?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Moringa oleifera Lam. (Moringa) plant, which belongs to the Moringaceae family, is an important crop in Asia and Africa. Moringa has been investigated for its health benefits, which are related to several bioactive components found in the plant, including flavonoids, vitamins, phenolic acids, isothiocyanates, saponins, and tannins. The leaves of moringa have been researched extensively and hence proven to be effective in various chronic illnesses such as insulin resistance, hypercholesterolemia, diabetes, cancer, nonalcoholic liver disease, high blood pressure, and general inflammation. Moringa includes a variety of dietary phytonutrients, notably flavonoids, the secondary plant metabolites which have health-enhancing effects, such as avoiding damage to normal cellular DNA and stimulating the death of cancer cells, therefore reducing the prevalence of non-communicable disorders (NCDs). Flavonoids are phenolic chemicals, which are plant elements that correlate to the non-energy portion of a person’s diet. Flavonoids are often removed chemically using solvents and conventional techniques that, in addition to being costly, take a long time and have an impact on the bioactivity of these substances. As a consequence, there is no universally applicable extraction procedure, and each best practice is adapted to the specific plants involved. To be selective, an extraction technique must have combined an ideal solvent or mixture of solvents with an optimum procedure. In this study, we provide information on the improved and standardized extraction process from Moringa leaf extract concerning flavonoids and its good consequences on immune system efficacy that have been documented.
Introduction
Moringa dry leaves are rich in polyphenol components such as phenolic acids and flavonoids. In plants, microbial infections produce flavonoids which have a ring structure like benzo-γ-pyrone[Citation1]. C-1: References should be number format? Flavonoids as secondary metabolites are tiny molecules that plants produce in response to various biotic and abiotic conditions, and a wide range of well-documented health-promoting properties have been shown by these chemical compounds.[Citation2] Flavonoids were initially recognized as vitamin P (owing to its propensity to promote capillary permeability) and vit. C2 due to some of their vitamin C-like properties. Conversely, the idea that flavonoids are vitamins has not been validated and these designations remained in use until roughly 1950.[Citation3] Flavonoids are divided into six groups based on their structural distinctions: isoflavones, flavan-3-ol, flavanone, anthocyanidin, flavanone flavonol and each of which has distinct properties.[Citation3] shows the basic structure of flavonoids and their different classes.[Citation4] Owing to their wide range health benefits, optimization of their extraction methods has been an intensive research area since then. Flavonoids are often removed chemically using solvents and conventional techniques that, in addition to being costly, take a long time and have an impact on the bioactivity of these components. Like heating, refluxing and boiling are commonly employed methods for flavonoid extraction, certain target components are wasted while extracting them due to extended processing times or chemical reactions, such as hydrolysis or oxidation.[Citation5] Advanced techniques and strategies are continuously being developed to overcome the limitations of traditional methods for extracting these compounds. These techniques include ultrasonically assisted extraction (UAE), supercritical fluid extraction (SFE), and microwave-assisted extraction (MAE). Recent biotechnological attempts for decreasing or eliminating the usage of harmful solvents, saving processing time while retaining extracted compounds’ bioactivity, have been described.[Citation3]
In a study by Chávez-González et al.,[Citation3] using subcritical ethanol extraction, a large-scale procedure for moringa leaf flavonoid extraction was devised, then extracts were HPLC–MS analyzed to determine their components qualitatively HPLC–UV–MS analysis of moringa leaves for phenolic components revealed 12 flavonoids as main ingredients, including kaempferol glucosides, quercetin and glucoside malonates.[Citation2] Flavonoids, in addition to their recognized antioxidant qualities, have additional capabilities, such as the activation of communication via gap junctions.[Citation3] Quercetin is a powerful antioxidant with a variety of medicinal benefits.[Citation6] In vitro flavonoids’ significant antioxidant activity is mostly owing to their ability to directly capture free radicals via transferring electrons or hydrogen atoms or metal chelation.[Citation7] The antioxidant activity of flavonoid components in moringa leaves has been studied in vitro by employing several techniques like ABTS radical scavenging tests 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid that have been employed for moringa leaf extracts to confirm their antiradical activity.[Citation6] Many phytochemicals have been found to have biological activity, and they may work together to prevent cancer. Flavonoids may potentially have a positive effect by preventing carcinogens from bioactivating.[Citation8] In the human diet, flavonoids are phenolic secondary plant metabolites that have a broad spectrum of pharmacological actions, including the potential to serve as anti-cancer drugs.[Citation9] Anti-CRC properties are found in flavonoids. Flavanols have a negligible effect on CRC.[Citation10] According to emerging data, dietary flavonoids do have the capacity to boost human memory and neurocognitive efficiency by preserving vulnerable neurons, encouraging neuronal regeneration, and improving existing neuronal function.[Citation3] Because flavonoids and their metabolite forms can affect cell oxidation reactions in the CNS, the current review investigates their potential to boost memory and immunity as well as establish a comparison of several variables such as extraction time, cost, extraction loss, bioactivity of the extracted flavonoid compounds from moringa leaves by conventional and advanced methods because determining the best extraction conditions is crucial to maximizing the extraction rate of these active chemicals. In this study, we provide information on the improved and standardized extraction process from Moringa leaf extract concerning flavonoids and its good consequences on immune system efficacy that have been documented.
Extraction techniques for flavonoidS
To acquire the chemicals found in moringa leaves, an optimal extraction process must be designed that allows for control of the quality of the final extract, which must be high in flavonoids. Flavonoids are lost in significant amounts throughout several food-processing procedures.[Citation11] There is a standard process for isolating, extracting, and identifying phytochemicals from plant sources that consists of three phases. The initial stage in the preparation of a sample utilizes filtration, centrifuge, or drying procedures among others.[Citation3] When the goal is to selectively separate the flavonoid fraction free of pigments, lipids, terpenoids, alkaloids, saponins and so on – the preexisting extraction technique must be modified into a new one. In this regard, scientific understanding in this field might be quite beneficial.[Citation1]
Because natural antioxidants seem to be advantageous for treating human diseases, an accurate assessment of moringa’s flavonoid content is essential for the creation of ways to support healthy eating.[Citation7] There are two types of extraction methods: traditional procedures (reflux, maceration and Soxhlet extraction) and contemporary approaches (supercritical extraction, extraction by ultrasound, microwave extraction).[Citation1]
The time required to attain equilibrium in all extraction procedures can be accelerated in a variety of ways.[Citation12]
Direct heating to increase molecular mobility and hence yield of extraction
Microwave heating unlike the conventional heating begins within the plant material.
High-speed agitation that effectively disturbs the static layer of solvent surrounding the sample that would otherwise impede extraction
Using an electrical discharge to disturb the stationary layer, however this is an uncommon procedure.
The application of ultrasonic energy, which helps with extraction different from all above-mentioned ways.
