Abstract
The aim of this study was to evaluate the effect of osmotic dehydration of ginger on the properties of color, total polyphenol content (TPC) and antioxidant capacity (AC). Fresh samples were pretreated with scalding (100°C/1.0 minute). Water loss (WL) and solid gain (SG) kinetics were performed using three sucrose concentrations: 35%, 50% and 65% at three temperatures: 40°C, 55°C and 70°C. WL and SG simulated kinetics by Diffusion model for cubes averaged a R2 of 0.95. The diffusivities for water and solid by Fick’s Law obtained maximum values of 6.81 × 10−7 and 2.65 × 10−7 m2/s, respectively. WL results were obtained up to 60.83% and SG 32.24%. Ginger treatments without blanching (GWB) at 40°C with 35% and 50% sucrose, respectively, showed a similar fresh ginger color. The GWB treatments showed the higher TPC using 50% sucrose at 40°C (753 μg GAE/mL) and AC (341.96 mg AAE/mL).
El objetivo de este estudio fue evaluar el efecto de la deshidratación osmótica de jengibre sobre las propiedades del color, contenido de polifenoles totales (CPT) y la capacidad antioxidante (CA). Las muestras frescas fueron pretratadas con escaldado (100°C/1.0 minuto). Las cinéticas de pérdida de agua (PA) y ganancia de solidos (GS) se realizaron con tres concentraciones de sacarosa: 35, 50 y 65% a tres temperaturas: 40, 55 y 70°C. Las cinéticas de PA y GS simuladas por el modelo de Difusión para cubos promediaron una R2 de 0,95. Los difusividades de agua y sólidos calculadas por la Ley de Fick obtuvieron valores máximos de 6,81 × 10−7 y 2,65 × 10−7 m2/s, respectivamente. Los resultados de PA obtuvieron hasta un 60,83% y la GS hasta un 32,24%. Los tratamientos de jengibre sin escaldar (GSE) a 40°C con 35 y 50% de sacarosa respectivamente, mostraron un color similar al jengibre fresco. Los tratamientos GSE mostraron el mayor CPT utilizando 50% de sacarosa a 40°C (753 mg EAG/mL) y CA (341,96 mg EAA/mL).
Introduction
Osmotic dehydration (OD) is a process that involves immersing a solid food in a hypertonic aqueous solution, which leads to the loss of water and a solids gain from the solution into the food. The process of osmotic solutes transferring from the solution into the product is directly related to the water exchange from the product into the osmotic solution (Barbosa Júnior, Cordeiro-Mancini, and Dupas-Hubinger (Citation2013). The driving force for this process originates from the solids concentration gradients through the activity of the sample and the solution interface. The variables affecting the rates of water removal and solute impregnation are the composition and the concentration of the osmotic solutes, the temperature of the osmotic solution, the immersion time, the level of agitation, the specific characteristics of the food, and the solution-to-food ratio (Fernandes, Rodrigues, Law, & Mujumdar, Citation2011). The OD of common fruits and vegetables, such as banana, pineapple, guava, papaya, and carrot, has been described by several authors (Jain, Verma, Murdia, Jain, & Sharma, Citation2011; Silva, Fernandes, & Mauro, Citation2014). Additionally, OD is gaining considerable attention as a method of minimal processing because of advantages such as energy savings and low temperatures. Moreover, OD is a drying process that provides better control of flavor loss and tissue damage as well as improved color and nutrient retention (Nowacka, Tylewicz, Laghi, Dalla-Rosa, & Witrowa-Rajcher, Citation2014). Sugars and salts are the two most commonly used solutes for OD, with relevance to sodium chloride and sucrose (Jokić, Gyura, Lević, & Zavargó, Citation2007). Because of its many advantages, OD has been widely used in various foods and can be used in tubers and leaves, such as Chinese ginger (An et al., Citation2013). Ginger (Zingiber officinale var. Grand Cayman) has been extensively used as a traditional medicine in the East. The main bioactive components of ginger are the gingerols, which possess antioxidant, anticancer, and anti-inflammatory attributes (Ghasemzadeh, Jaafar, & Rahmat, Citation2010). However, the high moisture content (70–75%) of ginger makes it susceptible to microbial contamination and insect infestation, resulting in significant loss and deterioration of product quality, drying could be a useful process to reduce product damage. Conventional drying reduces the moisture content and thus increases the shelf life of the product; however, it often results in the loss of nutrients and has an adverse effect on the flavor and appearance (color) of the product. Therefore, OD may provide an option for removing water without any adverse effects on the food and has been conducted in several countries (China, Poland, Brazil and Argentina), and on different products (Chinese ginger, kiwifruit, pineapple and kiwifruit pericarp tissue) (An et al., Citation2013; Nowacka et al., Citation2014; Santagapita et al., Citation2013; Silva et al., Citation2014). However, until now there is not information about that OD has been applied to ginger rhizomes in Mexico. Thus, in this paper, the effect of OD on the physicochemical properties of Mexican ginger was studied with and without the use of scalding as a pretreatment.
