The strongest activity of scavenging hydroxyl and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals was obtained at ultrasonic treatment of 100 W for 20 min. SDF modified by ultrasound exhibited improved water holding capacity, oil holding capacity, swelling capacity and cholesterol binding capacity. Ultrasonic-treated SDF had lower viscosity than that of untreated SDF. Furthermore, atomic force microscopy (AFM) measurement showed that ultrasound degraded SDF into fragments with smaller particle size. Ultrasound treatment did not change the primary structure of SDF in the test conditions according to the result determined by FTIR. The overall results indicate that SDF from garlic straw treated by energy-gathered ultrasound has great potential to be used in functional foods.
Introduction
Garlic bulb is one of the edible plants that have attracted particular attention because of its widespread medicinal and nutritional values. With the trend of increased consumption of garlic bulb, the garlic agriculture produces a great amount of straw byproducts every year, which is simply disposed or thrown causing serious environmental problems in the community.[Citation1] The productive use of such by-products could offer substantial economic benefits.
In recent years, dietary fiber (DF) attracts extensive concern from consumers and researchers, due to its health benefits.[Citation2,Citation3] DF is generally classified into insoluble dietary fiber (IDF) and soluble dietary fiber (SDF) fractions based on their solubility in water. Many of the beneficial health benefits attributed to DF are related to SDF, as for reducing risks of hypertension, diabetes, obesity, cardiovascular and colorectal cancer diseases.[Citation4] SDF is not only desirable for its nutritional value but also popular for its bioactive properties and its superiority in physicochemical properties.[Citation5,Citation6] These properties include stronger antioxidative activity, higher capacity to form gels, better emulsifiers, and fat replacers, which contribute to the different applications in food design and manufacturing.[Citation7–Citation9] Nevertheless, the nature of SDF aggregation limits its manufacturing interest.[Citation10] Many chemical, physical, and biological technologies have been used to modify DF from different food sources. However, as far as no information was available on the modification of SDF from garlic straw. The main advantage of SDF from garlic straw was its similar compounds to garlic bulb, which will endow the products with more functional properties. Therefore, it is of great value to develop an effective modification method that improves garlic straw SDF quality and physicochemical properties.
Soluble dietary fiber mainly includes pectins, gums, and mucilages. It was reported that the physiological properties of guar gum can be improved by enzymatic hydrolysis and gamma radiation.[Citation11] Molecular weight of polysaccharide from Schizophyllum commune and Porphyra yezoensis Ueda can be degraded by ultrasound, resulting in high anti-inflammatory activity and high antioxidant activity.[Citation12,Citation13] Ultrasound technology has been widely used in the factory to improve the physicochemical and functional properties of food. Efficacies of different ultrasonic equipment were compared in terms of acoustic and hydrodynamic cavitation.[Citation14,Citation15] “Energy-gathered ultrasound” is realized by avoiding the waste of energy, due to the direction of the ultrasound wave contrary to that of the solution. This kind of machine is equipped with a probe which can generate different power. The periodic mechanical motions of ultrasonic probe transfer energy into the fluid medium and generate high temperatures, pressures, and shear forces. These effects can break glycosidic bonds in the main chain of carbohydrates, leading to the changes of structural, physicochemical, and functional properties of SDF.[Citation16] Therefore, it can be inferred that the functional properties of garlic straw SDF can be modified by energy-gathered ultrasound. Compared with other pretreatment methods, the ultrasonic method has advantages of saving energy and time and reducing the consumption of organic solvents.
In this paper, the effect of energy-gather ultrasound on the antioxidant activity of garlic straw SDF was investigated. The in vivo physiological effects of SDF before and after ultrasonic treatment were predicted by an in vitro determination of the physicochemical and functional properties. Atomic force microscopy (AFM) and Fourier-transformed infrared spectroscopy (FTIR) were used to characterize the structural changes of the SDF induced by ultrasound. The information obtained from this study is useful for the interpretation in the relationship between the structure of garlic straw SDF and its biological functions.