Conventional methods
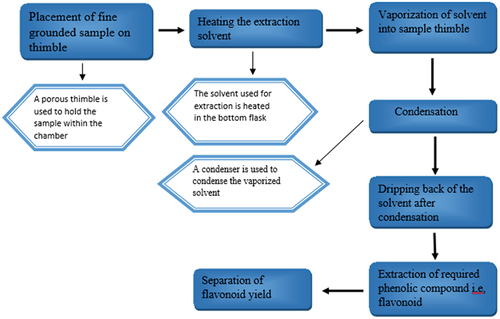
Various extraction procedures, such as percolation, maceration, boiling, reflux, soaking, hydro-distillation, and Soxhlet, have been proposed.[Citation5] Several solvents, including ethanol, chloroform, methanol, benzene, acetate and ethyl and others, have been tested to see how they affected extraction yields.[Citation3] Soxhle and Reflux extraction are both continuous and high temperature methods. Soxhlet extraction is performed with the Soxhlet apparatus: A porous “thimble” is used to hold the fine grounded sample within a chamber. In the bottom flask, extraction solvents are heated, vaporized into the sample thimble, a condenser used to condense it, and then it dripped back. These procedures, in comparison to maceration, use a lesser amount of solvent found that the Soxhlet extraction of moringa leaves resulted in a decreased yield of phenolic compounds, including flavonoids.[Citation1,Citation11]
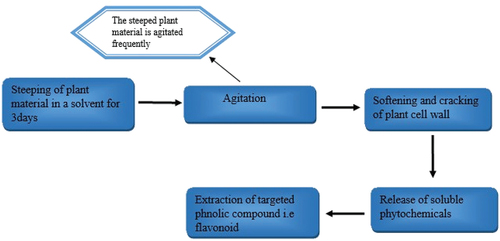
Maceration is a winemaking technique frequently used in medicinal plant research where plant materials steeped in a solvent at room temperature with frequent agitation result in softening and cracking of the plant’s cell wall, releasing soluble phytochemicals.[Citation10] Decoction- and infusion-like maceration involve steeping in cold or boiling water. For infusion, however, the time of maceration is shortened, and the sample is cooked in a predetermined amount of water 1:4 or 1:16 for a set duration for decoction.[Citation10] According to[Citation13] and,[Citation14] traditional extraction procedures are characterized by the need for a long time, a lot of energy, and a lot of toxic chemicals .
Modern techniques/advanced methods
Owing to the inadequacies of present extraction methods, such as more consumption of harmful chemicals, increased energy consumption, more than 70% of total process requires energy and more CO2 rejection, the food industry has been compelled to develop new “green” separating procedures that uses less energy and solvent in general such as, supercritical fluid extraction, microwave extraction, ultrasound extraction, flash distillation, ultrafiltration and the control of harmful chemicals.[Citation13] Polyamide chromatography, thin-layer chromatography (TLC) and paper electrophoresis are some of the most widely employed phenolic separation techniques in recent decades. Because TLC is a quick and simple technique of fractioning flavonoids, which is why it is still commonly used.[Citation15] The growing ultrasound-aided extraction (UAE) and microwave assisted extraction (MAE) technologies are two of the most extensively utilized ways for extracting flavonoids. However, supercritical fluid extraction is the most advanced one.
Ultrasound-assisted Extraction (UAE)
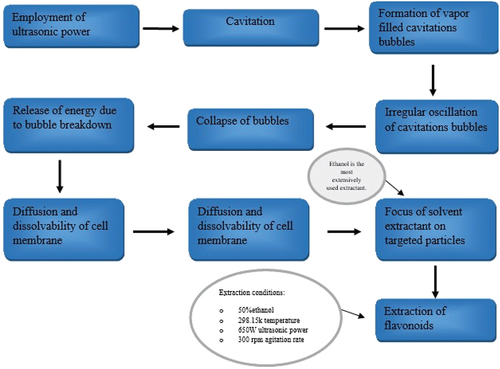
During the extraction process, UAE, also known as sonication, employs ultrasonic wave energy. Ultrasound causes cavitation, which helps the cell materials dissolve and diffuse faster. There are two forms of acoustic cavitation: transient and steady.[Citation13] The former happens when gas or vapor-filled cavitation bubbles oscillate irregularly and eventually collapse. Bubbles that oscillate in a predictable pattern over numerous sonic cycles are referred to as stable cavitation.[Citation13] The ultrasonic wave travels through the medium’s molecules, causing cavitation bubbles to develop if the power is strong enough . The breakdown of these bubbles releases energy, thus solvent jets focused on herbal particles more effectively extract the targeted components.[Citation11] This methodology’s cavitation effect not only allows the plant material’s cell walls to be destroyed but it also promotes particle size reduction, which helps the solvent – substrate interaction.[Citation13] The UAE boosted bioactive chemical yields, and flavonoid yields were found to be vary depending on extraction technique.
Extract of moringa leaf from UAE was analyzed using ultra-high-performance liquid chromatography (UHPLC), the following compounds were separately identified based on their molecular weight and retention time quercetin 3-glucoside, quercetin malonyl glucoside, quercetin hydroxy methyl glutaroyl glucoside, quercetin acetyl glycoside, kaempferol 3-glucoside, and isorhamnetin 3-glucoside with retention times of 5.316, 5.321, 5.338, 5.343, 5.363 and 5.408 min, respectively.
