ABSTRACT
Turmeric extract (0.5%) was applied to Sepia brevimana muscle to investigate its effects on muscle sensory evaluation, biochemical parameters, microbiological proliferation, and biogenic amine (BA) formation during storage at 4°C for 18 days. Sensory evaluation determined the shelf life of S. brevimana muscle to be 12 days for the controls and 15 days for the turmeric-treated samples. The biochemical quality index differences were significant in all instances, and the values were improved in the turmeric-treated samples compared to the control samples. Most microbiological counts were higher in the control samples than in the turmeric-treated samples after day 3. Additionally, the turmeric extract showed an inhibitory effect against some potential BA-forming bacteria. In conclusion, this study revealed the ability of turmeric extract to improve the shelf life and quality of S. brevimana muscle during chilled storage.
RESUMEN
Extracto de cúrcuma (0,5%) fue aplicado a músculo de Sepia brevimana con el fin de investigar sus efectos sobre la calidad sensorial, parámetros bioquímicos, calidad microbiológica, y la formación de aminas biogénicas durante 18 días de almacenamiento a 4°C. La evaluación sensorial determinó que la vida útil del músculo de S. brevimana alcanzó 12 días en el caso de los controles y 15 días para las muestras tratadas. Los parámetros relativos a la calidad bioquímica, fueron significativamente mejores en todos los casos en las muestras tratadas. La mayor parte de los parámetros microbiológicos mostraron mayores recuentos en las muestras controles en comparación con las muestras tratadas después del día 3. Además, el extracto de cúrcuma mostró un efecto inhibidor frente al desarrollo de bacterias potencialmente productoras de aminas biógenas. En conclusión, este estudio demostró que el extracto de cúrcuma mejora la vida útil y la calidad del músculo de S. brevimana durante el almacenamiento refrigerado.
PALABRAS CLAVE:
Introduction
The global cuttlefish catch has increased rapidly in recent years due to growing market demand from their increased consumption in countries that traditionally have not consumed them (FAO, Citation2007). However, similar to other marine fish, squid and cuttlefish are highly susceptible to rapid spoilage at ambient temperature and consequently are often preserved on ice or refrigerated to delay spoilage. The rate of deterioration during the storage of fish and fishery products varies with species, substrate concentration, metabolites in the tissues, microbial type and load, and storage conditions after catching (Aubourg, Losada, Gallardo, Miranda, & Barros-Velázquez, Citation2006).
The shelf life of fish can be estimated by sensory, microbiological, or chemical methods. Among chemical parameters, biogenic amines (BAs) are basic nitrogenous compounds of low molecular weight, which are mainly produced through the bacterial decarboxylation of specific amino acids in seafood products (Chong, Abu Bakar, Russly, Jamilah, & Mahyudin, Citation2011). Low levels of BAs in aquatic products do not present a serious risk to human health because amine oxidases in the human intestine can rapidly detoxify amines. However, the ingestion of aquatic foods containing high levels of BAs may result in severe toxicological symptoms (Mohamed, Abd Ei-Hameed, Nezam El-Din, & El-Din, Citation2010). Of all BAs, histamine and tyramine are the most dangerous for human health when their levels are high (Hu, Huang, Li & Yang, Citation2012). The continued consumption of these BAs causes a wide variety of symptoms, such as nausea, respiratory distress, vomiting, allergy, itching, burning sensations in the mouth, heart palpitations, headache, and hypertension or hypotension (Chong et al., Citation2011). The temperature, pH, water activity, and storage time mainly influence BA formation in fish and fishery products during postharvest processes (Chong et al., Citation2011). Previous research has determined that many bacterial species are known to possess histidine decarboxylase and produce histamine and other BAs (Fernandez-No, Bohme, Gallardo, Velazquez, Canas, & Mata, Citation2010; Kim, Mah, & Hwang, Citation2009). Although cephalopods are not commonly implicated as are some fish families such as Scombridae and Scomberesocideae, the presence of BAs has received attention in recent years (Hu et al., Citation2012; Kim et al., Citation2009; Prester, Citation2011).
BA formation can be controlled by inhibiting microbial growth and the decarboxylase activity of microbes, as well as by controlling several parameters such as the use of amine-oxidizing starter cultures for fermentation, enzymes to oxidize amines, packaging techniques, irradiation, or food additives (Naila, Flint, Fletcher, Bremer, & Meerdink, Citation2010). The use of natural preservatives, such as turmeric, garlic, and other spices, for marine seafood preservation has received immense interest among researchers. Moreover, the seafood industry is searching for natural preservatives to avoid adverse effects on fish meat and to extend shelf life (Gul & Bakht, Citation2015). Turmeric (Curcuma longa) is one of the spices most widely used as a preservative and as a color, antiseptic, anticancer, wound healing, and antibacterial agent in biological systems worldwide.
