Abstract
The present study was undertaken to investigate the antioxidant, anticancer, and cytotoxic properties of 18 ethanol extracts of the plant-based preparations described in Thai Pharmaceutical Textbook and locally used as rejuvenators. ‘Tri-Su-Ra-Phon’, ‘Tri-Sa-Mo’, ‘Jatu-Pha-La-Ti-Ga’, and ‘Nava-Kot’ extracts had much higher antioxidant activities than those of standard trolox and ascorbic acid. The percentage inhibitions, using 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) and 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)methods, of these extracts were found to be approximately 73.33 ± 6.01–93.50 ± 0.30% and 73.71 ± 2.77–101.86 ± 0.54%, respectively. ‘Tri-Su-Ra-Phon’ extract presented the highest phenolic and flavonoid contents of 1373.40 mg gallic acid equivalent/g of extract and 4290.60 mg catechin equivalent/g of extract, respectively. Moreover, ‘Jatu-Pha-La-Ti-Ga’, ‘Tri-Sa-Mo’, and ‘Tri-Su-Ra-Phon’ exhibited significant anticancer activity against human breast cancer cell lines with selectivity indexes of 3–12. Based on this information, it could be concluded that ‘Jatu-Pha-La-Ti-Ga’, ‘Tri-Sa-Mo’, and ‘Tri-Su-Ra-Phon’ have great potential to be used in the development of functional beverages that are currently in demand for health benefits.
El presente estudio se llevó a cabo para investigar las propiedades antitóxicas, anticancerígenas y citotóxicas de 18 extractos en etanol de las preparaciones herbales descritas en el Guía Farmacéutico Tailandés y utilizadas localmente como rejuvenecedoras. Los extractos de «Tri-Su-Ra-Phon», «Tri-Sa-Mo», «Jatu-Pha-La-Ti-Ga» y «Nava-Kot» presentaron actividades antioxidantes más elevadas que aquellas de trolox estándar y de ácido ascórbico. Los porcentajes de inhibición de dichos extractos obtenidos por los métodos DPPH y ABTS, fueron aproximadamente 73,33 ± 6,01–93,50 ± 0,30% y 73,71 ± 2,77–101,86 ± 0,54%, respectivamente. El extracto «Tri-Su-Ra-Phon» presentó contenidos de fenólico y de flavonoide de 1373,40 mg de ácido gálico equivalente/g de extracto y 4290,60 mg de catequinas equivalente/g de extracto, respectivamente. Además, «Jatu-Pha-La-Ti-Ga», «Tri-Sa-Mo» y «Tri-Su-Ra-Phon» mostraron tener actividad anticancerígena significativa contra líneas celulares de cáncer mamario con índices de selectividad de 3–12. Con base en esta información, puede concluirse que «Jatu-Pha-La-Ti-Ga», «Tri-Sa-Mo» y «Tri-Su-Ra-Phon» podrán utilizarse potencialmente para la elaboración de bebidas funcionales, las cuales están siendo demandadas actualmente por los beneficios que aportan a la salud.
Introduction
Reactive oxygen species and reactive nitrogen species are generated during metabolism and other activities in a biological system, such as reactions catalyzed by the electron carriers in the mitochondria, irradiation by ultraviolet light, X-rays, and gamma rays, reactions catalyzed by metals, and inflammation process, etc. They have sparked great interest in biomedical research because an over-production of these reactive species in biological systems beyond antioxidant defense systems results in oxidative stress and causes oxidative damage to biomolecules (Cárdenas-Rodríguez et al., Citation2013; Valko, Rhodes, Moncol, Izakovic, & Mazur, Citation2006). This imbalance between free radicals and the antioxidants has been proposed as one of the mechanisms that correlate with many chronic diseases, including cancer (Sosa et al., Citation2013), coronary heart diseases (Juni, Duckers, Vanhoutte, Virmani, & Moens, Citation2013), diabetes, neurodegenerative diseases, and aging (Gutowski & Kowalczyk, Citation2013). Therefore, several studies have claimed that the consumption of herbs, vegetables, and fruits containing antioxidants might protect the human body against these diseases (Govindarajan, Vijayakumar, & Pushpangadan, Citation2005; Lambert & Elias, Citation2010; Tripathi & Chandra, Citation2009).
There has been much interest in research on phytochemicals of medicinal plants and their roles in nutrition in recent years. In addition, it is generally accepted that antioxidants from natural products have efficacy and safety advantages when compared to synthetic products (Karre, Lopez, & Getty, Citation2013). In Thailand, a large number of plants that are consumed as food and herbs have been reported as sources of natural antioxidants. Previous studies have consistently shown that some of Thai edible and medicinal plants have strong antioxidant activity and may play a crucial role in the prevention of some chronic diseases. For example, ‘mao luang’ (Antidesma thwaitesianum), which is commonly consumed as juice and wine in Thailand, has been reported to have various cytoprotective effects along with antioxidant and antiinflammatory activities (Puangpronpitag, Areejitranusorn, Boonsiri, Suttajit, & Yongvanit, Citation2008; Puangpronpitag et al., Citation2011). Xanthones isolated from mangosteen (Garcinia mangostana) possess a wide spectrum of biological properties, including antioxidant and antitumor activities (Buelna-Chontal, Correa, Hernández-Reséndiz, Zazueta, & Pedraza-Chaverri, Citation2011; Kondo, Zhang, Ji, Kou, & Ou, Citation2009). Gynura pseudochina, Oroxylum indicum, and Muehlenbeckia platyclada, which are generally used in Thai antiinflammatory remedies, exhibited in vitro antiinflammatory, antiproliferative, and antioxidant activities (Siriwatanametanon, Fiebich, Efferth, Prieto, & Heinrich, Citation2010).