The main benefits of this technology are the decrease in extraction periods and increase in the biological qualities of the extracts.[Citation1] There are three distinct types of ultrasound applications:
Directly applying to the sample
Coupling to a Device
Immersion in an ultrasonic bath
Many variables can influence flavonoid extraction processes, and hence, the number of tests to optimize a process, experimental matrices are often employed to discover the parameters that promote the recovery of the greatest flavonoid content. Sonication may be performed using a variety of solvents and combinations, although ethanol was the most extensively utilized. However, using response surface methodology (RSM) approach, an ultrasonic-assisted extraction (UAE) process with chosen deep eutectic solvents (DES) as solvent was initially devised by[Citation14] to maximize the total flavonoid content/total phenolic (TFC/TPC) and bioactivity of moringa leaves extract. A study used response surface approach to construct a UAE procedure using chosen deep eutectic solvents (DES) as the solvent to concurrently maximize the total flavonoid content/total phenolic (TFC/TPC) and bioactivity of moringa leaves extract. Water concentration in DES was the most important component for RSM, whereas ultrasonic duration and liquid-to-solid ratio had no numerically significant influence on TPC.[Citation14] The flavonoid extraction from moringa leaves by UAE was further improved by using a Box–Behnken design, a type of response surface technique.[Citation5]
UAE extracts of dry leaves of Ocimum tenuiflorum employing RSM approach have superior in vitro thermo oxidative stability and bioactivity than traditional solvent extracts when applied ethanol concentration of 55.34%, sonication duration 11.71 min, and UED 0.26 W/cm3, as the ideal UAE conditions.[Citation16]
According to Vergara-Jimenez et al.[Citation1], the ideal extraction parameters for plant leaves extracts were found to be 50% ethanol as the extractant, 298.15 K temperature, 650 W ultrasonic power, and 300 rpm agitation rate. C-1: Avoid sentences starting with number? Please check all? The temperature degradability of the primary flavonoids present in moringa leaves was investigated, and it was discovered that there is no substantial degradation of these compounds in the temperature range investigated, hence a design temperature range of 10°C to 70°C was defined. In conclusion, as compared to Soxhlet extraction and maceration, UAE offers more advantages, such as conserving time and solvent, requiring lower temperatures, and yielding higher extract yields.[Citation1] Ultrasound radiation over 20 kHz, on contrary, may have an impact on active phytochemicals due to free radical’s formation.[Citation10] When the TFC/TPC yield of UAE was compared to the yield of the macerated extract, TFC/ TPC after UAE was considerably (p ≤ .05) greater. However, at 30°C for 10 min and 50°C for 30 min, the maceration extraction yielded greater TFC and TPC than the ultrasound-assisted extraction.[Citation14,Citation17]
Microwave assisted extraction (MAE)
Microwave extraction is a cutting-edge separation technique with the potential to revolutionize flavonoid extraction and analytical quantification.[Citation11] It is also superior to or similar to various modern extraction techniques in terms of yields, and it is simple to use for flavonoid extraction from plant materials with a variety of physicochemical properties.[Citation11,Citation18] Solvent characteristics, volume, dielectric properties, microwave power levels, exposure length, system features, and temperature are all critical factors that impact extraction efficiency.[Citation11,Citation19] Another significant component is the matrix:solvent ratio.[Citation20] The interaction between the solvent and the extraction matrix, the solubility of the intended analyte and the solvent’s microwave absorption capabilities all influence the solvent choice for MAE.[Citation16] In MAE, microwaves assist compounds of interest segregation from test sample into solvent.[Citation10]
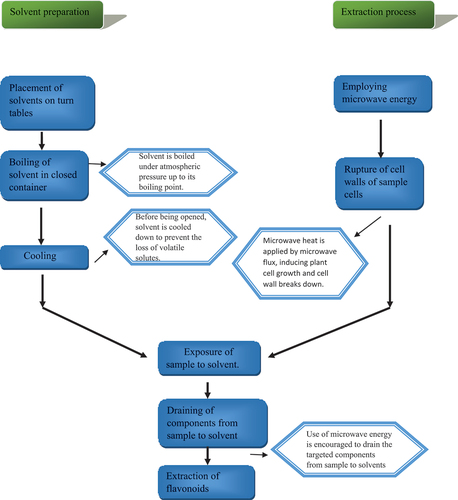
In the extraction procedure for MAE, the analytes are exposed to the solvent by rupturing the cells[Citation1,Citation20] used microwave energy to encourage the draining of target components from the sample to the solvent. Microwaves heat natural water in plant cells by microwave flux in a microwave environment, inducing plant cell growth and cell wall breakdown.[Citation19] Bagade et al. claim that the opening of cell walls causes the release of important components (constituents/secondary metabolites) from the plant sample.[Citation21] Rodríguez-Pérez used a multi-response surface methodology (RSM) to optimize microwave-assisted extraction (MAE) and pressurized liquid extraction (PLE) for the extraction of phenolic components from moringa leaves. As response variables, the extraction yield, total phenolic content (TPC), total flavonoid content (TF), DPPH scavenging method, and trolox equivalent antioxidant capacity (TEAC) assay were analyzed, while the effects of ethanol percentage, extraction time and temperature were investigated. Quantitatively,[Citation21] data demonstrate that MAE extracts quercetin, quercetin-3-O-glucoside, kaempferol, both kaempferol acetyl glycoside isomer (RT 32.39) and kaempferol-3-O-glucoside isomers better than PLE did, so MAE has advantage over PLE because these leading derivatives of quercetin and kaempferol are said to be more potent antioxidants than regular vitamins.[Citation22]
Forty-two percent ethanol, extraction time of 20 min, and 158°C were the MAE optimum conditions (Rodríguez‐Pérez et al., 2016)
For enhancing the efficiency and rate of extraction, the solvent is boiled under atmospheric pressure up to its boiling point in closed containers. Because numerous containers may be employed in a single extraction operation, this method enables temperature control as well as high sample throughput. To achieve even heating, they are placed on a turntable. Volatile solutes can segregate into headspace, which is the biggest disadvantage. To prevent the loss of volatile solutes, the containers are employed for cooling down at room temperature before being opened which lengthens the extraction process. The solvent boiling point at that pressure determines the maximum extraction temperature in open systems.[Citation23] This technique works on small molecules that can endure microwave heating. The oxidation of phenolics, notably flavanones, reduced the extraction rate with increasing MAE cycles.[Citation15]
As relatively small compounds like phenolic (ellagic acid and gallic acid), quercetin, trans-resveratrol, and isoflavin were microwave heat stable up to 100°C for 20 min[Citation15] this technique can be used to extract these molecules from moringa leaves. For extraction, separation, and purification, MAE in combination with high-speed countercurrent chromatography is an excellent choice.[Citation22] For example, it has been documented that using MAE as the primary solvent and distilled water as the secondary solvent allowed polysaccharides to be extracted from Potentilla anserina L.[Citation23]
MAE has a number of advantages over conventional extraction methods, including reduced extraction time, processing costs and energy, solvent quantity, and improved recovery. Additionally, it is among the most widespread and cost-effective extraction technologies accessible today, and various novel MAE processes and apparatus have been developed, such as solvent-free microwave-assisted extraction (SFMAE) and pressurized microwave-assisted extraction (PMAE), and have become available.[Citation20] Because this process relies on moisture inherent in the plant matrix, there is no need for a solvent.[Citation24,Citation25]
The extraction of flavonoid and phenolic compounds using MAE for 5 min was comparable to ultrasound-assisted extraction (UAE) for 60 min and conventional extraction for 24 h (). The extraction time at room temperature and in UAE was approximately 288 and 12 times longer than with MAE, respectively. MAE was more successful than conventional and UAE approaches due to the significant time savings.[Citation25] However, further cleanup is required when MAE extraction is complete. To employ the final analytical approach, some applications just require a filtering step, while others require additional liquid–liquid extraction or solid-phase extraction processes.[Citation22] MAE appears to have gotten over the hurdle and is quickly becoming the preferred extraction method for the twenty-first century.[Citation22]
Supercritical fluid extraction (SFE)
Supercritical fluids are substances having physio-chemical properties somewhere between gases and liquids at pressures and temperatures higher than their critical points.[Citation26] CO2 with critical point: 7.4 MPa and 32°C is the most often utilized supercritical fluid due to its lower critical temperature, nonflammable, nontoxic, nature, and excellent purity.[Citation26–28] Subcritical water, on the other hand, is frequently utilized as a substitute for supercritical CO2. Water in the subcritical area is strongly compressed water that is a static liquid state. The qualities of the fluid may be varied by adjusting the temperature between 150°C and 350°C and pressure between 15 and 200 bar to selectively remove natural components.
