ABSTRACT
The aim of the study was to evaluate the effect of probiotic bacteria strains (Lactobacillus rhamnosus LOCK900 and Bifidobacterium animalis ssp. lactis BB12) on antiradical activity of peptides isolated from dry-cured loins after fermentation and 4 months cold storage followed by in vitro gastrointestinal digestion. Studies have shown that the use of probiotic bacteria for the manufacture of dry-cured products affects the formation of protein proteolysis products with antiradical activity during cold storage and gastrointestinal enzyme digestion. Loins with the B. animalis ssp. lactis strain BB12 were characterised by the highest antiradical peptide activity among products after fermentation and cold storage (1.85 mg Trolox/mg peptides; 2.8 mg Trolox/mg peptides) than loins with a mixture of strains (1.17 mg Trolox/mg peptides; 3.36 mg Trolox/mg peptides).
RESUMEN
El objetivo de este estudio fue evaluar el efecto de cepas de bacteria probiótica (Lactobacillus rhamnosus LOCK900 y Bifidobacterium animalis ssp. lactis BB12) en la actividad antiradical de péptidos aisladas de lomo curado en seco después de una fermentación y almacenamiento en frío de 4 meses seguidos de digestión gastrointestinal in vitro. Algunos estudios han mostrado que el uso de bacteria probiótica para la elaboración de productos curados en seco afecta a la formación de productos de proteólisis proteínica con actividad antiradical durante el almacenamiento en frío y la digestión enzimática gastrointestinal. El lomo con cepa BB12 de Bifidobacterium animalis ssp. lactis se caracterizó por tener la mayor actividad de péptidos antiradicales entre los productos que habían estado fermentados y almacenados en frío (1,85 mg de Trolox/mg péptidos; 2,8 mg de Trolox/mg péptidos) por encima del lomo con mezcla de cepas (1,17 mg de Trolox/mg péptidos; 3,36 mg de Trolox/mg péptidos).
1. Introduction
A growing awareness of the relationship between diet, food components and health is leading to new insight into the effect of food ingredients on physiological function and health (Olmedilla-Alonso, Jiménez-Colmenero, & Sánchez-Muniz, Citation2013). Foods are considered functional because of their effects (scientifically demonstrated), not their origin, therefore the category of functional foods may include both natural (unmodified) foods and foods in which a component has been added, removed or modified (including bioavailability) via technological or biotechnological means (Diplock et al., Citation1999). This new approach to improving the health status is especially interesting for the meat industry. Functional foods constitute an excellent opportunity for the meat industry to improve both the quality and image of meat – not only to prevent the loss of market share that goes along with a negative perception of meats but also to achieve much-needed diversification in this sector’s activity through the development of products with health-beneficial properties (Olmedilla-Alonso et al., Citation2013). Most probiotic food products are categorised as functional foods and represent a significant part of these. It has been estimated that probiotic foods comprise between 60% and 70% of the total functional food market (Holzapfel & Schillinger, Citation2002; Kołożyn-Krajewska & Dolatowski, Citation2012; Tripathi & Giri, Citation2014). However, there are very few studies involving meat and meat products with probiotics to verify whether those modifications are of relevance to human health.
Biologically active peptides released during ageing and digestion of food proteins have an impact on several bodily functions. Bioactive peptides with antioxidant properties occur naturally in raw meat (glutathione, anserine, carnosine) or can be obtained by protein hydrolysis. Biological activity of peptides produced in dry-cured meat products, including their antioxidant effects, has been investigated (Escudero, Aristoy, Nishimura, Arihara, & Toldrá, Citation2012; Escudero, Mora, Fraser, Aristoy, & Toldrá, Citation2013). Antioxidant action of peptides in the product can be subjected to further modification and degradation during gastrointestinal enzyme digestion. The aim of the study was to evaluate the effect of probiotic bacteria strains (Lactobacillus rhamnosus LOCK900 and Bifidobacterium animalis subsp. lactis BB-12) on antiradical activity of peptides isolated from dry-cured loins after fermentation and 4 months cold storage followed by in vitro gastrointestinal digestion with pepsin and pancreatin.
2. Materials and methods
2.1. Starter cultures
Two different species, known and fulfilling the criteria required for probiotic strains, were used in this study: Lactobacillus rhamnosus LOCK900® (Lb. rhamnosus LOCK900) from the culture collection of the Technical University of Łódź in Poland (Aleksandrzak-Piekarczyk, Koryszewska-Bagińska, & Bardowski, Citation2013), Bifidobacterium animalis subsp. lactis BB-12® (B. animalis BB-12) from the culture collection of Chr. Hansen. In a study conducted by Neffe-Skocińska, Jaworska, Kolożyn-Krajewska, Dolatowski and Jachacz-Jówko (Citation2015), Wójciak, Dolatowski, Kołożyn-Krajewska and Trząskowska (Citation2012) and Jaworska, Neffe, Kołożyn-Krajewska and Dolatowski (Citation2011), it was demonstrated that these probiotic starter cultures have good technology properties for the production of dry fermented meat products.
2.2. Loins production
Pork loins (n = 9) with an average weight of 2.0 kg were obtained from the Large White Polish pig breed with a live weight of approx. 120–130 kg. The loins were dry-cured with 28 g of mixture (with the following content: 20 g of marine salt, 9.7 g of curing salt and 0.3 g of sodium nitrate)/kg of raw meat at a temperature of 2°C for 48 h. After 48-hour curing, bacteria strains (Lb. rhamnosus LOCK900, B. animalis BB-12) were transferred onto the meat surface in the amount of 6 CFU/g of meat and glucose (6 g/kg of meat), after which the loins were left in the fermentation chamber at a temperature of 16°C and relative air humidity of 70–75% for 30 days. Finished loins were vacuum-packed and stored at a temperature of 4°C for 4 months. Qualitative and quantitative tests of the proteolysis products were conducted immediately after fermentation (0) and after 4 months of cold storage. The loins were randomly divided in three experimental batches with three loins each:
C – without probiotic starter cultures, treated as the control one;
P1 – with probiotic starter culture Lb. rhamnosus LOCK900;
P2 – with probiotic starter culture B. animalis BB-12;
P3 – with a mixture of two starter cultures Lb. rhamnosus LOCK900 and B. animalis BB-12 (1:1).
