Abstract
The purpose of this paper was to analyze the utilization of pulp and residues from pineapple processing as substrates for alcoholic fermentation. The fruits were cleaned, ground, and hydrolyzed with pectinase, cellulase, hemicellulase, and amylase. Pineapple pulp and whole fruit were analyzed to determine the amounts of amide, hemicellulose, total reducing sugars, °Brix, pectin, cellulose, and lignin present. Fermentation experiments were conducted with 0, 100, 200, and 300 g/kg of peel. Wines were analyzed for total or volatile acidity, total reducing sugars, and ethanol. The assays with the highest fermentation kinetic parameters were analyzed to determine the secondary compounds in the wine. The results showed that the enzymatic hydrolysis decreased the polysaccharide amount and increased reducing sugars and soluble solids, thereby improving the fermentation process, mainly in integral samples. In pineapple fermentation, 100 g/kg of peel addition did not influence the kinetic parameters and secondary compounds analyzed from the pineapple wines were within the legal limits.
El propósito del presente estudio consistió en analizar el uso de la pulpa y de los residuales descartados durante el procesado de piña, como posibles sustratos para la fermentación de alcohol. Los frutos fueron lavados, molidos e hidrolizados con pectinasa, celulasa, hemicelulasa y amilasa. Se analizaron la pulpa de la piña y la fruta entera para determinar su contenido de amida, de hemicelulasa, de azúcares reductores totales, de °Brix, de pectina, de celulosa y de lignina. Las fermentaciones se realizaron con 0, 100, 200 y 300 g/kg de cáscara. Asimismo, se examinaron los vinos con el fin de determinar la cantidad de acidez total o volátil, de azúcares reductores totales y de etanol. Los ensayos con los parámetros de fermentación cinética más altos fueron analizados con el objetivo de determinar los compuestos secundarios presentes en el vino, constatándose que la hidrólisis enzimática redujo la cantidad de polisacáridos y elevó los niveles de concentración de azúcares reductores y los sólidos solubles, lo cual mejoró el proceso de fermentación, principalmente en las muestras integrales. Se concluye que si se agrega 100 g/kg de cáscara en la fermentación de piña, no se alteran los parámetros cinéticos y, además, los compuestos secundarios analizados en los vinos de piña se mantienen dentro de los límites legales.
Palabras claves:
Introduction
Fruit is an important crop of Brazil; however, there is a high amount of waste material. This waste includes season surplus, especially since it is a degradable raw material. Therefore, large amounts of bad fruits are daily discarded because of defects in the peels, sizes, color, and consistency, among other factors. Fruit processing generates a large amount of residues such as peels, wastes, and seeds, among other parts originated from each production step, varying according to the specific product. There is thus the need to develop new processes to minimize losses and increase the small cropper’s income. Finding viable alternative to agro-industrial residues is fundamental today. One of the alternatives is fermentation to produce vinegars and liquors.
Food industries produce waste that could be reused by man and benefit the environment. They are materials that can be a source of proteins, enzymes, essential oils, and other reusable products. The utilization of residues from fruit processing in the alcoholic fermentation step can generate special, more valuable products, besides avoiding waste. Generally, sweet raw materials are good fermentation substrates. Bortolini, Sant’anna, and Torres (Citation2001) have assessed acetic fermentation in kiwi juices. Nogueira, Santos, Paganini, and Wosiacki (Citation2005) have analyzed alcoholic fermentation in apple-waste extract. Asquieri, Silva, and Cândido (Citation2009) have produced jabuticaba spirit with peel and waste from fermented jabuticaba manufacture. Silva et al. (Citation2009) have produced banana spirit with banana peel and banana pulp and tested its physicochemical and sensorial quality. Further research needs to be developed using other fruits, such as pineapple, processed worldwide.
Products obtained from the fermentation of fruit residues and fruit exceedings are an alternative for fruit croppers to minimize vintage losses, although more research is necessary considering the fact that this is a new issue for study. The production of fruit wines is already well-established, and many tropical fruits are used in the process, such as orange (Corazza, Rodrigues, & Nozaki, Citation2001), sugar–apple, mangaba, ciriguela (Muniz, Borges, Abreu, Nassu, & Freitas, Citation2002), acerola (Santos, Almeida, Toledo, Santana, & Souza, Citation2005), and jaca (Assis Neto, Cruz, Braga, & Souza, Citation2010). This study aims to quantify the secondary compounds derivable from alcoholic beverages made from different fruits. The analysis of these compounds has the purpose of checking whether the characteristics of the product stay within legal limits (Garruti, Franco, Silva, Janzantti, & Alves, Citation2003).