Materials and methods
Materials
Garlic (Allium sativum L.) straw, collected after harvesting garlic, was offered by Shandong Jinxiang Chengong biotechnology Co., Ltd. (China). Heat stable α-amylase (enzyme activity≥50,000 U/ml) was purchased from Wuxi Xuemei Enzyme Preparation Sci-tech Co., Ltd. (Jiangsu, China). Amyloglucosidase (enzyme activity≥100,000 U/ml) and papain (enzyme activity≥50,000 U/g) were obtained from Gold Wheat Biotechnology Co., Ltd (Shanghai, China). 1,1-diphenyl-2-picrylhydrazyl (DPPH) was obtained from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade and purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China).
Preparation of soluble dietary fiber (SDF) from garlic straw
Garlic straw was cleaned, outdoor dried and then cut into small pieces (1–2 cm). The chopped straw was ground to pass through a 0.25 mm size screen. SDF was extracted from garlic straw according to the AOAC method 991.43, total, soluble and insoluble dietary fiber in foods with slight modifications.[Citation17] Garlic straw powder was mixed with 30 times volume of deionized water and hydrolyzed with 5% (enzyme/substrate, E/S, v/w) heat stable α-amylase at 95°C for 35 min. After the temperature of hydrolysates was decreased to 60°C, papain solution (50 mg/ml) was added with amount of 10% (E/S, v/w) and went further hydrolysis for 30 min at 60°C. The pH was adjusted to 4.5 with 3 M acetic acid and 10% (E/S, v/w) of amyloglucosidase was added, followed by stirring at 60°C for 30 min. In the end, the resulting slurry was heated in a boiling water bath for 10 min to terminate hydrolysis, and then centrifuged at 4500 g and 25°C for 15 min. The supernatant was collected and condensed to one-tenth in vacuum rotary evaporation system. The concentrated supernatant was mixed with 95% (1:4, v/v) ethanol at 4°C for 12 h and subjected to centrifugation at 4500 g for 15 min. The resulting residue was washed with 78% ethanol, 95% ethanol and dried in a cabinet dryer (DHG-9140A, Yiheng, Shanghai, China) at 50°C. The dried flocculate was milled and passed through a 0.25 mm sieve. The powder obtained was SDF and then stored at 4°C. The extraction yield of SDF from garlic straw was about 9.12%. The purity of SDF was measured according to the AOAC[Citation17] and the value was 85.6%.
Treatment of SDF by energy-gathered ultrasound
SDF powder was dispersed in water at 25°C under continuous magnetic stirring for 1 h to have a final concentration of 40 mg/ml. Two hundred milliliters of SDF suspension was put into a 250 ml beaker, and the beaker was placed in a thermostatic water bath at an initial temperature of 25°C for 10 min. Then, the suspension was sonicated in ultrasound processor (Model GA99-IID, Shangjia Biotechnology Co., Wuxi, China) with a 2.0 cm flat tip probe at 20 kHz. The on-time of pulsed ultrasound was 2 s and off-time was 2 s. One group was used to investigate the effect of ultrasonic power (0, 50, 100, 150 and 200 W) for 20 min. The other group was used to investigate the effect of ultrasound at different irradiation time (0, 5, 10, 20, and 30 min) at 100 W. After ultrasonic treatment, samples were centrifuged at 4500 g for 10 min. The supernatant was collected and lyophilized for further measurements.
Hydroxyl radical scavenging ability assay
The hydroxyl radical (OH) scavenging activity was determined according to the method of Smirnoff and Cumbes[Citation18] with slight modifications. SDF was dissolved in deionized water to obtain different concentrations. Two milliliters of SDF solution was mixed with 2 ml of 3 mM FeSO4, and then 2 ml of 3 mM H2O2 was added. The mixture was incubated in a water bath at 25°C for 10 min. Following this, 2 ml of 6 mM salicylic acid was added and incubated for further 15 min. Then, the mixture was centrifuged at 5000 g for 10 min. Absorbance of the supernatant was read at 510 nm against blank mixture. For the control, SDF was substituted by water. The ability to scavenge OH was calculated as follows:
where A1 and A0 were the absorbance of sample SDF and control, respectively. IC50 (50% inhibitory concentration) value (mg/ml) is the concentration at which hydroxyl radicals were scavenged by 50%.