2.3. In vitro gastro-pancreatic digestion
The process of gastrointestinal digestion was carried out according to the method of Clavel, Carlin, Lairon, Nguyen-The and Schmitt (Citation2004) and Marteau, Minekus, Havenaar and Huis (Citation1997). A sample of ground dry-cured loin (30 g) was mixed with 150 mL of buffer P (phosphoric buffer with 1% of pepsin from porcine gastric mucosa [0.7 FIP-U/mg] at pH 2.4 ± 0.2) and was incubated in a shaker at 37°C for 2 h. The sample was then mixed with PA buffer (50 mL of phosphate buffer with bile salts at pH 7.0 ± 0.2 and 1 Kreon 25,000 tablet containing 300 mg of pancreatin with the following enzyme content: 25,000 Ph. Eur. U of lipase, 18,000 Ph. Eur. U of amylase, 1,000 Ph. Eur. U of protease) in a 1:2 ratio and incubated in a shaker at a temperature of 37°C for 6 h. The digestion was stopped by 10-min incubation in a water bath at 95°C. The sample was quenched and filtered, and the resulting filtrate was used in subsequent assays.
2.4. Isolation of trichloroacetic acid (TCA) soluble peptides
Samples for analysis of peptides were prepared according to the modified method of Mikami, Nagao, Sekikawa, Miura and Hongo (Citation1994). A loin sample with a weight of 1 g was homogenised in 50 mL of distilled water (20°C) at a speed of 9500 rpm for 2 min. It was then filled up with distilled water to 100 mL and a 5 mL sample was taken. The sample was mixed with 5 mL of 4% TCA and left for 30 min at a temperature of 4°C. Then the sample was filtered through Whatman® Grade 1 filter paper. Two per cent TCA solubles were analysed for peptides content.
2.5. pH analysis
The pH was measured on 10 g of sample homogenised with 50 mL of distilled water using a digital pH meter CPC-501 (Elmetron, Zabrze, Poland) equipped with a combined electrode type ERH-111 (Hydromet, Gliwice, Poland). Each sample was analysed three times. Measurements were performed at 20°C.
2.6. Oxidation–reduction potential (ORP)
The ORP was measured on 10 g of sample homogenised with 50 mL of distilled water using a digital pH/conductivity meter CPC-501 (Elmetron, Zabrze, Poland) equipped with a combined electrode type ERPt-13 (Hydromet, Gliwice, Poland). Each sample was analysed three times. Measurements were performed at 20°C.
2.7. Water activity (aw)
The water activity (aw) was measured at 20 ± 1°C, using a LabMaster-aw instrument (Novasina AG, Lachen, Switzerland). Humidity standards based on saturated salt solutions were used for calibration.
2.8. Amino acids analysis
The contents of free amino acids were determined by the method reported by Stadnik and Dolatowski (Citation2015) using an automatic amino acid analyser AAA 400 (Ingos Ltd. Czech Republic). The free amino acid content was expressed as mg/g of dry matter (Bele, Matea, Bunea, Jecan, & Dulf, Citation2010).
2.9. Determination of the TCA soluble peptide concentration
The concentration of peptides was determined by the Lowry method using L-leucine as the standard (Lowry, Rosebrough, Farr, & Randall, Citation1951).
2.10. Antiradical activity
Free radical scavenging activity was measured using ABTS+· (2,2′-azobis(3-etylobenzotiazolino-6-sulfonian)) according to Re et al. (Citation1999) as the source of the free radicals. For the ABTS assay, 0.2 mL of TCA soluble peptide extracts were mixed with 2 mL solution of ABTS+·. Absorbance at 734 nm was measured in a UV–Vis spectrophotometer (Nicole Evolution 300, Thermo Elektron Corporation) immediately and after 6 min of incubation. The antiradical activity was related to Trolox (an analogue of vitamin E) and expressed as mg of Trolox per mg peptides. All assays were performed in triplicates.
2.11. Statistical analysis
Statistical characteristics of the sample were prepared (mean value, standard deviation) and ANOVA variance analysis was conducted by using SPSS software package version 22.0 for Windows (SPSS, Inc., Chicago, IL, U.S.A.). The significance of differences between the samples was defined by means of the Tukey test at a confidence level of 0.05. The experiment was carried out in three replicates. All measurements were performed in triplicate, and the data were expressed as mean ± standard error (SE).
3. Results
3.1. Physico-chemical properties of loins
Directly after fermentation () the lowest pH value was showed in the P2 sample (5.46) and the highest in the C sample (5.65). An increase in the pH values in all of the tested samples was recorded after 4 months of storage; the highest increase, of 0.3 units, was observed in the control and the lowest in the P1 sample (approx. 0.14 units). In the remaining P2 and P3 samples, an increase in the pH value was approx. 0.25 units. After 4 months of storage the control sample (5.95) had the highest pH, while the probiotic loins were characterised by a similar pH of 5.72–5.74. The pH of the probiotic loins showed no statistically significant differences between samples during cold storage.
Table 1. Physico-chemical parameters of the loins*.
Tabla 1. Parámetros fisicoquímicos del lomo*.
The results of oxidation–reduction potential (ORP) after fermentation () demonstrated the highest values in the control sample (377.2 mV), which were approx. 30–50 mV higher as compared with the probiotic loin samples (approx. 330 mV). After 4 months of storage the P2 sample had the highest value of ORP (364.8 mV), while the lowest values were observed in the P1 sample (336.47 mV). An increase in the ORP was confirmed after 4 months of storage in samples P2 (an increase of 32.2 mV) and P3 (19.2 mV), while a reduction was recorded in the C sample (by 21.27 mV) and P1 (by 8.3 mV).