This work aimed to study the reuse of residues from pineapple processing in alcoholic fermentation. The main analysis persists in evaluating the effect of enzymatic hydrolysis in raw material treatment with percent-determination of residuals added to the must, which would not interfere with alcoholic fermentation’s kinetic parameters besides determining the concentration of secondary compounds in the alcoholic beverages obtained.
Materials and methods
Source of pineapples
The pineapples used in this study were of the variety ‘pérola’ (Ananas comosus (L.) merril) in the last stage of maturation obtained from local markets in Belo Horizonte, Minas Gerais (Brazil).
Sanitization and raw-material processing
The pineapples were washed in tap water, left 15 min in chloride solution, and rinsed with distilled water. The pineapple-pulp sample (PP) was obtained from peeled fruits ground in an electric mixer, whereas the integral-pineapple sample (IP) was obtained after grinding unpeeled fruits in a mixer.
Enzymatic hydrolysis
Samples of PP and IP were enzymatically hydrolyzed, producing hydrolyzed pineapple pulp (HPP) and hydrolyzed integral pineapple (HIP). Commercial enzymes (Pectinex Ultra-SP enzyme, Celluclast R, Spring alfa, and Summer Xylan) were used as shown in . Later, enzymatic deactivation was carried out for 5 min in a water bath at 90°C.
Table 1. Pulp- and integral-pineapple enzymatic hydrolysis conditions.
Tabla 1. Condiciones de la hidrólisis enzimática de pulpa de piña e piña integral.
Thermo-gravimetric and physico-chemical analyses
The samples were analyzed through thermo-gravimetry. TG/DTG curves were obtained by means of a TGA-50H scale (Shimadzu). Between 11.0 and 17.0 mg sample were used in an aluminum crucible (Al2O3) under nitrogen dynamic atmosphere (50 mL/min flow) and 10°C/min heating ratio, up to 750°C.
Pineapple pulp and integral pineapple, having undergone or not enzymatic hydrolysis, were analyzed for amide amount (IAL, Citation1985), total reducing sugars (Miller, Citation1959), pH (AOAC, Citation1992), total soluble solids (AOAC, Citation1992), pulp amount (Koch, Citation1971), hemicelluloses, pectin, lignin, and cellulose (Schieber, Fügel, Henke, & Carle, Citation2005).
Experimental design
A completely randomized design was used with three replications.
Alcoholic fermentation
Alcoholic-fermentation tests were performed in flasks Erlenmeyers with 100 mL pineapple pulp, whether peeled or not (musts), which were incubated in a heating chamber at 30°C for 24 h. A bakery jammed-ferment (Itaiquara) constituted by Saccharomyces cerevisae at the concentration of 20 g/L was used as inoculate. Musts were fermented with 100, 200, and 300 g/kg of peel with and without enzymatic hydrolysis. All assays were performed in triplicate with 120 g/L total reducing sugars.
Musts samples were collected both at time zero and by the end of fermentation, and centrifuged for 5 min at 7462 G in a Janetski centrifuge; supernatant was analyzed for acidity, total reducing sugars (TRS), and ethanol amount.
Acidity was determined through titrimetry with 0.025 M sodium hydroxide solution, according to ABNT (Citation1997). As for the determination of initial total reducing sugars (must), as well as for the final TRS (wine), the DNS method (3,5 dinitrosalicilic acid), by Miller (Citation1959), was used.
Ethanol concentration was spectrographically determined through the modified potassium dichromate method (Salik & Povoh, Citation1993). In that reaction, in acid solution, ethanol is oxidated to acetic acid, and the solution turns green and greener proportionally to ethanol concentration in the sample. Yellow bichromate ion is reduced to green chromous ion absorbing at 600 nm. Absorption is proportional both to the concentration of chromous ion formed and to oxidated ethanol (Crowell, Citation1961).