DPPH radical scavenging activity assay
SDF powder was dissolved in deionized water to obtain different concentrations. Then, 3 ml of sample was mixed with 1 ml of 0.15 mM DPPH that was dissolved in 95% ethanol. After incubating at 25°C in the dark for 20 min, the mixture was centrifuged at 4000 g for 10 min and the absorbance of the supernatant was measured at 517 nm. For the control, distilled water was used instead of the sample. DPPH radical scavenging activity was calculated as:
where A0 was the absorbance of the control and A1 was the absorbance of the sample against a blank solution containing the sample without DPPH.
Water- and oil-holding capacities and swelling capacity
Water holding capacity (WHC): WHC was determined according to the method reported by Wang et al.[Citation19] 1 g of dry sample was mixed with 70 ml of deionized water in a 100 ml beaker. The mixture was stirred at 25ºC for 24 h and centrifuged at 4000 g for 10 min. The residue was weighed, and WHC was calculated by the following equation.
where M2 is the residue weight and M1 is the weight of dry SDF (g).
Oil holding capacity (OHC): Sample (1.0 g) was mixed with soybean oil (30 ml) for 16 h at 25°C and then centrifuged for 10 min at 4000 g. The residue was collected and weighed. OHC was expressed as the weight of oil absorbed by SDF.[Citation20]
where W2 and W1 are the residue weight and original weight of the dry sample (g), respectively.
Swelling capacity (SC):1.0 g of sample was placed in a test tube and hydrated with 25 ml of deionized water at 25°C for 4 h. The bed volume (ml) was recorded. SC was calculated by the EquationEq. (5(5)
(5) ).
where V is the bed volume occupied by SDF (ml), W is the weight of SDF (g).
Adsorption capacities of SDF for glucose and cholesterol
The adsorption properties of SDF including glucose adsorption capacity (GAC) and cholesterol binding capacity (CBC) were determined based on the previous method reported by Huang et al.[Citation21]
Determination of rheological properties
SDF solution (40 mg/ml) was loaded onto a stress-controlled rotational rheometer (DHR-1, TA Instrument, USA) equipped with peltier concentric cylinder conical DIN system. The SDF solution was equilibrated in a cup for 30 s prior to measurements in order to obtain a temperature of 25°C. Flow ramp analyses were performed with the shear rate from 0.01 s−1 to 100 s−1 at 25°C. Apparent viscosity and stress were recorded as functions of shear rate, respectively.[Citation22]
Atomic force microscopy (AFM) characterization
A 5 μl drop of SDF suspension (40 μg/ml) was deposited on a cleaved mica substrate. The substrate was placed in an incubator for 12 h at 25°C and the solvent was allowed to evaporate. AFM images were generated by an atomic force microscope (Multimode 8, Bruker Inc. Germany) using standard peak force-mode silicon cantilevers. Height and phase images were obtained simultaneously in tapping mode with a resonance frequency of 150–200 kHz and a force constant of 5 N/m. The scan size was 20 μm, and the scan rate was 1 Hz.
Fourier-transformed infrared spectroscopy (FTIR)
The functional groups of SDF were identified by a spectrophotometer (Nicolet 6700, Nicolet instrument Co., USA). SDF was completely mixed with KBr by the ratio of 1: 250 (w/w) and pelletized. FTIR spectra were obtained in the wave number region ranging from 4000 to 400 cm−1 and with a resolution of 1 cm−1. Taken KBr spectrum as background, spectra were obtained after baseline corrected.
Statistical analysis
Each experiment was carried out in triplicate. Statistical analyses were performed using SPSS statistics 19. Data were expressed as mean value ± standard deviation and analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s test. The confidence interval was 95% (P < 0.05).
Results and discussion
Effect of energy-gathered ultrasound on scavenging activities of hydroxyl and DPPH radicals
A limited amount of free radicals plays an inevitable role in regulating homeostasis and mediating stress responses in vivo.[Citation23] However, excessive free radicals might cause oxidative damage to proteins, lipids, and DNA molecules, leading to many degenerative diseases. Thus, exogenous antioxidants are highly important to prevent some diseases such as Alzheimer’s disease, cardiovascular disease and certain types of cancers.[Citation24] The hydroxyl and DPPH radicals scavenging activities of garlic straw SDF were expressed in terms of IC50, which was defined as 50% inhibitory concentration by plotting scavenging activity as a function of SDF concentrations.