The lowest water activity directly after fermentation () was observed in sample P1 (0.952), while after 4 months of storage in sample C (0.933). Water activity in the probiotic samples after fermentation and after 4 months of storage was similar (approx. 0.955 and 0.935, respectively).
3.2. Free amino acids after fermentation and storage
Free amino acid profile analysis in loins directly after fermentation showed that the sample containing a mixture of the bacterial strains Lb. rhamnosus LOCK900 and B. animalis BB-12 was characterised by the greatest amount of protein metabolism products (). Free amino acid profile analysis in the P3 sample after fermentation showed the highest content of alanine, glycine, histidine, isoleucine, glutamic acid, leucine, lysine, methionine, phenylalanine, serine, tyrosine and valine (1.12, 0.68, 0.90, 0.77, 1.08, 1.20, 1.98, 0.41, 0.72, 1.15, 0.58, 1.07 mg/g of dry matter, respectively). A high content of alanine and tyrosine (0.97 and 0.34 mg/g of dry matter, respectively) was found in the P3 loin sample (1.12 and 0.58 mg/g of dry matter, respectively) as compared with the control sample. It should be noticed that the highest content of arginine was recorded directly after the fermentation process in samples P1 and P2 with the addition of a single-species probiotic starter culture (0.35 and 0.46 mg/g of dry matter, respectively). The value was almost twofold higher than in the control (0.25 mg/g of dry matter) and in the P3 sample with the strain mixture (0.18 mg/g of dry matter). After 4 months of storage there was an increase in free amino acids in all of the samples, of which the highest values were observed for the sample with a mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 and the sample inoculated with Lb. rhamnosus LOCK900 cultures (). The highest histidine, isoleucine, glutamic acid, leucine, methionine, phenylalanine, proline, serine, tyrosine and valine values were observed in the P3 sample (1.13, 0.87, 2.25, 1.51, 0.55, 0.86, 0.53, 1.20, 0.55, 1.11 mg/g of dry matter, respectively). After 4 months of cold storage in anaerobic conditions, higher levels of glutamic acid were observed in the free amino acids profile of the dry-cured loin samples inoculated with a mixture of probiotic bacteria as compared with the control without the addition of starter cultures. Summing up the results, it can be concluded that the highest increase of all the free amino acids during 6 months of storage was observed in the P2 sample and amounted to 5.53 mg/g of dry matter, while the lowest increase, of 1.68 mg/g of dry matter, was measured in the control sample.
Table 2. Content (expressed as mg/g of dry matter) of free amino acids after fermentation and after 4 months of cold storage*.
Tabla 2. Contenido (expresado como mg/g de materia seca) de aminoácidos libres después de la fermentación y después de 4 meses de almacenamiento en frío*.
The arginine content after 4 months of storage was the highest in the probiotic loins (P1, P2) and was approx. 0.48 mg/g of dry matter, i.e. fourfold higher than in the control (0.11 mg/g of dry matter). Directly after fermentation the content of urea, as an amino acid degradation product, was the highest in the control (3.26 mg/g of dry matter), while after 4 months of storage it was the highest in the P1 sample (7.00 mg/g of dry matter).
3.3. TCA soluble peptide content in dry-cured loins
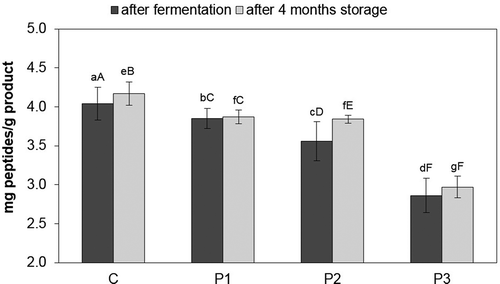
The highest content of TCA soluble peptides directly after fermentation () was recorded in the control (4.04 mg/g), while the lowest in the sample with the mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 (2.86 mg/g). The highest content of peptides after 4 months of storage was found in the control (4.17 mg/g), whereas the lowest, as after fermentation, was found in the P3 sample (2.97 mg/g). The highest increase in TCA soluble peptide content (of 0.28 mg/g) after 4 months of storage was observed in the probiotic loins as compared with the value directly after fermentation in the P2 sample, while the lowest was observed in the P1 sample (approx. by 0.02 mg/g). Quantitative analysis of the TCA soluble peptides demonstrated that the highest peptide content directly after fermentation and after 4 months of storage was observed in the control sample; it was higher by approx. 0.2–1.2 mg/g as compared with the probiotic loin samples.
Figure 1. Peptides content in dry-cured loins during cold storage.
a–g Within the same storage time, means followed by the common small letter do not differ significantly (P < 0.05)
A–F Within the same treatment, means followed by the common small letter do not differ significantly (P < 0.05)
C – sample without probiotic starter cultures, treated as the control one.
P1 – sample with probiotic starter culture Lb. rhamnosus LOCK900; P2 – sample with probiotic starter culture B. animalis BB-12; P3 – sample with a mixture of two starter cultures (Lb. rhamnosus LOCK900 and B. animalis BB-12).
Figura 1. Contenido de péptidos de lomo curado en seco durante el almacenamiento en frío.
a-g Durante el mismo tiempo de almacenamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
A-F En el mismo tratamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
C – muestra sin cultivos iniciadores probióticos, tratado como muestra control;
P1 – muestra con cultivo iniciador probiótico de Lb. rhamnosus LOCK900; P2 – muestra con cultivo iniciador probiótico de B. animalis BB-12; P3 – muestra con mezcla de dos cultivos iniciadores (Lb. rhamnosus LOCK900 y B. animalis BB-12).