Kinetic fermentation-parameters’ calculation
The calculated parameters were productivity (g/L∙h), ethanol production (%), and yeast efficiency (%). Productivity expresses the ethanol mass produced (g) over volume (L) of the fermenting mean by time unit (h), allowing for the determination of transformation speed from sugar to ethanol. Ethanol production was determined in relation to the theoretical ethanol amount expected for the must after its sugar amount determination. The expected sugar amount was calculated considering that 1g total reducing sugars produce 0.511 g ethanol (Cardoso, Citation2006; Hashizume, Citation2001). The efficiency of the conversion substrate (total reducing sugars) to ethanol by yeast, as percentage, expresses the amount of the ethanol produced relative to the theoretical quantity expected based on the sugar content of the must.
The pineapples wines, with 100 g/kg of added peel, and without peel-addition, underwent enzymatic hydrolysis and were analyzed to determine acidity, total soluble solids, pH, alcoholic amount, and secondary compounds.
The total soluble solids amount was measured through a 0–32° Brix hand-refractometer (Instrutemp, São Paulo, Brazil) (IAL, Citation1985). Total acidity was determined through titrimetry with sodium hydroxide, according to ABNT (Citation1997). pH was determined through a digital potentiometer (IAL, Citation1985). The real alcoholic amount was determined through densitometry (ABNT, Citation1997).
Total esters (ethyl-acetate, ethyl-lactate), aldehydes (acetaldehyde), total superior alcohols (n-propanol, isobuthanol, isoamilics), o-methanol, furfural, 1-buthanol, and 2-buthanol through chromatographic analyses (GC/FID), were determined, following the Vinegars and Liquors Operational Handbook (Brasil, Citation2005). The analyses were performed in gas-chromatographer, FID detection system, mark Intercom, model Generation 8000, carbowax column 0.53 mm X 30 m X 1.00 uM, with nitrogen flux 30 mL min–1, hydrogen flux 30 mL min–1, and air flux 300 mL min−1. The injector temperature was 140°C and the detector temperature was set to 180°C. The column temperature was held for 3 min at 35°C after injection, and then programmed at 5°C min−1 from 35 to 80°C, and at 6,07°C.min−1 from 80°C until 165°C, with a total run time of 30 min. The sample was previously distilled. Quantification was carried out by integration of the peak areas using the external standardization method. The standard curve of each compound was prepared by plotting the concentration against the area. 1-Pentanol was used as internal standard.
The analyses were performed in triplicate. The kinetic parameters’ results were compared in the ANOVA/Tukey test with 5% probability margin and the results for acidity, total soluble solids amount, ART, and pH, as well as the secondary compounds, were analyzed using Student’s t-test in the SISVAR software developed by Ferreira (Citation2011).
Results and Discussion
IP presented higher hemicellulose and lignin + cellulose amount than PP. HIP presented lower lignin + cellulose, amide, and pectin amount than non-hydrolyzed integral pineapple due to the action of the enzymes used. Non-hydrolyzed samples presented a lower amount of total soluble solids (°Brix) than the hydrolyzed samples, demonstrating °Brix increase whenever the raw material undergoes enzymatic hydrolysis ().
Table 2. Characterization of the main polysaccharides from pineapple samples undergoing different treatments.
Tabla 2. Caracterización de los principales polisacáridos a partir de muestras de piña de diferentes tratamientos.
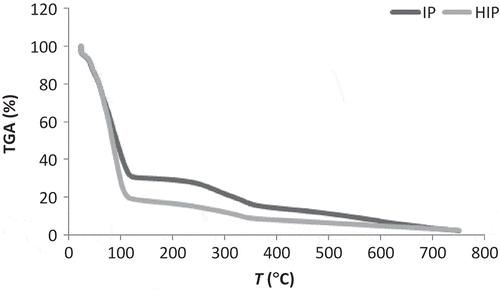
These gravimetric results indicate that both hydrolyzed and non-hydrolyzed samples undergo complete thermo-decomposition at 700°C. In the HPP curve, the hydrolysis effect was not detected, unlike HIPs, probably because of the integral sample’s higher polysaccharides concentration.