Even though herbal preparations are widely used and consumed in Thailand, there is very little evidence available to support their medical claims. ‘Phigut Ya’ is a group of traditional preparations consisting of equal parts of medical substances (‘Phigut’ means a group of medical substances and ‘Ya’ means medicines). There are 72 different types of remedies described in Thai Pharmaceutical Textbook. The herbal formulations are commonly consumed as tea or added to Thai ancient household remedies (Picheansoonthon & Jerawong, Citation2001).
In order to confirm the therapeutic potential of the preparations and to select effective preparations for future development as functional food and beverages, 18 preparations with health-rejuvenation and nourishment uses were selected from Thai Pharmaceutical Textbook and assessed for their antioxidant, anticancer, and cytotoxic effects. The presence of plant-derived bioactive chemicals, such as phenolics and flavonoids, was correlated with multiple biological and antioxidant effects. Therefore, the relationship between chemical constituents and antioxidant activity of these preparations was additionally determined.
Materials and methods
Plant materials and preparation of extracts
Plant parts () were purchased from a licensed traditional medical drug store, Triburi Orsot in Songkla, Thailand. Reference specimens of the materia medica were deposited at the Faculty of Traditional Thai Medicine, Prince of Songkla University, Thailand. This study reports 18 plant-based formulations containing 49 species belonging to 28 families, and the majority of these medicinal plants belong to Umbelliferae (8 species), Compositae (3 species), Lauraceae (3 species), Euphorbiaceae (3 species), Piperaceae (3 species), and Combretaceae (3 species) families. Different plant parts, such as root, rhizome, wood, fruit, mace, leaf, and flower, were used to prepare the remedies with fruits, flowers, and root being the most commonly used plant material. The most frequently mentioned medicinal plants used as household remedies were Nelumbo nucifera (found in four remedies: ‘Bua-Tung-Ha’, ‘Ga-Son-Tung-Ha’, ‘Ga-Son-Tung-Jed’, and ‘Tri-Ke-Son-Mat’) and Terminalia chebula (found in four remedies: ‘Tri-Chin-Tha-La-Ma-Ka’, ‘Tri-Sa-Mo’, ‘Nava-Kot’, and ‘Jatu-Pha-La-Ti-Ga’).
Table 1. Herbal components, medical application and extraction yield of selected Thai traditional ancient remedies.
Tabla 1. Componentes herbales, aplicación médica y rendimiento de extracción de algunos antiguos remedios tradicionales tailandeses.
These plants were cleaned by distilled water and dried at 60°C in an air blowing thermostatic oven. Described formulations that consist of equal amounts (150 g) of their medicinal plant components as shown in were finely powdered and passed through a 16 mesh sieve prior to extraction. The powdered medicine (200 g) was extracted with 95% ethanol (600 mL) for 7 days. After filtration through a Whatman No. 1 filter paper, ethanol filtrates were removed with a rotatory evaporator, kept at 55°C until they were completely dry. Yields (%; w/w) of each of the extracts were calculated as the ratio of the weight of the extract to the weight of the recipe powder and presented in . Dried extracts were stored in a sterile screw-capped bottle at −20°C until analyzed.
Determination of antioxidant activity using the DPPH method
DPPH (Merck, USA)-based radical scavenging activity of the extracts was determined utilizing the free-radical scavenging activity in accordance with the method of Yao, Sang, Zhou, and Ren (Citation2010) with modifications. Hydrogen-donating antioxidants were able to reduce purple-colored radical DPPH into the yellow-colored DPPH-H, resulting in a color change of the tested solution from purple to yellow. An aliquot of 20 μL of the formula extracts at different concentrations ranging from 6.25 to 100 µg/mL was mixed with 180 μL of DPPH solution and then incubated at room temperature for 30 min in the dark. The decrease in absorbance of the resulting solution was monitored at 492 nm after 10 min. The results were corrected for dilution and expressed as DPPH-based scavenging activities (%). These activities were calculated using the following equation: Scavenging activity (%) = [(A − B) × 100]/A, where A is the optical density of the control solution and B is the optical density of the test solution. All determinations were performed in triplicate (n = 3). Ascorbic acid (Sigma-Aldrich Chemic GmbH, Spain) was used as a positive control and 1% dimethylsulfoxide (DMSO; Sigma-Aldrich, USA) was added as a negative control.