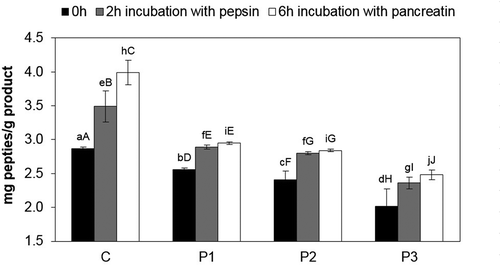
When analysing the quantitative peptide changes, the highest increase in peptides (2.87–3.49 mg/g) () during in vitro pepsin digestion was observed in dry-cured loins directly after fermentation as compared with the zero time in the control sample, while the lowest was observed in the P3 sample with Lb. rhamnosus LOCK900 and B. animalis BB-12 (2.02–2.36 mg/g – by 0.34 mg/g). After 6 h of pancreatin hydrolysis, the control sample had the highest content of peptides (3.99 mg/g) and the P3 sample had the lowest (2.48 m/g). After 6 h of pancreatin hydrolysis, the greatest increase in the TCA soluble peptide content was observed in the control sample, i.e. from 3.49 to 3.99 mg/g (0.49 mg/g), and the lowest in the sample with B. animalis BB-12, i.e. from 2.8 to 2.84 mg/g (0.04 mg/g).
Figure 2. Content of peptides during digestion pepsin and pancreatin in dry-cured loins after fermentation.
a–j Within the same storage time, means followed by the common small letter do not differ significantly (P < 0.05)
A–J Within the same treatment, means followed by the common small letter do not differ significantly (P < 0.05). C – sample without probiotic starter cultures, treated as the control one; P1 – sample with probiotic starter culture Lb. rhamnosus LOCK900; P2 – sample with probiotic starter culture B. animalis BB-12; P3 – sample with a mixture of two starter cultures (Lb. rhamnosus LOCK900 and B. animalis BB-12).
Figura 2. Contenido de péptidos durante la digestión de pepsina y pancreatina de lomo curado en seco después de la fermentación
a-j Durante el mismo tiempo de almacenamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
A-J En el mismo tratamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)C – muestra sin cultivos iniciadores probióticos, tratada como muestra control; P1 – muestra con cultivo iniciador probiótico de Lb. rhamnosus LOCK900; P2 – muestra con cultivo iniciador probiótico de B. animalis BB-12; P3 – muestra con mezcla de dos cultivos iniciadores (Lb. rhamnosus LOCK900 y B. animalis BB-12).
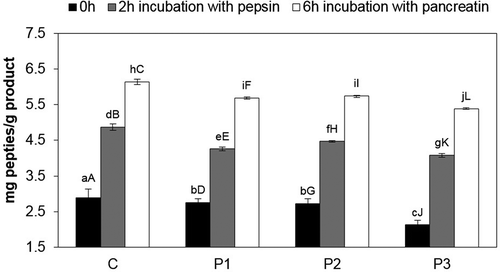
The results of in vitro digestion after 4 months of loin cold storage () showed that the highest content of TCA soluble peptides, after 2-h pepsin digestion, was present in the control sample (4.87 mg/g), while the lowest was present in the P3 sample (4.08 mg/g). The highest content of peptides after 6-h pancreatin treatment () was recorded in the control (6.14 mg/g), while the lowest in the sample with the mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 (5.39 mg/g). After 6 h of loin digestion with pancreatin, the highest increase in peptide content was recorded in the P1 sample (by 1.43 mg/g), and the lowest in the control (by 1.27 mg/g) and the P2 sample (by 1.27 mg/g) in comparison to the value after 2-h pepsin digestion. The highest significant (α ≤ 0.05) increase in the level of peptides in relation to the value after the zero time during the whole period of hydrolysis with gastrointestinal enzymes was detected in the sample with a mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 (2.13–5.39 mg/g – 3.26 mg/g) and in the control (2.89–6.14 mg/g – 3.25 mg/g), whereas the lowest was detected in the sample with the Lb. rhamnosus LOCK900 (2.76–5.69 mg/g – 2.93 mg/g).
Figure 3. Content of peptides during digestion pepsin and pancreatin in dry-cured loins after 4 months storage.
a–j Within the same storage time, means followed by the common small letter do not differ significantly (P < 0.05)
A–L Within the same treatment, means followed by the common small letter do not differ significantly (P < 0.05). C – sample without probiotic starter cultures, treated as the control one; P1 – sample with probiotic starter culture Lb. rhamnosus LOCK900; P2 – sample with probiotic starter culture B. animalis BB-12; P3 – sample with a mixture of two starter cultures (Lb. rhamnosus LOCK900 and B. animalis BB-12).
Figura 3. Contenido de péptidos durante la digestión de pepsina y pancreatina de lomo curado en seco después de 4 meses de almacenamiento
a-j Durante el mismo tiempo de almacenamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
A-L En el mismo tratamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)C – muestra sin cultivos iniciadores probióticos, tratada como muestra control; P1 – muestra con cultivo iniciador probiótico de Lb. rhamnosus LOCK900; P2 – muestra con cultivo iniciador probiótico de B. animalis BB-12; P3 – muestra con mezcla de dos cultivos iniciadores (Lb. rhamnosus LOCK900 y B. animalis BB-12).
3.4. Peptide antiradical activity
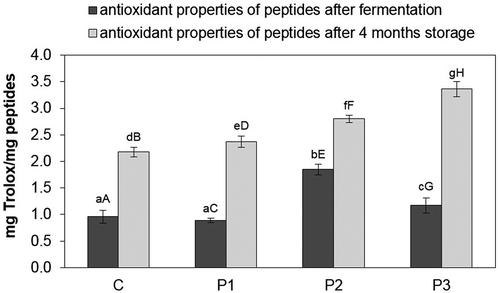
The antiradical properties of TCA soluble peptides in dry-cured loins during refrigerated storage were characterised by high variability (). The P2 sample demonstrated the highest antiradical activity directly after fermentation (1.85 mg Trolox/mg peptides), while the P1 and control sample had the lowest activity (0.89 mg Trolox/mg peptides and 0.96 mg Trolox/mg peptides, respectively). The greatest antiradical activity after 4 months of cold storage was present in the sample with a mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12, which amounted to 3.36 mg Trolox/mg peptides, while the control sample had the lowest (2.18 mg Trolox/mg peptides). A statistically significant (α ≤ 0.05) increase in the antiradical activity of TCA soluble peptides was found in the control between the value directly after fermentation and the value after 4 months of storage (1.22 mg Trolox/mg peptides), in the P1 sample (1.48 mg Trolox/mg peptides) and in the P3 sample (2.19 mg Trolox/mg peptides). In addition, statistically significantly (P < 0.05) higher antiradical activity was recorded directly after fermentation between samples P2 and P1 (a difference of 0.96 mg Trolox/mg peptides) and between the P2 sample and the control sample (a difference of 0.89 mg Trolox/mg peptides).