According to Aggarwal and Dollimore (Citation1998), more structural alterations occur during enzymatic hydrolysis, exposing a larger zone to heat action and consequently making thermo-degradation easier, thereby explaining the more accentuated thermo-degradation in HIP, as shown in and .
Figure 1. Mass loss from pineapple pulp (PP) and hydrolyzed pineapple pulp (HPP).
Figura 1. Pérdida de masa de la pulpa de piña (PP) y pulpa de piña hidrolizada (PPH).
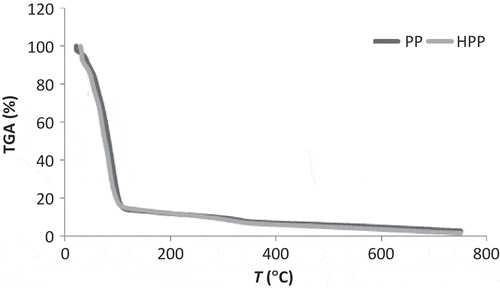
Figure 2. Mass loss from integral pineapple (IP) and hydrolyzed integral pineapple (HIP).
Figura 2. Pérdida de masa de la piña integral (PI) y piña integral hidrolizada (PIH).
Supernatant’s volumes obtained from HPP and HIP were greater than the ones obtained from PP treatments. Pulp amounts (supernatant’s volumes) from HPP and HIP exceeded those obtained from the same samples but not hydrolyzed, as shown in , demonstrating that hydrolysis decreases mean viscosity, allowing better productivity in centrifugation. This result indicates the necessity of hydrolyzing the raw material before fermentation, on behalf mainly of proceedings to be used in filtration and centrifugation.
Table 3. Supernatant’s volumes from the different treatments assessed.
Tabla 3. Volúmenes de sobrenadante de los diferentes tratamientos evaluados.
Initial must’s acidity values were 0.52 g acetic acid per 100 mL pineapple-pulp wine with 100 g/kg of peel pineapple pulp. As for fermentation with 200 and 300 g/kg of peel, acidity values were 5.5 and 5.4 g/L, respectively. Final acidity varied from 5.8 to 6.2 g/L between the treatments. Must with the largest peel amount presented slightly higher acidity values.
Total reducing sugars’ final amount varied between 6.0 and 11.3 g/L. For the wine obtained without peel, whether hydrolyzed or not, total soluble solids’ final amounts were lower than those for wines with peels addition, showing that peel makes total consummation of those solids harder during fermentation. For non-hydrolyzed must, total reducing sugars were 6.0, 6.8, 9.4, and 11.3 g/L, with 0, 100, 200, and 300 g/kg of peel, respectively. For hydrolyzed must, the concentrations were 6.7, 7.2, 8.1, and 7.1 g/L with 0, 100, 200, and 300 g/kg of peel, respectively. Musts with the largest amount of pineapple peel produced less ethanol and obtained more total reducing sugars at the end of fermentation, reflecting the yeast efficiency. shows that fermentations using the largest amounts of peel presented the lowest ethanol production.
Table 4. Means from kinetic parameters for pineapple fermentation.
Tabla 4. Medios de parámetros cinéticos para la fermentación de piña.
Fermentation’s kinetic parameters of hydrolyzed pulp, both with 100 g/kg of peel and without peel, did not differ significantly between each other, showing that 100 g/kg of pineapple-peel addition does not decrease ethanol production, not even yeast efficiency. In all fermentations, the values of kinetic parameters after hydrolysis exceeded those found for the same parameters whenever the must did not undergo hydrolysis, except for productivity after fermentation with 200 g/kg of peel.
It was observed that fermentative parameters for ethanol production and efficiency had similar results as those from sugarcane spirits (Oliveira, Rosa, Morgano, & Serra, Citation2004). Briones, Hernández, and Úbeda (Citation2002) produced melon spirits using as raw materials the whole melon, pulp, and seed, with a production lower than the production found in this work. Bortolini et al. (Citation2001) found ethanol production values between 38.65% and 47.23%, in a study on kiwi juice’s alcoholic fermentation.
The ethanol yield using commercial yeast was 94.06% in a study on banana juice’s alcoholic fermentation (Alvarenga et al., Citation2011).
The results of the analyses of acidity, total soluble solids amount, pH, and alcoholic amount are shown in .