Materials and methods
Fresh ginger (Zingiber officinale var. Grand Cayman), planted and harvested by farmers in the region of Chinantla in the State of Oaxaca, Mexico, was used. The rhizomes were prepared one day after being harvested; they were washed, peeled and cut into cubes of 1.5 cm, and blanched in water at 100°C for 1 min.
Experiment design and statistical analysis
A completely random design with a factorial arrangement: 3 × 3 × 2 was used with three solutions of sucrose concentration (35, 50 y 65%), three temperatures (40, 55 y 70°C) and two pretreatment: blanching and without blanching. The results were evaluated by analysis of variance (ANOVA) with a significance level of 95% (P < 0.05) using the StatGraphics Plus v5.1 software (2010).
Experimental OD kinetics
Three different concentrations of osmotic solution with sucrose were prepared (35, 50 and 65 g/100 g solution) and three temperatures, 40°C, 55°C and 70°C, were used. The ratio of solid:solution was 1:15 (w/v) for fresh and blanched samples. Heating and stirring (at about 200 rpm) of osmotic media was done with a digital hot plate stirrer (Thermo Scientific Cimarec) equipped with an external proportional-integral-derivate controller, where the osmotic solution temperature was directly acquired by an immersed sensor in order to achieve a precise temperature control (±0.5°C). Sampling was performed at different time intervals (0, 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 90, 110, 130, 160, 190, 220 minutes) until equilibrium was reached. The samples were allowed to drain, then the excess solution was washed off, and samples were blot-dried. Moisture content was determined using an oven official method (No. 925.10) AOAC (Citation2005). The data obtained were used for determining the kinetics of water loss (WL) and solid gain (SG) using Equations (1) and (2) (Beristain, Azuara, Cortés, & García, Citation1990). All treatments were random and performed in duplicate. The kinetic of OD was obtained by plotting the WL (%) and SG (%) versus time (min):
Prediction of WL and SG kinetics during OD
For the prediction of the experimental kinetics of WL and SG for fresh and pretreated ginger, Diffusion model for cubes (Equations (3) and (4)) was employed by nonlinear regression using Matlab 10.0 Statistics Toolbox (MathWorks Inc., Natick, MA, USA) software. The experimental data and values predicted by the Diffusion model for cubes were compared by the determination coefficient (R2):
Prediction of the effective diffusivity for the OD
For the prediction of the effective diffusivity of water and solids of fresh and blanched ginger, the second equation of Fick’s law (Equation (5)) was used with nonlinear regression using the Matlab Statistics Toolbox 10.0 software. Predicted values by the equation of Fick were analyzed by an ANOVA with P < 0.05 for its significance:
After the OD process, ginger cubes were dried at 40°C for 15 min in a convection oven (Binder ED 115 Oven, Germany). Then, they were ground in a blender (Osterizer blender 8 speed model) and passed through a sieve No. 40 (0.425 mm test sieve USA standard ASTM E-11 Specification WS Tyler, Milwaukee, WI, USA) as part of the conditioning for the following determinations.
Color determination
Color determination of samples was performed in triplicate on ginger powder of the different treatments using a HunterLab Colorimeter UltraScan Vis (Hunter Associates Laboratory Inc., USA). The results were expressed according to the CIELAB system with reference to illuminant D65 and a visual angle of 10°. Luminosity (L*), red/green chromaticity (a*), yellow/blue chromaticity (b*) were measured and chromaticity (C*), hue angle (h°) and total color difference (∆E) was calculated from these results. The color parameters were calculated through of the Equations (6)–(8):
Quantification of total polyphenols
The total polyphenol content (TPC) was determined according to the Folin–Ciocalteau method described by Singleton, Orthofer, and Lamuela-Raventos (Citation1999). The results were expressed in µg gallic acid equivalents per mL (µg GAE/mL).
Determination of antioxidant capacity
The antioxidant capacity (AC) or activity of the extract was measured by DPPH+ (2,2-diphenyl-1-picrylhydrazyl), according to the method of Cottele et al. (Citation1996). Results were expressed in µg of ascorbic acid equivalent per mL (AAE µg/mL).