Figure 4. Antiradical properties of peptides in dry-cured loins during cold storage.
a–g Within the same storage time, means followed by the common small letter do not differ significantly (P < 0.05)
A–H Within the same treatment, means followed by the common small letter do not differ significantly (P < 0.05)
C – sample without probiotic starter cultures, treated as the control one; P1 – sample with probiotic starter culture Lb. rhamnosus LOCK900; P2 – sample with probiotic starter culture B. animalis BB-12; P3 – sample with a mixture of two starter cultures (Lb. rhamnosus LOCK900 and B. animalis BB-12).
Figura 4. Propiedades antiradicales de péptidos de lomo curado en seco durante el almacenamiento en frío.
a-g Durante el mismo tiempo de almacenamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
A-H En el mismo tratamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
C – muestra sin cultivos iniciadores probióticos, tratada como muestra control; P1 – muestra con cultivo iniciador probiótico de Lb. rhamnosus LOCK900; P2 – muestra con cultivo iniciador probiótico de B. animalis BB-12; P3 – muestra con mezcla de dos cultivos iniciadores (Lb. rhamnosus LOCK900 y B. animalis BB-12).
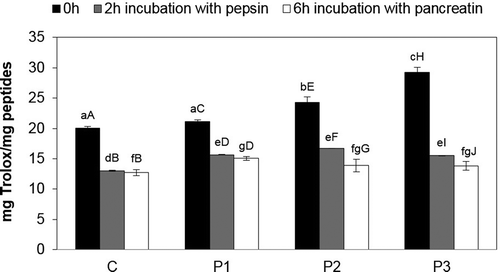
The results of antiradical peptide activity obtained during digestion with pepsin and pancreatin of dry-cured loins directly after fermentation indicated large variation in the antiradical peptide properties (). The highest antiradical activity after 2 h of pepsin digestion was observed in the sample with B. animalis BB-12 (17.78 mg Trolox/mg peptides), while the lowest was in the control sample (10 mg Trolox/mg peptides). The greatest antiradical activity after a 6-h hydrolysis was present in the sample with a mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 (16.75 mg Trolox/mg peptides), while the control sample had the lowest (11.03 mg Trolox/mg peptides). When analysing the obtained results, significantly higher antiradical activity was observed in the probiotic loins as compared with the control.
Figure 5. Antiradical properties of peptides during digestion of pepsin and pancreatin in dry-cured loins after fermentation.
a–i Within the same storage time, means followed by the common small letter do not differ significantly (P < 0.05)
A–I Within the same treatment, means followed by the common small letter do not differ significantly (P < 0.05)
C – sample without probiotic starter cultures, treated as the control one; P1 – sample with probiotic starter culture Lb. rhamnosus LOCK900; P2 – sample with probiotic starter culture B. animalis BB-12; P3 – sample with a mixture of two starter cultures (Lb. rhamnosus LOCK900 and B. animalis BB-12).
Figura 5. Propiedades antiradicales de péptidos durante la digestión de pepsina y pancreatina de lomo curado en seco después de la fermentación.
a-i Durante el mismo tiempo de almacenamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
A-I En el mismo tratamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
C – muestra sin cultivos iniciadores probióticos, tratada como muestra control; P1 – muestra con cultivo iniciador probiótico de Lb. rhamnosus LOCK900; P2 – muestra con cultivo iniciador probiótico de B. animalis BB-12; P3 – muestra con mezcla de dos cultivos iniciadores (Lb. rhamnosus LOCK900 y B. animalis BB-12).
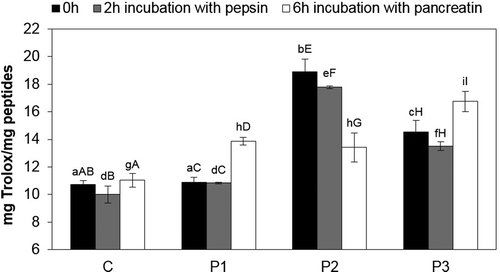
The analysis of the antiradical properties of the TCA soluble peptides obtained during pepsin hydrolysis of dry-cured loins after 4 months of storage () showed that the P2 sample was characterised by the highest antiradical peptide activity (Trolox 16.73 mg/mg peptides) after 2 h of incubation with pepsin, while the control sample showed the lowest activity (13 mg Trolox/mg peptides). After 6-h pancreatin digestion, the highest peptide antiradical activity was present in loins with the Lb. rhamnosus strain LOCK900 and amounted to 15.05 mg Trolox/mg peptides, whereas the lowest was recorded in the control loins (12.7 mg Trolox/mg peptides). After 2 h of pepsin digestion, the highest decrease in the antiradical activity of peptides was found in loins with a mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 (lower by 13.69 mg Trolox/mg peptides), whereas the lowest was in the sample with the Lb. rhamnosus LOCK900 (5.44 mg Trolox/mg peptides). The P1 sample demonstrated the lowest reduction of peptide antiradical activity during the entire period of gastrointestinal enzyme digestion in vitro (by 6.05 mg Trolox/mg peptides). In contrast, the sample with the mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 was characterised by the significantly (P ≤ 0.05) highest decrease in peptide antiradical activity during gastrointestinal enzyme digestion in vitro (by 15.38 mg Trolox/mg peptides).
