ABSTRACT
This study assessed the microbiological quality of coleslaw samples sold at restaurants in Ibadan, Oyo-state, Nigeria. Three hundred and sixty samples were analyzed over a 12-week period for aerobic mesophilic counts, psychrotrophic counts, levels of Enterobacteriaceae, yeasts and molds, total lactic acid bacteria and total anaerobes. The coleslaw samples were also analyzed for presumptive L. monocytogenes and Salmonella spp. Counts of up to 9.2, 8.2, 9.4, 9.0, 8.7, and 8.9 log CFU/g for aerobic mesophilic organisms, psychrotrophic counts, Enterobacteriaceae, yeasts and molds, total lactic acid bacteria and total anaerobes, respectively, were recovered from the coleslaw samples. Despite high counts of yeasts and molds and LAB (up to 9 and 8.7 log CFU/g, respectively) recovered from some samples, no visible spoilage was detected. The levels of microorganisms recovered from the coleslaw samples at different sample collection time (week 1–12) were significantly (P < 0.05) different for each restaurant. Similarly, microbial levels recovered from coleslaw samples collected from different restaurants differed significantly (P < 0.05) from one restaurant to the other. Thirty (30.5%) and 24.7% of the coleslaw samples were positive for presumptive L. monocytogenes and Salmonella, respectively, an indication of potential threats to food safety in the area. The study concluded that the roles of yeasts and molds, as well as LAB in the spoilage of coleslaw sold in the study area, need to be further investigated. Public health intervention strategies to enhance microbiological safety of RTE coleslaw are required in the city.
Introduction
Fresh produce and fresh-cut produce have been identified as important vehicles for the transmission of foodborne pathogens.[Citation1,Citation2] In advanced countries, vegetables and RTE vegetable salads have been either shown to be the source of infection, or strongly implicated as the source of foodborne illness outbreaks.[Citation3] The microbiological quality of fresh and fresh-cut produce has significant implications for not only food safety and public health, but also product shelf-life, since a significant percentage of cultivated produce is lost due to microbial spoilage between harvest and consumption.[Citation4,Citation5]
Due to certain peculiarities, fresh-cut vegetable salads may offer suitable conditions for microbial growth and multiplication. For example, compared to whole, intact vegetables, the preparation (slicing, shredding, grating, cutting, chopping, dicing and so on) of fresh-cut produce can damage plant tissue, or increase nutrient availability such as via the release of exudates, or other nutrient-laden liquids, thereby, creating conditions that are conducive for the growth and multiplication of microorganisms in the products.[Citation6,Citation7] Several studies have assessed the microbiological safety and quality of fresh-cut vegetables in various parts of the world.[Citation2,Citation5,Citation8–19] There is however, limited published data on the microbiological quality of fresh-cut Ready-To-Eat (RTE) vegetable salads in Nigeria. Coleslaw, composed majorly of finely shredded cabbage and carrots, finished with salad dressing (usually mayonnaise and or other types of salad cream) is a commonly consumed fresh-cut vegetable salad product in Nigeria. The vegetables are shredded using a food processor, hand-held or other types of graters, slicers, or knives and chopping boards, depending on the preparation scenarios. Coleslaw is usually eaten raw as a side dish or salad with other staples such as rice or as a stand-alone dish.
Although foodborne illness outbreaks specifically implicating coleslaw in Nigeria have not been reported, this might not be due to low incidence of associated illness but other factors such as poor surveillance and overall weak public health systems. Similarly, there is minimal documentation of spoilage, associated microbial agents, factors that influence the activity of spoilage microorganisms, as well as other relevant factors that influence microbial spoilage of coleslaw, making it difficult for stakeholders to plan and minimize microbial spoilage and associated losses. Therefore, the aim of this study was to evaluate the microbiological quality of coleslaw commercially retailed in Ibadan, Nigeria to assess potential food safety risks and determine levels of potential spoilage bacteria groups in coleslaw salads.
Materials and methods
Sample collection
A total of 360 samples were purchased from 30 restaurants in Ibadan, Oyo State, Nigeria over a period of 12 weeks in 2019. The samples were collected from restaurants every Thursday, during six weeks of the two distinct seasons in Nigeria i.e., rainy and dry season, such that week 1–6 falls within (peak) rainy season, while week 7–12 falls within the (peak) dry season. The restaurants are relatively modern, i.e., not indigenous/traditional, and are large-or-medium-scale operations, with some being part of large commercial food retail chains. All coleslaw salad samples were comprised of shredded carrots and cabbage, finished with salad dressing (mayonnaise). All samples were placed in sterile resealable plastic bags and transferred promptly to the lab on ice. Relevant details (particularly with respect to the microbiological quality of the samples including whether or not the vendor wore gloves, a mask, hat, apron, amongst other details), of the sample collection site (restaurant) were recorded systematically. Coleslaw sold in restaurants are minimally processed and prepared, but not packaged for retail; they are rather offered for sale in trays, which are placed in food display showcases, from where they are dispensed to consumers per purchase request. ‘Best-before’ date was therefore, not relevant/available in this case. All samples were however, collected within two hours of preparation and analyzed in not more than 24 h after collection. Before samples were processed, the surfaces of the sample packs were sterilized using ethanol swabs to minimize the chances of cross contamination. Samples that had fallen out, were damaged or visibly compromised were discarded. The pH of the samples was recorded using a portable pH meter (Thermo Scientific Elite pH pocket testers, Thermo Scientific™ 01 × 001535).
Assessment of the general microbiological quality of salad samples
To enumerate aerobic mesophilic and psychrotrophic plate counts, 25 g of sample were aseptically divided into sterile stomacher bags and homogenized with 225 mL of sterile maximum recovery diluent (MRD) (Oxoid, UK) (ISO 4833–1:2013). Suitable 1:10 dilutions of the resultant homogenate were prepared using MRD. Plate Count Agar (PCA) (HiMedia, India) plates were inoculated with 0.1 mL aliquot of selected dilutions and incubated at 37°C for 24 h (to enumerate aerobic mesophilic plate counts) (ISO 4833–1:2013), and 6°C for 5–7 d (for the enumeration of psychrotrophic plate counts), (ISO 17410:2019).
For the analyses of yeasts and molds, after suitable decimal dilutions, aliquot was inoculated onto dichloran rose Bengal chloramphenicol agar (DRBC) (Merck, Germany), incubated at 25°C and examined after 5 d (ISO 21527–1:2008). When there was no growth at this point, plates were re-incubated for another 48 h. Lactic acid bacteria were enumerated by pour plating in de man Rogosa Sharpe agar (MRS, Merck, Germany), with an overlayer added to achieve micro-aerophilic conditions and incubated at 37°C for 48 h (ISO 15214:1998).