Over the last two decades, there has been a lot of research on supercritical fluid extraction for phenolic chemical separation.[Citation29] According to Abhari and Khaneghah,[Citation28] nonpolar chemicals are often extracted via supercritical fluid extraction. However, if the intended compounds for extraction have a mid-polarity, the inclusion of co-solvents is occasionally required to improve SFE extraction efficiency.[Citation21] In the food industry, ethanol is the most often used solvent since it is easily accessible, affordable, 100% biodegradable, and Generally Recognized as Safe (GRAS) in concentrations ranging from 1% to 10%. It also encourages the extraction of polyphenols and flavanols from plant matrices.[Citation28]
With either pure carbon dioxide or carbon dioxide with the inclusion of polar or nonpolar enhancers, SFE has found use in the extraction of phenolic compounds.[Citation30] Hemolysis of human red blood cells was greatly reduced by a supercritical fluid extract of moringa leaves and its primary component cis-11-eicosenoic acid from S. aureus.[Citation3] The oil extracted by SC-CO2 was found to be of higher quality, with less thermal degradation and considerably higher nutritional and antioxidant activity (p < .01) and lesser anti-nutritional content than the solvent extracted and Soxhlet extracted oils.[Citation31] The oil extracted with SC-CO2 has the highest amount of bioactive chemicals (14 compounds).[Citation31] With the usual approach (hot water extraction), 1700 mg/kg of kaempferol was recovered from Moringa oleifera, which may be boosted with the CO2 supercritical extraction procedure.[Citation31] An ultrahigh-pressure supercritical fluid extraction method was devised to investigate the effects of direct pressure and temperature and indirect application (40–80 MPa and 40°C to 70°C, respectively) on the extracted oil amount from crushed moringa seeds. Extraction settings of 80 MPa and 57°C for pure CO2 produced 396 ± 23 mg oil per g of moringa seeds. shows the summary of SCF Extraction Puah et al., (2005) . According to[Citation3] subcritical ethanol extraction using 70% ethanol at 126.6°C and an extraction time of 2.05 h might give a highest flavonoid extraction yield of up to 2.60%. Flavonoid’s yield was enhanced by 26.7% under the optimized circumstances compared to the standard ethanol reflux approach, with an extraction time of just 2 h and significant energy savings. The amount of bioactive chemicals in essential oil recovered by SFE was found to be greater than that extracted by the soxhlet technique, indicating that SFE is more selective than soxhlet extraction. As a result, supercritical fluid extraction outperforms soxhlet extraction in terms of increasing the concentration of certain bioactive chemicals.[Citation29]
Table 1. Optimum extraction conditions for total flavonoids in methanolic UAE extracts from moringa leaves.
Table 2. A comparison of extraction techniques.
Health aspects of flavonoids
Flavonoids are essential entities in a number of nutraceutical, pharmacological, medical, and cosmetics because they have a wide range of health-promoting benefits. This is owing to their anti-inflammatory, antioxidative, anti-mutagenic, and anti-carcinogenic properties, as well as their capacity to influence critical cellular enzyme activity.[Citation34] Antioxidant and anti-inflammatory properties of flavonoid-rich diets have been shown to be beneficial in the prevention and treatment of diseases like diabetes, obesity, cardiovascular disease and neurodegenerative conditions, as well as cancer, as a result of their potent antioxidant capacity (free-radical scavenging capacity). Flavonoids have long been recognized as having critical biological effects on human cells.[Citation35]
Several brain boosting polyphenols, such as caffeine, quercetin, cocoa flavanols, and proteins like lectins, are nanoencapsulated. Some naturally occurring brain-boosting and antiviral compounds, such as quercetin, caffeine, banana lectins (Banlec), and cocoa flavonoids, may protect neurons from apoptosis and oxidative stress, provide anti-depressant effects (in the case of caffeine and lectin), and act as a neuroprotectant (in the case of cocoa flavonoid).[Citation36] Flavonoids decrease the synthesis and activity of eicosanoids, cytokines, C-reactive protein, adhesion molecules in cellular models and in vitro which are pro-inflammatory mediators, resulting in anti-inflammatory effects. The flavonoids that are most prevalent in the diet are not usually the ones that can reach the highest amounts in human circulation.[Citation37]
Research suggests that flavonoids in food may help enhance cognitive function by protecting vulnerable neurons, increasing current neuronal function, and inducing neuronal regeneration, all of which may help with memory and neuro-cognitive ability. The current study investigates the ability of flavonoids and their metabolite forms to increase memory and learning in human subjects by interacting with neural signaling pathways that drive LTP and memory.[Citation38] Natural chemicals like flavonoids have neuroprotective properties, which is likely due to their capacity to control inflammatory responses linked to neurodegenerative disorders. It has been shown that flavonoids (e.g., quercetin, epigallocatechin-3-gallate, hesperetin) or enriched-extracts have been demonstrated to down-regulate inflammatory indicators, limit the manufacturing of proinflammatory cytokines (TNF-a, IL-6, IL-1b, and COX-2) and down-regulate inflammatory indicators, hence preventing damage to the brain.[Citation39]
Flavonoids have grown in popularity as anti-cancer drugs, with promising results as anti-cancer cytotoxic chemicals that trigger cancer cell death.[Citation9] Natural flavonoids’ antioxidant properties are a major factor in defining their anti-inflammatory and anti-infectious disease effects.[Citation39] Flavonoids have multipotent therapeutic potential in the treatment of neurodegenerative disorders caused by oxidative stress and abnormal inflammatory responses.[Citation40] There is evidence that phytochemicals produced from fruits and vegetables, particularly flavonoids, can have favorable impacts on memory and learning.[Citation37]
Different forms of flavonoids, including hesperetin, can reverse the etiology and clinical effects of neurodegeneration. Hesperetin’s neuroprotective properties have been thoroughly investigated in a range of neurodegenerative disorder models.[Citation10] The most extensively researched flavonoids include hesperidin, quercetin, silibinin, rutin, naringin and anthocyanins, all of which are used to reduce oxidative stress and neuro-inflammation in a range of neurodegeneration conditions.[Citation37] The potential of phytochemicals to increase memory, learning, and general cognitive capacity is gaining popularity.[Citation38]
According to the data, dietary flavonoids can influence inflammatory reactions by triggering processes that activate antioxidant transcription and detoxifying defense mechanism-like glutathione peroxidase via the anti-oxidant responsive element.[Citation37] Flavonoids’ biological activities, particularly those on the brain, have long been attributed to their ability to act as antioxidants.[Citation37] The ability of dietary flavonoids can affect neuro-inflammation in the central nervous system, making them viable candidates for neuroprotection (CNS).[Citation41] Total flavonoids from Abelmoschus manihot were also shown to be cardioprotective in a rat model by inhibiting the NLRP3 inflammasome, which reduced myocardial I/R caused injury. In respect of their anti-inflammatory and antioxidative abilities, many flavonoids are being used and explored for their positive impact on infectious disorders.[Citation39] As a result, flavonoid metabolites’ beneficial effects in the brain are unlikely to be due to their capacity to outperform more abundant antioxidants such as ascorbate.[Citation38]