Evaluation of antioxidant activity using the ABTS˙+ method
The ABTS˙+ method is based on the ability of antioxidants to quench colored radical cation ABTS, and was previously used by Yao et al. (Citation2010) with modifications to evaluate the free-radical scavenging activity of the recipe extract. Briefly, the ABTS˙+ radical cation was produced by reacting distilled water-dissolved ABTS (Calbiochem, Germany) stock solution at a concentration of 7 mM with potassium persulphate (K2S2O8; Ajax Finechem, New Zealand) at a final concentration of 9.9 mM. The solution was kept at room temperature in the dark for 12–16 h before use. The resulting ABTS˙+ solution was diluted with distilled water to an absorbance of 0.70 (±0.02) at 734 nm. An aliquot of 30 µL of the tested extract at concentration of 0.63 mg/mL was added into 3 mL of the diluted ABTS˙+ solution, the absorbance at 734 nm was then taken exactly 6 min after initial mixing. ABTS˙+-based scavenging activities that resulted in a decrease of absorbance were corrected for dilution and reported as percentage of free-radical scavenging activity using the equation described above. All determinations were performed in triplicate (n = 3). Trolox (Merck, Germany) was used as a positive control.
Determination of total phenolic content
The total phenolic content was measured by Folin–Ciocalteu method. Briefly, a 360 µl of the methanol-dissolved extract (2.5 and 0.2 mg/mL) was mixed with 3 mL of Folin–Ciocalteu’s phenol reagent (Merck Chemical Supplies, Germany). After 5 min, Na2CO3 (Ajax Finechem, New Zealand) solution (3 mL; 20%w/v) was added, and the mixture was mixed thoroughly and kept in the dark at room temperature for 90 min before the absorbance was read at 725 nm. The total phenolic content was quantified from extrapolation of calibration curve of gallic acid (0.16–2.5 mg/mL; Sigma-Aldrich Chemie, Germany). The assessment of the phenolic compounds was carried out in triplicate and the mean of the results expressed as milligrams of gallic acid equivalents per gram of tested extract (Wojdylo, Oszmianski, & Czemerys, Citation2007).
Determination of total flavonoids
The total flavonoid content was determined by the Dowd method as modified by (Park et al., Citation2008). In a 10 mL volumetric flask, an aliquot of 1 mL of the methanol-dissolved extracts (100 or 60 µg/mL) was mixed with 4 mL of distilled water, 300 µL of 5% (w/v) of NaNO2 (Ajax Finechem, New Zealand), and 300 µL of 10% (w/v) aluminium trichloride (AlCl3·6H2O; Ajax Finechem, New Zealand). After 6 min, 2 mL of NaOH (1 M) was added to the mixture, followed by the addition of 2.4 mL of distilled water and mixed thoroughly. Absorption readings at 510 nm were taken after 10 min against the reagent blank. The standard curve for total flavonoids was made using catechin standard solution (20 to 100 µg/mL) under the same procedure as described earlier. The assessment of total flavonoids was carried out in triplicate and the mean of the results was expressed as milligrams of catechin equivalents per gram of tested extract.
Anticancer and cytotoxic effects
Cytotoxic activities of the recipe extracts against Vero cells and MCF-7 human breast cancer cells were determined by green fluorescent protein (GFP)-based assay at the National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency, Pathumthani, Thailand. Ellipticine, tomoxifen, and doxorubicin were used as positive controls (Isaka, Palasarn, Kocharin, & Saenboonrueng, Citation2005).
Results and discussion
The total antioxidant capacities of the herbal formulations
Plant-based remedies produce significant amount of phyto-antioxidants to prevent the oxidative stress that is implicated in the process of aging and the pathogenesis of diseases (Essa et al., Citation2012; Lofano et al., Citation2013). They represent a potential source for the development of antioxidant-rich beverages or food. Therefore, antioxidant properties of different polyherbal formulations described in Thai Pharmaceutical Textbook as rejuvenators are discussed below.
The results of DPPH and ABTS˙+-based scavenging activities by different remedy ethanol extracts are summarized in . There are remarkable differences in the percentages of scavenging capacity among the tested formulations ranging from 6 to 93 and 12 to 100 for DPPH and ABTS˙+ radicals, respectively. It is encouraging to note that 8 out of 18 extracts tested were found to exhibit potent free-radical scavenging activities, where six of these recipe extracts almost completely inhibited both DPPH and ABTS˙+ radicals (70–100%). Increasing the concentrations of the remedy extracts resulted in the increase of the radical scavenging activities. High DPPH radical scavenging activities were found in ‘Tri-Sa-Mo’, ‘Jatu-Pha-La-Ti-Ga’, and ‘Nava-Kot’, with the percentage inhibition at 93.50 ± 0.30, 90.96 ± 5.69, and 90.16 ± 0.45, respectively. ‘Tri-Sa-Mo’, ‘Jatu-Pha-La-Ti-Ga’, and ‘Tri-Su-Ra-Pon’ exhibited remarkable percentages of ABTS˙+ scavenging capacity at 100.23 ± 0.19, 101.86 ± 0.54, and 100.34 ± 0.22, respectively.
