ABSTRACT
Food packaging material is the primary element of the food industry; therefore, its consideration to sustain food quality and food safety is crucial. Several food-grade materials have been utilized for packaging food commodities for a long time. Still, these materials negatively influence safety, shelf life, texture, quality, and flavors of the food commodities. Concurrently, biotechnology introduces various techniques to produce several edible food packaging materials i.e. polysaccharides and protein-based films, intelligent and active packaging, which can preserve the food for a long period and inhibit the entry of biotic and abiotic components into the food. Various materials i.e. nisin, chitosan, cactus/mucilage, and bacterial nanocellulose, are being utilized to produce various kinds of edible packaging, including films, coatings, foams, with various kinds of edible active and intelligent packaging characteristics by biotechnological tools. The packaging material prepared by biotechnological applications is widely adopted and utilized in various food processing and preservation industries due to its higher safety levels and more nutritional components for consumption. The edible packaging material is currently utilized only for solid and semisolid processed products. However, there is an urgency to develop edible packaging material for liquid commodities such as products of the dairy and beverages industry by utilizing biotechnological techniques.
Introduction
Non-degradable plastics are listed among the global major issues. At the beginning of the 20th century, the development and utilization of plastic products worldwide have been tremendously enhanced. Previously, traditional and commercial food packaging materials were petroleum-derived plastics i.e. polypropylene (PP), Poly styrene (PS), and polyethylene (PE). [Citation1]]. The non-biodegradable synthetic polymers generated from petrochemicals are the main components of environmental pollution and these polymers are a big threat to wildlife when dispersed in surroundings.[Citation2] According to worldwide statistical data, 43% of marine species, 44% of seabirds, and 86% of sea turtle species are mainly receptive to marine plastic waste.[Citation3] However, scientists are looking for an alternative promising source of non-degradable plastic packaging materials because of their environmental pollution potential. Hence, degradable packaging films are consisting of bio-compatible macro-molecules with great film-framing attributes i.e. polysaccharides, proteins, nisin, and chitosan appear to be encouraging choices for plastic formation.[Citation4]
The edible film is a thin layer that can be applied precisely on the food surface or shaped independently as a slight film/sheet and folded around the food surface later. Edible films produced from edible ingredients can produce a cohesive and continuous network that can be consumed along with the coated foods which results in the reduction of disposal problems. Edible films are also quickly decomposed by the enzymatic activity of yeasts, microorganisms, and parasites, as edible films are biodegradable materials of packaging, they can decrease the prerequisite of landfills significantly.[Citation5] Frequently biopolymers utilized in edible packaging film i.e. chitosan,[Citation6] tragacanth/sodium alginate,[Citation7] soy protein,[Citation8] cellulose, gelatine,[Citation9] cactus-mucilage,[Citation10] tomato-based coating,[Citation11] chitosan and quinoa protein edible coating,[Citation12] banana starch,[Citation13] apple-based,[Citation14] and Aloe Vera.[Citation15]
Edible films are primarily produced by dry and wet processes. The casting process (wet process) usually depends upon solvent (Ethanol and water or their blend) for the diffusion of polymers on the surface and dried under controlled environmental conditions i.e. expulsion, infusion, blow-trim, and heat pressing which brings helps in film development.[Citation16,Citation17] To enhance the adaptability, toughness, and flexibility of these films, plasticizers are merged into the film matrix.[Citation18] Furthermore, some additives along with particular functionality could be supplemented into the film matrix i.e. colors, antimicrobial agents, and flavoring agents, according to the end uses of the edible coating.[Citation19] The dry method also known as the post-extrusion process includes film blowing, thermal compression, and injection molding that is required to produce characteristic film attribute.[Citation20]
The production of large-size films i.e. greater than 25 cm requires extended drying times such as 2–3 days and has low efficiency, as well as unreliable control of thickness, make the film formulation strategies. Furthermore, these large-size films are unacceptable for increasing modern industrial development. To make increased the development of edible packaging material, there should be essential to foster constant development of film with less development time along with high manufacturing rates by using new procedures and techniques.[Citation21] In the recent era, biotechnology manifests significant interest because of its anticipated use in various scientific areas. The biosynthesis of edible films by using biological structure is appraised as an environmentally friendly source that does not generate toxic wastes. Consequently, Bio-based film materials give physical protection as well as develop a significant physicochemical environment for commodities that are requisite for procuring a suitable shelf life.[Citation22] Microbial polymers such as polyhydroxyalkanoates (PHA) or polylactic acid (PLA), exopolysaccharide, Catus mucilage, Cyanobacterialwood-based polymers (starch, cellulose, lignin, and hemicellulose), and protein-based polymers (wheat gluten, gelatin, whey protein isolates, keratin, and soy protein) as well as bacterial nanocellulose are greatly used material for the forming of edible films. Subsequently, the production of an edible film by biotechnological application provides significant advantages over plant-derived and chemical-based development such as quick production, energy efficiency, independently of season and location, and the ability to utilize agricultural and industrial wastes as substrate.[Citation23]
This review describes the details of edible packaging that are produced through biotechnological application and provides a detailed description of the recent emerging trends in the forming of edible packaging, its properties and its application.
IO-Material-based packaging
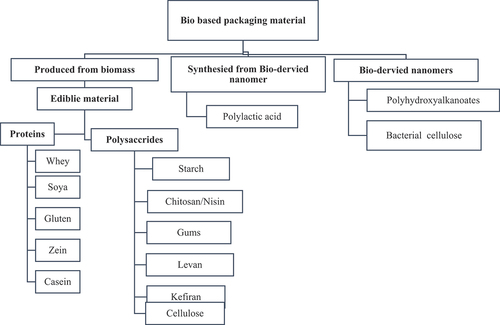
Edible film materials are a sub-group of biodegradable and bio-derived materials. These biodegradable and bio-based materials can be grouped into three major groups according to the source of their products such as:
Materials produce directly from biomass and natural sources such as proteins, lipids, and polysaccharides[Citation24]
Materials generated by microbes, generally essential to a certain form of polysaccharides[Citation25]
Compounds developed from bio-derived monomers[Citation26]
Polysaccharides, lipids, and proteins are significant polymers that are effectively utilized for the development of edible coating as well as films. The edible packaging developed from polysaccharides exhibits satisfactory gas barrier attributes and a weak barrier to water vapors as well as weak mechanical strength.[Citation27] However, protein-based films exhibit poor resistance to water vapors with good mechanical stability. In contrast to polysaccharides-derived films, lipids edible films have significant water vapors resistance properties but are not able of producing a self-sustaining network, that’s why they cannot be employed for the formation of edible films. Hence, lipids are accordingly utilized for layering applications or as a substrate to produce composite edible films of polysaccharides and protein.[Citation28] Different categories of Bio-based food packaging materials were shown in .
Microbial biopolymers application for edible coating and film production
Microbial exopolysaccharides (EPS) are the most compulsive compounds that can be produced by the activity of microbes including algae, fungi, and bacteria.[Citation29] These exopolysaccharides have significant film-formation properties and are also used as bioactive agents, structure enhancers as well as carriers of probiotics in the formation of edible film packaging. Concurrently, the edible film of exopolysaccharides improves the shelf file along with the quality of the commodities. Various Lactic acid bacteria (LAB) species incorporating Leuconostoc spp., Streptococcus spp., Lactococcus spp., and immensely 30 Lactobacillus species are entangled in the development of Exopolysaccharides. The biotechnological production of exopolysaccharides is a faster and more cost-effective technique in contrast to the plant-derived and chemically produced exopolysaccharides of the waste product process.[Citation30]
Microbial biopolymers are significant alternatives to synthetic and natural water-soluble polymers for different food applications. Their performance and functionality are mainly based on various characteristics such as water, carbon dioxide, and oxygen permeability and their color, flavor, opacity, and their resistance toward rupture and stretching,[Citation31] microbial biopolymers conceivably incorporated into edible coating with excellent oxygen and moisture barrier attributes as these films are microbiologically stable and have significant wettability, adhesion, cohesion, transparency, and solubility, along with mechanical attributes.[Citation32] Edible films of agar are an absolute origin for the addition of extracts, micro-nutrients as well as essential oils to produce material of biodegradable films that permit the release of antioxidant and antimicrobial compounds.[Citation33]
Consequently, Mantovan et al.[Citation34] produced an edible film of microbial levan that was synthesized by proving Bacillus subtilis natto (CCT 7712. Edible film levan develops a new biopolymer layer on the cassava starch to enhance its life span. Subsequently, the incorporation of levan in the edible film increased stretching strength, higher solubility, and elongation and decreases the moisture permeability of the film. Edible film of levan is an economical alternative since it empowers the utilization of levan in blends with a minimal cost and good accessibility material like starch. Subsequently, sodium alginate, agar, and chicory inulin along with glycerol have been utilized to produce edible films because of their biodegradability, thermoplastic character, and a moderate level of water resistance as well as biocompatibility. Hence agar has lower mechanical and thermal properties; therefore, to improve these properties, nanotechnology has been utilized to form edible films of biopolymer.[Citation35]
Piermaria et al.[Citation36] produced the edible coating of kefiran that consisted of Kluyveromyces marxianus (CIDCA 8154) and Lactobacillus plantarum (CIDCA 8327). When these microorganisms were incorporated in the glycerol plasticized, they maintained their viability in the film during storage. And the optical properties, moisture content, and compactness of edible film were not influenced by the addition of microorganisms. Subsequently, kefiran edible films are a significant source of probiotics. Likewise, sodium alginate can produce packaging material with significant prospective of strength, tasteless and odorless, water-solubility, and weak permeability to oils as well as for O2. Subsequently, a composite mixture of sodium alginate and glycerol has been utilized for coating cherries to hinder the deteriorating mechanism, and to maintain color, anthocyanins, and polyphenols after harvest. In combination with other hydrocolloids, sodium alginate, and glycerol form coatings films that have been utilized in various sections of the food industry[Citation37] Concurrently, Anis et al.[Citation38] proved that the supplementation of essential oils within matrices of the polysaccharide enhanced the mechanical and functional properties of the edible coating and films.
