Abstract
The total phenolic content (TPC) and antioxidant capacity as determined by 2,2-diphenyl-1-picrylhydrazyl, 2,2ʹ-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid and ferric reducing antioxidant power assays of spice extracts such as rosemary, lemon balm, hyssop, nettle and cumin seeds were investigated. The effect of spice extracts on the growth of selected lactic acid bacteria (LAB) was also analysed. Lemon balm extract exhibited the highest TPC and antioxidant capacity while the nettle and cumin seeds extracts were characterized by the lowest values. Spice extracts had no impact on the growth of the most tested LAB except for rosemary extract, which showed an inhibitory effect towards all strains of Lactobacillus acidophilus and L. delbrueckii. These results suggest that spice extracts may be considered as additives in dairy and meat products in which LAB are present.
Se investigaron el contenido fenólico total (CFT) y la capacidad antioxidante determinada mediante los métodos DPPH, ABTS y FRAP de los extractos de especias tales como romero, melisa, hisopo, ortiga y semillas de comino. También se analizó el efecto de extractos de especias en el crecimiento de determinadas bacterias ácido-lácticas. El extracto de melisa presentó el mayor contenido total de compuestos fenólicos y la mayor capacidad antioxidante, mientras que los extractos de ortiga y semillas de comino mostraron el menor CFT y la menor capacidad antioxidante. Los extractos de especias no tuvieron ningún impacto en el crecimiento de las bacterias ácido-lácticas examinadas, a excepción del extracto de romero, el cual presentó un efecto inhibidor con respecto a todas las cepas de Lactobacillus acidophilus y Lactobacillus delbrueckii. Estos resultados sugieren que los extractos de especias pueden ser considerados como aditivos en alimentos que contienen bacterias ácido-lácticas.
Introduction
Most consumers are looking for the minimally processed food with no addition of chemical preservatives. They accept the use of spices and herbs or other medicinal plants rather than synthetic antioxidants. Spices and herbs are well known for their antioxidant and antimicrobial activities and are useful for preventing lipid oxidation in living organisms as well as in foods (Ertürk, Citation2006; Kozłowska, Żbikowska, Gruczyńska, Żontała, & Półtorak, Citation2014). They are also a source of phenolic compounds that play an important role in human health and disease prevention. Phenolic compounds are a large class of plant secondary metabolites showing a diversity of structure from simple molecules to highly polymerized compounds. Among these, flavonoids and phenolic acids are well known for their antioxidant properties, possessing ideal structural chemistry for radical scavenging activity and being more effective than tocopherol and ascorbate. Some authors have demonstrated a linear correlation between the content of total phenolic compounds and their antioxidant activity (Djeridane, Yousfi, Nadjemi, Boutassouna, Stocker, & Vidal, Citation2006; Wong, Leong, & Koh, Citation2006). Extracts from spices and herbs are also effective in prolongation of the shelf life of food products through the reduction in growth of pathogenic and spoilage microorganisms (Elgayyar, Draughon, Golden, & Mount, Citation2001). Some spices such as garlic and cardamom are also used for the production of fermented foodstuffs, especially dairy and meat products (Verluyten, Leroy, & Vuyst, Citation2004). The addition of these to meat may accelerate lactic acid production by lactic acid bacterial starter culture. Lactic acid bacteria (LAB) are commonly used industrially in the fermentation of food and beverage products. LAB strains produce antimicrobial substances with activity against the homologous strain and microbicidal effects against gastric and intestinal pathogens and other microbes (Jacobsen et al., Citation1999). Various spices and herbal essential oils can influence the growth and activity of LAB in fermentative dairy and meat products. Elgayyar et al. (Citation2001) reported that essential oils obtained from oregano and cardamom inhibited the growth of Lactobacillus plantarum, while angelica and basil oils had no inhibitory effect. However, cumin and its essential oil stimulated the growth of L. plantarum and acid production (Souza, Stamford, Lima, Trajano & Filho, Citation2005). In the present study, aqueous ethanolic extracts from spices and herbs belonging to the Lamiaceae, Apiaceae and Urticaceae families were obtained and their antioxidant activity and total phenolics content (TPC) values evaluated. The aim of this study was to investigate their effect on the growth of selected LAB.