Table 2. DPPH and ABTS radical scavenging capacities, total phenolic and flavonoids contents of ethanol extracts of Thai traditional herbal formulations.
Tabla 2. Capacidad de eliminación de los radicales DPPH y ABTS, compuestos fenólicos totales y flavonoides totales en extractos en etanol de algunos antiguos remedios tradicionales tailandeses.
Although there is no evidence on antioxidant activity of herbal preparations consumed as rejuvenators in Thai traditional medicine, it has been shown in several studies that plant-based Rasayana medicines, which are rejuvenative recipes in the Indian traditional medical system, also known as Ayurveda, have immune-modulatory, antioxidant, antitumor effects, etc. (Govindarajan et al., Citation2005). ‘Tri-Sa-Mo’, ‘Tri-Su-Ra-Pon’, and ‘Jatu-Pha-La-Ti-Ga’ have been shown to be remarkable DPPH and ABTS free-radical scavengers. ‘Tri-Sa-Mo’ consists of Terminalia chebula, Terminalia arjuna, and Terminalia bellerica and ‘Jatu-Pha-La-Ti-Ga’ constists of the Terminalia species and Phyllanthus emblica. Some health benefits of the medicinal plants, such as their antioxidant, antiinflammatory, anti-mutagenic, cardioprotective, radioprotective, and hepatoprotective properties, have been reported (Bandyopadhyay, Pakrashi, & Pakrashi, Citation2000; Ingkaninan, Temkitthawon, Chuenchom, Yuyaem, & Thongnoi, Citation2003; Mahesh, Bhuvana, & Begum, Citation2009; Raghavan & Kumari, Citation2006). Medicinal plants have been used in Rasayana preparations, which were also found to contain potent free-radical scavengers such as Triphala (a composite Indian drug) (Naik et al., Citation2005), Vayasthapana Rasayana (Mukherjee et al., Citation2011), Brahma Rasayana (Guruprasad et al., Citation2012), and Amalakayas Rasayana (Samarakoon, Shukla, & Chandola, Citation2011). Furthermore, these in vivo studies demonstrated that the Rasayana preparations also possessed antiangiogenic and immunostimulatory properties. Various extracts of Terminalia spp. fruits were reported to be potent antioxidants and recently found to have in vitro iron chelating properties (Pfundstein et al., Citation2010). For example, hydroalcoholic extract of Terminalia chebula exhibited radical scavenging activity in DPPH assay with IC50 value of 42.14 µg/mL and contained phenolic concentration of 118.5 mg gallic acid equivalent/g of extract (Bag, Kumar Bhattacharyya, Kumar Pal, & Ranjan Chattopadhyay, Citation2013), which is lower than those of ‘Tri-Sa-Mo’ and ‘Jatu-Pha-La-Ti-Ga’. Therefore, it is possible that when all the ingredients are made into a single formulation, the ingredients may act synergistically in a potent phytochemical combination with more antioxidant activity than the activity of the isolated ingredients. Similarly, a previous study suggested that Triphala was more effective as a chemopreventive agent than its individual components, suggesting an additive effect of the medicinal plant (Deep, Dhiman, Rao, & Kale, Citation2005). Antagonistic effects may also occur; thus, the determination of antioxidant capacities of isolated compounds and compound combinations, which are currently being examined in our laboratory, may support the hypothesis.
This study for the first time has found promising antioxidant effects of ‘Tri-Su-Ra-Pon’, which is a polyherbal preparation composed of Cinnamomum bejolghota, Aquilaria malaccensis, and Cinnamomum porrectum. Antioxidant activities of its medicinal plant components have been studied in a few cases. Cinnamomum bejolghota, which is widely used in Thai foods, presented flavonoid and phenolic contents of 0.11 mg catechin equivalent/g sample and 1.34 mg gallic acid equivalent/g sample, respectively (Settharaksa, Jongjareonrak, Hmadhlu, Chansuwan, & Siripongvutikorn, Citation2012). Water extract of this plant yielded radical scavenging activity in DPPH assay of 17.40 mg gallic acid equivalent/g. Tea made from steamed leaves of Cinnamomum porrectum showed total phenolic content of 708.80 µmol gallic acid equivalent/g sample and displayed radical scavenging activity in DPPH assay of 407.68 µmol trolox/g sample (Pornthip, Worapong, & Sunisa, Citation2013). Huda, Munira, Fitrya, and Salmah (Citation2009) revealed that an ethanol extract made from leaves of Aquilaria malaccensis demonstrated DPPH free-radical scavenging activity with an IC50 value of 30 μg/ml. Though these plants generally possess antioxidant activity, no information is currently available regarding their in vivo and ex vivo antioxidant activities as well as possible toxicity.