Edible films of cassava starch (CS) were produced along with Lactobacillus plantarum and Pediococcus pentosaceus as well as sodium carboxymethyl cellulose by Li et al.[Citation39] After the supplementation of these probiotics and the antioxidant activity of the conglomerate coating layer was greatly increased and effectively reduced the penetration of water molecules or exhibited ultraviolet protection. Subsequently, dextran is an exopolysaccharide that was produced by the utilization of lactic acid bacteria including Leuconostoc, Streptococcus, and Lactobacillus. Davidović et al.[Citation40] formed edible films of dextran in addition to sorbitol. Both sorbitol and the application of dextran had a significant effect on extensible strength as well as elongation at break, although only sorbitol aggregation had a significant influence on Young’s modulus which showed that a film with 20.43 wt.% of sorbitol, and 3.40 wt% of dextran exhibited the lowest permeability for water vapor while showed the highest elasticity and tensile strength.
Inulin is a β fructan that is present in more than 3000 species of plant. It is soluble dietary fiber that is found in a wide variety of foods, such as asparagus, bananas, chicory, wheat, garlic, and onions. Inulin has a variety of health benefits, including aiding digestion, promoting gut health and boosting immunity. Inulin-based films not only showed good physical properties such as homogeneity, lightly sweet taste, high solubility capacity, well-defined margins, good optical properties, and complete solubilization but also exhibit significant mechanical properties incorporating good tensile strength and tremendously high expansion standards.[Citation41] Puscaselu et al.[Citation42] developed an edible film of inulin. Edible film of inulin can be utilized in food that requires solubilization and product that is sold in powder form such as coffee, powdered milk, spices, dehydrated fruits, teas as well as vegetables.
The immensely porous and light structure of EPS is not appropriate to produce edible films and requires being plasticized.[Citation43] Therefore, the smoothness, structural integrity as well as glittering of the surface can be controlled by LAB. LAB facilitating the supplication of EPS in the preparation of edible films and coating.[Citation44] Concurrently, Alizadeh-Sani et al.[Citation32] explained that microbial gums, because of their microbiological stability, cohesion, solubility, adhesion, transparency, wet ability, as well as mechanical characteristics could be exploited as edible coating and films. Moreover, these gums, like curdlan, bacterial cellulose xanthan, gellan, and pullulan are conceivably used in coalescence with bioactive components that increase the storage life of perishable food commodities. Vijayendra and Shamala[Citation45] prove that blow-modeled biopolymers such as polyhydroxyalkanoates (PHA) in combination with PLA (polylactic acid) can be chemically synthesized in vitro by practicing lactic acid. Lactic acid is generally synthesized by lactic acid bacteria by fermentation. PHA exhibits good moisture and oxygen barrier properties when incorporated into films. The addition of antimicrobials like bacteriocins and nanoparticles such as silver or copper can further increase the potency of polymer edible films, particularly in form of wrapping and coating of food
Streptococcus zooepidemicus produce Hyaluronic acid that is highly hydrophilic anionic heteropolysaccharide, and linear exhibits significant edible film-forming properties. Sgorla et al.[Citation46] produced Hyaluronic acid film by casting process by mixing bare and cross-linked biopolymer in the aqueous diffusion of ethylcellulose, at differentiable proportions and assessed biocompatibility along with the safety of films against HT29-MTX intestinal cells and Caco-2 (clone C2BBe1). In simulated gastric fluid, films of Hyaluronic acid showed lower hydration capability, and films lost weight partially, revealing the capability to prevent the release of drugs at gastric and higher intestinal pH. These results reflected that edible films of hyaluronic acid can be used as a coating film for oral compact dosage forms, and it can reduce the immature liberation of drugs in an acidic condition of the stomach.
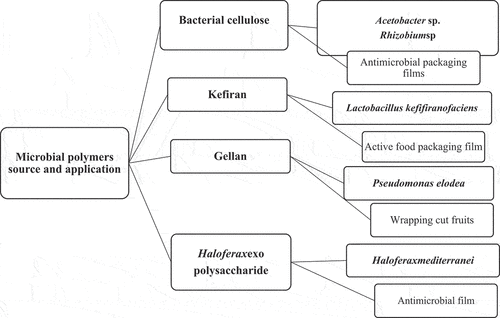
Several microorganisms produce biopolymers, incorporating polyhydroxyalkanoates, exo-polysaccharides, and endo-polysaccharides which are acidic and neutral in nature as well as exhibit a broad range of physical properties of films. Subsequently, these microbial polysaccharides are developing predominately carbohydrate moieties (glucose, rhamnose, mannose), and non-carbohydrate moieties (succinate, acetate, pyruvate, phosphate).[Citation47] The mechanism regarding utilization of microbial polymers in development of packaging films was shown in .
Protein-derived edible films
Edible Films which are derived from polysaccharides[Citation48] and proteins[Citation49] are being widely utilized for the packaging of food because of their renewable, biodegradable, and edible properties.[Citation50] In terms of potential origin, zein, gluten, and soy protein are significant plant protein sources for the development of edible films. Gelatin, keratin, collagen protein and casein are principal animal proteins. Fumarase, lactate dehydrogenase and Chymotrypsin are known as potential bacterial proteins source.[Citation51]
Egg yolk is one of the potential protein sources that are conceivably utilized to synthesize novel edible films. Sáez-Orviz et al.[Citation52] synthesized three different types of bioactive films by using lipid-free egg yolk, Lactobacillus plantarum (CECT 9567) (probiotic), and lactobionic acid (prebiotic). Results revealed that the addition of lactobionic acid enhanced the probiotic viability while showing a lower water solubility ratio. Therefore, results indicated that these types of edible coating can opt for food packaging. Likewise, Zubeldía et al.[Citation53] explained that bioplastic-derived films can be developed by plasticizing wheat gluten through explosive extrusion techniques including compression and molding process. Moreover, previously it was found that the pattern of plasticizer and its concentration had a captivating effect on water vapor permeability along with mechanical features. In addition, the effect of the expended plasticizer like glycerol is strongly based on the natural latency of water in commercial gluten that is utilized for the formation of the film.
Sharma et al.[Citation54] prepared an edible film with coalescence of hydrogen, disulfide, and hydrophobic interactions. Disulfide bonds were stabilized by the presence of sulfhydryl groups. Resulted films showed good gas barrier properties, mechanical strength, and homogeneity. While Chiou et al.[Citation55] concluded that the heating process results in the denaturation of polymer and the wreckage of native disulfide and hydrophobic groups. During the drying process, new disulfide bonds are usually generated through the process of oxidation of gluten protein. Moreover, the clarity of the film primarily depends upon the gluten purity level and on the type of casting medium such as whether it is acidic or alkaline. Besides gluten protein, whey protein also has potential film-forming functional properties. Whey protein-derived films have been widely studied because of their good transparency, flexibility, and oil/gas/barrier functionality at low humidity levels by Marashi et al.[Citation56] Hence, whey protein-derived films usually showed poor water barrier attributes. But the supplementation of essential lipids/oil has resulted to enhance water barrier attributes of whey-derived films.[Citation57]
Çakmak et al.[Citation58] developed whey protein-derived edible films with protein glycerol plasticizer. They reported that lemon and bergamot essential oils act as functional ingredients of bioplastic films. Findings reflected that whey protein-based films had good antimicrobial potential properties as a consequence of the addition of essential oils in films. Moreover, compelling oxygen/water vapor permeability was reported. Hence, the evaluation of the interaction between different biopolymers which as usually incorporated during the formation of coatings is mandatory to synthesize novel packaging films with the most desired properties and functionality. Likewise, potatoes, carrots, and freshly cut apples were wrapped in a blended pectin film/whey protein, which was prepared by the addition of transglutaminase that decreases the loss of fruit weight during storage conditions, preserves their total phenolic and carotenoids content, and also prevented the growth of microorganism.[Citation59] Otoni et al.[Citation60] prepared soy protein-derived bioplastic films for food packaging requisition. They concluded that soy proteins-derived bioplastic films were cost-effective, transparent, flexible and smoother as compared to other protein-derived bioplastic films. In addition, soy protein-derived films exhibit also showed oxygen barrier attributes under a low moisture environment.
In contrast to the good oxygen barrier properties, soy-protein-derived films have weak mechanical strength, allergenicity, and low heat stability than LDP films. Moreover, isolates of soy protein don’t have film formation properties on SST plates under high-temperature conditions. The formation of film by using various sources of protein including pumpkin cake oil, canola protein, pea protein and pistachio globulin-type protein also has been studied by different scientists.[Citation61–63]
Active food packaging of Nisin and Chitosan composites
The unique properties of chitosan-based biofilms have highlighted the use of these films in different foods.[Citation64] Chitosan is a linear polysaccharide of (1,4)-linked 2-aminodeoxy-β-d-glucan.[Citation65] The waste of several processing industries including shrimps, crabs, lobsters, and krill provides the raw material for chitosan-based biofilms.[Citation66] The excellent biocompatible, film-forming, nontoxic, antimicrobial and biodegradable properties make chitosan popular.[Citation67] The antimicrobial properties were investigated of fungal chitosan that was extracted from Aspergillus brasiliensis (niger)[Citation68]
Koc et al.[Citation69] used the fungal specie Tricholomaterreum to obtain fungal chitosan for the formation of chitosan-derived films. The antimicrobial, hydrophobicity, elasticity, and antioxidant properties of chitosan-based biofilms increased by the addition of fungal chitosan. Gentamicin, a commonly used antibiotic was compared with fungal chitosan and observed that the antimicrobial and anti-quorum sensing properties of gentamicin were lower. Likewise, the utilization of fungal chitosan from pomegranate peel extract and Aspergillusniger in the coating of food for Oreochromis niloticus (Nile tilapia) was investigated by Alsaggaf et al.[Citation70] During storage, the overall microbial count was reduced by fillet coating.