Table 5. Acidity amount, °Brix, pH, and alcoholic amount of the pineapple wines.
Tabla 5. Tasas de la acidez, ° Brix, pH y tasa alcohólica de los fermentados de piña.
The initial acidity averages of the wines analyzed did not differ between one another, not even the acidity analyzed at the end of the process. These values stay above the acidity found for the cajá wine (2.0 g/L), assessed by Dias, Schwan, and Lima (Citation2003) and below the results found by Corazza et al. (Citation2001) for the acidity of the orange wine (8.1 g/L).
Regarding the fermented beverages of fruits, the total acidity amount must range between 3.3 and 7.8 g/L (Rizzon, Zanuz, & Manfredini, Citation1994). Comparing total acidity at the beginning and at the end of the process, it was observed that the parameter did not duplicate its value. According to Almeida, Silva, Conrado, Mota, and Freire (Citation2011), this fact indicates that there was no excessive production of organic acids; in other words, there was no bacterial contamination.
Soluble solids at the end of fermentation did not differ significantly from wines with and without pineapple peel (). Non-fermentable substances dissolved in the must can explain this result at the end of fermentation. According to Corazza et al. (Citation2001), the soluble solids are not necessarily constituted by the total amount of sugars. Similar results were found by Muniz et al. (Citation2002) after assessing the fermentation of ata, ciriguela, and mangaba.
According to , pH at the beginning and at the end of fermentation did not differ significantly (p > 0.05) for the musts assessed. The fruit wine’s pH usually oscillates between 3.0 and 4.0 (Hashizume, Citation2001), as found in this work. According to Hashizume (Citation2001), the pH value at the end of the pineapple wine fermentation without the peel is considered ideal to lessen bacterial contamination problems. Corazza et al. (Citation2001) obtained a final pH of 3.3 in orange wine. Jabuticaba pulp wines reached a final pH of 3.3 (Asquieri, Candido, Damiani, & Assis, Citation2004). As for the mangaba, ata, and ciriguela wine, Muniz et al. (Citation2002) obtained pH values of 3.21, 4.12, and 3.06 respectively. Lopes and Silva (Citation2006) obtained for fig-of-India a final pH of 3.5.
Pineapple wine added with 100 g/kg of peel presented a higher alcoholic amount. Both pineapple wines presented results within the Brazilian legal limits for liquors (Brasil, Citation1997). Muniz et al. (Citation2002) found 8.4, 9.8, and 10.1°GL for the ata, mangaba, and ciriguela wine, respectively. Arruda, Casimiro, Garruti, and Abreu (Citation2003), in a paper on banana wine processing, obtained alcoholic graduation going from 8.9 to 9.1% v/v. Reddy and Reddy (Citation2009) obtained similar results with mango wine.
The esters, aldehyde, superior alcohols, n-butylic alcohol, sec-butylic alcohol, methanol, and furfural amount are described in .
Table 6. Secondary compounds from pineapple wines.
Tabla 6. Compuestos secundarios de fermentados de piña.
Ethyl acetate concentration was higher in the pineapple wine with 100 g/kg of peel (), and the results found in this study were below those found in the fermented beverage of cajá (Dias et al., Citation2003). The high ethyl acetate amount provides an acetic taste to the wines, spoiling their quality (Rizzon et al., Citation1994). Ethyl acetate represents around 80% of the wines’ volatile esters (Almeida et al., Citation2011). Ethyl acetate concentrations between 50 and 80 mg/L contribute to the product’s aroma (Garruti et al., Citation2003). Considering the referred range suggested by Garruti et al. (Citation2003), only the alcoholic pineapple beverage with peel in the fermentation has the ideal ethyl concentration to contribute to the product’s aroma. Oliveira, Cardello, Jeronimo, Souza, and Serra (Citation2005) state that ethyl acetate concentrations below 200 mg/L provide the wine with an agreeable odor.
The acetic aldehyde amount in the wines did not differ significantly between one another (p > 0.05). Concentrations below those of the present study were found in the mandacaru-fruit bre (154.33 mg/L) assessed by Almeida et al. (Citation2011), and in the caju wine (Torres Neto, Silva, Silva, Swarnakar, & Silva, Citation2006), with a concentration of 690 mg/L. Acetaldehyde, formed along the alcoholic fermentation, is a product of the primary fermentation metabolism, unchained after the amino acids in the fermentative mean and after the ethanol oxidation. Garruti et al. (Citation2003) state that the high amount of acetaldehyde is responsible for the oxidation odor.