Correlation of antioxidant activity and bioactive compounds
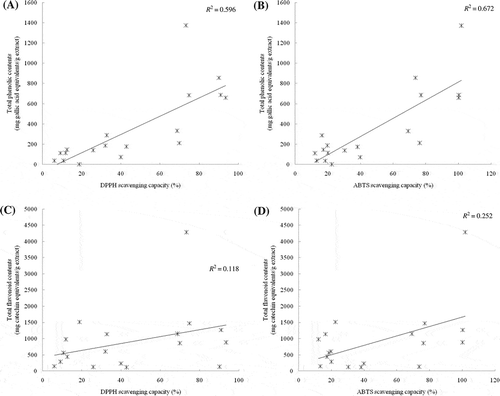
Since the structural features of flavonoids and phenolic compounds are reportedly responsible for antioxidant activity, measurements of total phenolic and flavonoid content in these extracts may be related to their antioxidant properties. As shown in , total phenolic and flavonoid content varied widely in different recipes and ranged from 0.99 to 1373.40 mg gallic acid equivalent/g of extract for phenolic content and 134.62 to 4290.60 mg catechin equivalent/g of extract for flavonoids. The ethanol extract of Tri-Su-Ra-Pon, which consists of Cinnamomum bejolghota, Aquilaria malaccensis, and Cinnamomum porrectum, possesses the highest phenolic and flavonoid content. shows the correlation between the antioxidant activities and the total phenolic compounds and flavonoid content of the 18 recipes tested in this study. Results revealed a good correlation between free-radical scavenging activities and the total phenolic compounds (R2 = 0.596 and 0.672 for DPPH and ABTS˙+ radical scavenging activities, respectively). However, this work reported weak correlation between free-radical scavenging activities and flavonoid content (R2 = 0.118 and 0.252 for DPPH and ABTS˙+ radical scavenging activities, respectively).
Figure 1. Correlation between free-radical scavenging activity (DPPH and ABTS) and total phenolic ((A) and (B)) and flavonoid ((C) and (D)) contents of selected Thai traditional ancient remedies.
Figura 1. Correlación entre la actividad de eliminación de radicales libres (DPPH y ABTS) y el contenido total de fenólicos ((A) y (B)) y de flavonoides ((C) y (D)) de algunos antiguos remedios tradicionales tailandeses.
As reported by Cai, Sun, Xing, Luo, and Corke (Citation2006), the presence of different categories of phenolics resulted in differences in radical scavenging activities. The activity of phenolic acids depends on the number and position of hydroxyl (–OH) groups and methoxy (–OCH3) substituents, while the activity of tannins depends on galloyl group and ortho-hydroxyl structure. Furthermore, both condensed and hydrolyzable tannins demonstrated a stronger antioxidant activity than that of quinones, isoflavones, and lignans (Cai et al., Citation2006). Phenolics are also known to play an important role in stabilizing lipids against peroxidation and inhibiting various types of oxidizing enzymes (Serafini, Laranjinha, Almeida, & Maiani, Citation2000). The roles of flavonoids as antioxidants are well described in food systems and clinical studies, as reviewed by Nijveldt et al. (Citation2001). Flavonoids exhibit wide variations in free-radical scavenging activities. Flavanols that contain five to eight hydroxyl groups, especially with ortho-(3′,4′-)dihydroxy groups in the B-ring and 3-hydroxyl group and/or 3-galloyl group in the C-ring, have very strong activity (Nijveldt et al., Citation2001). However, flavanones and isoflavones which have the 2, 3-double bond in the C-ring and do not have the 3–OH group in the C-ring appeared to be inactive (Cai et al., Citation2006). Additionally, many reports on antioxidant constituents found that flavonol aglycones such as quercetin, myricetin, and kaempferol have higher antioxidant activity than their glycosides such as rutin, myricitrin, and astragalin (Cai, Luo, Sun, & Corke, Citation2004; Cai et al., Citation2006).
A good correlation between total phenolic contents and the antioxidant activity of tested extracts was observed, but we found a weak correlation between total flavonoid contents and the activity of the extracts. This implies that the antioxidant activity of these herbal formulations should be attributed to the presence of total phenolics, not to that of total flavonoids. This weak correlation between total flavonoid contents and free-radical scavenging activity might be attributed to the fact that the flavonoids in these herbal formulations are in the glycoside form, flavanones or isoflavones (Cai et al., Citation2004, Citation2006). Similar results were also found in Atractylodes macrocephala extract which exhibit weak correlation with total flavonoid content in radical-scavenging assays, while show good correlation in the chelating assays (Li et al., Citation2012).