A thermoplastic polyester named poly L-lactic acid (PLLA) was developed from renewable materials.[Citation71] PLLA is a nontoxic, biodegradable, biocompatible polyester[Citation72] same as chitosan. Hence, in active food packaging chitosan and poly (L- lactic acid is considered antimicrobial entity carrier. Subsequently, several active components are carried in chitosan films as natural biopolymers. These compounds may be polyphenols that can dispense their sustained liberation to food commodities during storage and also may protect them from spoilage and oxidation. An extract from chokeberry pomace extract has better antioxidant properties when added to chitosan. The UV – Visible light barrier and also water vapors barrier properties of CS films can be increased by chokeberry extract and can also reduce oxygen permeability.[Citation73]
Bonilla and Sobral[Citation74] manufactured boldo extract containing edible gelatin-chitosan blended films. These films were coated on sliced cheese Prato to investigate the properties changes during storage up to 10 days at 4°C. Their pH and fat content remained unchanged in these films. The findings were correlated with the control sample and the results showed significant protection against oxidation. The growth of psychrotrophic microorganisms was also inhibited and substantially low growth of coliforms was intimated in sliced Prato cheese samples.
Likewise, Razavi et al.[Citation75] developed double-layer edible films of cellulose paper-chitosan by the incorporation of epsilon-poly-l-lysine (ε-PL). The chicken meat was studied for antimicrobial activity against Listeria monocytogenes. There was no antimicrobial activity in cellulose paper-chitosan films without epsilon-poly-l-lysine. However, the introduction of epsilon-poly-l-lysine induced significant effects. During the storage period of 12 days of meat at 4°C, it was shown that cellulose-chitosan films containing 1% epsilon-poly-l-lysine showed 1.5 log10 CFU/g reductions in the L. monocytogenes population. However, the brittleness and moisture barrier nature of pure chitosan films have limited their application as food fresh-keeping films.[Citation76]). The blending of chitosan with other functional polymers is the only solution to overcome this problem, particularly at the level of microscopic to develop CS-derived composite films like CS/starch,[Citation77] CS/cellulose,[Citation78] and CS/poly vinyl alcohol (PVA)[Citation79] were studied.
A Nisin (antimicrobial peptide) synthesized by strains of Lactococcuslactis subsp. Lactis has a huge spectrum of antimicrobial properties against spoilage and gram-positive bacteria. The antimicrobial efficacy could be enhanced by the addition of nisin into alginate/chitosan, chitosan/carrageenan to develop a composite mixture of chitosan known as CS-40 and while nisin marked as CS-40/nisin,[Citation80] chitosan plus nisin/carageenannanocapsules[Citation81] as well as a combination of nisin with chitosan/alginate edible film.[Citation82]
Lan et al.[Citation83] embedded the Lactococcuslactis in a coating layer of corn starch and carboxymethyl cellulose by adopting the casting method. After storage of 8 days, good results were shown by the film with 1.5% L. lactis reflecting the increased liberation of nisin (3.35 mgmL−1). The antibacterial activity against Staphylococcus aureus about 53.53% was also shown by this film. Hence, foods having low moisture content can use these films as a viable substitute for the antimicrobial activity for active food packaging. Consequently, chitosan film blended with natamycin lowered the consumption of O2 in fruit like strawberries. It also delayed different changes in TSS, microbial counts, pH, and water activity.[Citation84] Another edible film was prepared which depended on konjac glucomannan by the addition of nisin and chitosan as potential bioactive packaging. To improve antimicrobial efficacy valuable work was done.[Citation83] The assessment of the antimicrobial exertion of chitosan films embracing potassium sorbate, nisin, and garlic oil was done. Nisin blended chitosan films were prepared to study the antimicrobial activity against different food-pathogenic bacteria such as S. aureus, E. coli, L. monocytogenes, B. cereus, and S. typhimurium.[Citation85]
Simultaneously, the chitosan blended films with natamycin and nisin controlled the growth of both microorganisms (Saccharomyces cerevisiae and Listeria innocua) which were present on the cheese surface.[Citation86] Divsalar et al.[Citation87] use nisin, chitosan, and cellulose to develop a new antimicrobial bilayer film. After a storage period at 4°C for 14 days, they resolved that the initial (∼5 log10 CFU/g) counts of L. monocytogenes were inactivated by the films with 1000 μg/mL of nisin on the surface of Ultra-filter white cheese. Hence, the nano-composite film of chitosan-cellulose supplemented with nisin has unique antibacterial activity and it has vast applications for the packaging of cheese. Nisin and Chitosan composites film formation and its properties has been provided in
Table 1. Nisin and Chitosan composites film formation and its properties.
Bacterial nanocellulose for the formation of intelligent and active packaging
A bottom-up method was used to produce microbial cellulose (MC), bacterial cellulose (BC), and bacterial nanocellulose (BNC).[Citation8] Bacterial nanocellulose (BNC) is made up of β-1, 4-glycosidic linked glucose units and it is an unbranched polymer with nanofibrils. [Citation92] Bacterial cellulose is produced through oxidative fermentation by acetic acid bacteria in both non-synthetic and synthetic mediums. A most studied bacterium; Acetobacterxylinum is the most efficient bacterial chitosan producer. It can yield a high level of cellulose and manage to assimilate various sugars in a liquid medium.[Citation93] Correspondingly, in a nutritional culture medium bacterial nanocellulose was synthesized by acetic acid bacteria by oxidative fermentation by Fang et al.[Citation94] Some other bacteria can produce bacterial chitosan such as Rhizobium, Agrobacterium, Pseudomonas, and Sarcinaas as reported by Campano et al.[Citation95] and Komagataeibacterxylinus.[Citation96] The high degree of crystallinity, excellent mechanical strength, high water absorbency, large surface area, and permeability are some outstanding chemical and physical properties of bacterial nanocomposites that makes BNC a promising material for the formation of an improved food packaging spectrum. The higher reinforcing effects of bacterial nanocomposites such as the extent of polymerization and greater tensile strength make them suitable for supplication in packaging material.[Citation97]
Similarly, the nano-composite films were developed by Vilela et al.[Citation98] by one-pot polymerization of sulfobetaine methacrylate in the latency of ethylene glycol which is used as a cross-linking agent. PSBMA and bacterial nanocomposites have UV-barrier properties, antimicrobial activity toward pathogenic microorganisms, and moisture scavenging ability. It can also use for intelligent food packaging for monitoring food humidity levels due to the proton motion. Antimicrobial activities showed that there was a 7 and 3-log CFU/mL reduction in Escherichia coli O157:H7 count in raw beef and liquid form. It suggests that at least 10 days of storage at 4°C, these films were able to prevent the growth of naturally present bacteria. Similarly, Sharma et al.[Citation99] study provide an industrially symbolic approach to the production of eco-friendly and cost-effective bacterial nanocellulose (BNC) films. The static intermittent fed-batch technology was used by using a cost-effective medium i.e., fermented black tea. A simple immersion technique was used for the modification of BNC films with a biopolymer – chitosan.
However, the drawbacks of these biofilms may be a lack of antimicrobial and antioxidant activity and poor mechanical attributes. These limitations can be effectively reduced by the addition of different bioactive compounds. Recently, there are so many studies that show that the incorporation of bacterial nanocomposites could enhance the features of EFP films. These characteristics include competence, water sensitivity, optical, barrier, antioxidant and mechanical properties, and antimicrobial attributes.[Citation100] Ludwicka et al.[Citation101] proved that the supplementation of different agents of bacterial nanocomposite matrices allows obtaining such as antimicrobial membranes and pH, antioxidant-releasing films, moisture absorbers, freshness and humidity, and other biosensors. Moreover, bacterial nanocomposite when mixed with other materials such as silver nanoparticles can also act as a reducing agent in the formation of nanoparticles and different stabilizers.[Citation102]
However, the limitation of bacterial nanocomposite is its lack of flexibility. The reason for the limitation of bacterial nanocomposites is their non-stretchability. Thus, to obtain better mechanical properties, adaption of BNC with a polymer matrix is required. To overcome this limitation, Wang et al.[Citation92] developed an antimicrobial biofilm that used silver nanoparticles and BNC and the film was environmentally friendly. The methods which were used for the modification were reduction and UV-assisted methods. Hence, these exhibited significant oxygen barrier capacity; however, vapor permeability was slightly improved. Bacterial nanocellulose for the development of edible films and their properties are shown in .
Table 2. Bacterial nanocellulose for the development of edible films.