In pineapple wines with and without 100 g/kg of peel, the superior alcohols concentrations were below 400 mg/L, thus benefiting the product’s aroma. Concentrations above 400 mg/L contribute negatively to the wine quality, since they produce disagreeable odors (Garruti et al., Citation2003). According to the results presented in , pineapple wine without peel addition presented higher n-propilic and isobutylic amount, and a lower concentration of isoamilic alcohol. Superior alcohol concentrations in the pineapple wine were lower than the concentrations found for mandacaru wine (1905.63 mg/L) (Almeida et al., Citation2011). Superior alcohols are formed along the fermentation, out of amino acids such as leucin, isoleucin, and valin, present in the musts or resulting from the hydrolysis of yeast cells’ proteins (Garruti et al., Citation2003). The pineapple wines analyzed in this study presented lower isoamilic alcohol concentration compared with the cajá wine (Dias et al., Citation2003).
The pineapple wine without peel presented a higher methanol amount (). Normally, the methanol amounts found in fruit wines are higher in the wines from musts with higher pectin amount, since methanol originates from pectin degradation (Cardoso, Citation2006). In the case of pineapple, the pectin amount from integral pineapple (with peel) was statistically equal to the pulp pectin amount (). Therefore, methanol amount was not an issue whenever the peel was used in fermentation. Methanol concentrations were 356.77 mg/L in the mandacaru wine analyzed by Almeida et al. (Citation2011), 100 mg/L in the cashew wine (Torres Neto et al., Citation2006), and 140.5 mg/L in the grape wine (Manfroi, Miele, Rizzon, & Barradas, Citation2006).
Methanol is one of the most important components in wine, but its production is not desirable because of its toxicity (Torres Neto et al., Citation2006). Methanol is toxic to humans, severely affecting the respiratory system and may lead to blindness and death (Cardoso, Citation2006). In case its production occurs, it must not exceed the limit of 350 mg/L wine or 500 mg methanol/100 mL anhydric alcohol (Salton, Daudt, & Rizzon, Citation2000).
Conclusions
Enzymatic hydrolysis decreased polysaccharide amount and increased reducing sugars and wine’s soluble solids, thereby improving the fermentation process, mainly in integral samples. In pineapple fermentation, 100 g/kg of peel addition did not influence ethanol production and yeast’s efficiency, which indicates the possibility of using pineapple processing residues without quality losses along the alcoholic fermentation.
All the secondary compounds analyzed from the pineapple wine were within the legal limits. The 100 g/kg of peel added to the wine did not interfere negatively in the physico-chemical quality of the fermented product.
Therefore, pineapple peel can be used both by small croppers and by big processing industries to obtain distillate, vinegar, and ethanol.
Acknowledgment
The authors thank FAPEMIG for the financial support.
References
- ABNT. Associação Brasileira de Normas Técnicas. (1997). NBR 13856: acidez titulável total, volátil total e fixa. São Paulo, SP: ABNT.
- Aggarwal, P., & Dollimore, D. (1998). A thermal analysis investigation of partially hydrolyzed starch. Thermochimica Acta, 319, 17–25. doi:10.1016/S0040-6031(98)00355-4
- Almeida, M. M., Silva, F. L. H., Conrado, L. S., Mota, J. C., & Freire, R. M. M. (2011). Estudo cinético e caracterização da bebida fermentada do cereus jamacaru p. dc. Revista Verde (GVAA), 6, 176–183.
- Alvarenga, R. M., Geocze, A. C., Silva, C. M., & Oliveira, E. S. (2011). Potential application of saccharomyces cerevisiae strains for the fermentation of banana pulp. African Journal of Biotechnology, 10, 3608–3615. doi:10.5897/AJB10.1366
- AOAC. Association of official agricultural chemists. (1992). Official methods of analysis of the association of the agricultural chemists (12th ed.). Washington: AOAC.