Results and discussion
Experimental and simulated kinetics of WL and solid gain for OD
The experimental and simulated (using the Diffusion model for cubes) kinetic results of WL of ginger samples osmotically dehydrated with and without blanching, using sucrose at 35%, 50% and 65% (w/w) at temperatures of 40°C, 55°C and 70°C, are shown in –. The increasing concentration and temperature of the osmotic solution increased the WL of osmotically dehydrated ginger. It is noteworthy that the maximum concentration of sucrose was 65% because higher concentrations of this component are difficult to dissolve at 40°C, causing the solution to become very viscous and difficult to stir. Furthermore, according to Ferrari and Hubinger (Citation2008), when working with high sucrose concentrations above 50%, excess sucrose forms a surface crust on the product and could act as a barrier to not only the transfer of solids but also the diffusion of water. An increase in the temperature produces an increase in WL, which could lead to changes in cell membrane permeability and the fluidity of the osmotic solution. High temperatures cause swelling and plasticity of the cellular membranes, making them more permeable and reducing the viscosity of the osmotic solution, which likely favors greater transfer of water across the surface of the product and through its interior. Similarly, according to values reported by Cao, Zhang, Mujumdar, Du, and Sun (Citation2006) on the OD of kiwi, an increase in the concentration of sucrose could result in changes in the osmotic pressure of the medium, enabling greater WL. Thus, it was observed that within the sucrose concentration range of 35–65% and the temperature range of 40–70°C used in the osmotic solution, the WL ranged from 34.15% to 58.66% in the ginger without blanching and 29.42–62.38% in the blanched ginger. Similar results were obtained by An et al. (Citation2013) in Chinese ginger without blanching using a ternary solution of water–sucrose–sodium chloride at different concentrations and temperatures. The average degree of correlation obtained between the experimental and simulated kinetics was R2 = 0.95; thus, the Diffusion model for cubes adequately predicted the experimental behavior of WL in the kinetics of OD under all of the sucrose concentration and temperature conditions employed. In , the lowest concentration of sucrose caused the WL to be even lower in the treatments of the blanched ginger than in those of the ginger without blanching at the same temperatures. When the solute concentration was increased, the WL increased, especially in treatments with blanched ginger in comparison to those without blanching, as shown in and . The SG experimental kinetics and those simulated by the Diffusion model for cubes are shown in – for the different working conditions employed. The concentration and temperature of the osmotic solution’s influence over SG in the OD samples are of the same form as those found in the WL kinetics. The SG kinetics behaved similarly to the WL kinetics. The greatest rate of SG occurred in the first 60 min of the kinetics, but the equilibrium was achieved at longer times (more than 200 min in blanching treatments). The simulated kinetics of unblanched ginger samples had SG values 2.85%, 5.75% and 14.18% for 35% sucrose; 4.84%, 10.40% and 17.24% for 50% sucrose; and 10.65%, 13.33% and 27.10% for 65% sucrose at 40°C, 55°C and 70°C, respectively. However, for treatments where scalding was employed, higher SG values were obtained from the simulated kinetics: 19.42%, 21.50%, and 25.27% for 35% sucrose; 22.78%, 23.89% and 27.30% for 50% sucrose; and 25.23%, 26.05% and 32.53% for 65% sucrose at 40°C, 55°C and 70°C, respectively. This result is because the scalding using the highest temperature allowed for greater penetration of solutes into the ginger, which was also observed for the effective diffusivity of solids in the blanched ginger (). Castillo and Cornejo (Citation2007) observed a similar behavior for star fruit. Employing sucrose solutions at different concentrations of 35–65% at temperatures of 40–70°C, SG was achieved from 2.85% to 27.10% for ginger without blanching and 19.42–32.53% for blanched ginger samples, both with OD treatment. An et al. (Citation2013) also reported the highest SG in Chinese ginger when the concentration of the osmotic solution was increased. However, they did not use blanching as a pretreatment to increase the solid transfer in ginger, as shown in this work. Greater SG has been observed in experiments where blanched ginger was used (Martinez, Calero, Ayala-Aponte, Chiralt, & Fito, Citation2011). The average correlation coefficient obtained between the experimental and simulated kinetics was R2 = 0.91; thus, the Diffusion model for cubes adequately predicted the experimental Page behavior of SG in OD kinetics under all of the sucrose concentration and temperature conditions employed.
Figure 1. Experimental and simulated kinetics of WL during ginger OD using: (a) 35%, (b) 50% and (c) 65% sucrose solution with and without blanching at different temperatures.