Enterobacteriaceae were determined according to ISO 21528–2:2017: briefly, 25 g of samples were homogenized in sterile buffered peptone water (HiMedia, India), serially diluted and pour plated using Violet Red Bile Glucose agar (VRBDA, Merck, Germany) and incubated at 37°C for 24 h. Subsequently, biochemical testing including oxidase reaction (done using 1% Kovac’s oxidase reagent, Merck Germany) and fermentation test in glucose OF medium (Neogen, US) were conducted. A minimum of five, but up to nine typical colonies were selected, streaked on to nutrient agar (Oxoid, UK) and subsequently used for the biochemical tests. To determine total anaerobic counts, suitable decimal dilution series prepared from the primary dilution were pour plated in Reinforced Clostridial Agar (Oxoid, UK), with an overlayer and incubated anaerobically in anaerobic jars (2.5 L, Thermo Scientific Oxoid AnaeroJar AG0025A) with anaerogen sachets (Thermo Scientific, UK).[Citation20,Citation21]
Detection of presumptive Listeria monocytogenes in salad samples
To detect presumptive L. monocytogenes, coleslaw sample (25 g) was prepared in 225 mL of demi Fraser broth (3 M, United States) and incubated at 30°C for 24 h (EN ISO 11290–1:2017). From the primary enrichment, 0.1 mL of culture was added to 10 mL Fraser broth (3 M, United States) and incubated at 37°C for 24 h and up to 48 h. From both primary and secondary enrichment, an aliquot was streaked unto (a) Listeria selective agar (Oxford formulation) (Oxoid, UK) prepared with Listeria selective supplement (Oxford formulation) (Oxoid, UK) incubated for 24 h at 37°C and unto (b) Listeria identification agar (PALCAM) prepared with Listeria selective supplement (PALCAM) (HiMedia, India) and incubated for 24 h and up to 48 h at 37°C. Typical colonies (up to seven) were streaked unto nutrient agar (Neogen, UK) and further testing procedures including Gram, catalase, oxidase, indole, methyl red reaction, rhamnose test (Rhamnose broth, BioRad), as well as assessment of hydrogen sulfide production, arginine (ammonia production), and heamolysis test on blood agar base No. 2 (Oxoid, UK) were conducted.
Detection of presumptive Salmonella in RTE salad samples
For detection of presumptive Salmonella in the coleslaw samples, 25 g of salad sample was aseptically inoculated into sterile buffered peptone water (225 mL) (HiMedia, India), homogenized gently and then incubated for 18 h at 37°C. Subsequently, 0.1 mL was inoculated into 10 mL Rappaport-Vassiliadis with soya (RVS broth) (Neogen, US), while 1 mL BPW was inoculated into 10 mL Mueller Kaufmann tetrathionate-novobiocin broth (MKTTn broth) (HiMedia, India) prepared with novobiocin selective supplement (Oxoid, UK) and incubated at 41.5°C for 24 h and 37°C for 24 h respectively (EN ISO 6579–1:2017). Post-incubation, culture from respective enrichment tube were plated unto Mannitol Lysine Crystal Violet Brilliant Green Agar (MLCB) (Oxoid, UK) agar, Bismuth Sulphite Agar (Neogen, MI), and Xylose Lysine Deoxycholate (HiMedia, India) and incubated at 35°C for 24 h, 35°C for 24 h and 37°C for 24 h, respectively. Typical or suspect Salmonella colonies (based on growth characteristics/colony morphology) were selected for biochemical analyses including urease test [Urea Agar base (Christensen), KASVI, Italy], color of slant and butt, as well as hydrogen sulfide and gas production on Kliger’s iron agar (Oxoid, England) slant, citrate utilization on Simmons citrate agar (Himedia, India) and indole reaction (Kovac’s reagent, Merck Germany). Finally, a minimum of five typical colonies were further tested (using slide agglutination) (EN ISO 6579–1:2017), where a drop of antiserum (BD Difco Salmonella Antiserum Poly A- I & Vi) was placed unto appropriately prepared microbial suspension and gently homogenized. In the case of negative agglutination results, additional four marked colonies were tested.
Statistical analyses
Average of duplicate plate counts was calculated and expressed as log CFU/g. Two-way ANOVA was used to determine if microbial log counts recovered from coleslaw samples at the different sampling time (weeks) varied significantly (p value < 0.05 was taken to be statistically significant). One sample t and Wilcoxon test was performed to determine whether microbial log counts recovered from the restaurants per sampling time differed significantly from one another. Microbial log counts recovered from samples collected from all restaurants during all sampling periods (week 1 − 12) were subjected to descriptive statistical analyses to determine minimum, maximum, range, mean, standard deviation and standard error of the mean of duplicate weekly counts. Similarly, the datasets were subjected to frequency distribution to determine the percentage of samples with counts in respective indicated interval(s). Two-way ANOVA was applied to determine whether the pH of coleslaw samples collected during the 12 weeks from respective restaurants were significantly different (P < .05). One sample t and Wilcoxon test were used to determine whether pH values of coleslaw samples collected per sampling time from different restaurants were significantly different from one another (P < .05). Statistical analyses and graphical illustration were done by GraphPad Prism (version 9.1) for Windows, GraphPad software, San Diego, California, USA, www.graphpad.com.
Results and discussion
Fresh produce is fundamentally predisposed to microbial contamination since they are cultivated in open fields. Agricultural input including irrigation water, (contaminated) soil and raw or improperly composted manure, biosolids, and other biological soil amendments can introduce microorganisms to produce such as carrots and cabbage at the preharvest stage.[Citation3,Citation22,Citation23] Carrots may be particularly susceptible to microbial contamination, since they are subterranean crops and are prone to contamination via splash or otherwise close contact with soil and water.[Citation8] Post-harvest, processing such as cutting, shredding, slicing, grating and other forms of handling are potential sources of contamination or could create circumstances such as the secretion of juices, which could encourage the proliferation of microbial pathogens.[Citation6] Temperature abuse conditions could also encourage the growth of microorganisms during marketing/retail. In this study, majority of coleslaw vendors (at least 98%) did not retail/hold coleslaw under low temperature conditions (data not shown). Most restaurants held coleslaw in serving pans at room temperature (average of 27–28°C and 24–26°C, during the rainy and dry season, respectively). Factors such as storage temperature, culinary practices and physicochemical properties of the samples can also influence the microbial population detected on foods.[Citation24]
Physicochemical properties of coleslaw samples
The pH values of coleslaw samples collected at different sampling time (weeks) ranged between 5.0 ± 0.07 and 6.9, and were significantly different from one another (P < .05), according to two-way ANOVA. The factors that influence the pH of the coleslaw samples might include the constituent ingredients of salad dressing (in the sample area, this is typically mayonnaise), as well as the vegetables, themselves. The factors that dictate the pH of coleslaw and how the pH impacts the incidence and levels of microorganisms in the samples, merit further investigation.
General microbiological quality of coleslaw samples
The results of aerobic mesophilic (AM) counts, aerobic psychrotrophic bacteria, Enterobacteriacaeae, total LAB, yeasts and molds, and total anaerobes are presented in , while the mean of duplicate microbial counts together with standard deviation values are presented in Tables S1-6. Variations in microbial counts recovered from the 30 restaurants at different sampling time can be appreciated by viewing heatmaps provided in . Overall, there were significant variations (p < .05) in counts recovered from samples collected from different restaurants and at various sample collection time. Microbiological quality of different batches of coleslaw samples may vary due to factors such as plant related traits (cultivars and varieties), geographical location of the farm, agricultural practices, and postharvest handling including transport and holding temperature.[Citation25]
Figure 1. pH of coleslaw samples collected from 30 restaurants in Ibadan, Oyo-State, Nigeria. A = pH of coleslaw samples collected during week 1–6, B = pH of coleslaw samples collected during week 7–12.