Flavonoids’ many features and actions influence cell activation, maturation, signaling, cytokine synthesis, and secretory processes in a variety of immune cells.[Citation42] Citrus flavonoids have pharmacological actions as antioxidants, anti-inflammatory agents, blood lipid-lowering agents, and cholesterol reducing compounds.[Citation34] Flavonoids have the ability to affect the transition from a procarcinogen to a carcinogen. Flavonoids have been recognized for a long time to have essential biological effects on human cells.[Citation35]
Immune boosting properties of flavonoids
Flavonoids stimulate gene expression and neuronal signaling in the brain. The brain’s neurogenesis and synaptic plasticity may be affected by these mechanisms, influencing memory, learning, and cognition.[Citation39] Naringenin and hesperidin, two citrus flavonoids that function as antioxidants and may pass the blood–brain barrier, are shown to be useful in the control of neurodegenerative illnesses.[Citation10] Flavonoids protect plants from outside agents (ultraviolet radiation, parasites, and viruses), regulate cell metabolic enzymes, and have antioxidant characteristics.[Citation39] Hesperetin is a neuroprotective protein that gives protection against neurodegeneration of brain. Studies on nerve cell models, particularly neuroblastoma SH-SY5Y, PC12 cells and hippocampus HT22 cells in mice, have explored the effects of hesperidin on several types of neurodegeneration and nerve cell models.[Citation43]
Polyphenols and proteins, among other natural substances, have been investigated for years to see if they have antiviral or brain-stimulating properties. Due to its capacity to inhibit reverse transcriptase, quercetin has antiviral efficacy against RNA viruses.[Citation36] As a neuroprotective strategy, flavonoids prevent b amyloid-induced neuron damage and death via modulating pro-inflammatory cytokines and ROS generation, as well as suppressing microglia and astrocyte activation.[Citation44] Chrysin (CH) is a possible preventative drug in immunopathological and physicochemical injuries, similar to other flavonoids, due to its anti-inflammatory actions and anti-oxidative ability.[Citation45]
Flavonoids have been shown to have anti-cancer properties in breast, lung, and prostate malignancies.[Citation40] Flavonoids have been shown to be a significant influence on cancer risk alleviation.[Citation35] Food flavonoid analysis has been more popular in recent decades as flavonoids have become recognized as significant bioactive substances with substantial physiological impacts on human health.[Citation41] Protein tyrosine kinase, which is similarly involved in cell proliferation, is inhibited by genistein and quercetin.[Citation8] In the adaptive immune system, lymphocyte subpopulations play a critical role in eliciting the proper immunological responses. In all aspects of immunological responses, T helper (Th) cells play a crucial role.[Citation45]
Flavonoids are extensively recognized for their biological and pharmacological activities, including antiviral, anticarcinogenic, antioxidant, antibacterial, anti-inflammatory, anti-angiogenic, and anti-thrombogenic capabilities, among these natural bioactive substances.[Citation44] Most forms of oxidizing molecules, including singlet oxygen and other free radicals, have been proven to be highly effective scavengers of flavonoids, which may be involved in DNA damage and tumor promotion.[Citation8] Flavonoids have a wide range of cellular effects and may protect neurons from injury by modulating key neuronal signaling pathways involved in neuronal death and survival.[Citation8]
One of the most well-known flavonoids is quercetin. Quercetin has anticancer effects, according to.[Citation40] A well-known anti-inflammatory and antioxidant flavonoid, quercetin, has been demonstrated in mice with the COPD phenotype to reduce the development of rhinovirus-induced lung illness in mice, according to the study. In gastrointestinal disease, the antioxidant quercetin has shown a protective function in gastrointestinal illness by decreasing the NLRP3 stimulation after E. coli O157: H7 infection by maintaining mitochondrial viability and suppressing mitochondrial ROS, interleukin-1β and interleukin-18 release.[Citation10] In diverse models of dementia, hesperetin has significant antioxidant, anti-inflammatory, and neuroprotective properties. Citrus flavonoid like naringenin, polyphenols, hesperetin and dietary anthocyanin are absorbed by brain cells, according to Khan et al.[Citation43] Citrus peel is high in polymethoxy flavones such as tangeretin and nobiletin, and they are involved in a diverse spectrum of biological processes, including control of cancer formation caused by inflammation.[Citation44]
In vivo and in vitro studies have shown quercetin’s preventive capabilities in the treatment of neurodegeneration and cerebrovascular illness. Preventing LDL (bad) cholesterol from oxidizing is one-way quercetin defends from coronary heart disease (CHD).[Citation46] Many types of cerebrovascular disorders, particularly those linked to stroke and dementia, may be prevented by flavonoids. Peripheral blood flow and endothelial function may be affected by flavonoids.[Citation39]
The ability of flavonoids for interacting with this physiological and molecular apparatus appears to play a role in their capacity to affect this memory system.[Citation39] Flavonoids may influence the survival of cells in the peripheral nerve system (PNS) and in the central nervous system (CNS).[Citation47] Some of the flavonoids’ action methods in an inflammatory environment include acting as antioxidants, regulating gene expression (i.e., adhesion molecules, cytokines) and increasing enzyme activity.[Citation48] Due to their phenolic character, flavonoids may play a moderating function in the treatment of neurodegenerative illnesses by disrupting cellular oxidative processes in the central nervous system.[Citation49] The capacity to alter antioxidative defense in the brain, resulting in preventative benefits, was first ascribed to anthocyanins.[Citation50] Pure substances belonging to the flavonoids subclasses’ potential neuroprotective and/or therapeutic activities against neuroinflammation in neurodegenerative disorders.[Citation39] Flavonoids are found in many foods, particularly fruits and veggies, and have been proven to have a variety of biological functions for human health, including anti-inflammatory properties.[Citation44] Astrocytes, once thought to be only neuronal support cells, are now acknowledged as critical cells in the nervous system’s homeostatic and functional control.[Citation47]