- Arruda, A. R., Casimiro, A. R. S., Garruti, D. S., & Abreu, F. A. P. (2003). Processamento de bebida fermentada de banana. Revista Ciência Agronômica, 34, 161–167.
- Asquieri, E. R., Candido, M. A., Damiani, C., & Assis, E. M. (2004). Fabricacíon de vino blanco y tinto de jabuticaba (Myrciaria jaboticaba Berg) utilizando la pulpa y la cáscara respectivamente. Alimentaria, 355, 97–109.
- Asquieri, E. R., Silva, A. G. M., & Cândido, M. A. (2009). Aguardente de jabuticaba obtida da casca e borra da fabricação de fermentado de jabuticaba. Ciência E Tecnologia De Alimentos, 29, 896–904. doi:10.1590/S0101-20612009000400030
- Assis Neto, E. F., Cruz, J. M. P., Braga, A. C. C., & Souza, J. H. P. (2010). Elaboração de bebida alcoólica fermentada de jaca (Artocarpus heterophyllus Lam.). Revista Brasileira De Tecnologia Industrial, 4, 186–197. doi:10.3895/S1981-36862010000200007
- Bortolini, F., Sant’anna, E. S., & Torres, R. C. (2001). Behaviour of alcoholic and acetic fermentations of kiwi mashes (Actinidia deliciosa); composition of mashes and production methods. Ciência E Tecnologia De Alimentos, 21, 236–243. doi:10.1590/S0101-20612001000200020
- Brasil, Decreto nº 2314. (1997). Dispõe sobre a padronização, a classificação, o registro, a inspeção, a produção e a fiscalização de bebidas. Diário Oficial da República Federativa do Brasil.
- Brasil, Instrução normativa n.24 (2005). Aprova o manual operacional de bebidas e vinagres. Brasília: Ministério da Agricultura, Pecuária e Abastecimento.
- Briones, A. I., Hernández, L. F., & Úbeda, J. F. (2002). Elaboración de aguardiente de melón. Alimentación Equipos Y Tecnología, 171, 47–52.
- Cardoso, M. G. (2006). Produção de aguardente de cana (2nd ed.). Lavras, MG: UFLA.
- Corazza, M. L., Rodrigues, D. G., & Nozaki, J. (2001). Preparação e caracterização do vinho de laranja. Química Nova, 24, 449–452. doi:10.1590/S0100-40422001000400004
- Crowell, E. A. (1961). Techniques for studying the mechanism of higher alcohol formation by yeast. American Journal of Enology and Viticulture, 12, 111–116.
- Dias, D. R., Schwan, R. F., & Lima, L. C. O. (2003). Metodologia para elaboração de fermentado de cajá (Spondias mombin L.). Ciência E Tecnologia De Alimentos, 23, 342–350. doi:10.1590/S0101-20612003000300008
- Ferreira, D. F. (2011). Sisvar: um sistema computacional de análise estatística. Ciência E Agrotecnologia, 35, 1039–1042. doi:10.1590/S1413-70542011000600001
- Garruti, D. S., Franco, M. R. B., Silva, M. A. A. P., Janzantti, N. S., & Alves, G. L. (2003). Evaluation of volatile flavour compounds from cashew apple (Anacardium occidentale L) juice by the osme gas chromatography/olfactometry technique. Journal of the Science of Food and Agriculture, 83, 1455–1462. doi:10.1002/jsfa.1560
- Granotec do Brasil, S. A. (2009). Nutrição e Biotecnologia. Pesquisa e Desenvolvimento. Regulamentação técnica. Curitiba: Granotec. 20p.
- Hashizume, T. (2001). Tecnologia do vinho. In E. Aquarone, W. Borzani, W. Schmidell, & U. A. Lima (Coords.), Biotecnologia industrial: Biotecnologia na produção de alimentos. São Paulo: Edgard Blucher.
- IAL – Instituto Adolfo Lutz. (1985). Normas analíticas do instituto adolfo lutz: métodos químicos e físicos para análise de alimentos (3rd ed.). São Paulo: IAL.
- Koch, J. H. D. (1971). Determination of falsified orange juice. Deutsche Lebensmittel-Rundschau, 6, 185–195.
- Lopes, R. V. V., & Silva, F. L. H. (2006). Elaboração de fermentados a partir de figo-da-índia. Revista De Biologia E Ciência Da Terra, 6, 305–315.