Cactus/Mucilage for film development
Earlier, natural biopolymers have been greatly used for the formation of biodegradable films. Among these natural biopolymers, cactus mucilage is attaining more interest among all plant organs that are used for the derivation of bioactive compounds. Cactus mucilage is known as a functional biopolymer carbohydrate and is characterized as a hydrophilic compound. Cactus mucilage is usually sticky due to the sticky plant extract and plant microorganisms.[Citation107] Zeng and Lai[Citation108]utilized proteinase enzyme for the extraction of protein components from mucilage compounds. These compounds are leading to improve solubility and viscosity of this compound. While Chiang and Lai[Citation109] extracted mucilage yield by glucanase from A. australasicum and xylanase. Mucilage is an exceedingly branched heteropolysaccharide that has been investigated earlier because of its significant biotechnological applications, structural properties, and chemical composition, along with its requisition in the different packaging industries by developing edible films.[Citation110,Citation111]
During the last decades, mucilage from chia, flax, quince, okra fruits, cactus, and Balangu, Dracocephalummoldavica seeds have been employed to prepare edible films and coating with unique attributes[Citation112,Citation113] Gheribi et al.[Citation112] developed a plasticizer sorbitol-based edible film. These films showed good water vapor barrier characteristics, while, polyethylene glycol plasticized edible films exhibit a high glass transition temperature of about 49 ◦C while having thermal stability at 171 ◦C. Moreover, novel edible films were formed by Gheribi et al.[Citation10] by preparing a composite mixture of cactus mucilage (CM) with polyvinyl alcohol (PVA). PVA/CM composite film exhibits good UV and visible light barrier attributes with improved thermal variability. The cactus mucilage films usually have good gas barrier characteristics with good flexibility and heat resistance. Hence, their disadvantage includes weak mechanical resistance and good propinquity to water.[Citation114] The functional features of cactus mucilage edible films are usually based on the composition of polysaccharides and their structure.[Citation115] For these reasons, previously various studies were undertaken to develop composite film materials with cactus mucilage and other biodegradable polymers. Cactus mucilage coating was used to coat guava fruits[Citation116] strawberries,[Citation117] tomatoes,[Citation118] minimally processed yam,[Citation119] Mango,[Citation120] and strawberry,[Citation121] etc.
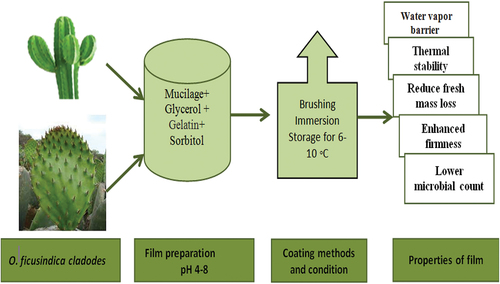
Pereskia aculeata leaf mucilage comprised of an arabinogalactan biopolymer. The arabinogalactan-based films have smooth surfaced, thermally stability, biodegradable, and flexible.[Citation122] Dick et al.[Citation123] developed chia-seed mucilage-based edible films. These films showed good thermally stable and low solubility and good UV light barrier properties. Likewise, previously developed balangu seed mucilage films showed high barrier and mechanical attributes.[Citation124] Despite their gas barrier properties, the weak stretching strength along with poor vapor barrier attributes of mucilage-derived films decrease the ratio of their application as independent wrapping films.[Citation112] To reduce these drawbacks, different considerations such as the addition of additives including cross-linkers and nanoparticles, and the use of a composite blend of bio-derived polymers can be embraced.[Citation125] Utilization of Cactus/mucilage for edible film development presented in .
Consumer acceptance and safety acceptance
Consumer perception of any edible product is the most important factor that decides the success or failure of any industrialist’s venture. A consumer only invests in edible foils if he/she is convinced that these are not toxic or unhygienic and are healthy to eat.[Citation126] Creating the right awareness about edible foils and their benefits convince consumers that their use is not only safe but also provides several benefits, and conversely, a lack of awareness can lead to less acceptance.[Citation127]). Functional properties along with factors including film appearance, cost, marketing strategies, and organoleptic properties, decide the degree of consumer acceptability. The nutritional value and sensory properties of food packaged in edible foils should not suffer because of the edible foil.[Citation128]
The permissibility of edible films of animal origin is influenced by many religious reasons. Vegetarians do not eat animal-based edible films, which reduces their acceptance. The threat of allergies can contribute to lower acceptance rates. Correct labeling of all ingredients especially allergens is considered mandatory by regulatory authorities.[Citation129] The motive is to avert any allergic reaction that may occur if people with certain allergies consume edible foils containing allergens.EU Regulations (EC No. 1935/2004) provided four fundamental requisites for the development of edible materials having direct contact with food (i) do not alter the food composition intolerably (ii) do not alter the texture, smell or taste of commodity (iii) do not imperil the human health (iv) good manufacturing practice (GMPs) should be considered during its development. The toxicological impact of nanomaterials in food packaging is not formulated comprehensively.[Citation130,Citation131]
Price plays an important factor in edible films acceptability by consumers. Edible films are 10–15 times more expensive in comparison to plastic films derived from petroleum. The edible film production at small scales is because of the development period that gives leverage and the price is not considered a negative point. The adaptability of edible films can be justified by proper cost-benefit analysis. However, guidelines are given for the relationship between the cost of a black-and-white product and packaging. The total packaging material cost must be below 10% of the the original price of a commodity. The same or lower price of edible films compared to petroleum-derived plastic films will attract consumers more toward edible films.[Citation132]
Limitation and recommendation
The satisfaction of practical applications is not properly ensured by the performance of edible films containing pure matrix. For example, the properties of chitosan-based film and coating i.e., mechanical properties, antioxidant aspects and barrier properties, are highly unsatisfactory. To provide functional properties and improve existing properties, film scientists are working hard to find a suitable additive.[Citation73] The production of an edible film is still in the spectrum of laboratory, despite all the advantages, due to many problems such as safety and health problems, high cost, lack of poor elongation, and processing obstacles. A great increase in the properties of films was observed because of the inclusion of plasticizers, and the production of multilayer materials and composites. However, seminal research on key factors is still unexplored[Citation10] Problems (long drying time, imprecise thickness control, impossibility to form continuous films) occurring at the laboratory scale must be solved before starting production at the industrial stage.[Citation21]
The brittleness and inferior film properties of unplasticized edible film differentiate them from petroleum-derived plastics, but the gas barrier properties and thermal weld ability are comparable to plastics. Further research is needed to achieve superior film properties.[Citation125] Improved functional properties due to the application of nanotechnology are evident, however, research on nanomaterials and their lethal effects need more attention.[Citation51] Several elements play a significant role in consumer acceptance and food safety, e.g. absence of evidence for edibility, toxicological, health issues, biodegradability, poor marketing strategies, cultural consideration, etc. for the successful production of edible films on a larger industrial scale and to ensure the viability of the industry. Further research must cover all the above barriers enabling the processors to manufacture multifunctional edible films and increase their compatibility with modern packaging technologies. The need of the hour is extensive research that will focus on all the ignored factors and features of the film.
Conclusion
Biotechnology is prevailing drastically in the production of food packaging edible material. The thin layers of films used in developed countries to preserve fresh fruits and vegetables are prepared by biotechnological tools. However, these films are not commercialized globally because of their higher rates of production cost i.e. 10–50 times greater as compared to petroleum-plastic films. Moreover, research about toxicological, health impact, biodegradability, lack of awareness, and cultural consideration of these films and coating is further required which has a significant influence on food quality, food safety, and customer acceptance. The development of cheaper techniques and sources of edible packaging is the need of time. Researchers and investigators need to find out the various cost-effective edible raw materials (i.e. whey and buttermilk waste of the dairy industry) for the formation of edible packaging material.
Acknowledgment
The principal author would like to thank all of the co-authors for their contribution to writing the original draft of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kunwar, B.; Cheng, H. N.; Chandrashekaran, S. R.; Sharma, B. K. Plastics to Fuel: A Review. Renew. Sust. Energ. Rev. 2016, 54, 421–428. DOI: 10.1016/j.rser.2015.10.015.
- Dwivedi, P.; Mishra, P. K.; Mondal, M. K.; Srivastava, N. Non-Biodegradable Polymeric Waste Pyrolysis for Energy Recovery. Heliyon. 2019, 5(8), e02198. DOI: 10.1016/j.heliyon.2019.e02198.
- Zhang, W.; Jiang, W. Antioxidant and Antibacterial Chitosan Film with Tea Polyphenols-Mediated Green Synthesis Silver Nanoparticle via a Novel One-Pot Method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. DOI: 10.1016/j.ijbiomac.2019.11.093.
- Zhang, W.; Li, X.; Jiang, W. Development of Antioxidant Chitosan Film with Banana Peels Extract and Its Application as Coating in Maintaining the Storage Quality of Apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. DOI: 10.1016/j.ijbiomac.2019.10.275.
- Hassan, B.; Chatha, S. A. S.; Hussain, A. I.; Zia, K. M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. DOI: 10.1016/j.ijbiomac.2017.11.097.
- Homez-Jara, A.; Daza, L. D.; Aguirre, D. M.; Muñoz, J. A.; Solanilla, J. F.; Váquiro, H. A. Characterization of Chitosan Edible Films Obtained with Various Polymer Concentrations and Drying Temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. DOI: 10.1016/j.ijbiomac.2018.03.057.
- Bahrami, A.; Mokarram, R. R.; Khiabani, M. S.; Ghanbarzadeh, B.; Salehi, R. Physico-Mechanical and Antimicrobial Properties of Tragacanth/Hydroxypropyl Methylcellulose/Beeswax Edible Films Reinforced with Silver Anoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. DOI: 10.1016/j.ijbiomac.2018.09.045.
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, Properties and Applications of Soy-Protein-Based Materials: A Review. Int. J. Biol. Macromol. 2018, 120, 475–490. DOI: 10.1016/j.ijbiomac.2018.08.110.
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H. W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life. 2020, 26, 100551–100571. DOI: 10.1016/j.fpsl.2020.100551.