Figure 6. Antiradical properties of peptides during digestion of pepsin and pancreatin in dry-cured loins after 4 months storage.
a–g Within the same storage time, means followed by the common small letter do not differ significantly (P < 0.05)
A–J Within the same treatment, means followed by the common small letter do not differ significantly (P < 0.05)
C – sample without probiotic starter cultures, treated as the control one; P1 – sample with probiotic starter culture Lb. rhamnosus LOCK900; P2 – sample with probiotic starter culture B. animalis BB-12; P3 – sample with a mixture of two starter cultures (Lb. rhamnosus LOCK900 and B. animalis BB-12).
Figura 6. Propiedades antiradicales de péptidos durante la digestión de pepsina y pancreatina de lomo curado en seco después de 4 meses de almacenamiento.
a-g Durante el mismo tiempo de almacenamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
A-J En el mismo tratamiento, los promedios seguidos de la misma letra minúscula no difieren significativamente (P < 0.05)
C – muestra sin cultivos iniciadores probióticos, tratada como muestra control; P1 – muestra con cultivo iniciador probiótico de Lb. rhamnosus LOCK900; P2 – muestra con cultivo iniciador probiótico de B. animalis BB-12; P3 – muestra con mezcla de dos cultivos iniciadores (Lb. rhamnosus LOCK900 y B. animalis BB-12).
4. Discussion
Sample pH values during storage were at a similar level, and differences between the samples were approx. 0.2 units. Lücke (Citation2000) found that the stage at which the pH value during fermentation stops decreasing and begins to rise depends largely on the microbiological quality of the raw material, on the technology and the dynamics of microbial growth conditioned by the process parameters. The action of probiotic bacterial cultures used in this study, and those described in the literature, in the production of dry-cured products involves acidification of the production environment with different substances, which results in the elimination of other microflora, including pathogenic groups of a given environment (Mora, Fraser, & Toldrá, Citation2013). This was particularly evident in the probiotic loins after 4 months of cold storage. Higher participation of bacterial culture enzymes in proteolytic changes can also be observed during ageing (storage) of dry-cured sausages (Aro Aro et al., Citation2010). Probiotic strains change the quantitative and qualitative levels of amino acids in samples. Changes may be induced by inhibiting the activity of natural environmental microflora that occur in raw meat (Freiding, Gutsche, Ehrmann, & Vogel, Citation2011; Latorre-Moratalla, Bover-Cid, Bosch-Fusté, Veciana-Nogués, & Vidal-Carou, Citation2014). The Bifidobacterium and Lactobacillus genera, which were applied in the present technological process conditions, require certain amino acids for their development, i.e. arginine, isoleucine, leucine, tyrosine, cysteine and valine (Fadda et al., Citation1999; Sanz et al., Citation1999a, Citation1999b). The results obtained here showed that these were present in the product as free amino acids or in peptides of low molecular weight. Poch and Bezkorovainy (Citation1991), Gomes, Malcata and Klaver (Citation1998) and Belkaaloul, Chekroun, Ait-Abdessalam, Saidi and Kheroua (Citation2010) suggested that Lactobacillus acidophilus Ki bacteria hydrolyse milk proteins to low-molecular-weight peptides that can be used for bacterial growth and acidification of the environment, which in turn increases the number of other bacteria species or strains of B. animalis ssp. lactis Bo. Similar interactions, i.e. as those in the present study, were observed with other bacterial strains of Bifidobacterium and Lactobacillus by Gomes et al. (Citation1998) and Altier, Bevilacqua, D’Amato, Del Nobile and Sinigaglia (Citation2008). This phenomenon was confirmed by Aro Aro et al. (Citation2010), who found no significant difference in pH of the product between samples after 21 days of fermentation of sausages with different bacterial strains (Lactobacillus sakei D-1001, Staphylococcus carnosus SB-61, Staphylococcus xylosus and Pediococcus pentosaceus), while there was a significant increase in free amino acid content in these samples. The content of free amino acids in the product has an influence on their degradation rate to biogenic amines by respective enzymes, the action of which is in turn inhibited by the salt content and low water activity (Berge et al., Citation2001; Ruiz-Ramirez, Arnau, Serra, & Gou, Citation2005). Similar correlations were observed by Flores, Aristoy and Toldrá (Citation1997), Toldrá, Rico and Flores (Citation1992), Ruiz-Ramirez et al. (Citation2005) and Schivazappa et al. (Citation2002). The results indicate the advisability of applying probiotic bacteria to the production of dry-cured meat products due to altered muscle protein degradation. Similar correlations were observed by Casaburi et al. (Citation2007), who reported an increase in glutamic acid and alanine during changes in free amino acid contents in sausages aged with strains of S. xylosus and Lactobacillus curvatus. Fadda et al. (Citation1999) found in their study that the LAB bacteria contribute, in varying degrees, to the release of certain amino acids. They showed that Lb. curvatus stimulates the formation of large quantities of glutamic acid, alanine, histidine, arginine and lysine, and that Lb. sakei affects the level of glutamic acid, alanine, gamma-aminobutyric acid, threonine, leucine, phenylalanine and ornithine. The studies of other authors also report similar changes in the amounts of free amino acids in the compositions of traditional fermented sausages caused, as in the current study, by starter cultures with a specific bacteria composition (Casaburi et al., Citation2008; Latorre-Moratalla et al., Citation2014).
The pH of probiotic loins after 4 months of storage was lower by 0.2 units as compared with the control sample. The peptide content after fermentation and after 4 months of storage in probiotic loins produced with a single strain was similar to the control and higher than in loins with a mixture of two bacterial strains. The lowest content of peptides in the sample with a mixture of strains Lb. rhamnosus LOCK900 and B. animalis BB-12 may suggest inhibition of proteolytic changes in the analysed loins. The current study elucidates the effects of selected strains of probiotic bacteria on meat proteolysis during fermentation, storage and digestion in vitro (pepsin and pancreatin) of the dry-ageing product. The use of probiotic bacteria in dry-cured loins increased the effectiveness of gastrointestinal enzymes in vitro (pepsin, pancreatin) in the degradation of meat protein. A twofold increase in the peptide content was recorded after 4 months of storage after pepsin digestion with a mixture of probiotic strains.