Cerebral cortex, hippocampus, striatum and cerebellum, all crucial for memory and learning formation, have been revealed to contain flavonoid metabolites, which are also affected by aging and neurodegenerative diseases.[Citation50] Lowering blood pressure, increasing the bioavailability of nitric oxide, and boosting arterial flow-mediated dilation are all linked to lower cardiovascular risk in flavonoid-rich diets.[Citation51] The interaction of flavonoids with receptor molecules in the treatment of acute and chronic illnesses is a crucial field of research.[Citation34] When flavonoids were coupled with synthetic anticancer medicines, additive effects were seen.[Citation35] When flavonoids were mixed with synthetic anticancer medicines, additive effects were seen.[Citation48] Flavonoids, which are found in a variety of pharmaceutical and medical supplements, have a number of health benefits, according to.[Citation43]
Many flavonoids have low bioavailability because they are quickly digested before they can exert their antioxidant properties. Recent research reveals that flavonoids, at physiologically achievable quantities, may have a variety of functions that directly affect disease-causing pathways in neurodegenerative diseases.[Citation49] The cerebral cortex, hippocampus, striatum, and cerebellum, all crucial for memory and learning formation, have been revealed to contain flavonoid metabolites, which are also affected by neurodegenerative diseases and aging.[Citation52] Numerous studies indicate that flavonoids may play a role in diabetes therapy and also have hypoglycemic effects in a variety of animal models and therapies.[Citation53] The ingestion of flavanol-rich cocoa has the potential to significantly increase cerebral blood circulation in relation to cognitive demands.[Citation51]
Flavonoids can protect cells from injury by directly scavenging free radicals and thereby preventing their harmful effects. Endogenous antioxidants can be boosted by flavonoids, preventing the generation of reactive oxygen species (ROS) and consequent cell damage.[Citation54] Flavonoids are thought to be implicated in the role performed by plant diets in disease prevention via modulating immune function.[Citation55] The immune system is responsible for defending the human body against infectious illnesses and cancer. Flavonoids contain immuno-modulator properties and can impact the immune system response.[Citation56] Several flavonoids have been demonstrated to prevent the development of the eosinophilic inflammatory response by inhibiting these granulocytic cells’ chemoattractant factors.[Citation57] Flavonoids can reduce the oxidation of low-density lipoprotein (LDL) and hence may help to avoid atherosclerosis.[Citation54]
Flavonoids have particular immunomodulatory actions that may be important in treating a variety of malignancies. Flavonoids effect the immune system’s various compartments, including innate and acquired immune cells, antibody formation, cytokine release, and nuclear transcription factor activity.[Citation56] Additionally, we urge additional clinical trials and laboratory investigations to uncover intracellular action routes, ascertain the quantity of flavonoids necessary to achieve these effects, and ascertain the specific action of each flavonoid or flavonoid extract.[Citation48] Flavonoids have been proposed as herbal therapeutics to prevent harm caused by neutrophils, which act as the core cells in acute inflammatory processes. Anti-inflammatory cytokines aim to decrease inflammation and improve healing, while pro-inflammatory cytokines regulate host responses to immune responses and worsen the disease.[Citation45]
At the transcriptional level, the production and regulation of inflammatory cytokines can either increase or prevent the inflammatory mechanism.[Citation37] The primary mechanism by which flavonoids shield from neurodegeneration seems to be the inhibition of neuroinflammation mediated by microglia.[Citation39] To defend themselves from hazardous ROS, cells have antioxidant systems, and antioxidants must be always present in large concentrations on manufacturing facilities.[Citation49] Hesperidin, a glycosidic flavanone, can cause progenitor cells in neurons to differentiate and protect them from death by activating the MAPK and PI3K pathways.[Citation47] The ability of several flavonoids to prevent allergic reactions has been studied. Human neutrophil elastase has been demonstrated to be inhibited by a number of flavonoids. Chalcones inhibit the synthesis of elastase, NO, and superoxide anion, making them effective anti-inflammatory chemicals.[Citation57] Because flavonoid molecules impede arachidonic acid metabolism and have antiradical capabilities, they are known to have anti-inflammatory characteristics.[Citation18] According to Al-Ishaq et al.,[Citation52] flavonoids’ anti-diabetic properties help to regulate insulin signaling, carbohydrate digestion, insulin production, adipose deposition and glucose absorption.
Conclusion
It is concluded that no single extraction technique is adequate, and that every extraction methodology must be tailored to the individual plants under consideration. The use of previously optimized approaches may be used to aid the selection of suitable procedures for a certain situation. Higher yields, improved product quality, and less environmental impact may all be achieved via the use of newly developed extraction methods. We reviewed innovative extraction methods, such as UAE, MAE, SCF, and their combinations in this review (RSM combined UAE, SFMAE, PMAE, SC-CO2). Moringa leaves have been shown to protect against neurological illnesses, modulate immunological responses, Parkinson’s disease, Alzheimer’s disease, hypertension, and other disorders in animals, thanks to the bioactive components’ activity in avoiding lipid buildup and inflammation. To assess the effects of phytochemicals like flavonoids in moringa on enhancing immunity, cooperation between nutritionists, chemists, epidemiologists and physicians, food engineers, and other plant metabolism specialists is required.
Ethical approval
The study does not involve any human or animal testing.
Acknowledgments
The authors acknowledge Government College University, Faisalabad-Pakistan, for providing the facilities used for the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
References
- Vergara-Jimenez, M.; Almatrafi, M.; Fernandez, M. Bioactive Components in Moringa Oleifera Leaves Protect Against Chronic Disease. Antioxidants. 2017, 6(4), 91. DOI: 10.3390/antiox6040091.
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes. 2020, 8(10), 1222. DOI: 10.3390/pr8101222.
- Chávez-González, M. L.; Sepúlveda, L.; Verma, D. K.; Luna-García, H. A.; Rodríguez-Durán, L. V.; Ilina, A.; Aguilar, C. N. Conventional and Emerging Extraction Processes of Flavonoids. Processes. 2020, 8(4), 434. DOI: 10.3390/pr8040434.
- Nishiumi, S.; Miyamoto, S.; Kawabata, K.; Ohnishi, K.; Mukai, R.; Murakami, A.; Ashida, H.; Terao, J. Dietary Flavonoids as Cancer-Preventive and Therapeutic Biofactors (Scholar edition). Front. Biosci. 2011, 3(1), 1332–1362. DOI: 10.2741/229.
- Hamed, Y.; Abdin, M.; Akhtar, H.; Chen, D.; Wan, P.; Chen, G.; Zeng, X. Extraction, Purification by Macrospores Resin and in vitro Antioxidant Activity of Flavonoids from Moringa oliefera Leaves. South African J. Bot. 2019, 124, 270–279. DOI: 10.1016/j.sajb.2019.05.006.
- Vázquez-León, L. A.; Páramo-Calderón, D. E.; Robles-Olvera, V. J.; Valdés-Rodríguez, O. A.; Pérez-Vázquez, A.; García-Alvarado, M. A.; Rodríguez-Jimenes, G. C. Variation in Bioactive Compounds and Antiradical Activity of Moringa Oleifera Leaves: Influence of Climatic Factors, Tree Age, and Soil Parameters. Eur. Food Res. Technol. 2017, 243(9), 1593–1608. DOI: 10.1007/s00217-017-2868-4.