- Gheribi, R.; Gharbi, M. A.; El Ouni, M.; Khwaldia, K. Enhancement of the Physical, Mechanical and Thermal Properties of Cactus Mucilage Films by Blending with Polyvinyl Alcohol. Food Packag. Shelf Life. 2019, 22, 100386. DOI: 10.1016/j.fpsl.2019.100386.
- Du, W. X.; Olsen, C. W.; Avena‐bustillos, R. J.; McHugh, T. H.; Levin, C. E.; Mandrell, R.; Friedman, M. Antibacterial Effects of Allspice, Garlic, and Oregano Essential Oils in Tomato Films Determined by Overlay and Vapor‐phase Methods. J. Food Sci. 2009, 74(7), 390–397. DOI: 10.1111/j.1750-3841.2009.01289.x.
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymolnanoemulsions Incorporated in Quinoa Protein/Chitosan Edible Films; Antifungal Effect in Cherry Tomatoes. Food Chem. 2018, 246, 211–219. DOI: 10.1016/j.foodchem.2017.11.032.
- García-Ramón, J. A.; Carmona-García, R.; Valera-Zaragoza, M.; Aparicio-Saguilán, A.; Bello-Pérez, L. A.; Aguirre-Cruz, A.; Alvarez-Ramirez, J. Morphological, Barrier, and Mechanical Properties of Banana Starch Films Reinforced with Cellulose Nanoparticles from Plantain Rachis. Int. J. Biol. Macromol. 2021, 187, 35–42. DOI: 10.1016/j.ijbiomac.2021.07.112.
- Kadzińska, J.; Bryś, J.; Ostrowska-Ligęza, E.; Estéve, M.; Janowicz, M. Influence of Vegetable Oils Addition on the Selected Physical Properties of Apple–Sodium Alginate Edible Films. Polym. Bullet. 2020, 77(2), 883–900. DOI: 10.1007/s00289-019-02777-0.
- Maan, A. A.; Ahmed, Z. F. R.; Khan, M. K. I.; Riaz, A.; Nazir, A. Aloe Vera Gel, an Excellent Base Material for Edible Films and Coatings. Trends Food Sci. Technol. 2021, 116, 329–341. DOI: 10.1016/j.tifs.2021.07.035.
- Suhag, R.; Kumar, N.; Petkoska, A. T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food. Res. Int. 2020, 136, 109582. DOI: 10.1016/j.foodres.2020.109582.
- Aguirre-Joya, J. A.; De Leon-Zapata, M. A.; Alvarez-Perez, O. B.; Torres-León, C.; Nieto-Oropeza, D. E.; Ventura-Sobrevilla, J. M.; Aguilar, C. N. Basic and Applied Concepts of Edible Packaging for Foods. In Food Packaging and Preservation. Academic Press. 2018, 1–6. DOI: 10.1016/B978-0-12-811516-9.00001-4.
- Murrieta-Martínez, C.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Ramírez-Wong, B.; AndMárquez-Ríos, E.; Santos-Sauceda, I.; Olibarría-Rodríguez, G.; Márquez-Ríos, E. Effect of Different Polyalcohols as Plasticizers on the Functional Properties of Squid Protein Film (DosidicusGigas). Coatings. 2019, 9(2), 77–89. DOI: 10.3390/coatings9020077.
- Sharma, P.; Shehin, V. P.; Kaur, N.; Vyas, P. Application of Edible Coatings on Fresh and Minimally Processed Vegetables: A Review. Int. J. Veg. Sci. 2019, 25(3), 295–314. DOI: 10.1080/19315260.2018.1510863.
- Jeevahan, J. J.; Chandrasekaran, M.; Venkatesan, S. P.; Sriram, V.; Joseph, G. B.; Mageshwaran, G.; Durairaj, R. B. Scaling Up Difficulties and Commercial Aspects of Edible Films for Food Packaging: A Review. Trends Food Sci. Technol. 2020, 100, 210–222. DOI: 10.1016/j.tifs.2020.04.014.
- Zhang, S.; Gu, W. C.; Cheng, Z. Y.; Li, Y.; Gu, W. J. Development of Edible Packaging Materials.In Advanced Materials Research. Trans. Tech. Publications Ltd. 2014, 904, 189–191. DOI: https://doi.org/10.4028/www.scientific.net/AMR.904.189 .
- Martău, G. A.; Mihai, M.; Vodnar, D. C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers. 2019, 11(11), 1837–1865. DOI: 10.3390/polym11111837.
- Barcelos, M. C.; Vespermann, K. A.; Pelissari, F. M.; Molina, G. Current Status of Biotechnological Production and Applications of Microbial Exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60(9), 1475–1495. DOI: 10.1080/10408398.2019.1575791.
- Saklani, P.; Das, S. K.; Singh, S. M.; Singh, S. M. A Review of Edible Packaging for Foods. Int. J. Curr. Microbiol.App. Sci. 2019, 8(7), 2885-5. DOI: 10.20546/ijcmas.2019.807.359.
- Regubalan, B.; Pandit, P.; Maiti, S.; Nadathur, G. T.; Mallick, A. Potential bio-based edible films, foams, and hydrogels for food packaging. In Bio-based Materials for Food Packaging. Springer, 2018, 105–123
- Ramos, Ó. L.; Pereira, R. N.; Cerqueira, M. A.; Martins, J. R.; Teixeira, J. A.; Malcata, F. X.; Vicente, A. A. Bio-Based Nanocomposites for Food Packaging and Their Effect in Food Quality and Safety. In Food Packaging and Preservation; Academic Press: 2018; pp. 271–306. doi:10.1016/B978-0-12-811516-9.00008-7
- Jeevahan, J.; Chandrasekaran, M.; Durairaj, R.; Mageshwaran, G.; Joseph, G. B. A Brief Review on Edible Food Packing Materials. J. Global Eng. Prob. Solut. 2017, 1(1), 9–19.
- Jeevahan, J.; Chandrasekaran, M. Nanoedible Films for Food Packaging: A Review. J. Mater. Sci. 2019, 54(19), 12290–12318. DOI: 10.1007/s10853-019-03742-y.
- Freitas, F.; Torres, C. A.; Reis, M. A. Engineering Aspects of Microbial Exopolysaccharide Production. Bioresources Technol. 2017, 245, 1674–1683. DOI: 10.1016/j.biortech.2017.05.092.
- Saadat, Y. R.; Khosroushahi, A. Y.; Gargari, B. P. A Comprehensive Review of Anticancer, Immunomodulatory and Health Beneficial Effects of the Lactic Acid Bacteria Exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. DOI: 10.1016/j.carbpol.2019.04.025.
- Zikmanis, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Segliņa, D.; Krasnova, I.; Kolesovs, S.; Semjonovs, P.; Šilaks, A.; Semjonovs, P. Microbial Polymers in Edible Films and Coatings of Garden Berry and Grape: Current and Prospective Use. Food Bioprocess. Technol. 2021, 14(8), 1432–1445. DOI: 10.1007/s11947-021-02666-3.
- Alizadeh-Sani, M.; Ehsani, A.; Moghaddas Kia, E.; Khezerlou, A. Microbial Gums: Introducing a Novel Functional Component of Edible Coatings and Packaging. Appl. Microbiol. Biotechnol. 2019, 103(17), 6853–6866. DOI: 10.1007/s00253-019-09966-x.
- Basumatary, K.; Daimary, P.; Das, S. K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia Speciosa Fruit-Mediated Synthesis of Silver Nanoparticles and Its Application as Filler in Agar Based Nanocomposite Films for Antimicrobial Food Packaging Food Packag. Shelf Life. 2018, 17, 99–106. DOI: 10.1016/j.fpsl.2018.06.003.
- Mantovan, J.; Bersaneti, G. T.; Faria-Tischer, P. C.; Celligoi, M. A. P. C.; Mali, S. Use of Microbial Levan in Edible Films Based on Cassava Starch. Food Packag. Shelf Life. 2018, 18, 31–36. DOI: 10.1016/j.fpsl.2018.08.003.
- Sagnelli, D.; Kirkensgaard, J. J.; Giosafatto, C. V. L.; Ogrodowicz, N.; Kruczała, K.; Mikkelsen, M. S.; Blennow, A.; Lourdin, D.; Mortensen, K.; Blennow, A. All-Natural Bio-Plastics Using Starch-Betaglucan Composites. Carbohydr. Polym. 2017, 172, 237–245. DOI: 10.1016/j.carbpol.2017.05.043.
- Piermaria, J.; Diosma, G.; Aquino, C.; Garrote, G.; Abraham, A. Edible Kefiran Films as Vehicle for Probiotic Microorganisms. IFSET. 2015, 32, 193–199. DOI: 10.1016/j.ifset.2015.09.009.
- Chiabrando, V.: Giacalone, G. Effects of Alginate Edible Coating on Quality and Antioxidant Properties in Sweet Cherry During Postharvest Storage. Ital J. Food Saf. 2015, 27(2), 173–180. DOI: 10.14674/1120-1770/ijfs.v184.
- Anis, A.; Pal, K.; Al-Zahrani, S. M. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers. 2021, 13(4), 575–607. DOI: 10.3390/polym13040575.
- Li, S.; Ma, Y.; Ji, T.; Sameen, D. E.; Ahmed, S.; Qin, W.; Liu, Y.; Li, S.; Liu, Y. Cassava Starch/Carboxymethylcellulose Edible Films Embedded with Lactic Acid Bacteria to Extend the Shelf Life of Banana. Carbohydr. Polym. 2020, 248, 116805–116817. DOI: 10.1016/j.carbpol.2020.116805.