Figura 1. Cinéticas experimentales y simuladas de la pérdida de agua durante la deshidratación osmótica de jengibre usando: (a) 35%, (b) 50% y (c) 65% de solución de sacarosa con y sin escaldado a diferentes temperaturas.
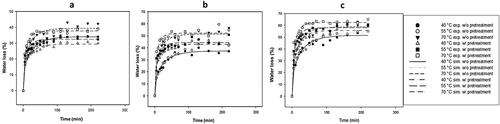
Figure 2. Experimental and simulated kinetics of SG during ginger OD using: (a) 35%, (b) 50% and (c) 65% sucrose solution with and without blanching at different temperatures.
Figura 2. Cinéticas experimentales y simuladas de la ganancia de sólidos durante la deshidratación osmótica de jengibre usando: (a) 35%, (b) 50% y (c) 65% de solución de sacarosa con y sin escaldado a diferentes temperaturas.
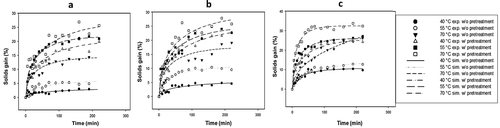
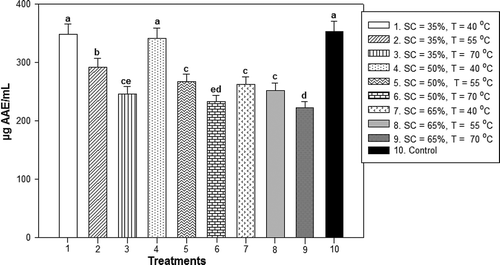
Figure 3. AC obtained of unblanched ginger and osmotically dehydrated with sucrose concentrations of 35%, 50% and 65% at temperatures of 40°C, 55°C and 70°C.Notes: * Data are expressed as mean ± standard derivation of three determinations. Different letters in the same column indicate significant difference (P < 0.05). SC = Sucrose concentration and T = Temperature.
Figura 3. Capacidad antioxidante obtenida de jengibre sin escaldar y deshidratado osmóticamente con concentraciones de sacarosa de 35, 50 y 65% a temperaturas de 40, 55 y 70°C.
Prediction of the effective diffusivity for OD
Diffusion is a temperature-dependent phenomenon that promotes an increase in WL at higher temperatures because of the increased plasticity of the cell membrane (Barbosa Júnior et al., Citation2013). Therefore, an increase in the concentration of the osmotic agent and the temperature produce a greater diffusion rate for water and solids, as shown in and , respectively. In , the effective diffusivity of water (Dew) is obtained during the OD for blanched ginger and ginger without blanching. The Dew values determined in this study were similar to those reported in fruits and vegetables in the literature, which ranged from 1 × 10−6 to 1 × 10−9 m2/s, as reported by Waliszewski, Delgado, and García (Citation2002) for pineapple and Alakali, Ariahu, and Nkpa (Citation2006) for mango. It was noted in the sucrose concentration range of 35–65% and the temperature range of 40–70°C used in the osmotic solution that the water diffusion rates ranged from 1.1188 × 10−7 to 3.5235 × 10−7 m2/s in ginger without blanching and 3.3980 × 10−7–6.8118 × 10−7 m2/s in scalded ginger. These data suggest that blanching may be more effective in increasing Dew. The scalding-type pretreatment was found to be important as it resulted in open pores or channels within the tissue of the product, allowing for faster effective diffusivity of water and thus greater WL during OD. All treatments of scalded ginger and ginger without scalding had significant differences at P < 0.05. The diffusivity coefficients of solid (Des) obtained during OD of the scalded ginger and ginger without blanching are shown in . High concentrations of sucrose exerted a greater osmotic pressure and thus faster solid transfer. The values of effective diffusivity obtained for the sucrose solutions at concentrations of 35–65% and temperatures of 40–70°C were 0.4449 × 10−7–1.2481 × 10−7 m2/s for ginger without blanching and 0.6075 × 10−7–2.6504 × 10−7 m2/s for blanched ginger. In all treatments, except for two blocks, there were statistically significant differences for both unblanched and blanched ginger at P < 0.05. The first of the two blocks was unblanched ginger in sucrose at 40°C with concentrations of 50% and 65%, and the second block was blanched ginger in sucrose at 50% at temperatures of 40–55°C. The values of solid diffusivity coefficients were much lower than the values obtained for the diffusivity of water. Furthermore, the values were also observed to be related to the lower SG with greater WL during OD. Sutar and Gupta (Citation2007) previously reported this behavior for onion slices.