Materials and methods
Materials
Plant materials used were dried leaves of rosemary (Rosmarinus officinalis L.), lemon balm (Melissa officinalis L.), hyssop (Hyssopus officinalis L.) and nettle (Urtica dioica L.) and cumin seeds (Cuminum cyminum L.) purchased from a local market in Warsaw (Poland). Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,2ʹ-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and 2,4,6-tripyridyl-s-triazine (TPTZ) were obtained from Sigma-Aldrich Chemicals (Poznań, Poland). Potassium persulphate, sodium carbonate and chemical solvents were obtained from POCh (Gliwice, Poland). All solvents and reagents were of the highest purity and were used without further purification.
Microorganisms
The LAB strains used in this study were as follows: Lactobacillus acidophilus ATCC 4356, L. acidophilus La14, L. acidophilus La-5, L. acidophilus NCFM, L. casei 431, L. casei ATCC 393, L. casei 01, L. paracasei AD 200, L. rhamnosus GG, L. rhamnosus ATCC 7469, L. rhamnosus 573, L. plantarum NCAIM B.01834, L. plantarum NCAIM B.01149, L. plantarum 299 v, L. delbrueckii subsp. lactis ATCC 4797, L. delbrueckii subsp. bulgaricus ATCC 11,842 and L. delbrueckii subsp. bulgaricus LB58. All strains were supplied by the Museum of Clean Cultures of the Division of Milk Biotechnology of Warsaw University of Life Sciences.
Sample preparation
Aqueous ethanolic extracts from plant materials were prepared according to Kozłowska et al. (Citation2010). In the case of cumin extract, before extraction with ethanol (70%), 20 g of seeds were ground to a fine powder in a household grinder and mixed with 200 mL hexane at ambient temperature for 6 h. Then, the solvent was filtrated and the residue dried at room temperature, and extracted with 70% ethanol. The yields of spice extracts were calculated following solvent evaporation to dryness under reduced pressure at 40°C by a rotary evaporator, and were as follows: rosemary 18.02%, lemon balm 23.67%, hyssop 26.06%, nettle 13.32% and cumin 10.98%.
To determine antioxidant activity and TPC, the spice extracts were re-dissolved in 70% ethanol to yield solutions containing 0.5–2.0 mg of extract per mL.
Total phenolics content
TPC was estimated using the Folin–Ciocalteu method described previously (Singleton & Rossi, Citation1965) with slight modification. Briefly, the appropriately diluted spice extract was mixed with deionized water (20 mL) and Folin–Ciocalteu reagent (0.5 mL). After 30 s, 5 mL of a Na2CO3 (20%, v/v) was added. Then, the solution was incubated at 25ºC for 1 h and the absorbance at 765 nm was measured. TPC was expressed as mg gallic acid equivalents per gram of extract using a standard gallic acid calibration curve in the range 1.2–29.4 µmol/L.
DPPH radical scavenging activity
Free radical scavenging activity of the spice extracts was determined by the method of Yen and Chen (Citation1995) with modification. One millilitre of 0.3 mmol/L freshly prepared DPPH-methanol solution was mixed with 0.2 mL of the extract and 3.8 mL of methanol. After 10 min of incubation in the dark at room temperature, the absorbance at 517 nm was measured. Antioxidant capacity based on the DPPH free radical scavenging ability of the extract was expressed as mmol Trolox equivalents per gram of dry matter of extract. A standard curve was obtained using the Trolox standard in the range 8–40 µmol/L.
ABTS assay
Total antioxidant capacity assay was performed according to the method developed by Re et al. (Citation1999). ABTS (14 mmol/L) was mixed with 4.9 mmol/L of potassium persulphate and kept for 12–16 h in the dark at room temperature. For the analysis, the solution was diluted in water to an absorbance of 0.7 ± 0.02 at 734 nm. Then, 40 µL of spice extract and 4 mL of ABTS∙+ working solution were mixed. After 6 min, the absorbance of samples at 734 nm was recorded and compared to that from the calibrated Trolox standard. Results were expressed as mmol Trolox equivalents per gram of dry matter of extract.
Ferric reducing antioxidant power assay
The ferric reducing antioxidant power (FRAP) assay was conducted according to Benzie and Strain (Citation1996) with some modification. The FRAP reagent consists of 20 mmol/L FeCl3 solution, 300 mmol/L acetate buffer (pH 3.6) and 10 mmol/L TPTZ solution in 40 mmol/L HCl in the proportions 1:10:1 (v/v/v). An appropriate concentration of spice extract was allowed to react with 3 mL of FRAP solution for 10 min at room temperature and the absorbance was measured at 593 nm. Results were expressed as mmol Trolox equivalents per gram of dry matter of extract. A standard curve was prepared using Trolox in the range 80–500 µmol/l.