Cytotoxic effects of the herbal recipes
It is well recognized that phenolics that have their own antioxidant activity also have anticancer activity (Darvesh & Bishayee, Citation2013; Deep et al., Citation2005). Therefore, the ethanol extracts of tested plant-based formulations were evaluated for their antiproliferative activity on both breast cancer cell line (MCF-7) and normal mammalian cells (Vero cells). Positive controls, ellipticine exhibited cytotoxicity against Vero cells with an IC50 value of 0.48 μg/mL. Tomoxifen and doxorubicin displayed cytotoxicity on human breast cancer cells with IC50 values of 8.96 and 9.49 μg/mL, respectively. Five extracts including ‘Tri-Chin-Tha-La-Ma-Ka’, ‘Tri-Sa-Mo’, ‘Tri-Su-Ra-Pon’, ‘Jatu-Pha-La-Ti-Ga’, and ‘Nava-Kot’ demonstrated growth inhibitory activity on the MCF-7 with IC50 values of 4.63, 5.06, 16.39, 4.01, and 15.07 µg/mL, respectively, indicating that these recipes are relatively active on the cancer cells (). With exception of Tri-Chin-Tha-La-Ma-Ka, these formulations exhibited activity against the normal cells with an IC50 value of >50 µg/mL, which was higher than the IC50 values obtained with the cancer cell lines and higher than that of the drugs ellipticine, tomoxifen, and doxorubicin. The selectivity index (SI) values for the MCF-7 cells of ‘Tri-Sa-Mo’, ‘Tri-Su-Ra-Pon’, ‘Jatu-Pha-La-Ti-Ga’, and ‘Nava-Kot’ were at least 9.88, 3.05, 12.45, and 3.31, respectively, suggesting that the recipes show a promising specificity for the breast cancer cell lines. Similarly, Mahavorasirikul, Viyanant, Chaijaroenkul, Itharat, and Na-Bangchang (Citation2010) have demonstrated that a Thai folklore recipe, ‘Pra-Sa-Prao-Yhai’, and its medicinal plant components, Atractylodes lancea, Zingiber officinal, Piper chaba, also show promising and selective cytotoxic activity with SI of 8.6–12.5 against cholangiocarcinoma and HepG2 cells. Moreover, the IC50 value of ‘Tri-Sa-Mo’ and ‘Jatu-Pha-La-Ti-Ga’ against MCF-7 cells was found to be lower than that of some medicinal plants such as Elaeis guineensis (Vijayarathna & Sasidharan, Citation2012), Ecbolium viride, and Glinus oppositifolius (Akter, Uddin, Grice, & Tiralongo, Citation2014).
Table 3. Cytotoxicity and anticancer activity against human breast adenocarcinoma cell line (MCF-7) obtained from ethanol extracts of selected Thai traditional ancient remedies.
Tabla 3. Citotoxicidad y actividad anticancerígena contra líneas celulares de cáncer mamario adenocarcinoma (MCF-7) obtenidas de extractos en etanol de algunos antiguos remedios tradicionales tailandeses.
Conclusions
One of the main findings in this study was that Thai traditional polyherbal formulations, in particular ‘Tri-Su-Ra-Phon’, show strong in vitro antioxidant activity, contain the highest phenolic and flavonoid contents, and have anticancer with low toxicity. ‘Tri-Su-Ra-Phon’ has great potential to be used as a source of natural antioxidants and beneficial chemopreventive agent in the development as functional beverages for commercial exploration. In vivo toxicity and sensory evaluation of herbal tea made from ‘Tri-Su-Ra-Phon’ as well as effects of this tea in both healthy and obese volunteers are currently being studied in our laboratory.
Acknowledgments
This work was funded by the Establishment Project of Halal Food Science Center, Department of Food Science and Nutrition, Faculty of Science and Technology, Prince of Songkla University (SAT-Hc56-ST07; 2013). We are thankful to Miss Stefania Vignotto for editing the manuscript.
References
- Akter, R., Uddin, S. J., Grice, I. D., & Tiralongo, E. (2014). Cytotoxic activity screening of Bangladeshi medicinal plant extracts. Journal of Natural Medicines, 68, 246–252. doi:10.1007/s11418-013-0789-5
- Bag, A., Kumar Bhattacharyya, S., Kumar Pal, N., & Ranjan Chattopadhyay, R. (2013). Anti-inflammatory, anti-lipid peroxidative, antioxidant and membrane stabilizing activities of hydroalcoholic extract of Terminalia chebula fruits. Pharmaceutical Biology, 51, 1515–1520. doi:10.3109/13880209.2013.799709
- Bandyopadhyay, S. K., Pakrashi, S. C., & Pakrashi, A. (2000). The role of antioxidant activity of Phyllanthus emblica fruits on prevention from indomethacin induced gastric ulcer. Journal of Ethnopharmacology, 70, 171–176. doi:10.1016/S0378-8741(99)00146-4
- Buelna-Chontal, M., Correa, F., Hernández-Reséndiz, S., Zazueta, C., & Pedraza-Chaverri, J. (2011). Protective effect of α-mangostin on cardiac reperfusion damage by attenuation of oxidative stress. Journal of Medicinal Food, 14, 1370–1374. doi:10.1089/jmf.2010.0238
- Cai, Y., Luo, Q., Sun, M., & Corke, H. (2004). Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences, 74, 2157–2184. doi:10.1016/j.lfs.2003.09.047
- Cai, Y.-Z., Sun, M., Xing, J., Luo, Q., & Corke, H. (2006). Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sciences, 78, 2872–2888. doi:10.1016/j.lfs.2005.11.004
- Cárdenas-Rodríguez, N., Coballase-Urrutia, E., Rivera-Espinosa, L., Romero-Toledo, A., Sampieri, A. I., Ortega-Cuellar, D., … Carmona-Aparicio, L. (2013). Modulation of antioxidant enzymatic activities by certain antiepileptic drugs (valproic acid, oxcarbazepine, and topiramate): Evidence in humans and experimental models. Oxidative Medicine and Cellular Longevity, 2013, 1–8. doi:10.1155/2013/598493
- Darvesh, A. S., & Bishayee, A. (2013). Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutrition and Cancer, 65, 329–344. doi:10.1080/01635581.2013.767367
- Deep, G., Dhiman, M., Rao, A. R., & Kale, R. K. (2005). Chemopreventive potential of Triphala (a composite Indian drug) on benzo(a)pyrene induced forestomach tumorigenesis in murine tumor model system. Journal of Experimental & Clinical Cancer Research, 24, 555–563.