The low redox potential of the probiotic loins directly after fermentation suggests the inhibition of oxidative changes as a result of the accumulation of large quantities of antioxidant products (in this case peptides and amino acids). An increase in redox potential after 4 months of storage in the P2 and P3 samples may indicate that other oxidation mechanisms occurred during this period of storage in the tested samples. By analysing the changes in peptide antiradical activity in dry-cured loins during storage, the lowest capacity to quench the ABTS radical cation was measured for the control sample and for the sample with Lb. rhamnosus LOCK900 directly after fermentation. These loins also had the highest TCA soluble peptide content during the 4-month storage. It can be assumed that the activity of peptides was blocked in them by other compounds or that breakdown of biologically active groups occurred. In contrast, the sample with the B. animalis BB-12 and the sample with a mixture of strains L. rhamnosus LOCK900 and B. animalis BB-12 demonstrated high antiradical activity throughout the storage period. Peptide antiradical activity during in vitro digestion with pepsin decreased in all of the samples. These changes were probably caused by interactions of the resulting hydrolysis products (or their degradation products). It can also be assumed that products limiting ABTS radical quenching formed during hydrolysis.
5. Conclusions
The use of probiotic bacteria for the manufacture of dry-cured products affects the formation of protein proteolysis products with antiradical activity during cold storage and gastrointestinal enzyme digestion. Loins with the B. animalis ssp. lactis strain BB12 were characterised by the highest antiradical peptide activity among products after fermentation and cold storage.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aleksandrzak-Piekarczyk, T., Koryszewska-Bagińska, A., & Bardowski, J. (2013). Genome sequence of the probiotic strain Lactobacillus rhamnosus (formerly Lactobacillus casei) LOCK900. Genome Announcements, 4, e00640–13. doi: 10.1128/genomeA.00640-13
- Altier, C., Bevilacqua, A., D’Amato, D., Del Nobile, M.A., & Sinigaglia, M. (2008). Modelling the survival of starter lactic acid bacteria and Bifidobacterium bifidum in single and simultaneous cultures. Food Microbiology, 25, 729–734. doi: 10.1016/j.fm.2008.03.005
- Aro Aro, J.M., Nyam-Osor, P., Tsuji, K., Shimada, K., Fukushima, M., & Sekikawa, M. (2010). The effect of starter cultures on proteolytic changes and amino acid content in fermented sausages. Food Chemistry, 119, 279–285. doi: 10.1016/j.foodchem.2009.06.025
- Bele, C., Matea, C., Bunea, A., Jecan, C., & Dulf, F. (2010). Determination of amino acid content in romanian salami by ion-exchange chromatography. Research Journal of Agricultural Science, 42, 211–215.
- Belkaaloul, K., Chekroun, A., Ait-Abdessalam, A., Saidi, D., & Kheroua, O. (2010). Growth, acidification and proteolysis performance of two co-cultures (Lactobacillus plantarum-Bifidobacterium longum and Streptococcus thermophilus-Bifidobacterium longum). African Journal of Biotechnology, 9, 1463–1469. doi: 10.5897/AJB09.1090
- Berge, P., Ertbjerg, P., Larsen, L. M., Astruc, T., Vignon, X. & Møller, A. (2001). Tenderization of beef by lactic acid injected at different times post mortem. Meat Science, 57, 347–357.
- Casaburi, A., Aristoy, M., Cavella, S., Di Monaco, R., Ercolini, D., Toldra, F., & Villani, F. (2007). Biochemical and sensory characteristics of traditional fermented sausages of Vallo di Diano (Southern Italy) as affected by the use of starter cultures. Meat Science, 76, 295–307. doi: 10.1016/j.meatsci.2006.11.011
- Casaburi, A., Di Monaco, R., Cavella, S., Toldrá, F., Ercolini, D., & Villani, F. (2008). Proteolytic and lipolytic starter cultures and their effect on traditional fermented sausages ripening and sensory traits. Food Microbiology, 25, 335–347. doi: 10.1016/j.fm.2007.10.006
- Clavel, T., Carlin, F., Lairon, D., Nguyen-The, C., & Schmitt, P. (2004). Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. Journal of Applied Microbiology, 97, 214–219. doi: 10.1111/jam.2004.97.issue-1
- Diplock, A.T., Aggett, P., Ashwell, M., Bornet, F., Fern, E., & Roberfroid, M. (1999). The European comission concerted action on functional foods science in Europe (FUFOSE). Scientific concepts of functional foods in Europe. British Journal of Nutrition, 81, S1–S27.
- Escudero, E., Aristoy, M., Nishimura, H., Arihara, K., & Toldrá, F. (2012). Antihypertensive effect and antioxidant activity of peptide fractions extracted from Spanish dry-cured ham. Meat Science, 91, 306–311. doi: 10.1016/j.meatsci.2012.02.008
- Escudero, E., Mora, L., Fraser, P., Aristoy, M., & Toldrá, F. (2013). Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chemistry, 138, 1282–1288. doi: 10.1016/j.foodchem.2012.10.133
- Fadda, S., Sanz, Y., Vignolo, G., Aristoy, M.C., Oliver, G., & Toldrá, F. (1999). Hydrolysis of pork muscle sarcoplasmic proteins by Lactobacillus curvatus and Lactobacillus sake. Applied and Environmental Microbiology, 65, 578–584.