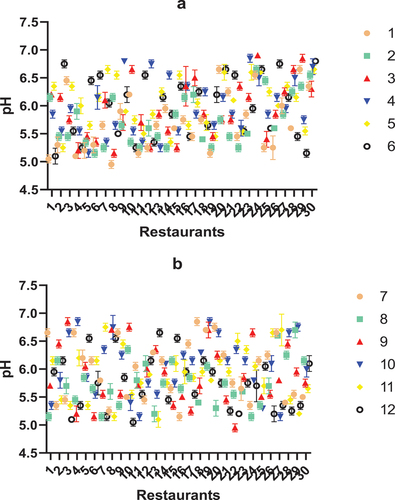
Table 1. Summary results of aerobic mesophiles enumerated from coleslaw samples.
Table 2. Summary results of psychrotrophic bacteria enumerated from coleslaw samples
Table 3. Summary results of Enterobacteriaceae enumerated from coleslaw samples.
Table 4. Summary results of LAB enumerated from coleslaw samples
Table 5. Summary results of total yeasts and moulds enumerated from coleslaw samples
Table 6. Summary results for total anaerobes recovered from coleslaw samples.
The maximum AM count recovered from coleslaw samples was 9 log CFU/g (), while the lowest count was 3 log CFU/g (, Table S1). Similar studies conducted in other parts of the world have reported counts recovered from fresh-cut vegetables to be within the same range (102–108).[Citation2,Citation9,Citation10,Citation18,Citation26–30] High AM counts (> 6 log CFU/g) detected in some cases in this study, could be an indication that more stringent adherence to good hygienic codes, as well as other safe food handling recommendations at all points of production and preparation might be necessary. It is however, noteworthy that AM levels can serve as an indicator of general microbial quality and predictor/assessor of shelf-life duration of food products, and may or may not specifically correlate with food safety hazards.[Citation18,Citation31] In fact, some members may not be pathogenic, or have any significant food safety or public health relevance, and some may be spoilage microorganisms, or epiphytic microorganisms.[Citation17]
Levels of psychrotrophic microorganisms recovered from coleslaw samples ranged between 2.8 log CFU/g and 8.2 log CFU/g (). These levels are somewhat similar with levels (<3 – 7 log CFU/g) Seow et al[Citation2] recovered from fresh-cut vegetables retailed in Singapore. Studies have suggested that psychrotrophic microorganisms can multiply in fresh-cut vegetables during retail, especially when products are stored at abusive temperatures.[Citation2,Citation8]
The levels of Enterobacteriaceae recovered from the coleslaw samples at different sample collection time (week 1–12) were significantly (P < .001) different from one another. Levels ranged between 2 and 9.5 log CFU/g (). Similar studies conducted in other parts of the world have reported levels of Enterobacteriacae ranging between <1.0 and 8.0 log CFU/g in fresh-cut vegetables.[Citation10,Citation30,Citation32] The Enterobacteriaceae are pathogens and commensals of the mammalian gastrointestinal tract, but they are also ubiquitous in most moist environments notably soil, water as well as the domestic environment.[Citation33] Although some, such as E. coli are typical indicators of fecal contamination,[Citation9,Citation34] several others are environmental strains and are thus, part of the normal flora of raw vegetables. Some studies have reported high numbers of Enterobacteriaceae in raw vegetables,[Citation35] and RTE vegetable salads,[Citation18] while others have isolated specific members: Rahnella aquatilis, Enterobacter agglomerans, Serratia spp. and Erwinia hericola from minimally-processed vegetables,[Citation36,Citation37] as well as Citrobacter freundii from parsley, fennel, radish and tomatoes.[Citation38] Counts of up to 9.5 log CFU/g detected in coleslaw in this study (), might thus, not necessarily be an indication of fecal contamination or otherwise inferior microbiological quality.
Counts of total LAB ranged between 0 and 9 log CFU/g (). Several other monitoring studies have detected LAB in different types of vegetable salads including carrots,[Citation8,Citation39] mixed vegetable salads,[Citation8,Citation39] as well as other minimally processed vegetable salad products.[Citation8,Citation9,Citation18] Despite these relatively high levels recovered from the coleslaw samples, none of the samples exhibited any visible signs of spoilage and no off-odors were perceived by the point of microbiological analyses. Lactic acid bacteria form part of the normal flora of vegetables, and vegetables may harbor wide diversity of LAB.[Citation40,Citation41] Lactic acid bacteria may however, also be introduced during post-harvest handling; during preparation or in the processing facility.[Citation20] Some members are known spoilage agents and have been associated with spoilage in sliced or shredded carrots and cabbage.[Citation6,Citation42] Specific members of LAB genus such as Leuconostoc mesenteroides have been associated with spoilage of carrots.[Citation42–44] Some other members of Leuconostoc, also seem to have spoilage potential, as they have been implicated in food spoilage,[Citation45–47] and have been isolated from food products that were categorized as unfit before the end of their shelf-life.[Citation48–51] It is however, apparent that not all LAB trigger spoilage in minimally processed RTE vegetable salads, and studies have in fact, elucidated intra-species variation in the potential for LAB strains to cause produce spoilage.[Citation20,Citation52] Future studies should explore strain-level distinctions among LAB with regards to their potential to cause spoilage. Based on findings of this and previously published papers in the literature, it is apparent that there are no simple correlations between spoilage and enumerated cell numbers of LAB. Consolidated research and regulatory efforts to determine the cell-number (at the very least, a suitable estimate/range), beyond which RTE vegetable salads such as coleslaw can be considered spoiled, are required. Although, some studies have posited LAB counts of 6 log CFU/g, to be sufficiently high to trigger or induce spoilage,[Citation37,Citation53] even with counts of up to 9 log CFU/g, recovered from some samples, no detectable or significant deterioration was observed in the coleslaw samples. The occurrence of high numbers (say, >6 log CFU/g) of LAB in RTE salads may not correspond to visual defects.