Table 1. Effective diffusivity of water (Dew) in ginger with and without blanching using sucrose at different concentrations and temperatures.
Tabla 1. Difusividad efectiva de agua (Dew) en jengibre con y sin escaldado usando sacarosa a diferentes concentraciones y temperaturas.
Table 2. Effective diffusivity of solid (Des) in ginger with and without blanching using sucrose at different concentrations and temperatures.
Tabla 2. Difusividad efectiva de sólido (Des) en jengibre con y sin escaldado usando sacarosa a diferentes concentraciones y temperaturas.
Color determination
Experimental results of brightness (L*), chroma (C*), hue angle (h°) and total color difference (∆E) are shown in . The characteristic yellow color of ginger is placed at approximately 80 h°. Osmotically dehydrated samples of ginger generally showed a greenish-yellow color with a Hue angle > 80°. However, there were no significant differences at P < 0.05 in treatments No. 1, 4, and 7 with sucrose concentrations at 35%, 50% and 65%, respectively, at 40°C for samples without blanching because the low temperature employed did not affect the natural pigments of ginger. However, it was observed that blanching had an adverse effect on the hº parameter, showing statistically significant differences in all of the treatments at P < 0.05 because of the high temperatures used in the blanching. In , was also observed that C* was not significantly different (P < 0.05) compared with the control. Other than the treatments where high temperatures of 70°C were used, there was a significant difference at P < 0.05 in osmotically dehydrated ginger with and without blanching (treatments Nos. 3, 6 and 9). Moreover, yellow chroma saturation for the rhizome in ginger was presented, in accordance with Munsell (Citation2013). These values are recorded under normal reflectance materials for foods and are not used for those materials in which higher values are obtained for fluorescent objects. The color scale begins at zero for neutral colors. The scale of C* for normal reflectance materials extends beyond the numerical value of 20 in some cases, whereas fluorescent materials may have a chroma above 30. Similar to the brightness, the treatments where higher temperatures were used (70°C for treatments 6 and 9 with unblanched samples) and where sucrose concentrations of 65% were used at all temperatures (treatments 7, 8 and 9 with scalding) showed statistical significance at P < 0.05 in comparison to the control. This finding could be a result of the long duration of the OD in which nonenzymatic browning reactions, such as oxidation of ascorbic acid, could occur (Zambrano, Valera, Maffei, Materano, & Quintero, Citation2008).
Table 3. Experimental results of L*, C*, h°and ΔE in ginger with and without blanching and osmotically dehydrated with sucrose (35%, 50% and 65%) and temperature at 40°C, 55°C and 70°C.
Tabla 3. Resultados experimentales de L*, C*, h° y ΔE en jengibre con y sin escaldado y deshidratado osmóticamente con sacarosa (35, 50 y 65%) y temperatura a 40, 55 y 70°C.
The total difference of color (ΔE) in treatments without blanching was found to rise when the temperature and sucrose concentration were increased with respect to the control. However, no significant differences were found among treatments Nos. 1, 2 and 3 compared with the control. The highest values of ΔE were found in the blanched treatments except in the treatments with sucrose concentrations of 65%. These results agree with those of other reports on the thermal treatments of fruit (Chutintrasri & Noomhorm, Citation2007; Manayay, Ibarz, Castillo, & Palacios, Citation2013).
Total polyphenol content
Experimental results for TPC are presented in . The different OD treatments applied to ginger without blanching showed a variation of 348–823 µg GAE/mL, obtaining statistically significant differences at P < 0.05 compared to the control (fresh ginger), which had a value of 1101.33 µg GAE/mL. Higher temperatures and sucrose concentrations showed the highest TPCs in ginger. Conversely, in the treatments where lower temperatures and osmotic solution concentrations were used, higher TPCs were obtained. However, during the OD, the change in color (h°) did not appreciate in the unblanched ginger samples at the lower temperature (40°C), as established in , which is the first indication of oxidative degradation of polyphenols (Rodríguez-Amado, Escalona-Arranz, & Lafourcade-Prada, Citation2010). Therefore, the decrease in TPC in this case could also be related to the amount of time employed during the OD. A different behavior was observed by An et al. (Citation2013) for Chinese ginger, where the TPC was found to increase with increasing temperature. The same behavior was observed when the different OD treatments were applied to ginger with blanching, showing a variation of 319.67–673.00 µg GAE/mL, with significant differences observed at P < 0.05 compared to the control. The higher temperatures and sucrose concentrations affected the phenolic compounds in ginger as well. Additionally, in , higher TPCs were obtained in treatments without scalding in comparison to treatments with scalding with significant differences. Zambrano et al. (Citation2008) obtained similar results in the analysis of scalded mango pulp. Thus, the treatments with higher TPC were Nos. 1, 4 and 7 for unblanched and blanched ginger with values of 823.00, 753.00 and 736.33 µg GAE/mL, respectively, for unblanched ginger and 673.00, 666.33 and 638.00 µg GAE/mL, respectively, for blanched ginger, at a temperature of 40°C for every sucrose concentration employed. These results significantly demonstrate that blanching induced the decrease in TPC because of the high temperature employed. The TPCs obtained in this investigation were higher than those obtained by Chan et al. (Citation2009), where a value of 187 mg µg GAE/mL was obtained in leaves and rhizomes of different ginger species.