- Davidović, S.; Miljković, M.; Tomić, M.; Gordić, M.; Nešić, A.; Dimitrijević, S. Response Surface Methodology for Optimisation of Edible Coatings Based on Dextran from Leuconostoc mesenteroides T3. Carbhydr. Polym. 2018, 184, 207–213. DOI: 10.1016/j.carbpol.2017.12.061.
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of Edible Films and Coatings from Alginates and Carrageenans. Carbohydr.Polym. 2016, 137, 360–374. DOI: 10.1016/j.carbpol.2015.10.074.
- Puscaselu, R.; Gutt, G.; Amariei, S. Biopolymer-Based Films Enriched with Stevia Rebaudiana Used for the Development of Edible and Soluble Packaging. Coatings. 2019, 9(6), 360–373. DOI: 10.3390/coatings9060360.
- Hasheminya, S. M.; Mokarram, R. R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H. S.; Dehghannya, J. Development and Characterization of Biocomposite Films Made from Kefiran, Carboxymethyl Cellulose and Satureja Khuzestanica Essential Oil. Food Chem. 2019, 289, 443–452. DOI: 10.1016/j.foodchem.2019.03.076.
- Ahmed, Z.; Wang, Y.; Anjum, N.; Ahmad, H.; Ahmad, A.; Raza, M. Characterization of New Exopolysaccharides Produced by Coculturing of L. Kefiranofaciens with Yoghurt Strains. Int. J. Biol. Macromol. 2013, 59, 377–383. DOI: 10.1016/j.ijbiomac.2013.04.075.
- Vijayendra, S. V. N.; Shamala, T. R. Film Forming Microbial Biopolymers for Commercial Applications—A Review. Crit. Rev. Biotechnol. 2014, 34(4), 338–357. DOI: 10.3109/07388551.2013.798254.
- Sgorla, D.; Almeida, A.; Azevedo, C.; Cavalcanti, B.; Sarmento, O. A.; Cavalcanti, O. A. Development and Characterization of Crosslinked Hyaluronic Acid Polymeric Films for Use in Coating Processes. Int. J. Pharm. 2016, 511(1), 380–389. DOI: 10.1016/j.ijpharm.2016.07.033.
- Donot, F.; Fontana, A.; Baccou, J. C.; Schorr-Galindo, S. Microbial Exopolysaccharides: Main Examples of Synthesis, Excretion, Genetics and Extraction. Carbohydr. Polym. 2012, 87(2), 951–962. DOI: 10.1016/j.carbpol.2011.08.083.
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements-A Review. Carbohydr. Polym. 2021, 2, 100024. DOI: 10.1016/j.carpta.2020.100024.
- Kumari, N.; Bangar, S. P.; Petrů, M.; Ilyas, R. A.; Singh, A.; Kumar, P. Development and Characterization of Fenugreek Protein-Based Edible Film. Foods. 2021, 10(9), 1976. DOI: 10.3390/foods10091976.
- García, A.; Pérez, L. M.; Piccirilli, G. N.; Verdini, R. A. Evaluation of Antioxidant, Antibacterial and Physicochemical Properties of Whey Protein-Based Edible Films Incorporated with Different Soy Sauces. LWT. 2020, 117, 108587. DOI: 10.1016/j.lwt.2019.108587.
- Malathi, A. N.; Santhosh, K. S.; Nidoni, U. Recent Trends of Biodegradable Polymer: Biodegradable Films for Food Packaging and Application of Nanotechnology in Biodegradable Food Packaging. CTTS. 2014, 3(2), 73–79.
- Sáez-Orviz, S.; Marcet, I.; Rendueles, M.; Díaz, M. Bioactive Packaging Based on Delipidated Egg Yolk Protein Edible Films with Lactobionic Acid and Lactobacillus Plantarum CECT 9567: Characterization and Use as Coating in a Food Model. Food Hydrocoll. 2021, 119, 106849–106859. DOI: 10.1016/j.foodhyd.2021.106849.
- Zubeldía, F.; Ansorena, M. R.; Marcovich, N. E. Wheat Gluten Films Obtained by Compression Molding. Polym. Test. 2015, 43, 68–77. DOI: 10.1016/j.polymertesting.2015.02.001.
- Sharma, N.; Khatkar, B. S.; Kaushik, R.; Sharma, P.; Sharma, R. Isolation and Development of Wheat Based Gluten Edible Film and Its Physicochemical Properties. Int. Food Res. J. 2017, 24(1), 94–101.
- Chiou, B. S.; Cao, T.; Bilbao-Sainz, C.; Vega-Galvez, A.; Glenn, G.; Orts, W. Properties of Gluten Foams Containing Different Additives. Ind. Crops Prod. 2020, 152, 112511–112520. DOI: 10.1016/j.indcrop.2020.112511.
- Marashi, S. M. H.; Hashemi, M.; Berizi, E.; Raeisi, M.; Noori, S. M. A. Elaboration of Whey Protein-Based Films in Food Products: Emphasis on the Addition of Natural Edible Bio-Nanocomposites with Antioxidant and Antimicrobial Activity. JJNP. 2022, 17(2). DOI: 10.5812/jjnpp.117046.
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S. M.; Abdollahi, M. Whey Protein Concentrate Edible Film Activated with Cinnamon Essential Oil. J. Food Process Preserv. 2014, 38(3), 1251–1258. DOI: 10.1111/jfpp.12086.
- Çakmak, H.; Özselek, Y.; Turan, O. Y.; Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey Protein Isolate Edible Films Incorporated with Essential Oils: Antimicrobial Activity and Barrier Properties. Polym. Degrad. Stab. 2020, 179, 1–11. DOI: 10.1016/j.polymdegradstab.2020.109285.
- Marquez, G. R.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C. V.; Porta, R. Fresh-Cut Fruit and Vegetable Coatings by Transglutaminase-Crosslinked Whey Protein/Pectin Edible Films. LWT. 2017, 75, 124–130. DOI: 10.1016/j.lwt.2016.08.017.
- Otoni, C. G.; Avena-Bustillos, R. J.; Olsen, C. W.; Bilbao-Sáinz, C.; McHugh, T. H. Mechanical and Water Barrier Properties of Isolated Soy Protein Composite Edible Films as Affected by Carvacrol and Cinnamaldehyde Micro and Nanoemulsions. Food Hydrocoll. 2016, 57, 72–79. DOI: 10.1016/j.foodhyd.2016.01.012.
- Acquah, C.; Zhang, Y.; Dubé, M. A.; Udenigwe, C. C. Formation and Characterization of Protein-Based Films from Yellow Pea (Pisum sativum) Protein Isolate and Concentrate for Edible Applications. CRFS. 2020, 2, 61–69. DOI: 10.1016/j.crfs.2019.11.008.
- Umaraw, P.; Verma, A. K. Comprehensive Review on Application of Edible Film on Meat and Meat Products: An Eco-Friendly Approach. Crit. Rev. Food Sci. Nutr. CRIT REV FOOD SCI. 2017, 57(6), 1270–1279. DOI: 10.1080/10408398.2014.986563.
- Zhang, Y.; Liu, Q.; Rempel, C. Processing and Characteristics of Canola Protein-Based Biodegradable Packaging. Crit. Rev. Food Sci. Nutr. 2018, 58(3), 475–485. DOI: 10.1080/10408398.2016.1193463.
- Shitu, A.; Zhang, Y.; Danhassan, U. A.; Li, H.; Tadda, M. A.; Ye, Z.; Zhu, S. Synergistic Effect of Chitosan-Based Sludge Aggregates CS@ NGS Inoculum Accelerated the Start-Up of Biofilm Reactor Treating Aquaculture Effluent: Insights into Performance, Microbial Characteristics, and Functional Genes. Chemosphere. 2022, 303, 135097. DOI: https://doi.org/10.1016/j.chemosphere.2022.135097.
- Wu, Z.; Li, Y.; Tang, J.; Lin, D.; Qin, W.; Loy, D. A.; Li, S.; Chen, H.; Li, S. Ultrasound-Assisted Preparation of Chitosan/nano-Silica Aerogel/Tea Polyphenol Biodegradable Films: Physical and Functional Properties. Ultrason. Sonochem. 2022, 87, 106052. DOI: 10.1016/j.ultsonch.2022.106052.
- Lionetto, F.; Esposito Corcione, C. Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers. 2021, 13(14), 2337. DOI: 10.3390/polym13142337.
- Rezaei, F. S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan Films and Scaffolds for Regenerative Medicine Applications: A Review. Carbohydr. Polym. 2021, 273, 118631. DOI: 10.1016/j.carbpol.2021.118631.
- El-Gendi, H.; Saleh, A. K.; Badierah, R.; Redwan, E. M.; El-Maradny, Y. A.; El-Fakharany, E. M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi. 2021, 8(1), 23. DOI: 10.3390/jof8010023.
- Koc, B.; Akyuz, L.; Cakmak, Y. S.; Sargin, I.; Salaberria, A. M.; Labidi, J.; Kaya, M.; Cekic, F. O.; Akata, I.; Kaya, M. Production and Characterization of Chitosan-Fungal Extract Films. Food Biosci. 2020, 35, 100545–100554. DOI: 10.1016/j.fbio.2020.100545.
- Alsaggaf, M. S.; Moussa, S. H.; Tayel, A. A. Application of Fungal Chitosan Incorporated with Pomegranate Peel Extract as Edible Coating for Microbiological, Chemical and Sensorial Quality Enhancement of Nile Tilapia Fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. DOI: 10.1016/j.ijbiomac.2017.03.017.
- Celen, O.: Kocer, H. B. Spinnability and Characterization of Poly (D‐lactic Acid) Blended Poly (L‐lactic Acid) Filament Yarns. J. Appl. Polym. Sci. 2022, 139(15), 51916. DOI: 10.1002/app.51916.