- Essa, M. M., Vijayan, R. K., Castellano-Gonzalez, G., Memon, M. A., Braidy, N., & Guillemin, G. J. (2012). Neuroprotective effect of natural products against Alzheimer’s disease. Neurochemical Research, 37, 1829–1842. doi:10.1007/s11064-012-0799-9
- Govindarajan, R., Vijayakumar, M., & Pushpangadan, P. (2005). Antioxidant approach to disease management and the role of ‘Rasayana’ herbs of Ayurveda. Journal of Ethnopharmacology, 99, 165–178. doi:10.1016/j.jep.2005.02.035
- Guruprasad, K. P., Subramanian, A., Singh, V. J., Sharma, R. S., Gopinath, P. M., Sewram, V., … Satyamoorthy, K. (2012). Brahmarasayana protects against ethyl methanesulfonate or methyl methanesulfonate induced chromosomal aberrations in mouse bone marrow cells. BMC Complementary and Alternative Medicine, 12, 113. doi:10.1186/1472-6882-12-113
- Gutowski, M., & Kowalczyk, S. (2013). A study of free radical chemistry: Their role and pathophysiological significance. Acta Biochimica Polonica, 60, 1–16.
- Huda, A., Munira, M., Fitrya, S. D., & Salmah, M. (2009). Antioxidant activity of Aquilaria malaccensis (thymelaeaceae) leaves. Pharmacognosy Research, 1, 270–273.
- Ingkaninan, K., Temkitthawon, P., Chuenchom, K., Yuyaem, T., & Thongnoi, W. (2003). Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. Journal of Ethnopharmacology, 89, 261–264. doi:10.1016/j.jep.2003.08.008
- Isaka, M., Palasarn, S., Kocharin, K., & Saenboonrueng, J. (2005). A cytotoxic xanthone dimer from the entomopathogenic fungus Aschersonia sp. BCC 8401. Journal of Natural Products, 68, 945–946. doi:10.1021/np058028h
- Juni, R. P., Duckers, H. J., Vanhoutte, P. M., Virmani, R., & Moens, A. L. (2013). Oxidative stress and pathological changes after coronary artery interventions. Journal of the American College of Cardiology, 61, 1471–1481. doi:10.1016/j.jacc.2012.11.068
- Karre, L., Lopez, K., & Getty, K. J. (2013). Natural antioxidants in meat and poultry products. Meat Science, 94, 220–227. doi:10.1016/j.meatsci.2013.01.007
- Kondo, M., Zhang, L., Ji, H., Kou, Y., & Ou, B. (2009). Bioavailability and antioxidant effects of a xanthone-rich Mangosteen (Garcinia mangostana) product in humans. Journal of Agricultural and Food Chemistry, 57, 8788–8792. doi:10.1021/jf901012f
- Lambert, J. D., & Elias, R. J. (2010). The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Archives of Biochemistry and Biophysics, 501, 65–72. doi:10.1016/j.abb.2010.06.013
- Li, X., Lin, J., Han, W., Mai, W., Wang, L., Li, Q., … Chen, D. (2012). Antioxidant ability and mechanism of rhizome Atractylodes macrocephala. Molecules, 17, 13457–13472.
- Lofano, K., Principi, M., Scavo, M. P., Pricci, M., Ierardi, E., & Di-Leo, A. (2013). Dietary lifestyle and colorectal cancer onset, recurrence, and survival: Myth or reality? Journal of Gastrointestinal Cancer, 44, 1–11. doi:10.1007/s12029-012-9425-y
- Mahavorasirikul, W., Viyanant, V., Chaijaroenkul, W., Itharat, A., & Na-Bangchang, K. (2010). Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complementary and Alternative Medicine, 10, 55. doi:10.1186/1472-6882-10-55
- Mahesh, R., Bhuvana, S., & Begum, V. M. (2009). Effect of Terminalia chebula aqueous extract on oxidative stress and antioxidant status in the liver and kidney of young and aged rats. Cell Biochemistry and Function, 27, 358–363. doi:10.1002/cbf.1581
- Mukherjee, S., Pawar, N., Kulkarni, O., Nagarkar, B., Thopte, S., Bhujbal, A., & Pawar, P. (2011). Evaluation of free-radical quenching properties of standard Ayurvedic formulation Vayasthapana Rasayana. BMC Complementary and Alternative Medicine, 11, 38. doi:10.1186/1472-6882-11-38
- Naik, G. H., Priyadarsini, K. I., Bhagirathi, R. G., Mishra, B., Mishra, K. P., Banavalikar, M. M., & Mohan, H. (2005). In vitro antioxidant studies and free radical reactions of Triphala, an ayurvedic formulation and its constituents. Phytotherapy Research, 19, 582–586. doi:10.1002/ptr.1515
- Nijveldt, R. J., van Nood, E., van Hoorn, D. E., Boelens, P. G., van Norren, K., & van Leeuwen, P. A. (2001). Flavonoids: A review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition, 74, 418–425.