- Manfroi, L., Miele, A., Rizzon, L. A., & Barradas, C. I. N. (2006). Composição físico-química do vinho cabernet franc proveniente de videiras conduzidas no sistema lira aberta. Ciência E Tecnologia De Alimentos, 26, 290–296. doi:10.1590/S0101-20612006000200010
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428. doi:10.1021/ac60147a030
- Muniz, C. R., Borges, Mr. F., Abreu, F. A. P., Nassu, R. T., & Freitas, C. A. S. (2002). Bebidas fermentadas a partir de frutos tropicais. Boletim Do CEPPA, 20, 309–322.
- Nogueira, A., Santos, L. D., Paganini, C., & Wosiacki, G. (2005). Avaliação da fermentação alcoólica do extrato de bagaço de maçã. Semina Ciências Agrárias, 6, 187–194. doi:10.5433/1679-0359.2005v26n2p187
- Oliveira, E. S., Cardello, H. M. A. B., Jeronimo, E. M., Souza, E. L. R., & Serra, G. E. (2005). The influence of different yeasts on the fermentation, composition and sensory quality of cachaça. World Journal of Microbiology and Biotechnology, 21, 707–715. doi:10.1007/s11274-004-4490-4
- Oliveira, E. S., Rosa, C. A., Morgano, M. A., & Serra, G. E. (2004). Fermentation characteristics as criteria for selection of cachaça yeast. World Journal of Microbiology and Biotechnology, 20, 19–24. doi:10.1023/B:WIBI.0000013286.30695.4e
- Reddy, L. V. A., & Reddy, O. V. S. (2009). Effect of enzymatic maceration on synthesis of higher alcohols during mango wine fermentation. Journal of Food Quality, 32, 34–47. doi:10.1111/j.1745-4557.2008.00234.x
- Rizzon, L. A., Zanuz, M. C., & Manfredini, S. (1994). Como elaborar vinho de qualidade na pequena propriedade (3 rd ed.). Embrapa: Bento Gonçalves.
- Salik, F. L. M., & Povoh, N. P. (1993). Método espectrofotométrico para determinação de teores alcoólicos em misturas hidroalcoólicas. Congresso Nacional da Stab, Águas de São Pedro.Piracicaba, Brasil, Anais do Congresso Nacional da Stab. p. 262–266.
- Salton, M. A., Daudt, C. E., & Rizzon, L. A. (2000). Influência do dióxido de enxofre e cultivares de videira na formação de alguns compostos voláteis e na qualidade sensorial do destilado de vinho. Ciência E Tecnologia De Alimentos, 20, 302–308. doi:10.1590/S0101-20612000000300005
- Santos, S. C., Almeida, S. S., Toledo, A. L., Santana, J. C. C., & Souza, R. R. (2005). Elaboração e análise sensorial do fermentado de acerola. Brazilian Journal of Food Technology, 5a SIPAL, 47–50.
- Schieber, A., Fügel, R., Henke, M., & Carle, R. (2005). Determination of the fruit content of strawberry fruit preparations by gravimetric quantification of hemicellulose. Food Chemistry, 91, 365–371. doi:10.1016/j.foodchem.2004.09.003
- Silva, M. B. L., Chaves, J. B. P., Lelis, V. G., Alvarenga, L. M., Zuim, D. R., & Silva, P. H. A. (2009). Qualidade fisico quimica e sensorial de aguardentes de polpa de banana e banana integral submetidas à hidrólise enzimática. Alimentos & Nutrição, 20, 217–221.
- Torres Neto, A. B., Silva, M. E., Silva, W. B., Swarnakar, R., & Silva, F. L. H. (2006). Cinética e caracterização físico-química do fermentado do pseudofruto do caju (Anacardium occidentale L.). Química Nova, 29, 489–492. doi:10.1590/S0100-40422006000300015
- Yuliarti, O., Matia-Merino, L., Goh, K. T., Mawson, J., & Brennan, C. (2011). Effect of Celluclast 1.5L on the Physicochemical Characterization of Gold Kiwifruit Pectin. International Journal of Molecular Sciences, 12, 6407–6417. doi:10.3390/ijms12106407