- Fitzgerald, R.; Bass, L. M.; Goldberg, D. J.; Graivier, M. H.; Lorenc, Z. P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet. Surg. J. 2018, 38(suppl_1), S13–17. DOI: 10.1093/asj/sjy012.
- Sadyt, S.; Błaszczyk, A.; Kozak, W.; Boryło, P.; Szindler, M. Quality Assessment of Innovative Chitosan-Based Biopolymers for Edible Food Packaging Applications. Food Pack. Shelf Life. 2021, 30, 100756–100764. DOI: 10.1016/j.fpsl.2021.100756.
- Bonilla, J.; Sobral, P. J. Gelatin‐chitosan Edible Film Activated with Boldo Extract for Improving Microbiological and Antioxidant Stability of Sliced Prato Cheese. Int. J. Food Sci. Technol. 2019, 54(5), 1617–1624. DOI: 10.1111/ijfs.14032.
- Razavi, R.; Tajik, H.; Moradi, M.; Molaei, R.; Ezati, P. Antimicrobial, Microscopic and Spectroscopic Properties of Cellulose Paper Coated with Chitosan Sol-Gel Solution Formulated by Epsilon-Poly-L-Lysine and Its Application in Active Food Packaging. Carbohydr. Res. 2020, 489, 107912–107921. DOI: 10.1016/j.carres.2020.107912.
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Li, J.; Zhou, L.; Zou, L.; Liu, Y., et al. Carboxymethyl Chitosan-Pullulan Edible Films Enriched with Galangal Essential Oil: Characterization and Application in Mango Preservation. Carbohydr. Polym. 2021, 256, 117579. DOI: 10.1016/j.carbpol.2020.117579.
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-Based Ph-Sensitive Smart Packaging Films for Monitoring Food Freshness. J. Agric. Res. 2022, 9, 100340. DOI: 10.1016/j.jafr.2022.100340.
- Ambaye, T. G.; Vaccari, M.; Prasad, S.; van Hullebusch, E. D.; Rtimi, S. Preparation and Applications of Chitosan and Cellulose Composite Materials. J. Environ. Manage. 2022, 301, 113850. DOI: 10.1016/j.jenvman.2021.113850.
- Ebrahimzadeh, S.; Bari, M. R.; Hamishehkar, H.; Kafil, H. S.; Lim, L. T. Essential Oils-Loaded Electrospun Chitosan-Poly (Vinyl Alcohol) Nonwovens Laminated on Chitosan Film as Bilayer Bioactive Edible Films. LWT. 2021, 144, 111217. DOI: 10.1016/j.lwt.2021.111217.
- Settier-Ramírez, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Broadening the Antimicrobial Spectrum of Nisin-Producing Lactococcus lactis Subsp. Lactis to Gram-Negative Bacteria by Means of Active Packaging. Int. J. Food Microbiol. 2021, 339, 109007. DOI: 10.1016/j.ijfoodmicro.2020.109007.
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Surfactant Assisted Nisin Loaded Chitosan-Carageenannanocapsule Synthesis for Controlling Food Pathogens. Food Control. 2014, 37, 158–164. DOI: 10.1016/j.foodcont.2013.09.024.
- Maresca, D.; Mauriello, G. Development of Antimicrobial Cellulose Nanofiber-Based Films Activated with Nisin for Food Packaging Applications. Foods. 2022, 11(19), 3051. DOI: 10.3390/foods11193051.
- Lan, W.; Zhang, R.; Ji, T.; Sameen, D. E.; Ahmed, S.; Qin, W.; Liu, Y.; He, L.; Liu, Y. Improving Nisin Production by Encapsulated Lactococcus lactis with Starch/Carboxymethyl Cellulose Edible Films. Carbohydr. Polym. 2021, 251, 117062. DOI: 10.1016/j.carbpol.2020.117062.
- Duran, M.; Aday, M. S.; Zorba, N. N. D.; Temizkan, R.; Büyükcan, M. B.; Caner, C. Potential of Antimicrobial Active Packaging ‘Containing Natamycin, Nisin, Pomegranate and Grape Seed Extract in Chitosan Coating’to Extend Shelf Life of Fresh Strawberry. Food Bioprod. Process. 2016, 98, 354–363. DOI: 10.1016/j.fbp.2016.01.007.
- Wang, H.; Guo, L.; Liu, L.; Han, B.; Niu, X. Composite Chitosan Films Prepared Using Nisin and Perilla Frutescense Essential Oil and Their Use to Extend Strawberry Shelf Life. Food Biosci. 2021, 41, 101037. DOI: 10.1016/j.fbio.2021.101037.
- Resa, C. P. O.; Gerschenson, L. N.; Jagus, R. J. Natamycin and Nisin Supported on Starch Edible Films for Controlling Mixed Culture Growth on Model Systems and Port Salut Cheese. Food Control. 2014, 44, 146–151. DOI: 10.1016/j.foodcont.2014.03.054.
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of Cellulosic Paper Coated with Chitosan-Zinc Oxide Nanocomposite Containing Nisin and Its Application in Packaging of UF Cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. DOI: 10.1016/j.ijbiomac.2017.11.145.
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial Food Packaging: Potential and Pitfalls. Front. Microbiol. 2015, 6, 611. DOI: 10.3389/fmicb.2015.00611.
- Pranoto, Y.; Rakshit, S. K.; Salokhe, V. M. Enhancing Antimicrobial Activity of Chitosan Films by Incorporating Garlic Oil, Potassium Sorbate and Nisin. LWT-JFST. 2005, 38(8), 859–865. DOI: 10.1016/j.lwt.2004.09.014.
- Chang, S. H.; Chen, Y. J.; Tseng, H. J.; Hsiao, H. I.; Chai, H. J.; Shang, K. C.; Tsai, G. J.; Tsai, G. -J. Applications of Nisin and EDTA in Food Packaging for Improving Fabricated Chitosan-Polylactate Plastic Film Performance and Fish Fillet Preservation. Membranes. 2021, 11(11), 852–867. DOI: 10.3390/membranes11110852.
- Zhang, L.; Wang, H.; Jin, C.; Zhang, R.; Li, L.; Li, X.; Jiang, S. Sodium Lactate Loaded Chitosan-Polyvinyl Alcohol/Montmorillonite Composite Film Towards Active Food Packaging. IFSET. 2017, 42, 101–108. DOI: 10.1016/j.ifset.2017.06.007.
- Wang, W.; Yu, Z.; Alsammarraie, F. K.; Kong, F.; Lin, M.; Mustapha, A. Properties and Antimicrobial Activity of Polyvinyl Alcohol-Modified Bacterial Nanocellulose Packaging Films Incorporated with Silver Nanoparticles. Food Hydrocoll. 2020, 100, 105411–105421. DOI: 10.1016/j.foodhyd.2019.105411.
- Esa, F.; Tasirin, S. M.; AbdRahman, N. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Proced. 2014, 2, 113–119. DOI: 10.1016/j.aaspro.2014.11.017.
- Fang, L.; Catchmark, J. M. Characterization of Cellulose and Other Exopolysaccharides Produced from Gluconacetobacter Strains. Carbohydr. Polym. 2015, 115, 663–669. DOI: 10.1016/j.carbpol.2014.09.028.
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the Fermentation Process and Properties of Bacterial Cellulose: A Review. Cellulose. 2016, 23(1), 57–91. DOI: 10.1007/s10570-015-0802-0.
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H. W.; Licciardello, F.; Pulvirenti, A. Characterization of Bio-Nanocomposite Films Based on Gelatin/Polyvinyl Alcohol Blend Reinforced with Bacterial Cellulose Nanowhiskers for Food Packaging Applications. Food Hydrocoll. 2021, 113, 106454–106464. DOI: 10.1016/j.foodhyd.2020.106454.
- Salari, M.; Khiabani, M. S.; Mokarram, R. R.; Ghanbarzadeh, B.; Kafil, H. S. Development and Evaluation of Chitosan Based Active Nanocomposite Films Containing Bacterial Cellulose Nanocrystals and Silver Nanoparticles. Food Hydrocoll. 2018, 84, 414–423. DOI: 10.1016/j.foodhyd.2018.05.037.
- Vilela, C.; Moreirinha, C.; Domingues, E. M.; Figueiredo, F. M.; Almeida, A.; Freire, C. S. Antimicrobial and Conductive Nanocellulose-Based Films for Active and Intelligent Food Packaging. J. Nanomater. 2019, 9(7), 980–996. DOI: 10.3390/nano9070980.
- Sharma, C.; Bhardwaj, N. K.; Pathak, P. Static Intermittent Fed-Batch Production of Bacterial Nanocellulose from Black Tea and Its Modification Using Chitosan to Develop Antibacterial Green Packaging Material. J. Clean. Prod. 2021, 279, 123608–123622. DOI: 10.1016/j.jclepro.2020.123608.
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the Performance of Edible Food Packaging Films by Using Nanocellulose as an Additive. Int. J. Biol. Macromol. 2021, 166, 288–296. DOI: 10.1016/j.ijbiomac.2020.10.185.
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers. 2020, 12(10), 2209–2232. DOI: 10.3390/polym12102209.
- Yang, G.; Xie, J.; Hong, F.; Cao, Z.; Yang, X. Antimicrobial Activity of Silver Nanoparticle Impregnated Bacterial Cellulose Membrane: Effect of Fermentation Carbon Sources of Bacterial Cellulose. Carbohydr. Polym. 2012, 87(1), 839–845. DOI: 10.1016/j.carbpol.2011.08.079.