Antimicrobial activity
Antimicrobial activity was carried out using the agar diffusion method (Bonev, Hooper, & Parisot, Citation2008). Lactobacilli were revived in MRS broth at either 30 or 37°C for 18 h. Petri dishes were loaded with 0.5 mL of the culture broth cultured to a concentration of about 106 CFU mL−1, which was then covered with MRS agar medium at 45°C. After solidification, the wells were cut to a diameter of 6 mm and each was loaded with 20 µL of spice extracts dissolved in dimethyl sulfoxide (DMSO). The Petri dishes were incubated at either 30 or 37°C under anaerobic conditions for 72 h and the respective zone of inhibition was measured. The inhibition tests were done in quadruplicate. The wells containing DMSO but no spice extracts were used as a negative control.
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical evaluations were performed using Statgraphics Plus version 4 software. The data were subjected to one-way analysis of variance and the significant differences between means were calculated by Tukey`s test. Differences at P ≤ 0.05 were considered significant.
Results and discussion
Total polyphenol content
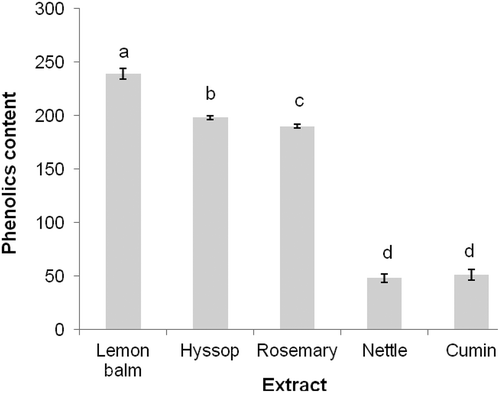
Total phenols determined in the spice extracts are shown in . The highest content was recorded in lemon balm extract (239 mg/g of extract as gallic acid equivalents), followed by hyssop and rosemary (198 and 190 mg/g, respectively), with nettle extract the lowest (48 mg/g). A similar TPC value for rosemary leaves was obtained by Dorman, Peltoketo, Hiltunen and Tikkanen (Citation2003). However, Gramza-Michałowska, Sidor and Hes (Citation2011) found a lower TPC value for rosemary extract (161.26 mg/g). Gallego, Gordon, Segovia, Skowyra and Almajano (Citation2013) reported a value of 219 mg/g for lyophilized rosemary leaf extract, a value higher than that found in the present study (). A slightly higher TPC value in lemon balm extract was recorded by Marcinčák, Popelka, and Šoltysová (Citation2008). The TPC value for cumin seed extract determined in the present study is different to that determined by Mariod, Ibrahim, Ismail, and Ismail (Citation2009), because they estimated TPC among different fractions of black cumin seedcake. However, El-Ghorab, Nauman, Anjum, Hussain, and Nadeem (Citation2010) reported that in regard to the extraction of polyphenols from cumin, methanol proved a better solvent than hexane. The solvent polarity used for extraction and method of extraction may affect variation in TPC of plant extracts and thus their antioxidant activity.
Figure 1. Total phenolic content of spice extracts (mg/g of extract as gallic acid equivalent).
Figura 1. Contenido de fenoles totales en extractos de especias (mg/g de extracto como equivalente ácido gálico).