) illustrates the effect of ultrasonic power on hydroxyl and DPPH radicals scavenging activities of SDF. It can be seen that at all intensities tested, ultrasound produced a positive effect on the free radical scavenging activity of SDF comparing to that of control (without exposure to ultrasonic irradiation). For hydroxyl radical scavenging activity, the IC50 value decreased with the increase of ultrasonic power from 50 to 100 W, while increased when ultrasonic power extended to 200 W. No significant difference was observed between 100 W and 150 W (P> 0.05). As DPPH radical scavenging activity was concerned, similar IC50 values were observed from ultrasonic power 100 W to 200 W. When the ultrasonic power was 100 W, IC50 values of hydroxyl and DPPH radicals scavenging activities were decreased by 59.2% and 49.0% compared to those values of control.
Figure 1. Effects of different ultrasonic power (a) and time (b) on hydroxyl and DPPH radicals scavenging activities of SDF
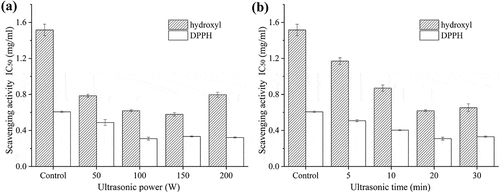
The hydroxyl and DPPH radicals scavenging activities of SDF exposed with different ultrasonic durations at 100 W were displayed in ). It is observed that the IC50 value of hydroxyl radical scavenging activity was decreased from 1.17 mg/ml to 0.62 mg/ml as the ultrasonic time increased from 5 min to 20 min. However, the significant difference in hydroxyl radical scavenging activity between 20 min and 30 min was not detected. ) also shows that the lowest IC50 value of DPPH scavenging activity was obtained at ultrasonic time of 20 min. These results illustrate that ultrasonic pretreatment is an effective way for improving the antioxidant activity of SDF. It was reported that lower molecular weight of polysaccharides had more pronounced scavenging capacity on radical.[Citation25] Therefore, increase of SDF antioxidant activity may be due to ultrasonic degradation. However, the real mechanism of this result needs to be further researched.
Changes in physicochemical and functional properties of SDF with different treatments
The physicochemical and functional properties of ultrasonic-treated SDF were determined in comparison with control. The ability of SDF to retain oil is important to prevent fat loss during cooking, and also is beneficial for flavor retention.[Citation26] As shown in , ultrasonic power and time both showed significant influence on the OHC. The OHC of SDF treated by ultrasound was increased from 5.40 g/g to 6.01 g/g. Ultrasonic-treated SDF also exhibited significantly higher WHC and SC than control. The highest values of WHC and SC were increased by 26.9% and 65.0% compared to those of control. These high values of modified SDF might be attributed to porosity and specific surface area of dietary fiber induced by ultrasound.[Citation20] These results were in agreement with previous reports on the effect of ultrasound on the properties of IDF.[Citation21]
Table 1. Physicochemical and functional properties of garlic straw SDF modified by ultrasounda.
Glucose and cholesterol adsorption capacities are useful in vitro indices to predict the effect of SDF on the decrease of glucose and cholesterol absorption in vivo. shows that the highest value of CBC of ultrasonic-treated SDF was 9.40 mg/g, which was only increased by 7.92% over the untreated SDF (P < 0.05). However, GAC values of treated-SDF were all significantly decreased over control. Gupta et al.[Citation11] reported that glucose dialysis retardation index was positively correlated with Mw and viscosity of dietary fiber. Therefore, the decrease of the GAC might mainly be due to the degradation of SDF induced by ultrasound.