Levels of total yeasts and molds recovered from coleslaw samples ranged between 0 and 9 log CFU/g (). Yeasts are ubiquitous in the environment and may be part of the normal flora of vegetables.[Citation42,Citation54,Citation55] The natural yeast populations in the raw vegetables, as well as environmental contamination during post-harvest handling are potential routes of yeasts to the coleslaw commodities.[Citation24] Equipment and other manufacturing facilities are also major niches for yeast contamination. Other possible sources of contamination include air, vectors, and water (Kurtzman, 2011). Environmental conditions in various manufacturing settings are unique and highly variable, and the factors that predispose salads to contamination as well as specific sources of contamination will vary.[Citation56]
Post contamination, yeasts can contribute to spoilage of salad products via several mechanisms including production of lytic enzymes (proteases, lipases and cellulases) and gas, formation of off-flavors, discolouration, and utilization of organic acids.[Citation24,Citation42] Properties of food including water activity, sugar content, pH and acidity, as well as temperature significantly influence the extent of growth and proliferation of yeasts.[Citation57] While stress imposed by each of these factors may limit the survival and growth of yeasts, the exact extent to the limitation/prevention of growth and proliferation is not easy to define, since the impact of one factor may interact with the impact exerted by another factor. Despite high levels of yeasts and molds recovered from some samples (), no visible spoilage was detected, and thus, the roles of yeasts and molds in the spoilage of coleslaw should be further explored. The incidence of molds in fresh cut RTE vegetable salads may represent significant threats to public health safety since some molds may produce mycotoxins, and others could cause allergies and respiratory problems.[Citation58–60]
Levels of total anaerobes recovered from coleslaw samples ranged between 0 and 9 log CFU/g (, Table S6). Some anaerobes particularly anaerobic sulfite-reducing bacteria are generally considered as indicators of fecal or clostridial contamination.[Citation61,Citation62] However, since this study did not apply any downstream phenotypic and typing techniques to identify Clostridium spp in particular, it is impossible to categorically describe enumerated bacteria as Clostridium or fecal indicator bacteria. Anaerobic microorganisms may be introduced to vegetables at the pre-harvest stage, as they have been detected in soil, marine sediment, biological soil amendment (especially poorly composted amendments) and decaying vegetation.[Citation63–66] They may also be introduced to the product via human handling since some studies have suggested that humans may serve as important reservoirs for some anaerobic bacteria such as Clostridium.[Citation67]
Overall, it seems that the range of total microbial counts that can induce significant changes in sensory quality factors in minimally processed vegetables, to the extent where the product is rejected is in the range of 7–8 log CFU/g.[Citation37] Levels of microbial groups analyzed that exceeded this threshold in this study did not however, translate to visual defects. This could be attributed to the fact that factors that contribute to produce spoilage are not only microbiological, other factors such as produce physiological activity play roles in spoilage of fresh-cut vegetable salad products.[Citation37]
Prevalence of presumptive L. monocytogenes in coleslaw samples
In this study, 30.5% of the coleslaw samples studied were positive for presumptive L. monocytogenes (). Coleslaw has been implicated or strongly suspected to be the vehicle of L. monocytogenes in many Listeriosis outbreaks in various parts of the world.[Citation23,Citation68–72] Studies have also detected/characterized L. monocytogenes from retailed coleslaw samples in different parts of the world.[Citation19,Citation73] The methodology used to characterize absence/presence of (presumptive) L. monocytogenes in this study is limited, and future studies should explore the use of advanced molecular detection/quantification approaches to identify and quantify the levels of L. monocytogenes in coleslaw salads. Temperature conditions and pH parameters of the coleslaw samples may have contributed to the presence of L. monocytogenes in the samples. George and Levett[Citation74] described the effect of temperature and pH on the survival of L. monocytogenes in coleslaw, reporting that at pH 4.0, L. monocytogenes was not detectable in fresh coleslaw after incubation for five days. In their study, coleslaw also did not support the survival of L. monocytogenes at 4, 15 and 25°C, at pH 5. At 25°C and pH 6, however, viable counts of L. monocytogenes were detectable for up to 25 days, post inoculation. In this study, the pH of the tested coleslaw samples ranged between 5. 0 and 6.9, while the average environmental temperature was between 24–28°C. Although L. monocytogenes was not detected in some samples with pH values ≥ 6 (i.e., negative (-)/25 g of samples), several of the samples where presumptive L. monocytogenes were detected per 25 g of sample had pH of 6 or higher (; ). A few examples include: samples collected in week 3, 7 and 12 from restaurant 1, week 3, 6, 8 and 9 from restaurant 2, week 2 and 5 from restaurant 4, as well as those collected in week 6 and 12 from restaurant 15 (; ).
Figure 2. Heatmaps showing microbial counts recovered from coleslaw samples collected from 30 restaurants in Ibadan over a 12-week period. A = aerobic mesophilic counts, B = aerobic psychrotrophs, C = Enterobacteriaceae, D = Lactic Acid Bacteria, E = yeasts and molds, F = total anaerobes.
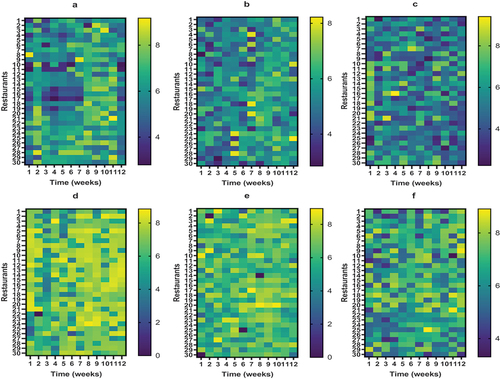
Table 7. Presence/absence of presumptive L. monocytogenes in coleslaw samples
There are several possible routes of L. monocytogenes to the coleslaw samples, especially since L. monocytogenes are ubiquitous in nature; occurring in soil, surface waters and other cultivation input. For example, listeriosis outbreaks linked to fresh produce (cantaloupe) in other parts of the world implicated environmental and equipment contamination in the processing plant as an important source of L. monocytogenes.[Citation75] Future studies should use advanced testing such as metagenomic approaches and high throughput sequencing (HTS) techniques or other advanced molecular techniques that facilitate in-depth tracking of microbial species to survey farms, preparation and retail environments to verify the sources and routes of L. monocytogenes to coleslaw in the area. Proper identification of the exact sources will aid the management and control of contamination, and improve overall safety.
Prevalence of presumptive Salmonella in coleslaw samples
In this study, 24.7% of coleslaw samples were positive for presumptive Salmonella (). Other studies have associated Salmonella contamination with coleslaw,[Citation6] as well as other types of fresh-cut RTE vegetable salads.[Citation13,Citation76] Raw materials (cabbage/carrots) may be contaminated with Salmonella via several routes including via contaminated biosolids or other types of biological fertilizers.[Citation77] Irrigation water might also be a source to vegetables on-field.[Citation78] Studies have shown that Salmonella may evade post-harvest decontamination since it has the capacity to form biofilms, and internalize into the edible portions of produce.[Citation3] Salmonella is an important foodborne pathogen, and its incidence in RTE coleslaw salad samples constitutes a major public health hazard.[Citation79] However, like the methodology used to analyze for L. monocytogenes, the screening approach adopted herein is limited and future studies should use advanced techniques to verify the incidence and levels of Salmonella in coleslaw samples.
Table 8. Presence/absence of presumptive Salmonella in coleslaw samples
Effect of mayonnaise on the microbiological quality of coleslaw samples
Certain peculiarities such as application of dressing (mayonnaise), may influence detected microbial levels. Addition of mayonnaise can either inhibit or bolster the growth of microorganisms in the coleslaw samples.[Citation80] The pH and ingredients of some brands/formulations may inhibit the growth of majority of the microbial groups, or even be lethal to some.[Citation80] The effect of pH (if any, since we did not assess the impact of mayonnaise addition on pH, and by extension, microbial levels) may create suitable conditions for the growth of some microbial groups such as yeasts. For example, Brocklehurst et al.[Citation81] showed that vegetable (carrot and cabbage) tissue absorbed acetic acid when mayonnaise was added during coleslaw preparation, creating conditions that encouraged the growth of spoilage yeasts. For reference, the pH of the coleslaw samples purchased at the point of retail ranged between 5.0 and 6.9 ().