Antioxidant capacity
The results of antioxidant activity measured by different assays are shown in . In the ABTS, DPPH and FRAP assays, lemon balm extract demonstrated the highest antioxidant capacity followed by hyssop and rosemary extracts, while nettle and cumin extracts exhibited the lowest activity. Among the five tested spices, lemon balm, hyssop and rosemary belong to the Lamiaceae family, cumin to the Apiaceae family and nettle to the Urticaceae family. Herbs from the Lamiaceae family are well known for their antioxidant activity because they are a rich source of biologically active substances, especially phenolic compounds classified into three groups: phenolic diterpenes, flavonoids and phenolic acids (Shan, Cai, Sun, & Corke, Citation2005). Zgórka and Głowniak (Citation2001) found that among plant extracts from the Lamiaceae family, hyssop extract contained the highest content of chlorogenic, ferulic and protocatechuic acids that are implicated as natural antioxidants. Babovic, Djilas, Jadranin, Vajs, Ivanovic, Petrovic, and Zizovic (Citation2010) reported that rosemary, sage and thyme extracts obtained by fluid extraction with carbon dioxide demonstrated high DPPH radical scavenging activity in comparison to hyssop extract. Spiridon, Bodirlau and Teaca (Citation2011) showed that extracts from Origanum vulgare, Lavandula angustifolia and Melissa officinalis demonstrated the highest antioxidant activity of all plants tested in their study. The presence of phenolic compounds, especially flavonoids and caffeic acid derivatives, in nettle extract was found to influence its antioxidant effect although significantly less so than plants from the Lamiaceae family (Pinelli et al., Citation2008). In our study, the extract from cumin seeds presented the lowest value of antioxidant activity in all assays, with the exception of the FRAP assay (0.024 mM/g dm of extract; ). Seed extracts of cumin (Altrooz, Citation2013) were able to neutralize free radicals over a period of 60 min in a DPPH assay. The ability of ethanol cumin extract to scavenge DPPH radicals and changes in the FRAP of the tested cumin extracts were observed by Kim et al. (Citation2009). Various authors have reported a correlation between antioxidant activity and TPC in selected plants. Our results also indicate that TPC significantly correlated with DPPH (r2 = 0.97), ABTS (r2 = 0.99) and FRAP (r2 = 0.99). It can therefore be concluded that the antioxidant capacity of the spice extracts tested is mainly due to the presence of phenolic compounds.
Table 1. Antioxidant activity of spice extracts determined by DPPH, ABTS and FRAP assays (mM/g dm of extract).
Tabla 1. Actividad antioxidante de extractos de especias determinada mediante los métodos DPPH, ABTS y FRAP (mM/g dm de extracto).
Antimicrobial activity
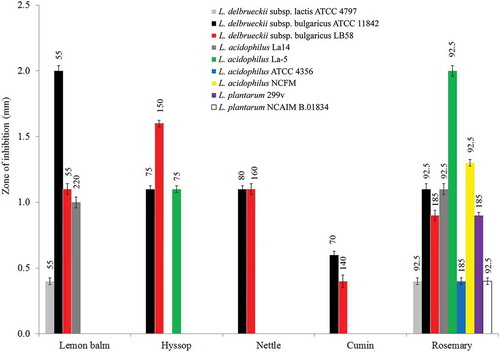
The spice extracts exhibited an inhibitory effect towards 9 of 17 LAB strains tested. Nettle and cumin seed extracts were active against only two strains of L. delbrueckii subsp. bulgaricus (ATCC 11842 and LB58) (). These bacterial strains were also susceptible to hyssop and lemon balm extracts. Additionally, hyssop extract in the concentrations used was active against L. acidophilus La-5 while lemon balm extract inhibited the growth of L. acidophilus La14 and L. delbrueckii subsp. lactis ATCC 4797. Rosemary extract showed the strongest inhibitory effect towards all strains of L. acidophilus and L. delbrueckii, as well as two strains of L. plantarum. Zaika, Kissinger and Wasserman (Citation1983) investigated the effect of herbs on the growth and acid production of L. plantarum and Pediococcus acidilactici. They found that increasing concentrations of oregano and rosemary gradually delayed the growth of these two microorganisms, especially P. acidilactici. However, Saguibo and Elegado (Citation2012) observed the resistance of the probiotic L. plantarum BS against all plant part extracts including methanol extracts of avocado (Persea americana Mill.) and malunggay (Moringa oleifera Lam.) leaves. Other workers investigating LAB (Pediococcus acidilactici 4E5, P. acidilactici AA5a, P. acidilactici 3G3, P. acidilactici K3A2-2, P. pentosaceous K2A2-3, L. plantarum BS) showed selective sensitivity to various herb and vegetable extracts. In general, methanol plant extracts exhibited greater inhibitory effect than aqueous and ethanol extracts. Methanol avocado seed extract was found to be a very good growth inhibitor of P. acidilactici 4E5. All LAB tested with guava leaf extract were sensitive, apart from P. acidilactici, and malunggay leaf extract had an inhibitory effect towards P. acidilactici 4E5 and P. acidilactici AA5A (Saguibo & Elegado, Citation2012). Verluyten, Leroy, and Vuyst (Citation2004) studied the effect of different spices applied in fermented sausage manufacture on the growth of L. curvatus LTH 1174. They found that pepper, nutmeg, rosemary and garlic decreased the maximum specific growth rate of L. curvatus while paprika was the only spice that increased its growth. Essential oils obtained from coriander, angelica and parsley were not effective against L. plantarum (Elgayyar, Draughon, Golden, & Mount, Citation2001), while the essential oils of five different varieties of basil possessed mild antimicrobial activity (Lachowicz et al., Citation1998).