Table 4. TPC obtained with and without blanching ginger and osmotically dehydrated with sucrose (35, 50 and 65%) and temperatures at 40, 55 and 70°C.
Tabla 4. Contenido total de polifenoles obtenido con y sin escaldado del jengibre y deshidratado osmóticamente con sacarosa (35, 50 y 65%) y temperaturas a 40, 55 y 70°C.
Antioxidant capacity
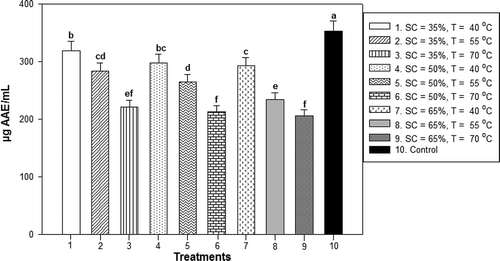
Experimental results for the AC are shown in . The AC exhibited similar behavior to TPC for the different treatments of OD with or without scalding of the ginger. The higher temperatures and sucrose concentrations affected the AC of ginger. Therefore, in the treatments where the lower temperatures (40°C) and osmotic solution concentrations (35%) were used, higher AC values were obtained. A decrease in the content of ascorbic acid equivalents was observed for high temperatures and sucrose concentrations, which is associated with the decreased TPC by the same effect. The different treatments of OD for ginger without blanching showed a variation in antioxidant activity of 222.39–348.74 µg AAE/mL, most of which showed significant differences compared to the control (fresh ginger), which had an AC of 353.01 µg AAE/mL. However, treatments 1 and 4, where sucrose solutions of 35% and 50% were used, did not show significant differences at P < 0.05 compared with the control. The AC values of treatments 1 and 4 were determined to be 348.74 and 341.96 µg AAE/mL, respectively, which were both obtained at 40°C. In addition, this reduction may be a result of solids transfer with AC of the ginger to the osmotic solution by leaching. Experimental results for AC of osmotically dehydrated ginger with blanching are presented in . The OD treatments using scalding exhibited the same behavior as the OD treatments without scalding, that is, a decrease in the content of ascorbic acid equivalents was observed for high temperatures and sucrose solution concentrations. In addition, scalding had an adverse effect on the trapping capacity of the radical DPPH+ at the high temperature used. All treatments with scalding showed significant differences compared to the control at P < 0.05. The antioxidant activity ranged from 206.76 to 319.53 µg AAE/mL. Treatments 1, 4 and 7 obtained the highest AC results of 319.53, 298.28 and 292.68 µg AAE/mL, respectively, at 40°C. The employment of the lowest temperature (40°C) exerted the least damage to AC for each sucrose concentration employed during OD. Treatments without blanching exhibited higher values of AC than those corresponding to blanched samples. Finally, a correlation of TPC with respect to AC was observed: reducing the TPC decreased the ginger AC. Cui, Xu, and Sun (Citation2004) established a similar behavior in the analysis of carotenoids in fresh and blanched carrot samples.
Figure 4. AC obtained of blanched ginger osmotically dehydrated with sucrose concentrations of 35%, 50% and 65% at temperatures of 40°C, 55°C and 70°C.Note: * Data are expressed as mean ± standard derivation of three determinations. Different letters in the same column indicate significant difference (P < 0.05). SC = Sucrose concentration and T = Temperature.
Figura 4. Capacidad antioxidante obtenida de jengibre escaldado y deshidratado osmóticamente con concentraciones de sacarosa de 35, 50 y 65% a temperaturas de 40, 55 y 70°C.