Rheological property of garlic straw SDF
The rheological property of SDF treated by ultrasound at 100 W for 20 min was measured in comparison with control. Viscosity is an important physicochemical property that is related to the ability of dietary fiber to absorb water and form a gelatinous mass.[Citation4] ) shows viscosity profiles of garlic straw SDF which was plotted as shear rate dependence of apparent viscosity. It was observed that SDF exhibit typical shear-thinning behavior (also termed pseudoplastic) properties with high viscosities at low shear rates. The rheological properties always depend on some structural parameters, including distributions of molecular weight and substituents.[Citation27] Ultrasound treated-SDF exhibited a significant decrease in viscosity. At a shear rate of 10 s−1, the viscosity of treated-SDF and control were 0.010 and 0.037 Pa•s, respectively. The decrease in apparent viscosity of treated SDF solution can be mainly attributed to the reduction in particle size or changes in the particle shape induced by ultrasound treatment. Since the viscosity of viscous SDF plays an important role on physiological responses such as controlled postprandial glucose and re-absorption of bile acids[Citation5,Citation28], the decrease in viscosity of ultrasound treated-SDF might elicit the decreases in the binding capacities of glucose shown in .
Figure 2. Rheological properties of SDF from garlic straw. (a) Viscosity as a function of the shear rate; (b) Shear stress as a function of shear rate
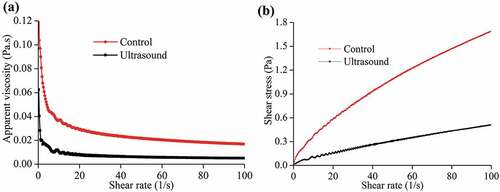
The stress profiles are shown in ). It can be seen that the shear stress exhibited a practically linear dependence on shear rate and the ultrasound treated-SDF had lower stress than control. Lundberg et al.[Citation29] reported that lower shear stress correlated with smaller particle size for any given shear rate, which was expected due to decreased particle interactions with smaller fiber length.
AFM analysis
Atomic force microscope (AFM) is a powerful tool to measure the surface morphology, particle size and distribution, which are always related to functional properties of biological molecules .[Citation30,Citation31] Therefore, the AFM images of ultrasonic-treated SDF and control were compared and results are shown in . Particle size was analyzed with Nanoscope Analysis software, and then Gaussian curve was fitted to size histograms of height distribution as shown in .
Figure 3. Effect of ultrasound pretreatment on the surface morphology of SDF. (a) Control; (b) SDF treated by ultrasound at power of 100 W and time of 20 min
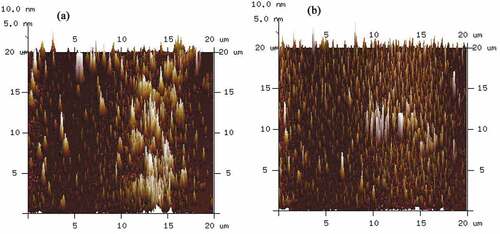
Figure 4. Effect of ultrasound pretreatment on the height of SDF. (a) Control; (b) SDF treated by ultrasound at power of 100 W and time of 20 min
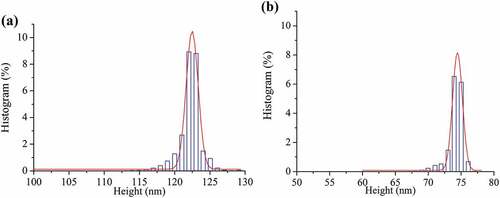
It can be seen that the aggregate particles of control showed a larger size with 122 nm height () and )). When SDF was treated by ultrasound, well-distributed and smaller nano-aggregates were formed on the mica sheet surface due to the cavitation and mechanical effects of ultrasound ()).[Citation32] The height of the ultrasonic treated-SDF particle was ranged from 70 nm to 77 nm ()). The micro-morphologies and particle height of SDF proved that ultrasonic treatment made garlic straw SDF degrade into smaller molecular fragments, which was in agreement with the result of rheological properties. This finding is also consistent with the report of Wang et al.[Citation4] as well as Brummer et al.[Citation33] stipulating that a smaller particle size of SDF can result in less particle interactions and thus lower apparent viscosity.