Figure 2. Antimicrobial activity of spice extracts (zone inhibition).
Figura 2. Actividad antimicrobiana de extractos de especias (zona de inhibición).
Conclusions
Plant extracts used in the present study had an impact on the growth of 9 of 17 LAB tested. LAB are frequently often used as starter cultures in fermented foods, playing an important role in maintaining and promoting human health. Many spices and herbs have multiple biological effects including antioxidant activity, especially when these contain polyphenolic compounds. In the present study, no relationship between phenolic content in spice extracts and their capacity to inhibit LAB growth was observed. Therefore, plant extracts may be successfully used as a functional additive in fermented foods.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Altrooz, O. M. (2013). The effects of Cuminum cyminum L. and Carum carvi L. seed extracts on human erythrocyte hemolysis. International Journal of Biology, 5, 57–63. doi:10.5539/ijb.v5n2p57
- Babovic, N., Djilas, S., Jadranin, M., Vajs, V., Ivanovic, J., Petrovic, S., & Zizovic, I. (2010). Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innovative Food Science and Emerging Technologies, 11, 98–107. doi:10.1016/j.ifset.2009.08.013
- Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Analytical Biochemistry, 239, 70–76. doi:10.1006/abio.1996.0292
- Bonev, B., Hooper, J., & Parisot, J. (2008). Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. Journal of Antimicrobial Chemotherapy, 61, 1295–1301. doi:10.1093/jac/dkn090
- Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P., & Vidal, N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry, 97, 654–660. doi:10.1016/j.foodchem.2005.04.028
- Dorman, H. J. D., Peltoketo, A., Hiltunen, R., & Tikkanen, M. J. (2003). Characterization of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chemistry, 83, 255–262. doi:10.1016/S0308-8146(03)00088-8
- Elgayyar, M., Draughon, F. A., Golden, D. A., & Mount, J. R. (2001). Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. Journal of Food Protection, 64, 1019–1024. Retrieved from http://www.anndraughon.com/publications/journals/2001_jfp_64-7_1019-1024_elgayyar.pdf
- El-Ghorab, A. H., Nauman, M., Anjum, F. M., Hussain, S., & Nadeem, M. A. (2010). A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). Journal of Agricultural and Food Chemistry, 58, 8231–8237. doi:10.1021/jf101202x
- Ertürk, Ö. (2006). Antibacterial and antifungal activity of ethanolic extracts from eleven spice plants. Biologia, Bratislava, 61, 275–278. doi:10.2478/s11756-006-0050-8
- Gallego, M. G., Gordon, M. H., Segovia, F. J., Skowyra, M., & Almajano, M. P. (2013). Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. Journal of the American Oil Chemists’ Society, 90, 1559–1568. doi:10.1007/s11746-013-2303-3
- Gramza-Michalowska, A., Sidor, A., & Hes, M. (2011). Herb extract influence on the oxidative stability of selected lipids. Journal of Food Biochemistry, 35, 1723–1736. doi:10.1111/j.1745-4514.2010.00497.x
- Jacobsen, C. N., Rosenfeldt, N. V., Hayford, A. E., Møller, P. L., Michaelsen, K. F., Paerregaard, A., … Jakobsen, M. (1999). Screening of probiotic activities of forty-seven strains of Lactobacillus spp. By in vitro techniques and evaluation of the colonizationability of five selected strains in humans. Applied and Environmental Microbiology, 65, 4949–4956. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10543808
- Kim, J. H., Shin, M.-H., Hwang, Y.-J., Srinivasan, P., Kim, J. K., Park, H. J., … Lee, J. W. (2009). Role of gamma irradiation on the natural antioxidants in cumin seeds. Radiation Physics and Chemistry, 78, 153–157. doi:10.1016/j.radphyschem.2008.08.008
- Kozłowska, M., Laudy, A. E., Starościak, B. J., Napiórkowski, A., Chomicz, L., & Kazimierczuk, Z. (2010). Antimicrobial and antiprotozoal effect of isolates from sweet marjoram (Origanum majorana L.). Acta Scientiarum Polonorum Hortorum Cultus, 9, 133–141.