Conclusions
WL and SG were found to increase when the osmotic solution concentration and temperature were increased. Blanching pretreatment was also a major factor in increasing these values. The effective diffusivities of water and solid were within the range of foods, such as ginger, with or without blanching. The Diffusion model for cubes adequately predicted the kinetics of WL and SG during OD with an average of R2 of 0.95. OD treatments where temperatures of 40°C and sucrose concentrations of 35% and 50% were employed in the unblanched ginger showed a color close to that of fresh ginger with regard to parameters such as L*, C*, h° and ΔE. However, OD affected the TPC and the AC in all treatments where ginger was pretreated by blanching. TPC and AC were retained in treatments of ginger without blanching using a sucrose solution of 35% at 40°C. Finally, blanching had a greater effect on WL of ginger during OD. The ideal OD treatments were determined to be lower temperatures (40°C) and sucrose solutions (35%) for blanched and unblanched ginger, which led to the greatest color, TPC and AC retention.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Irving I. Ruiz-López ![]() http://orcid.org/0000-0002-6592-6838
http://orcid.org/0000-0002-6592-6838
Cecilia E. Martínez-Sánchez ![]() http://orcid.org/0000-0001-8098-9610
http://orcid.org/0000-0001-8098-9610
Jesús Rodríguez-Miranda ![]() http://orcid.org/0000-0002-1290-5670
http://orcid.org/0000-0002-1290-5670
Juan G. Torruco-Uco ![]() http://orcid.org/0000-0002-0689-8152
http://orcid.org/0000-0002-0689-8152
Erasmo Herman-Lara ![]() http://orcid.org/0000-0001-9766-0563
http://orcid.org/0000-0001-9766-0563
References
- Alakali, J. S., Ariahu, C. C., & Nkpa, N. N. (2006). Kinetics of osmotic dehydration of mango. Journal of Food Processing and Preservation, 30(5), 597–607. doi:10.1111/j.1745-4549.2006.00080.x
- An, K., Ding, S., Tao, H., Zhao, D., Wang, X., Wang, Z., & Hu, X. (2013). Response surface optimisation of osmotic dehydration of Chinese ginger (Zingiber officinale Roscoe) slices. International Journal of Food Science & Technology, 48, 28–34. doi:10.1111/j.1365-2621.2012.03153.x
- Association of Official Analytical Chemists. (2005). Official methods of analysis (18th ed.). W. Horwitz & G. W. Latimer (Eds.). Gathersburg, MD: AOAC International.
- Barbosa Júnior, J. L., Cordeiro-Mancini, M., & Dupas-Hubinger, M. (2013). Mass transfer kinetics and mathematical modelling of the osmotic dehydration of orange fleshed honeydew melon in corn syrup and sucrose solutions. International Journal of Food Science & Technology, 48(12), 2463–2473. doi:10.1111/ijfs.12237
- Beristain, C. I., Azuara, E., Cortés, R., & García, H. S. (1990). Mass transfer during osmotic dehydration of pineapple rings. International Journal of Food Science & Technology, 25, 576–582. doi:10.1111/j.1365-2621.1990.tb01117.x
- Cao, H., Zhang, M., Mujumdar, A. S., Du, W., & Sun, J. (2006). Optimization of osmotic dehydration of kiwi fruit. Drying Technology, 24(1), 89–94. doi:10.1080/07373930500538741
- Castillo, M., & Cornejo, F. (2007). Estudio del efecto del proceso de deshidratación osmótica en la obtención de trozos secos de carambola (Averroha carambola L.). Revista Tecnológica ESPOL, 20, 183–188. Retrieved from http://www.rte.espol.edu.ec/index.php/tecnologica
- Chan, E. W. C., Lim, Y. Y., Wong, S. K., Lim, K. K., Tan, S. P., Lianto, F. S., & Yong, M. Y. (2009). Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chemistry, 113, 166–172. doi:10.1016/j.foodchem.2008.07.090
- Chutintrasri, B., & Noomhorm, A. (2007). Color degradation kinetics of pineapple puree during thermal processing. LWT-Food Science and Technology, 40(2), 300–306. doi:10.1016/j.lwt.2005.11.003
- Cottele, N., Bernier, J. L., Catteau, J. P., Pommery, P., Wallet, J. C., & Gaydou, E. M. (1996). Antioxidant properties of hydroxy-flavones. Free Radical Biology & Medicine, 20, 35–43. Retrieved from http://www.journals.elsevier.com/free-radical-biology-and-medicine/