One of the major limitations of this study is that we did not compare the microbiological quality of raw materials with that of the salads, which might have offered some insights into the impact of mayonnaise application, as well as handling or other potential post-harvest sources. Unfortunately, in this study, it was not possible to independently test the ingredients and pH of the mayonnaise applied to the salad samples. As far as we can tell, coleslaw is typically not retailed/served without the salad dressing in most parts of Ibadan, Oyo-State, Nigeria. We only acknowledged the potential influence of mayonnaise on the pH and microbial levels in hindsight. A future study could perhaps involve efforts to liaise with the producers and restaurateurs to obtain the shredded vegetable salad samples prior to the addition of mayonnaise for comparison. Certainly, the propensity for mayonnaise to exert any antimicrobial or other relevant effect will depend on a variety of factors such as the type, the quantity applied and transport/storage temperature, amongst other factors. It might be interesting for studies to assess how mayonnaise brand variety and application processes influence the pH of samples as well as microbial growth in coleslaw.
In general, it is key for future efforts to trace and understand the origin and routes of microbial contamination to coleslaw, so as to determine control protocols that are specific to coleslaw production and handling practices. Adoption of standard procedures and good manufacturing practices can minimize the chances for microbial contamination along the production distribution chain. It is useful for studies to assess the efficaciousness of post-harvest decontamination protocols (likely washing) applied, and determine the microbial log reduction it achieves. Another potentially useful research area will be to assess the growth of microorganisms, specifically pathogenic microbes after preparation, during retail.
Conclusion
This study assessed the microbiological quality of RTE coleslaw samples using culture dependent methods. The levels of aerobic mesophilic organisms, aerobic psychrotrophic counts, Enterobacteriaceae, yeasts and molds, total lactic acid bacteria and total anaerobes recovered from the samples ranged between 2.8, 2.8, 2.2, 0, 0, 3.3 to 9.2, 8.2, 9.4, 9.0, 8.7 and 8.9 log CFU/g, respectively. There were no significant peculiarities among the restaurants surveyed with respect to levels of detected microbial groups. No coleslaw samples, even those from which high microbial counts were recovered were visibly spoiled. Of the samples analyzed, 30.5 and 24.7% were positive for presumptive L. monocytogenes and Salmonella, respectively. Although it is recommended that future studies use advanced microbial typing techniques to confirm the incidence of L. monocytogenes and Salmonella in coleslaw samples, the detection of presumptive L. monocytogenes and Salmonella is a cause for some concern. In general, steps to enhance microbiological safety of RTE coleslaw in the area might be necessary.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10942912.2023.2173775
References
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C. M. Microbial Contamination of Fresh Produce: What, Where, and How? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. DOI: 10.1111/1541-4337.12487.
- Seow, J.; Ágoston, R.; Phua, L.; Yuk, H.-G. Microbiological Quality of Fresh Vegetables and Fruits Sold in Singapore. Food Control. 2012, 25, 39–44. DOI: 10.1016/j.foodcont.2011.10.017.
- Alegbeleye, O. O.; Singleton, I.; Sant’Ana, A. S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. DOI: 10.1016/j.fm.2018.01.003.
- Alegbeleye, O.; Odeyemi, O. A.; Strateva, M.; Stratev, D. Microbial Spoilage of Vegetables, Fruits and Cereals. Appl. Food Res. 2022, 2, 100122. DOI: 10.1016/j.afres.2022.100122.
- Korir, R. C.; Parveen, S.; Hashem, F.; Bowers, J. Microbiological Quality of Fresh Produce Obtained from Retail Stores on the Eastern Shore of Maryland, United States of America. Food Microbiol. 2016, 56, 29–34. DOI: 10.1016/j.fm.2015.12.003.
- Erickson, M. C.;. Microbial Risks Associated with Cabbage, Carrots, Celery, Onions, and Deli Salads Made with These Produce Items. Compr. Rev. Food Sci. Food Saf. 2010, 9, 602–619. DOI: 10.1111/j.1541-4337.2010.00129.x.
- Koukkidis, G.; Haigh, R.; Allcock, N.; Jordan, S.; Freestone, P. Salad Leaf Juices Enhance Salmonella Growth, Colonization of Fresh Produce, and Virulence. Appl. Environ. Microbiol. 2017, 83, e02416. DOI: 10.1128/AEM.02416-16.
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Microbiological, V. I. Quality of Fresh, Minimally-Processed Fruit and Vegetables, and Sprouts from Retail Establishments. Int. J. Food Microbiol. 2008, 123, 121–129. DOI: 10.1016/j.ijfoodmicro.2007.12.013.
- Caponigro, V.; Ventura, M.; Chiancone, I.; Amato, L.; Parente, E.; Piro, F. Variation of Microbial Load and Visual Quality of Ready-to-Eat Salads by Vegetable Type, Season, Processor and Retailer. Food Microbiol. 2010, 27, 1071–1077. DOI: 10.1016/j.fm.2010.07.011.
- Cardamone, C.; Aleo, A.; Mammina, C.; Oliveri, G.; Di Noto, A. M. Assessment of the Microbiological Quality of Fresh Produce on Sale in Sicily, Italy: Preliminary Results. J. Biol. Res. 2015, 22, 3. DOI: 10.1186/s40709-015-0026-3.
- Esteban-Cuesta, I.; Drees, N.; Ulrich, S.; Stauch, P.; Sperner, B.; Schwaiger, K.; Gareis, M.; Gottschalk, C. Endogenous Microbial Contamination of Melons (Cucumis Melo) from International Trade: An Underestimated Risk for the Consumer? J. Sci. Food Agric. 2018, 98, 5074–5081. DOI: 10.1002/jsfa.9045.
- Fröder, H.; Martins, C. G.; de Souza, K. L. O.; Landgraf, M.; Franco, B. D. G. M.; Destro, M. T. Minimally Processed Vegetable Salads: Microbial Quality Evaluation. J. Food Prot. 2007, 70, 1277–1280. DOI: 10.4315/0362-028X-70.5.1277.
- Jeddi, M. Z.; Yunesian, M.; Gorji, M. E.; Noori, N.; Pourmand, M. R.; Khaniki, G. R. J. Microbial Evaluation of Fresh, Minimally-Processed Vegetables and Bagged Sprouts from Chain Supermarkets. J. Health Popul. Nutr. 2014, 32, 391–399.
- Johannessen, G. S.; Loncarevic, S.; Kruse, H. Bacteriological Analysis of Fresh Produce in Norway. Int. J. Food Microbiol. 2002, 77, 199–204. DOI: 10.1016/S0168-1605(02)00051-X.
- Łepecka, A.; Zielińska, D.; Szymański, P.; Buras, I.; Kołożyn-Krajewska, D. Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health? Int. J. Environ. Res. Public. Health. 2022, 19, 1582. DOI: 10.3390/ijerph19031582.