- Kozłowska, M., Żbikowska, A., Gruczyńska, E., Żontała, K., & Półtorak, A. (2014). Effects of spice extracts on lipid fraction oxidative stability of cookies investigated by DSC. Journal of Thermal Analysis and Calorimetry, 118, 1697–1705. doi:10.1007/s10973-014-4058-y
- Lachowicz, K. J., Jones, G. P., Briggs, D. R., Bienvenu, F. E., Wan, J., Wilcock, A., & Coventry, M. J. (1998). The synergistic preservative effects of the essential oils of sweet basil (Ocimum basilicum L.) against acid-tolerant food microflora. Letters in Applied Microbiology, 26, 209–214. doi:10.1046/j.1472-765X.1998.00321.x
- Marcinčák, S., Popelka, P., & Šoltysová, L. (2008). Polyphenols and antioxidative capacity of extracts from selected Slovakian plants. Acta Scientiarum Polonorum Medicina Veterinaria, 7, 9–14. Retrieved from http://www.acta.media.pl/pl/main.php?p=8&sub=0&act=10&s=5&no=176&lang=endf
- Mariod, A. A., Ibrahim, R. M., Ismail, M., & Ismail, N. (2009). Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chemistry, 116, 306–312. doi:10.1016/j.foodchem.2009.02.051
- Pinelli, P., Ieri, F., Vignolini, P., Bacci, L., Baronti, S., & Romani, A. (2008). Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. Journal of Agricultural and Food Chemistry, 56, 9127–9132. doi:10.1021/jf801552d
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26, 1231–1237. doi:10.1016/S0891-5849(98)00315-3
- Saguibo, J. D., & Elegado, F. B. (2012). Resistance profile of probiotic lactic acid bacteria against inhibitory effects of selected plant extracts. The Philippine Agricultural Scientist, 95, 22–32. Retrieved from http://www.google.pl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CCAQFjAA&url=http%3A%2F%2Fwww.pasuplbca.edu.ph%2Fdownload.php%3Fid%3D90&ei=AqpgVLOpN8j1OICogNgO&usg=AFQjCNG0PMiMC0JyveAGmxQ5fHQN3seQRw
- Shan, B., Cai, Y. Z., Sun, M., & Corke, H. (2005). Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry, 53, 7749–7759. doi:10.1021/jf051513y
- Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic –phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158. Retrieved from http://www.ajevonline.org/cgi/content/abstract/16/3/144
- Souza, E. L., Stamford, T. L. M., Lima, E. O., Trajano, V. N., & Filho, J. M. B. (2005). Antimicrobial effectiveness of spices: An approach for use in food conservation systems. Brazilian Archives of Biology and Technology, 48, 549–558. doi:10.1590/S1516-89132005000500007
- Spiridon, I., Bodirlau, R., & Teaca, C.-A. (2011). Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Central European Journal of Biology, 6, 388–396. doi:10.2478/s11535-011-0028-6
- Verluyten, J., Leroy, F., & Vuyst, L. (2004). Effects of different spices used in production of fermented sausages on growth of and curvacin a production by Lactobacillus curvatus LTH 1174. Applied and Environmental Microbiology, 70, 4807–4813. doi:10.1128/AEM.70.8.4807-4813.2004
- Wong, S. P., Leong, L. P., & Koh, J. H. W. (2006). Antioxidant activities of aqueous extracts of selected plants. Food Chemistry, 99, 775–783. doi:10.1016/j.foodchem.2005.07.058
- Yen, G.-C., & Chen, H.-Y. (1995). Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry, 43, 27–32. doi:10.1021/jf00049a007
- Zaika, L. L., Kissinger, J. C., & Wasserman, A. E. (1983). Inhibition of lactic acid bacteria by herbs. Journal of Food Science, 48, 1455–1459. doi:10.1111/j.1365-2621.1983.tb03515.x
- Zgórka, G., & Głowniak, K. (2001). Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. Journal of Pharmaceutical and Biomedical Analysis, 26, 79–87. doi:10.1016/S0731-7085(01)00354-5