- Lin, M.; Zhang, J.; Chen, X. Bioactive Flavonoids in Moringa Oleifera and Their Health-Promoting Properties. J. Funct. Foods. 2018, 47, 469–479. DOI: 10.1016/j.jff.2018.06.011.
- Le Marchand, L. Cancer Preventive Effects of Flavonoids—A Review. Biomed. Pharmacother. 2002, 56(6), 296–301. DOI: 10.1016/s0753-3322(02)00186-5.
- Batra, P.; Sharma, A. K. Anti-Cancer Potential of Flavonoids: Recent Trends and Future Perspectives. 3 Biotech. 2013, 3(6), 439–459. DOI: 10.1007/s13205-013-0117-5.
- Owona, B. A.; Abia, W. A.; Moundipa, P. F. Natural Compounds Flavonoids as Modulators of Inflammasomes in Chronic Diseases. Int. Immunopharmacol. 2020, 84, 106498. DOI: 10.1016/j.intimp.2020.106498.
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess. Technol. 2011, 5(2), 409–424. DOI: 10.1007/s11947-011-0573-z.
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAc Trends Anal. Chem. 2017, 97, 159–178. DOI: 10.1016/j.trac.2017.09.002.
- Chemat, F.; E-Huma, Z.; Khan, M. K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18(4), 813–835. DOI: 10.1016/j.ultsonch.2010.11.023.
- Dadi, D. W.; Emire, S. A.; Hagos, A. D.; Eun, J. B. Effect of Ultrasound-Assisted Extraction of Moringa Stenopetala Leaves on Bioactive Compounds and Their Antioxidant Activity. Food Technol. Biotechnol. 2019, 57(1), 77–86. DOI: 10.17113/ftb.57.01.19.5877.
- Orsat, V.; Routray, W. Microwave-Assisted Extraction of Flavonoids. Water Extraction Of Bioactive Compounds. 2017, 221–244. DOI: 10.1016/b978-0-12-809380-1.00008-5.
- Upadhyay, R.; Nachiappan, G.; Mishra, H. N. Ultrasound-Assisted Extraction of Flavonoids and Phenolic Compounds from Ocimum tenuiflorum Leaves. Food Sci. Biotechnol. 2015, 24(6), 1951–1958. DOI: 10.1007/s10068-015-0257-y.
- Zahari, N. A. A. R.; Chong, G. H.; Abdullah, L. C.; Chua, B. L. Ultrasonic-Assisted Extraction (UAE) Process on Thymol Concentration from PlectranthusAmboinicus Leaves: Kinetic Modeling and Optimization. Processes. 2020, 8(3), 322. DOI: 10.3390/pr8030322.
- Routray, W.; Orsat, V. Variation of Dielectric Properties of Aqueous Solutions of Ethanol and Acids at Various Temperatures with Low Acid Concentration Levels. Phys. Chem. Liq. 2013, 52(2), 209–232. DOI: 10.1080/00319104.2013.812022.
- Bagade, S. B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2019, 51(2), 138–149. DOI: 10.1080/10408347.2019.1686966.
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S. D. Microwave-Assisted Extraction in Natural Products Isolation. Methods In Molecular Biology Natural Products Isolation. 2012, 89–115. DOI: 10.1007/978-1-61779-624-1_5.
- Rodríguez-Pérez, C. 2016. Emerging Green Technologies for the Extraction of Phenolic Compounds from Medicinal Plants. Recent Progress In Medicinal Plants. 41, 81–104. Analytical and Processing Techniques
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K. M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants. 2022, 11(19), 2623. DOI: 10.3390/plants11192623.
- Teo, C. C.; Chong, W. P.; Ho, Y. S. Development and Application of Microwave-Assisted Extraction Technique in Biological Sample Preparation for Small Molecule Analysis. Metabolomics. 2013, 9(5), 1109–1128. DOI: 10.1007/s11306-013-0528-7.
- Luque-Garcı́a, J.; Castro, M. L. Focused Microwave-Assisted Soxhlet Extraction: Devices and Applications. Talanta. 2004, 64(3), 571–577. DOI: 10.1016/j.talanta.2004.03.054.
- Gharekhani, M.; Ghorbani, M.; RASOULNEJAD, N. 2022. Microwave-Assisted Extraction of Phenolic and Flavonoid Compounds from Eucalyptus Camaldulensis Dehn Leaves as Compared with Ultrasound-Assisted Extraction. Latin American Applied Research Pesquisaaplicadalatino americana = Investigaciónaplicadalatinoamericana. 42, 305–310.
- Rodríguez-Pérez, C.; Mendiola, J.; Quirantes-Piné, R.; Ibáñez, E.; Segura-Carretero, A. Green Downstream Processing Using Supercritical Carbon Dioxide, CO2-Expanded Ethanol and Pressurized Hot Water Extractions for Recovering Bioactive Compounds from Moringa Oleifera Leaves. J. Supercrit. Fluids. 2016, 116, 90–100. DOI: 10.1016/j.supflu.2016.05.009.
- Belo, Y. N.; Al-Hamimi, S.; Chimuka, L.; Turner, C. Ultrahigh-Pressure Supercritical Fluid Extraction and Chromatography of Moringa Oleifera and Moringa Peregrina Seed Lipids. Anal. Bioanal. Chem. 2019, 411(16), 3685–3693. DOI: 10.1007/s00216-019-01850-x.
- Abhari, K.; Khaneghah, A. M. Alternative Extraction Techniques to Obtain, Isolate and Purify Proteins and Bioactive from Aquaculture and By-Products. In Advances in Food and Nutrition Research Aquaculture and By-Products: Challenges and Opportunities in the Use of Alternative Protein Sources and Bioactive Compounds, 2020; pp. 35–52. DOI: 10.1016/bs.afnr.2019.12.004.
- Dinesha, B. L.; Nidoni, U.; Ramachandra, C. T.; Naik, N. Qualitative and Quantitative Analysis of Bioactive Compounds from Supercritical Fluid and Soxhlet Extracted Moringa (Moringa Oleifera Lam.) Seed Kernel Oil. Res. J. Agric. Sci. 2016, 7, 339–343.
- Dinesha, B. L.; Nidoni, U.; Ramachandra, C. T.; Naik, N.; Sankalpa, K. Effect of Extraction Methods on Physicochemical, Nutritional, Antinutritional, Antioxidant and Antimicrobial Activity of Moringa (Moringa Oleifera Lam.) Seed Kernel Oil. J. Appl. Nat. Sci. 2018, 10(1), 287–295. DOI: 10.31018/jans.v10i1.1619.