- Choo, K. W.; Dhital, R.; Mao, L.; Lin, M.; Mustapha, A. Development of Polyvinyl Alcohol/Chitosan/Modified Bacterial Nanocellulose Films Incorporated with 4-Hexylresorcinol for Food Packaging Applications. Food Packag. Shelf Life. 2021, 30, 100769–100780. DOI: 10.1016/j.fpsl.2021.100769.
- Costa, S. M.; Ferreira, D. P.; Teixeira, P.; Ballesteros, L. F.; Teixeira, J. A.; Fangueiro, R. Active Natural-Based Films for Food Packaging Applications: The Combined Effect of Chitosan and Nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. DOI: 10.1016/j.ijbiomac.2021.02.105.
- Maliha, M.; Herdman, M.; Brammananth, R.; McDonald, M.; Coppel, R.; Werrett, M.; Batchelor, W.; Batchelor, W. Bismuth Phosphinate Incorporated Nanocellulose Sheets with Antimicrobial and Barrier Properties for Packaging Applications. J. Clean. Product. 2020, 246, 119016–119028. DOI: 10.1016/j.jclepro.2019.119016.
- Jipa, I. M.; Stoica-Guzun, A.; Stroescu, M. Controlled Release of Sorbic Acid from Bacterial Cellulose Based Mono and Multilayer Antimicrobial Films. LWT. 2012, 47(2), 400–406. DOI: 10.1016/j.lwt.2012.01.039.
- Kamel, R.; Afifi, S. M.; Kassem, I. A.; Elkasabgy, N. A.; Farag, M. A. Arabinoxylan and Rhamnogalacturonan Mucilage: Outgoing and Potential Trends of Pharmaceutical, Environmental, and Medicinal Merits. Int. J. Biol. Macromol. 2020, 165, 2550–2564. DOI: 10.1016/j.ijbiomac.2020.10.175.
- Zeng, W. W.; Lai, L. S. Characterization of the Mucilage Extracted from the Edible Fronds of Bird’s Nest Fern (Asplenium australasicum) with Enzymatic Modifications. Food Hydrocoll. 2016, 53, 84–92. DOI: 10.1016/j.foodhyd.2015.03.026.
- Chiang, C. F.; Lai, L. S. Effect of Enzyme-Assisted Extraction on the Physicochemical Properties of Mucilage from the Fronds of Asplenium australasicum (J. Sm.) Hook. Int. J. Biol. Macromol. 2019, 124, 346–353. DOI: 10.1016/j.ijbiomac.2018.11.181.
- Stintzing, F. C.; Carle, R. Cactus Stems (Opuntia Spp.): A Review on Their Chemistry, Technology, and Uses. Mol. Nutr Food Res. 2005, 49(2), 175–194. DOI: 10.1002/mnfr.200400071.
- Qamar, S. A.; Junaid, M.; Riasat, A.; Jahangeer, M.; Bilal, M.; Mu, B. Z. Carrageenan‐based Hybrids with Biopolymers and Nano‐structured Materials for Biomimetic Applications. Starch - Stärke. 2022, 2200018, 2200018. DOI: 10.1002/star.202200018.
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of Plasticized Edible Films from Opuntia ficus-Indica Mucilage: A Comparative Study of Various Polyol Plasticizers. Carbohydr. Polym. 2018, 190, 204–211. DOI: 10.1016/j.carbpol.2018.02.085.
- De Alvarenga Pinto Cotrim, M.; Mottin, A. C.; Ayres, E. Preparation and Characterization of Okra Mucilage (Abelmoschus esculentus) Edible Films. Macromol. Sym. 2016, 367(1), 90–100. DOI: 10.1002/masy.201600019.
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The Effectiveness of Opuntia ficus-Indica Mucilage Edible Coating on Post-Harvest Maintenance of ‘Dottato’fig (Ficus carica L.) Fruit. Food Packag. Shelf Life. 2017, 12, 135–141. DOI: 10.1016/j.fpsl.2017.04.010.
- Guadarrama-Lezama, A. Y.; Castaño, J.; Velázquez, G.; Carrillo-Navas, H.; Alvarez-Ramírez, J. Effect of Nopal Mucilage Addition on Physical, Barrier and Mechanical Properties of Citric Pectin-Based Films. J. Food Sci. Techol. 2018, 55(9), 3739–3748. DOI: 10.1007/s13197-018-3304-x.
- Zambrano, J.; Valera, A. M.; Materano, W.; Maffei, M.; Quintero, I.; Ruiz, Y.; Marcano-Belmonte, D. Effect of Edible Coatings Based on Cactus (Opuntia Elatior Mill.) Mucilage on the Physicochemical and Sensory Properties of Guava Fruits (Psidium Guajava L.) Under Controlled Storage. Rev. de la Fac. de Agron. 2018, 35(4), 476–495.
- Liguori, G.; Gaglio, R.; Settanni, L.; Inglese, P.; D’Anna, F.; Miceli, A.; Genovese, F. Effect of Opuntia ficus-indica Mucilage Edible Coating in Combination with Ascorbic Acid, on Strawberry Fruit Quality During Cold Storage. J. Food Qual. 2021, 2021, 1–8. DOI: 10.1155/2021/9976052.
- Bernardino-Nicanor, A.; Montañez-Soto, J. L.; Conde-Barajas, E.; Negrete-Rodríguez, M. D. L. L. X.; Teniente-Martínez, G.; Vargas-León, E. A.; Juárez-Goiz, J.; Acosta-García, G.; González-Cruz, L. Spectroscopic and Structural Analyses of Opuntia Robusta Mucilage and Its Potential as an Edible Coating. Coatings. 2018, 8(12), 466–477. DOI: https://doi.org/10.3390/coatings8120466.
- dos Santos Morais, M. A.; Fonseca, K. S.; Viégas, E. K. D.; de Almeida, S. L.; Maia, R. K. M.; Silva, V. N. S.; Do NascimentoSimões, A. Mucilage of Spineless Cactus in the Composition of an Edible Coating for Minimally Processed Yam (Dioscorea Spp.). J. Food Meas. Charact. 2019, 13(3), 2000–2008. DOI: 10.1007/s11694-019-00120-9.
- Abera, N. G.; Kebede, W.; Wassu, M. Effect of Aloe Gel and Cactus Mucilage Coating on Chemical Quality and Sensory Attributes of Mango (Mangifera indica L.). Int. J. Postharvest Technol. Innov. 2019, 7(2), 31–43.
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M. J. Development of a Cactus-Mucilage Edible Coating (Opuntiaficusindica) and Its Application to Extend Strawberry (Fragaria ananassa) Shelf-Life. Food Chem. 2005, 91(4), 751–756. DOI: 10.1016/j.foodchem.2004.07.002.
- Oliveira, N. L.; Rodrigues, A. A.; Neves, I. C. O.; Lago, A. M. T.; Borges, S. V.; de Resende, J. V. Development and Characterization of Biodegradable Films Based on Pereskia aculeata Miller Mucilage. Ind. Crops Prod. 2019, 130, 499–510. DOI: 10.1016/j.indcrop.2019.01.014.
- Dick, M.; Costa, T. M. H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Flôres, S. H. Edible Film Production from Chia Seed Mucilage: Effect of Glycerol Concentration on Its Physicochemical and Mechanical Properties. Carbohydr. Polym. 2015, 130, 198–205. DOI: 10.1016/j.carbpol.2015.05.040.
- Sadeghi-Varkani, A.; Emam-Djomeh, Z.; Askari, G. Physicochemical and Microstructural Properties of a Novel Edible Film Synthesized from Balangu Seed Mucilage. Int. J. Biol. Macromol. 2018, 108, 1110–1119. DOI: 10.1016/j.ijbiomac.2017.11.029.
- Marvdashti, L. M.; Koocheki, A.; Yavarmanesh, M. Alyssum Homolocarpum Seed Gum-Polyvinyl Alcohol Biodegradable Composite Film: Physicochemical, Mechanical, Thermal and Barrier Properties. Carbohydr. Polym. 2017, 155, 280–293. DOI: 10.1016/j.carbpol.2016.07.123.
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings. 2020, 10(2), 166. DOI: 10.3390/coatings10020166.
- Singh, A.; Gu, Y.; Castellarin, S. D.; Kitts, D. D.; Pratap-Singh, A. Development and Characterization of the Edible Packaging Films Incorporated with Blueberry Pomace. Foods. 2020, 9(11), 1599. DOI: 10.3390/foods9111599.
- Rawdkuen, S., Edible Films Incorporated with Active Compounds: Their Properties and Application, Active Antimicrobial Food Packag., 2019, Isıl, V.; Uzunlu, S.; Eds, 71–85.
- Naskar, A.; Chakraborty, I.; Roy, S. R.; Bhattacharya, T. Edible Film on Food with Smart Incorporation of Health‐friendly Supplements. Nanotech. in Intell. Food Pkg. 2022, 361–382. DOI: 10.1002/9781119819011.ch15.
- Raybaudi-Massilia, R.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Combinational Edible Antimicrobial Films and Coatings. Antimicrobial. Food Pkg. 2016, 633–646. DOI: 10.1016/B978-0-12-800723-5.00052-8.
- Restuccia, D.; Spizzirri, U. G.; Parisi, O. I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU Regulation Aspects and Global Market of Active and Intelligent Packaging for Food Industry Applications. Food Control. 2010, 21(11), 1425–1435. DOI: https://doi.org/10.1016/j.foodcont.2010.04.028.
- Mihindukulasuriya, S. D. F.; Lim, L. T. Nanotechnology Development in Food Packaging: A Review. Trends Food Sci. Technol. 2014, 40(2), 149–167. DOI: 10.1016/j.tifs.2014.09.009.