- Flores, M., Aristoy, M.C., & Toldrá, F. (1997). Curing agents affect aminopeptidas activity from porcine skeletal muscle. Zeitschrift Fur Lebensmittel Untersuchung Und Forschung A, 205, 343–346. doi: 10.1007/s002170050177
- Freiding, S., Gutsche, K.A., Ehrmann, M.A., & Vogel, R.F. (2011). Genetic screening of Lactobacillus sakei and Lactobacillus curvatus strains for their peptidolytic system and amino acid metabolism, and comparison of their volatilomes in a model system. Systematic and Applied Microbiology, 34, 311–320. doi: 10.1016/j.syapm.2010.12.006
- Gomes, A.M., Malcata, F.X., & Klaver, F.A. (1998). Growth enhancement of Bifidobacterium lactis Bo and Lactobacillus acidophilus Ki by milk hydrolyzates. Journal of Dairy Science, 81, 2817–2825. doi: 10.3168/jds.S0022-0302(98)75840-0
- Holzapfel, W.H., & Schillinger, U. (2002). Introduction to pre- and probiotics. Food Research International, 35, 109–116. doi: 10.1016/S0963-9969(01)00171-5
- Jaworska, D., Neffe, K., Kołożyn-Krajewska, D., & Dolatowski, Z. (2011). Survival during storage and sensory effect of potential probiotic lactic acid bacteria Lactobacillus acidophilus Bauer and Lactobacillus casei Bif3ʹ/IV in dry fermented pork loins. International Journal of Food Science and Technology, 46, 2491–2497. doi: 10.1111/j.1365-2621.2011.02772.x
- Kołożyn-Krajewska, D., & Dolatowski, Z.J. (2012). Probiotic meat products and human nutrition. Processing Biochemistry, 47, 1761–1772. doi: 10.1016/j.procbio.2012.09.017
- Latorre-Moratalla, M., Bover-Cid, S., Bosch-Fusté, J., Veciana-Nogués, M.T., & Vidal-Carou, M.C. (2014). Amino acid availability as an influential factor on the biogenic amine formation in dry fermented sausages. Food Control, 36, 76–81. doi: 10.1016/j.foodcont.2013.07.038
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., & Randall, R.J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
- Lücke, F.K. (2000). Utilization of microbes to process and preserve meat. Meat Science, 56, 105–115. doi: 10.1016/S0309-1740(00)00029-2
- Marteau, P., Minekus, M., Havenaar, R., & Huis, J.H.J. (1997). Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine, validation and the effects of bile. Journal of Dairy Science, 80, 1031–1037. doi: 10.3168/jds.S0022-0302(97)76027-2
- Mikami, M., Nagao, M., Sekikawa, M., Miura, H., & Hongo, Y. (1994). Effects of electrical stimulation on the peptide and free amino acid contents of beef homogenate and sarcoplasma during storage. Animal Science and Technology, 11, 1034–1043.
- Mora, L., Fraser, P.D., & Toldrá, F. (2013). Proteolysis follow-up in dry-cured meat products through proteomic approaches. Food Research International, 54, 1292–1297. doi: 10.1016/j.foodres.2012.09.042
- Neffe-Skocińska, K., Jaworska, D., Kolożyn-Krajewska, D., Dolatowski, Z., & Jachacz-Jówko, L. (2015). The effect of LAB as probiotic starter culture and green tea extract addition on dry fermented pork loins quality. Biomed Research International, 2015, 1–9. Article ID 452757, 9 pages. doi: 10.1155/2015/452757
- Olmedilla-Alonso, B., Jiménez-Colmenero, F., & Sánchez-Muniz, F.J. (2013). Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Science, 95, 919–930. doi: 10.1016/j.meatsci.2013.03.030
- Poch, M., & Bezkorovainy, A. (1991). Bovine milk-casein trypsin digest is a growth enhancer for the genus Bifidobacterium. Journal of Agricultural and Food Chemistry, 39, 73–77. doi: 10.1021/jf00001a013
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidants activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
- Ruiz-Ramirez, J., Arnau, J., Serra, X., & Gou, P. (2005). Relationship between water content, NaCl content, pH and texture parameters in dry-cured muscles. Meat Science, 70, 579–587. doi: 10.1016/j.meatsci.2005.02.007
- Sanz, Y., Fadda, S., Vignolo, G., Aristoy, M., Oliver, G., & Toldra, F. (1999a). Hydrolysis of muscle myofibrillar proteins by Lactobacillus curvatus and Lactobacillus sake. International Journal of Food Microbiology, 53, 115–125. doi: 10.1016/S0168-1605(99)00134-8
- Sanz, Y., Fadda, S., Vignolo, G., Aristoy, M.C., Oliver, G., & Toldrá, F. (1999b). Hydrolytic action of Lactobacillus casei CRL 705 on pork muscle sarcoplasmic and myofibrillar proteins. Journal of Agricultural and Food Chemistry, 47, 3441–3448. doi: 10.1021/jf981255n
- Schivazappa, C., Degni, M., Nanni Costa, L., Russo, V., Buttazzoni, L., & Virgili, R. (2002). Analysis of raw meat to predict proteolysis in Parma ham. Meat Science, 60, 77–83. doi: 10.1016/S0309-1740(01)00109-7
- Stadnik, J., & Dolatowski, Z.J. (2015). Free amino acids and biogenic amines content during ageing of dry-cured pork loins inoculated with Lactobacillus casei ŁOCK 0900 probiotic strain. Food Science and Technology Research, 21, 167–174. doi: 10.3136/fstr.21.167
- Toldrá, F., Rico, E., & Flores, J. (1992). Activities of pork muscle proteases in model cured meat systems. Biochimie, 74, 291–296. doi: 10.1016/0300-9084(92)90128-2
- Tripathi, M.K., & Giri, S.K. (2014). Probiotic functional foods: Survival of probiotics during processing and storage. Journal of Functional Foods, 9, 225–241. doi: 10.1016/j.jff.2014.04.030
- Wójciak, K.M., Dolatowski, Z.J., Kołożyn-Krajewska, D., & Trząskowska, M. (2012). The effect of the Lactobacillus casei ŁOCK 0900 probiotic strain on the quality of dry-fermented sausage during chilling storage. Journal of Food Quality, 35, 353–365. doi: 10.1111/j.1745-4557.2012.00458.x