- Maffei, D. F.; Silveira de, N. F. A.; Catanozi da, M. P. L. M. Microbiological Quality of Organic and Conventional Vegetables Sold in Brazil. Food Control. 2013, 29, 226–230. DOI: 10.1016/j.foodcont.2012.06.013.
- Oliveira, M.; Usall, J.; Viñas, I.; Anguera, M.; Gatius, F.; Abadias, M. Microbiological Quality of Fresh Lettuce from Organic and Conventional Production. Food Microbiol. 2010, 27, 679–684. DOI: 10.1016/j.fm.2010.03.008.
- Xylia, P.; Botsaris, G.; Chrysargyris, A.; Skandamis, P.; Tzortzakis, N. Variation of Microbial Load and Biochemical Activity of Ready-to-Eat Salads in Cyprus as Affected by Vegetable Type, Season, and Producer. Food Microbiol. 2019, 83, 200–210. DOI: 10.1016/j.fm.2019.05.013.
- Zhang, H.; Yamamoto, E.; Murphy, J.; Locas, A. Microbiological Safety of Ready-to-Eat Fresh-Cut Fruits and Vegetables Sold on the Canadian Retail Market. Int. J. Food Microbiol. 2020, 335, 108855. DOI: 10.1016/j.ijfoodmicro.2020.108855.
- Pothakos, V.; Snauwaert, C.; De Vos, P.; Huys, G.; Devlieghere, F. Monitoring Psychrotrophic Lactic Acid Bacteria Contamination in a Ready-to-Eat Vegetable Salad Production Environment. Int. J. Food Microbiol. 2014, 185, 7–16. DOI: 10.1016/j.ijfoodmicro.2014.05.009.
- International Organization for Standardisation, ISO 6579-1:2017. Microbiology of the Food Chain- Horizontal Method for the detection, enumeration and serotyping of Salmonella- Part 1 detection of Salmonella spp., 2017. https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/67/56712.html
- Alegbeleye, O. O.; Sant’Ana, A. S. Manure-Borne Pathogens as an Important Source of Water Contamination: An Update on the Dynamics of Pathogen Survival/Transport as Well as Practical Risk Mitigation Strategies. Int. J. Hyg. Environ. Health. 2020, 227, 113524. DOI: 10.1016/j.ijheh.2020.113524.
- Beuchat, L. R.;. Listeria Monocytogenes: Incidence on Vegetables. Food Control. 1996, 7, 223–228. DOI: 10.1016/S0956-7135(96)00039-4.
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M. J.; Villalobos, M. C.; Martín, A.; Córdoba, M. G. Spoilage Yeasts: What are the Sources of Contamination of Foods and Beverages? Int. J. Food Microbiol. 2018, 286, 98–110. DOI: 10.1016/j.ijfoodmicro.2018.07.031.
- Leff, J. W.; Fierer, N.; Berg, G. Bacterial Communities Associated with the Surfaces of Fresh Fruits and Vegetables. PLoS ONE. 2013, 8, e59310. DOI: 10.1371/journal.pone.0059310.
- Ailes, E. C.; Leon, J. S.; Jaykus, L.-A.; Johnston, L. M.; Clayton, H. A.; Blanding, S.; Kleinbaum, D. G.; Backer, L. C.; Moe, C. L. Microbial Concentrations on Fresh Produce are Affected by Postharvest Processing, Importation, and Season. J. Food Prot. 2008, 71, 2389–2397. DOI: 10.4315/0362-028x-71.12.2389.
- Nousiainen, -L.-L.; Joutsen, S.; Lunden, J.; Hänninen, M.-L.; Bacterial Quality, F.-A. M. Safety of Packaged Fresh Leafy Vegetables at the Retail Level in Finland. Int. J. Food Microbiol. 2016, 232, 73–79. DOI: 10.1016/j.ijfoodmicro.2016.05.020.
- Soriano, J. M.; Rico, H.; Moltó, J. C.; Mañes, J. Assessment of the Microbiological Quality and Wash Treatments of Lettuce Served in University Restaurants. Int. J. Food Microbiol. 2000, 58, 123–128. DOI: 10.1016/s0168-1605(00)00288-9.
- Valero, A.; Carrasco, E.; Pérez-Rodríguez, F.; García-Gimeno, R.; Blanco, C.; Zurera, G. Monitoring the Sensorial and Microbiological Quality of Pasteurized White Asparagus at Different Storage Temperatures. J. Sci. Food Agric. 2006, 86, 1281–1288. DOI: 10.1002/jsfa.2489.
- Ziegler, M.; Kent, D.; Stephan, R.; Guldimann, C. Growth Potential of Listeria Monocytogenes in Twelve Different Types of RTE Salads: Impact of Food Matrix, Storage Temperature and Storage Time. Int. J. Food Microbiol. 2019, 296, 83–92. DOI: 10.1016/j.ijfoodmicro.2019.01.016.
- Oliveira, M.; Usall, J.; Solsona, C.; Alegre, I.; Viñas, I.; Abadias, M. Effects of Packaging Type and Storage Temperature on the Growth of Foodborne Pathogens on Shredded ‘Romaine’ Lettuce. Food Microbiol. 2010, 27, 375–380. DOI: 10.1016/j.fm.2009.11.014.
- Tucci, P.; Centorotola, G.; Salini, R.; Iannetti, L.; Sperandii, A. F.; D’Alterio, N.; Migliorati, G.; Pomilio, F. Challenge Test Studies on Listeria Monocytogenes in Ready-to-Eat Iceberg Lettuce. Food Sci. Nutr. 2019, 7, 3845–3852. DOI: 10.1002/fsn3.1167.
- Österblad, M.; Pensala, O.; Peterzéns, M.; Heleniusc, H.; Huovinen, P. Antimicrobial Susceptibility of Enterobacteriaceae Isolated from Vegetables. J. Antimicrob. Chemother. 1999, 43, 503–509. DOI: 10.1093/jac/43.4.503.
- Wood, J. L.; Chen, J. C.; Friesen, E.; Delaquis, P.; Allen, K. J. Microbiological Survey of Locally Grown Lettuce Sold at Farmers’ Markets in Vancouver, British Columbia. J. Food Prot. 2015, 78, 203–208. DOI: 10.4315/0362-028X.JFP-14-199.
- Little, C.; Roberts, D.; Youngs, E.; de Louvois, J. Microbiological Quality of Retail Imported Unprepared Whole Lettuces: A PHLS Food Working Group Study. J. Food Prot. 1999, 62, 325–328. DOI: 10.4315/0362-028X-62.4.325.
- Nguyen‐the, C.; Carlin, F. The Microbiology of Minimally Processed Fresh Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 1994, 34, 371–401. DOI: 10.1080/10408399409527668.
- Ragaert, P.; Devlieghere, F.; Debevere, J. Role of Microbiological and Physiological Spoilage Mechanisms during Storage of Minimally Processed Vegetables. Postharvest Biol. Technol. 2007, 44, 185–194. DOI: 10.1016/j.postharvbio.2007.01.001.