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and Supercritical Fluid Extraction of Functional Ingredients from Different Natural Sources: Plants, Food-By-Products, Algae and microalgaeA Review. Food Chem. 2006, 98(1), 136–148. DOI: 10.1016/j.foodchem.2005.05.058.
- Karabegović, I.; Nikolova, M.; Veličković, D.; Stojičević, S. S.; Veljković, V. B.; Lazić, M. L. Comparison of Antioxidant and Antimicrobial Activities of Methanolic Extracts of the Artemisia sp. Recovered by Different Extraction Techniques. Chin. J. Chem. Eng. 2011, 19(3), 504–511. DOI: 10.1016/S1004-9541(11)60013-X.
- Rodríguez De Luna, S. L.; Ramírez-Garza, R. E.; Serna Saldívar, S. O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 1–38. DOI: 10.1155/2020/6792069.
- Panche, A. N.; Diwan, A. D.; Chandra, S. R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5. DOI: 10.1017/jns.2016.41.
- Bagwe-Parab, S.; Kaur, G.; Buttar, H. S.; Tuli, H. S. Absorption, Metabolism, and Disposition of Flavonoids and Their Role in the Prevention of Distinctive Cancer Types. Current Aspects Of Flavonoids: Their Role In Cancer Treatment. 2019, 125–137. DOI: 10.1007/978-981-13-5874-6_6.
- Noor, N.; Gani, A.; Gani, A.; Shah, A.; Ashraf, Z. U. Exploitation of Polyphenols and Proteins Using Nanoencapsulation for Anti-Viral and Brain Boosting Properties – Evoking a Synergistic Strategy to Combat COVID-19 Pandemic. Int. J. Biol. Macromol. 2021, 180, 375–384. DOI: 10.1016/j.ijbiomac.2021.03.028.
- Hošek, J.; Šmejkal, K. Flavonoids as Anti-Inflammatory Agents. Compendium Of Inflammatory Diseases. 2016, 482–497. DOI: 10.1007/978-3-7643-8550-7_19.
- Spencer, J. P. Food for Thought: The Role of Dietary Flavonoids in Enhancing Human Memory, Learning and Neuro-Cognitive Performance. Proc. Nutr. Soc. 2008, 67(2), 238–252. DOI: 10.1017/s0029665108007088.
- Spagnuolo, C.; Moccia, S.; Russo, G. L. Anti-Inflammatory Effects of Flavonoids in Neurodegenerative Disorders. Eur. J. Med. Chem. 2018, 153, 105–115. DOI: 10.1016/j.ejmech.2017.09.001.
- Koosha, S.; Alshawsh, M. A.; Looi, C. Y.; Seyedan, A.; Mohamed, Z. An Association Map on the Effect of Flavonoids on the Signaling Pathways in Colorectal Cancer. Int. J. Med. Sci. 2016, 13(5), 374–385. DOI: 10.7150/ijms.14485.
- Maleki, S. J.; Crespo, J. F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. DOI: 10.1016/j.foodchem.2019.125124.
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers. 2018, 11(1), 28. DOI: 10.3390/cancers11010028.
- Khan, A.; Ikram, M.; Hahm, J. R.; Kim, M. O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants. 2020, 9(7), 609. DOI: 10.3390/antiox9070609.
- Pan, M.; Lai, C.; Ho, C. Anti-Inflammatory Activity of Natural Dietary Flavonoids. Food Funct. 2010, 1(1), 15. DOI: 10.1039/c0fo00103a.
- Zeinali, M.; Rezaee, S. A.; Hosseinzadeh, H. An Overview on Immunoregulatory and Anti-Inflammatory Properties of Chrysin and Flavonoids Substances. Biomed. Pharmacother. 2017, 92, 998–1009. DOI: 10.1016/j.biopha.2017.06.003.
- Suganthy, N.; Devi, K. P.; Nabavi, S. F.; Braidy, N.; Nabavi, S. M. Bioactive Effects of Quercetin in the Central Nervous System: Focusing on the Mechanisms of Actions. Biomed. Pharmacother. 2016, 84, 892–908. DOI: 10.1016/j.biopha.2016.10.011.
- Matias, I.; Buosi, A. S.; Gomes, F. C. Functions of Flavonoids in the Central Nervous System: Astrocytes as Targets for Natural Compounds. Neurochem. Int. 2016, 95, 85–91. DOI: 10.1016/j.neuint.2016.01.009.
- Pérez-Cano, F.; Castell, M. Flavonoids, Inflammation and Immune System. Nutrients. 2016, 8(10), 659. DOI: 10.3390/nu8100659.
- Diniz, T. C.; Silva, J. C.; Lima-Saraiva, S. R. G. D.; Ribeiro, F. P. R. D. A.; Pacheco, A. G. M.; de Freitas, R. M.; Quintans-Júnior, L. J.; Quintans, J. D. S. S.; Mendes, R. L.; Almeida, J. R. G. D. S. The Role of Flavonoids on Oxidative Stress in Epilepsy. Oxid. Med. Cell. Longev. 2015, 2015, 1–9. DOI: 10.1155/2015/171756.
- Rendeiro, C.; Rhodes, J. S.; Spencer, J. P. The Mechanisms of Action of Flavonoids in the Brain: Direct versus Indirect Effects. Neurochem. Int. 2015, 89, 126–139. DOI: 10.1016/j.neuint.2015.08.002.
- Flanagan, E.; Müller, M.; Hornberger, M.; Vauzour, D. Impact of Flavonoids on Cellular and Molecular Mechanisms Underlying Age-Related Cognitive Decline and Neurodegeneration. Curr. Nutr. Rep. 2018, 7(2), 49–57. DOI: 10.1007/s13668-018-0226-1.
- Al-Ishaq, R. K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules. 2019, 9(9), 430. DOI: 10.3390/biom9090430.
- Zeka, K.; Ruparelia, K.; Arroo, R.; Budriesi, R.; Micucci, M. Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes. Diseases. 2017, 5(3), 19. DOI: 10.3390/diseases5030019.
- Mahmoud, A. M.; Bautista, R. J.; Sandhu, M. A.; Hussein, O. E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 1–19. DOI: 10.1155/2019/5484138.
- Peluso, I.; Miglio, C.; Morabito, G.; Ioannone, F.; Serafini, M. Flavonoids and Immune Function in Human: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2014, 55(3), 383–395. DOI: 10.1080/10408398.2012.656770.
- Jasprica, I.; Dumic, J.; Mornar, A.; Medic-Šaric, M. Immunomodulatory Effects of Flavonoids in vitro. Planta. Med. 2006, 72(11). DOI: 10.1055/s-2006-949979.
- Martínez, G.; Mijares, M. R.; Sanctis, J. B. Effects of Flavonoids and Its Derivatives on Immune Cell Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13(2), 84–104. DOI: 10.2174/1872213x13666190426164124.