FT-IR analysis
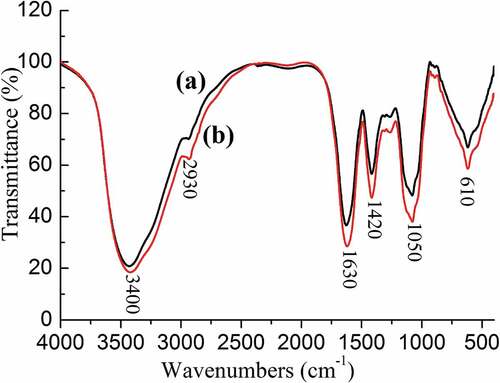
FTIR spectra of SDF samples were analyzed to check for any structural changes that might have occurred through ultrasound treatment at 100 W for 20 min. The results are presented in . Typical peaks could be seen from the spectra of ultrasonic-treated SDF and control, including peaks around 3400, 2930, and 1050 cm−1 for O-H, C-H, and C-O-C vibrations, respectively.[Citation34,Citation35] Two bands at 1630 cm−1and 1420 cm−1 corresponding to the stretching vibrations of the carboxylate groups (C = O & C-O).[Citation36] As shows, the FTIR spectra of ultrasound treated SDF has a spectrum superimposable over that of control SDF, implying that there are no changes in the functional groups and chemical structure during the treatment. The decrease in viscosity and particle size were mainly ascribed to breakage of glycosidic linkages which may be induced by free radicals or due to the physical effects of cavitation.[Citation34]
Conclusion
The results of the present work indicated that the ultrasonic degraded SDF from garlic straw exhibited stronger antioxidant activity than that of control. Ultrasound treatment also resulted in modified SDF with higher physicochemical properties, such as water holding capacity, oil holding capacity and swelling capacity. However, the GAC of ultrasonic-treated SDF was decreased over control, which was attribute to the decrease of viscosity induced by ultrasound treatment. The AFM results revealed that the garlic straw SDF was degraded into fragments of small particle size, which is correlated to the higher antioxidant activity and lower viscosity. FTIR spectra of control and ultrasound treated SDF showed a similar basic structure. This result ascertained ultrasound degradation did not change the primary structure of garlic straw SDF in the test conditions. Overall, the work suggested that ultrasound provided a viable alternative method for the modification of garlic straw SDF.
Acknowledgments
The authors are grateful for the support provided by National Natural Science Foundation of China (31701540) and Natural Science Foundation of Jiangsu Province (BK20170539) and A project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Additional information
Funding
References
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S. E. Isolation and Structural Characterization of Cellulose Nanocrystals Extracted from Garlic Straw Residues. Ind. Crops Prod. 2016, 87, 287–296. DOI: 10.1016/j.indcrop.2016.04.060.
- Xu, H.; Ding, Y.; Xin, X.; Wang, W.; Zhang, D. Dietary Fiber Intake Is Associated with a Reduced Risk of Ovarian Cancer: A Dose-Response Meta-Analysis. Nutr. Res.. 2018, 57, 1–11. DOI: 10.1016/j.nutres.2018.04.011.
- Vázquez-Sánchez, K.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Del Castillo, M. D.; Gaytán-Martínez, M.; Campos-Vega, R. In Vitro Health Promoting Properties of Antioxidant Dietary Fiber Extracted from Spent Coffee (Coffee arabica L.) Grounds. Food Chem. 2018, 261, 253–259. DOI: 10.1016/j.foodchem.2018.04.064.
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical Characterization of Five Types of Citrus Dietary Fibers. Biocatal. Agric. Biotechnol. 2015, 4(2), 250–258. DOI: 10.1016/j.bcab.2015.02.003.
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H. D. The Effect of in Vitro Digestive Processes on the Viscosity of Dietary Fibres and Their Influence on Glucose Diffusion. Food Hydrocolloids. 2014, 35(3), 718–726. DOI: 10.1016/j.foodhyd.2013.08.007.
- Chen, H.; Zhao, C.; Li, J.; Hussain, S.; Yan, S.; Wang, Q. Effects of Extrusion on Structural and Physicochemical Properties of Soluble Dietary Fiber from Nodes of Lotus Root. LWT- Food Sci. Technol. 2018, 93, 204–211. DOI: 10.1016/j.lwt.2018.03.004.
- Nandi, I.; Ghosh, M. Studies on Functional and Antioxidant Property of Dietary Fibre Extracted from Defatted Sesame Husk, Rice Bran and Flaxseed. Bioact Carbohydr. Dietary Fibre. 2015, 5(2), 129–136. DOI: 10.1016/j.bcdf.2015.03.001.