- Ben Said, L.; Jouini, A.; Klibi, N.; Dziri, R.; Alonso, C. A.; Boudabous, A.; Ben Slama, K.; Torres, C. Detection of Extended-Spectrum Beta-Lactamase (Esbl)-producing Enterobacteriaceae in Vegetables, Soil and Water of the Farm Environment in Tunisia. Int. J. Food Microbiol. 2015, 203, 86–92. DOI: 10.1016/j.ijfoodmicro.2015.02.023.
- Sant’Ana, A. S.; Barbosa, M. S.; Destro, M. T.; Landgraf, M.; Franco, B. D. G. M. Growth Potential of Salmonella Spp. and Listeria Monocytogenes in Nine Types of Ready-to-Eat Vegetables Stored at Variable Temperature Conditions during Shelf-Life. Int. J. Food Microbiol. 2012, 157, 52–58. DOI: 10.1016/j.ijfoodmicro.2012.04.011.
- Ponce, A. G.; Moreira, M. R.; Del Valle, C. E.; Roura, S. I. Preliminary Characterization of Bacteriocin-like Substances from Lactic Acid Bacteria Isolated from Organic Leafy Vegetables. LWT - Food Sci. Technol. 2008, 41, 432–441. DOI: 10.1016/j.lwt.2007.03.021.
- Trias, R.; Bañeras, L.; Badosa, E.; Montesinos, E. Bioprotection of Golden Delicious Apples and Iceberg Lettuce against Foodborne Bacterial Pathogens by Lactic Acid Bacteria. Int. J. Food Microbiol. 2008, 123, 50–60. DOI: 10.1016/j.ijfoodmicro.2007.11.065.
- Barth, M.; Hankinson, T. R.; Zhuang, H.; Breidt, F. Microbiological Spoilage of Fruits and Vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W. H., Doyle, M. P., Eds.; Food Microbiology and Food Safety; Springer: New York, NY, 2009; pp 135–183.
- Lampert, Y.; Dror, B.; Sela, N.; Teper-Bamnolker, P.; Daus, A.; Sela (Saldinger), S.; Eshel, D. Emergence of Leuconostoc Mesenteroides as a Causative Agent of Oozing in Carrots Stored under Non-Ventilated Conditions. Microb. Biotechnol. 2017, 10, 1677–1689. DOI: 10.1111/1751-7915.12753.
- Carlin, F.; Nguyen-The, C.; Cudennec, P.; Reich, M. Microbiological Spoilage of Fresh, Ready-to-Use Grated Carrots [Principal Component Analysis]. Sci. Aliments Fr. 1989, 9, 371–386.
- Lyhs, U.; Koort, J. M. K.; Lundström, H.-S.; Björkroth, K. J.; Gelidum, L. Leuconostoc Gasicomitatum Strains Dominated the Lactic Acid Bacterium Population Associated with Strong Slime Formation in an Acetic-Acid Herring Preserve. Int. J. Food Microbiol. 2004, 90, 207–218. DOI: 10.1016/s0168-1605(03)00303-9.
- Säde, E. Leuconostoc Spoilage of Refrigerated. Pack. Food. 2011, 57.
- Susiluoto, T.; Korkeala, H.; Björkroth, K. J. Leuconostoc Gasicomitatum Is the Dominating Lactic Acid Bacterium in Retail Modified-Atmosphere-Packaged Marinated Broiler Meat Strips on Sell-by-Day. Int. J. Food Microbiol. 2003, 80, 89–97. DOI: 10.1016/S0168-1605(02)00123-X.
- Björkroth, K. J.; Geisen, R.; Schillinger, U.; Weiss, N.; De Vos, P.; Holzapfel, W. H.; Korkeala, H. J.; Vandamme, P. Characterization of Leuconostoc Gasicomitatum Sp. Nov., Associated with Spoiled Raw Tomato-Marinated Broiler Meat Strips Packaged under Modified-Atmosphere Conditions. Appl. Environ. Microbiol. 2000, 66, 3764–3772. DOI: 10.1128/AEM.66.9.3764-3772.2000.
- Kato, Y.; Sakala, R. M.; Hayashidani, H.; Kiuchi, A.; Kaneuchi, C.; Ogawa, M. Lactobacillus Algidus Sp. Nov., a Psychrophilic Lactic Acid Bacterium Isolated from Vacuum-Packaged Refrigerated Beef. Int. J. Syst. Evol. Microbiol. 2000, 50(Pt 3), 1143–1149. DOI: 10.1099/00207713-50-3-1143.
- Sakala, R. M.; Hayashidani, H.; Kato, Y.; Kaneuchi, C.; Isolation, O. M. Characterization of Lactococcus Piscium Strains from Vacuum-Packaged Refrigerated Beef. J. Appl. Microbiol. 2002, 92, 173–179. DOI: 10.1046/j.1365-2672.2002.01513.x.
- Vihavainen, E. J.; Björkroth, K. J. Spoilage of Value-Added, High-Oxygen Modified-Atmosphere Packaged Raw Beef Steaks by Leuconostoc Gasicomitatum and Leuconostoc Gelidum. Int. J. Food Microbiol. 2007, 119, 340–345. DOI: 10.1016/j.ijfoodmicro.2007.08.029.
- Björkroth, K. J.; Vandamme, P.; Korkeala, H. J. Identification and Characterization of Leuconostoc Carnosum, Associated with Production and Spoilage of Vacuum-Packaged, Sliced, Cooked Ham. Appl. Environ. Microbiol. 1998, 64(9), 3313–3319. DOI: 10.1128/AEM.64.9.3313-3319.1998.
- García-Gimeno, R. M.; Zurera-Cosano, G. Determination of Ready-to-Eat Vegetable Salad Shelf-Life. Int. J. Food Microbiol. 1997, 36, 31–38. DOI: 10.1016/s0168-1605(96)01238-x.
- Qadri, O. S.; Yousuf, B.; Srivastava, A. K.; Fruits, F.-C.; Yildiz, F. Vegetables: Critical Factors Influencing Microbiology and Novel Approaches to Prevent Microbial Risks—A Review. Cogent Food Agric. 2015, 1, 1121606. DOI: 10.1080/23311932.2015.1121606.
- Kurtzman, C. P.;. Chapter 47 - Meyerozyma Kurtzman & M. Suzuki (2010). In The Yeasts, (Fifth ed.; Kurtzman, C. P., Fell, J. W., Boekhout, T., Eds.; Elsevier: London, 2011; pp 621–624.
- Garijo, P.; González-Arenzana, L.; López-Alfaro, I.; Garde-Cerdán, T.; López, R.; Santamaría, P.; Gutiérrez, A. R. Analysis of Grapes and the First Stages of the Vinification Process in Wine Contamination with Brettanomyces Bruxellensis. Eur. Food Res. Technol. 2015, 240, 525–532. DOI: 10.1007/s00217-014-2351-4.
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S. K.; Microbial Fermentation, K. S. Its Role in Quality Improvement of Fermented Foods. Fermentation. 2020, 6, 106. DOI: 10.3390/fermentation6040106.