- Cui, Z., Xu, S., & Sun, D. (2004). Effect of microwave-vacuum drying on the carotenoids retention of carrot slices and chlorophyll retention of Chinese chive leaves. Drying Technology, 22, 563–575. doi:10.1081/DRT-120030001
- Fernandes, F. A. N., Rodrigues, S., Law, C. L., & Mujumdar, A. S. (2011). Drying of exotic tropical fruits: A comprehensive review. Food and Bioprocess Technology, 4, 163–185. doi:10.1007/s11947-010-0323-7
- Ferrari, C. C., & Hubinger, M. D. (2008). Evaluation of the mechanical properties and diffusion coefficients of osmodehydrated melon cubes. International Journal of Food Science & Technology, 43(11), 2065–2074. doi:10.1111/j.1365-2621.2008.01824.x
- Ghasemzadeh, A., Jaafar, H. Z. E., & Rahmat, A. (2010). Antioxidant activities, total phenolics and flavonoids content in two varieties of malaysia young ginger (Zingiber officinale Roscoe). Molecules, 15, 4324–4333. doi:10.3390/molecules15064324
- Jain, S. K., Verma, R. C., Murdia, L. K., Jain, H. K., & Sharma, G. P. (2011). Optimization of process parameters for osmotic dehydration of papaya cubes. Journal of Food Science and Technology, 48, 211–217. doi:10.1007/s13197-010-0161-7
- Jokić, A., Gyura, J., Lević, L., & Zavargó, Z. (2007). Osmotic dehydration of sugar beet in combined aqueous solutions of sucrose and sodium chloride. Journal of Food Engineering, 78, 47–51. doi:10.1016/j.jfoodeng.2005.09.003
- Manayay, D., Ibarz, A., Castillo, W., & Palacios, L. (2013). Cinética de la diferencia de color y croma en el proceso térmico de pulpa de mango (Mangifera indica L.) variedad Haden. Scientia Agropecuaria, 4(3), 181–190. Retrieved from http://revistas.unitru.edu.pe/index.php/scientiaagrop/index
- Martinez, J., Calero, A., Ayala-Aponte, A., Chiralt, A., & Fito, P. (2011). Efecto del Escaldado sobre la Deshidratación Osmótica del Mango. Rev Ingeniería Y Competitividad, 4, 27–33. Retrieved from http://revistaingenieria.univalle.edu.co:8000/index.php/inycompe
- Munsell. (2013, January 24). How color notation works. Retrieved from http://munsell.com/about-munsell-color/how-color-notation-works/
- Nowacka, M., Tylewicz, U., Laghi, L., Dalla Rosa, M., & Witrowa-Rajchert, D. (2014). Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chemistry, 144, 18–25. doi:10.1016/j.foodchem.2013.05.129
- Rodríguez-Amado, J. R., Escalona-Arranz, J. C., & Lafourcade-Prada, A. (2010). Estudio de estabilidad química acelerada de las tabletas de Tamarindus indica L. Revista Cubana De Química, 23(9–16). Retrieved from. http://ojs.uo.edu.cu/index.php/cq
- Santagapita, P., Laghi, L., Panarese, V., Tylewicz, U., Rocculi, P., & Dalla Rosa, M. (2013). Modification of transverse NMR relaxation times and water diffusion coefficients of kiwifruit pericarp tissue subjected to osmotic dehydration. Food and Bioprocess Technology, 6(6), 1434–1443. doi:10.1007/s11947-012-0818-5
- Silva, K. S., Fernandes, M. A., & Mauro, M. A. (2014). Osmotic dehydration of pineapple with impregnation of sucrose, calcium, and ascorbic acid. Food and Bioprocess Technology, 7(2), 385–397. doi:10.1007/s11947-013-1049-0
- Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178. Retrieved from http://www.sciencedirect.com/science/bookseries/00766879
- Sutar, P. P., & Gupta, D. K. (2007). Mathematical modeling of mass transfer in osmotic dehydration of onion slices. Journal of Food Engineering, 78, 90–97. doi:10.1016/j.jfoodeng.2005.09.008
- Waliszewski, K. N., Delgado, J. L., & García, M. A. (2002). Equilibrium concentration and water and sucrose diffusivity in osmotic dehydration of pineapple slabs. Drying Technology, 20(2), 527–538. doi:10.1081/DRT-120002555
- Zambrano, J., Valera, A., Maffei, M., Materano, W., & Quintero, I. (2008). Efecto del escaldado y la adición de preservativos sobre la calidad de la pulpa de mango tipo ‘bocado’ almacenada bajo refrigeración. Agronomia Tropical, 58, 257–265. Retrieved from http://www.scielo.org.ve/revistas/at/iaboutj.htm