- Feng, T.; Su, Q.; Zhuang, H.; Ye, R.; Gu, Z.; Jin, Z. Ghost Structures, Pasting, Rheological and Textural Properties between Mesona Blumes Gum and Various Starches. J. Food Qual. 2014, 37(2), 73–82. DOI: 10.1111/jfq.2014.37.issue-2.
- Li, N.; Feng, Z.; Niu, Y.; Yu, L. Structural, Rheological and Functional Properties of Modified Soluble Dietary Fiber from Tomato Peels. Food Hydrocolloids. 2018, 77, 557–565. DOI: 10.1016/j.foodhyd.2017.10.034.
- Li, Q.; Liu, R.; Wu, T.; Zhang, M. Aggregation and Rheological Behavior of Soluble Dietary Fibers from Wheat Bran. Food Res. Int. 2017, 102, 291–302. DOI: 10.1016/j.foodres.2017.09.064.
- Gupta, S.; Saurabh, C. K.; Variyar, P. S.; Sharma, A. Comparative Analysis of Dietary Fiber Activities of Enzymatic and Gamma Depolymerized Guar Gum. Food Hydrocolloids. 2015, 48, 149–154. DOI: 10.1016/j.foodhyd.2015.02.013.
- Du, B.; Zeng, H.; Yang, Y.; Bian, Z.; Xu, B. Anti-Inflammatory Activity of Polysaccharide from Schizophyllum Commune as Affected by Ultrasonication. Int. J. Biol. Macromol. 2016, 91, 100–105. DOI: 10.1016/j.ijbiomac.2016.05.052.
- Zhou, C.; Yu, X.; Zhang, Y.; He, R.; Ma, H. Ultrasonic Degradation, Purification and Analysis of Structure and Antioxidant Activity of Polysaccharide from Porphyra Yezoensis Udea. Carbohydr. Polym. 2012, 87(3), 2046–2051. DOI: 10.1016/j.carbpol.2011.10.026.
- Wali, A.; Ma, H.; Hayat, K.; Ren, X.; Ali, Z.; Duan, Y.; Rashid, M.T. Enzymolysis reaction kinetics and thermodynamics of rapeseed protein with sequential dual-frequency ultrasound pretreatment. Int. J. Food Sci. Technol. 2018, 53(1), 72–80. DOI:10.1111/ijfs.13555.
- Ren, X.; Zhangi, X.; Liang, Q.; Hou, T.; Zhou, H. Effects of different working modes of ultrasound on structural characteristics of zein and ACE inhibitory activity of hydrolysates. J. Food Quality, 2017, 11, 1–8. DOI: 10.1155/2017/7896037.
- Liao, N.; Zhong, J.; Ye, X.; Lu, S.; Wang, W.; Zhang, R.; Xu, J.; Chen, S.; Liu, D. Ultrasonic-Assisted Enzymatic Extraction of Polysaccharide from Corbicula fluminea: Characterization and Antioxidant Activity. LWT - Food Sci. Technol. 2015, 60(2), 1113–1121. DOI: 10.1016/j.lwt.2014.10.009.
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, 2000.
- Smirnoff, N.; Cumbes, Q. J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry. 1989, 28, 1057–1060. DOI: 10.1016/0031-9422(89)80182-7.
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and Physicochemical Properties of Soluble Dietary Fiber from Orange Peel Assisted by Steam Explosion and Dilute Acid Soaking. Food Chem. 2015, 185, 90–98. DOI: 10.1016/j.foodchem.2015.03.112.
- Ma, M.; Mu, T. Modification of Deoiled Cumin Dietary Fiber with Laccase and Cellulase under High Hydrostatic Pressure. Carbohydr. Polym. 2016, 136, 87–94. DOI: 10.1016/j.carbpol.2015.09.030.
- Huang, L.; Ding, X.; Zhao, Y.; Li, Y.; Ma, H. Modification of Insoluble Dietary Fiber from Garlic Straw with Ultrasonic Treatment. J. Food Process. Preserv. 2018, 42(1), 1–8. DOI: 10.1111/jfpp.2018.42.issue-1.