- Park, Y.-S., Jung, S.-T., Kang, S.-G., Heo, B. G., Arancibia-Avila, P., Toledo, F., … Gorinstein, S. (2008). Antioxidants and proteins in ethylene-treated kiwifruits. Food Chemistry, 107, 640–648. doi:10.1016/j.foodchem.2007.08.070
- Pfundstein, B., El Desouky, S. K., Hull, W. E., Haubner, R., Erben, G., & Owen, R. W. (2010). Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry, 71, 1132–1148. doi:10.1016/j.phytochem.2010.03.018
- Picheansoonthon, C., & Jerawong, V. (2001). Thai pharmaceutical textbook no. 5 Materia medica (in Thai) (pp. 350). Bangkok: Amarin Printing & Publishing.
- Pornthip, S., Worapong, U., & Sunisa, S. (2013, September). Tea production from Cinnamomum porrectum (Kosterm) and its antioxidant activity as affected of pretreatment process and drying temperature. Paper presented at the 13th ASEAN Food Conference, Singapore Institute of Food Science & Technology, Singapore Expo, Singapore.
- Puangpronpitag, D., Areejitranusorn, P., Boonsiri, P., Suttajit, M., & Yongvanit, P. (2008). Antioxidant activities of polyphenolic compounds isolated from Antidesma thwaitesianum Mull. Arg. seeds and marcs. Journal of Food Science, 73, C648–C653. doi:10.1111/j.1750-3841.2008.00951.x
- Puangpronpitag, D., Yongvanit, P., Boonsiri, P., Suttajit, M., Areejitranusorn, P., Na, H.-K., & Surh, Y.-J. (2011). Molecular mechanism underlying anti-apoptotic and anti-inflammatory effects of Mamao (Antidesma thwaitesianum Mull. Arg.) polyphenolics in human breast epithelial cells. Food Chemistry, 127, 1450–1458. doi:10.1016/j.foodchem.2011.01.099
- Raghavan, B., & Kumari, S. K. (2006). Effect of Terminalia arjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian Journal of Physiology and Pharmacology, 50, 133–142.
- Samarakoon, S. M., Shukla, V. J., & Chandola, H. M. (2011). Evaluation of antioxidant potential of Amalakayas Rasayana: A polyherbal ayurvedic formulation. International Journal of Ayurveda Research, 2, 23–28. doi:10.4103/0974-7788.83186
- Serafini, M., Laranjinha, J. A. N., Almeida, L. M., & Maiani, G. (2000). Inhibition of human LDL lipid peroxidation by phenol-rich beverages and their impact on plasma total antioxidant capacity in humans. The Journal of Nutritional Biochemistry, 11, 585–590. doi:10.1016/S0955-2863(00)00124-8
- Settharaksa, S., Jongjareonrak, A., Hmadhlu, P., Chansuwan, W., & Siripongvutikorn, S. (2012). Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. International Food Research Journal, 19, 1581–1587.
- Siriwatanametanon, N., Fiebich, B. L., Efferth, T., Prieto, J. M., & Heinrich, M. (2010). Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. Journal of Ethnopharmacology, 130, 196–207. doi:10.1016/j.jep.2010.04.036
- Sosa, V., Moliné, T., Somoza, R., Paciucci, R., Kondoh, H., & LLeonart, M. E. (2013). Oxidative stress and cancer: An overview. Ageing Research Reviews, 12, 376–390. doi:10.1016/j.arr.2012.10.004
- Tripathi, U. N., & Chandra, D. (2009). The plant extracts of Momordica charantia and Trigonella foenum-graecum have anti-oxidant and anti-hyperglycemic properties for cardiac tissue during diabetes mellitus. Oxidative Medicine and Cellular Longevity, 2, 290–296. doi:10.4161/oxim.2.5.9529
- Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M., & Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions, 160, 1–40. doi:10.1016/j.cbi.2005.12.009
- Vijayarathna, S., & Sasidharan, S. (2012). Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pacific Journal of Tropical Biomedicine, 2, 826–829. doi:10.1016/S2221-1691(12)60237-8
- Wojdylo, A., Oszmianski, J., & Czemerys, R. (2007). Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry, 105, 940–949. doi:10.1016/j.foodchem.2007.04.038
- Yao, Y., Sang, W., Zhou, M., & Ren, G. (2010). Phenolic composition and antioxidant activities of 11 celery cultivars. Journal of Food Science, 75, C9–C13. doi:10.1111/j.1750-3841.2009.01392.x