- Hardin, B. D.; Kelman, B. J.; Saxon, A. Adverse Human Health Effects Associated with Molds in the Indoor Environment. J. Occup. Environ. Med. 2003, 45, 470–478. DOI: 10.1097/00043764-200305000-00006.
- Mendell, M. J.; Mirer, A. G.; Cheung, K.; Tong, M.; Respiratory, D. J. Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health Perspect. 2011, 119, 748–756. DOI: 10.1289/ehp.1002410.
- Ratnaseelan, A. M.; Tsilioni, I.; Theoharides, T. C. Effects of Mycotoxins on Neuropsychiatric Symptoms and Immune Processes. Clin. Ther. 2018, 40, 903–917. DOI: 10.1016/j.clinthera.2018.05.004.
- Cabral, J. P. S.; Microbiology, W. Bacterial Pathogens and Water. Int. J. Environ. Res. Public. Health. 2010, 7, 3657–3703. DOI: 10.3390/ijerph7103657.
- Prevost, S.; Cayol, J.-L.; Zuber, F.; Tholozan, J.-L.; Remize, F. Characterization of Clostridial Species and Sulfite-Reducing Anaerobes Isolated from Foie Gras with respect to Microbial Quality and Safety. Food Control. 2013, 32, 222–227. DOI: 10.1016/j.foodcont.2012.11.030.
- Dharmasena, M.; Jiang, X.; Dudley, E. G. Isolation of Toxigenic Clostridium Difficile from Animal Manure and Composts Being Used as Biological Soil Amendments. Appl. Environ. Microbiol. 2018, 84, e00738–18. DOI: 10.1128/AEM.00738-18.
- Humbert, S.; Tarnawski, S.; Fromin, N.; Mallet, M.-P.; Aragno, M.; Zopfi, J. Molecular Detection of Anammox Bacteria in Terrestrial Ecosystems: Distribution and Diversity. ISME J. 2010, 4, 450–454. DOI: 10.1038/ismej.2009.125.
- Jung, Y.; Jang, H.; Matthews, K. R. Effect of the Food Production Chain from Farm Practices to Vegetable Processing on Outbreak Incidence. Microb. Biotechnol. 2014, 7, 517–527. DOI: 10.1111/1751-7915.12178.
- Schmid, M. C.; Risgaard-Petersen, N.; Van De Vossenberg, J.; Kuypers, M. M. M.; Lavik, G.; Petersen, J.; Hulth, S.; Thamdrup, B.; Canfield, D.; Dalsgaard, T., et al. Anaerobic Ammonium-Oxidizing Bacteria in Marine Environments: Widespread Occurrence but Low Diversity. Environ. Microbiol. 2007, 9, 1476–1484. DOI: 10.1111/j.1462-2920.2007.01266.x.
- Lindström, M.; Heikinheimo, A.; Lahti, P.; Korkeala, H. Novel Insights into the Epidemiology of Clostridium Perfringens Type A Food Poisoning. Food Microbiol. 2011, 28, 192–198. DOI: 10.1016/j.fm.2010.03.020.
- Garner, D.; Fresh Produce-Associated, K. S. Listeriosis Outbreaks, Sources of Concern, Teachable Moments, and Insights. J. Food Prot. 2016, 79, 337–344. DOI: 10.4315/0362-028X.JFP-15-387.
- Buchanan, R. L.; Gorris, L. G. M.; Hayman, M. M.; Jackson, T. C.; Whiting, R. C. A Review of Listeria Monocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control. 2017, 75, 1–13. DOI: 10.1016/j.foodcont.2016.12.016.
- Clark, C. G.; Farber, J.; Pagotto, F.; Ciampa, N.; Doré, K.; Nadon, C.; Bernard, K.; Ng, L.-K. Surveillance for Listeria Monocytogenes and Listeriosis, 1995–2004. Epidemiol. Infect. 2010, 138, 559–572. DOI: 10.1017/S0950268809990914.
- Schlech, W. F.;. New Perspectives on the Gastrointestinal Mode of Transmission in Invasive Listeria Monocytogenes Infection. Clin. Investig. Med. Med. Clin. Exp. 1984, 7, 321–324.
- Schlech, W. F., III; Acheson, D. Foodborne Listeriosis. Clin. Infect. Dis. 2000, 31, 770–775. DOI: 10.1086/314008.
- Ieren, I.; Bello, I.; Kwaga, M.; Occurrence, J. K. P. Antibiotic Resistance Profile of Listeria Monocytogenes in Salad Vegetables and Vegetable Salads Sold in Zaria, Nigeria. Afr. J. Food Sci. 2013, 7, 334–338. DOI: 10.5897/AJFS2013.1036.
- George, A. E.; Levett, P. N. Effect of Temperature and PH on Survival of Listeria Monocytogenes in Coleslaw. Int. J. Food Microbiol. 1990, 11, 345–349. DOI: 10.1016/0168-1605(90)90028-4.
- Rakic Martinez, M.; Ferguson, M.; Datta, A. R.; Palusinska-Szysz, M. Virulence Assessment of Listeria Monocytogenes Grown in Different Foods Using a Galleria Mellonella Model. PLoS ONE. 2020, 15, e0232485. DOI: 10.1371/journal.pone.0232485.
- Meldrum, R. J.; Little, C. L.; Sagoo, S.; Mithani, V.; McLauchlin, J.; de Pinna, E. Assessment of the Microbiological Safety of Salad Vegetables and Sauces from Kebab Take-Away Restaurants in the United Kingdom. Food Microbiol. 2009, 26, 573–577. DOI: 10.1016/j.fm.2009.03.013.
- García, S.; Heredia, N., Microbiological Safety of Fruit and Vegetables in the Field, During Harvest, and Packaging: A Global Issue, In Global Food Security and Wellness; Barbosa-Cánovas, G. V., María Pastore, G., Candoğan, K., Medina Meza, I. G., Caetano da Silva Lannes, S., Buckle, K., Yada, R. Y., Rosenthal, A. Eds.; Springer: New York, NY. 2017; 27–48.
- Alegbeleye, O. O.; Sant’Ana, A. S. Risks Associated with the Consumption of Irrigation Water Contaminated Produce: On the Role of Quantitative Microbial Risk Assessment. Curr. Opin. Food Sci. 2021, 41, 88–98. DOI: 10.1016/j.cofs.2021.03.013.
- Schierstaedt, J.; Jechalke, S.; Nesme, J.; Neuhaus, K.; Sørensen, S. J.; Grosch, R.; Smalla, K.; Schikora, A. Salmonella Persistence in Soil Depends on Reciprocal Interactions with Indigenous Microorganisms. Environ. Microbiol. 2020, 22, 2639–2652. DOI: 10.1111/1462-2920.14972.
- Smittle, R. B.;. Microbiology of Mayonnaise and Salad Dressing: A Review. J. Food Prot. 1977, 40, 415–422. DOI: 10.4315/0362-028X-40.6.415.
- Brocklehurst, T. F.; White, C. A.; Dennis, C. The Microflora of Stored Coleslaw and Factors Affecting the Growth of Spoilage Yeasts in Coleslaw. J. Appl. Bacteriol. 1983, 55, 57–63. DOI: 10.1111/j.1365-2672.1983.tb02647.x.