- Genccelep, H.; Saricaoglu, F. T.; Anil, M.; Agar, B.; Turhan, S. The Effect of Starch Modification and Concentration on Steady-State and Dynamic Rheology of Meat Emulsions. Food Hydrocolloids. 2015, 48, 135–148. DOI: 10.1016/j.foodhyd.2015.02.002.
- Li, L.; Li, X.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Hu, C.; Yuan, M. Ultrasonic-Assisted Enzymatic Extraction and Antioxidant Activity of Polysaccharides from Setaria Viridis. Sep. Sci. Technol. 2016, 51(11), 1798–1805. DOI: 10.1080/01496395.2016.1178287.
- Fiaschi, T.; Chiarugi, P. Oxidative Stress, Tumor Microenvironment, and Metabolic Reprogramming: A Diabolic Liaison. Int. J. Cell Biol. 2012, 2012(1), 1–8. DOI: 10.1155/2012/762825.
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Zhong, Z., Ji, X.; Li, P. Relevance of Molecular Weight of chitosan-N-2-hydroxypropyl Trimethyl Ammonium Chloride and Their antioxidant Activities. Eur. J. Med. Chem. 2008, 43(2), 336–340. DOI: 10.1016/j.ejmech.2007.03.025.
- Pérez-López, E.; Mateos-Aparicio, I.; Rupérez, P. Okara Treated with High Hydrostatic Pressure Assisted by Ultraflo ®; L: Effect on Solubility of Dietary Fibre. Innovative Food Sci. Emerg. Technol. 2016, 33, 32–37. DOI: 10.1016/j.ifset.2015.12.017.
- Shen, X.; Fang, T.; Gao, F.; Guo, M. Effects of Ultrasound Treatment on Physicochemical and Emulsifying Properties of Whey Proteins Pre- and Post-Thermal Aggregation. Food Hydrocolloids. 2017, 63, 668–676. DOI: 10.1016/j.foodhyd.2016.10.003.
- Qi, J.; Li, Y.; Masamba, K. G.; Shoemaker, C. F.; Zhong, F.; Majeed, H.; Ma, J. The Effect of Chemical Treatment on the Invitro, Hypoglycemic Properties of Rice Bran Insoluble Dietary Fiber. Food Hydrocolloids. 2016, 52, 699–706. DOI: 10.1016/j.foodhyd.2015.08.008.
- Lundberg, B.; Pan, X.; White, A.; Chau, H.; Hotchkiss, A. Rheology and Composition of Citrus Fiber. J. Food Eng. 2014, 125(1), 97–104. DOI: 10.1016/j.jfoodeng.2013.10.021.
- Guo, Y.; Liu, W.; Wu, B.; Wu, P.; Duan, Y.; Yang, Q.; Ma, H. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018, 268, 550–557. DOI: 10.1016/j.foodchem.2018.06.047.
- Wang, B.; Zhang, Y.; Venkitasamy, C.; Wu, B.; Pan, Z.; Ma, H. Effect of pulsed light on activity and structural changes of horseradish peroxidase. Food Chem. 2017, 234, 20–25. DOI: 10.1016/j.foodchem.2017.04.149.
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in Food Processing. Ultrason. Sonochem. 2012, 19(5), 975–983. DOI: 10.1016/j.ultsonch.2012.01.010.
- Brummer, Y.; Kaviani, M.; Tosh, S. M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas and Chickpeas. Food Res. Int. 2015, 67, 117–125. DOI: 10.1016/j.foodres.2014.11.009.
- Prajapat, A. L.; Gogate, P. R. Depolymerization of Guar Gum Solution Using Different Approaches Based on Ultrasound and Microwave Irradiations. Chem. Eng. & Process. Process Intensifi. 2015, 88, 1–9. DOI: 10.1016/j.cep.2014.11.018.
- Li, F.; Wang, W.; Wang, X.; Yu, J. The Changes of Structure and Property of Alkali Soluble Hydroxyethyl Celluloses (Hecs) and Their Regenerated Films with the Molar Substitution. Carbohydr. Polym. 2014, 114, 206–212. DOI: 10.1016/j.carbpol.2014.08.015.
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound Effects on the Degradation Kinetics, Structure and Rheological Properties of Apple Pectin. Ultrason. Sonochem. 2013, 20(1), 222–231. DOI: 10.1016/j.ultsonch.2012.07.021.