Like many fish species, cuttlefishes are prone to rapid spoilage (Prester, Citation2011). In contrast to fish, the enhancement of shelf life and BA formation control in cephalopods using food additives, such as turmeric, has not been well established. Hence, this study aimed to investigate the dual effect of temperature and turmeric to control the formation of BA and enhance the shelf life of S. brevimana. In addition, the antibacterial effect of turmeric was tested against biogenic amine-forming bacteria (BAF).
Materials and methods
Sample collection, treatment, and storage
Twenty shortclub cuttlefish (S. brevimana) samples (average 545.14 ± 32.38 g weight and 28.43 ± 0.89 cm total length) were collected from the Thondi coast, Palk Bay, southeast India and brought to the laboratory within 15 min in sterile polyethylene bags containing ice. The rhizomes of turmeric (C. longa) were powdered and diluted with triple-deionized water. Ten S. brevimana samples were washed in tap water, beheaded, eviscerated, and then washed thoroughly again in potable water and divided into two lots. One lot was given a dip treatment in 0.5% (w/v) turmeric extract for 30 min and another lot received no turmeric treatment, as a control. The samples were then placed in separate low-density polyethylene covers, sealed, and placed into a polypropylene box with ice that was maintained at 4°C (18 days) in aseptic conditions. The samples were analyzed for sensory attributes, microbiological quality, and chemical changes during chilled storage.
Sensory determinations
The sensory analysis of S. brevimana was based on the quality index method (QIM), as per Vaz-Pires and Seixas (Citation2006). The samples were divided into four categories: high quality (0), good quality (1), fair quality (2), and unacceptable quality (3). The samples were individually scored by five panelists who stated whether the S. brevimana samples were acceptable. Samples were considered unacceptable when reaching a total score higher than 12 (scale 0–17). Any samples rejected by the sensory panelists were not further analyzed.
Biochemical analysis
The total volatile base nitrogen (TVB-N) and trimethylamine-nitrogen (TMA-N) contents of the S. brevimana samples were measured by Conway’s dish method (Cobb, Aonaiz, & Thompson, Citation1973). For TVB-N, each ground sample (10 g) of S. brevimana meat was extracted with 20 ml of 6% trichloroacetic acid (TCA) and filtered through filter paper. The TCA extract of the S. brevimana samples was absorbed by boric acid and then titrated with 0.02 N HCl. The TVB-N content was expressed as mg/100 g. For TMA-N, 1 ml of 10% potassium carbonate was added to the TCA extract, and neutralized formalin was pipetted into the solution to react with ammonia, thereby allowing only the TMA-N to diffuse over the unit. The TMA-N content was expressed as mg/100 g.
Lipids were extracted from the white muscles of S. brevimana as described by Bligh and Dyer (Citation1959), using a single-phase solubilization at a 1:1 chloroform-to-methanol ratio. The results were expressed as g lipid/kg of muscle. The free fatty acids’ (FFAs) content of the lipid extracted from the muscle was determined according to Lowry and Tinsley (Citation1976). This method is based on the formation of an FFA/cupric acetate–pyridine complex and spectrophotometric (UV-VIS 2450 spectrophotometer, Shimadzu, Tokyo, Japan) measurement at 715 nm. The results were expressed as % oleic acid.
Lipid oxidation was determined by the peroxide value (PV) and thiobarbituric acid index (TBA-i). The PV was determined using peroxide reduction and ferric thiocyanate treatment of the lipid extract, according to Chapman and Mckay (Citation1949). The results were expressed as meq active oxygen/kg lipid. The TBA-i was determined according to Vyncke (Citation1970), based on the reaction between a TCA extract and TBA. The content of TBA-reactive substances was measured spectrophotometrically at 532 nm and calculated from a standard curve using 1,1,3,3-tetraethoxy-propane (TEP). The values were expressed as mg malondialdehyde (MA)/kg of muscle.
Microbiological analysis
S. brevimana samples (10 g) were aseptically dissected and homogenized with sterile 0.1% peptone water (HiMedia, Mumbai, India) in a stomacher. Serial dilutions were performed using sterile 0.1% peptone water and microbiologically analyzed. Total aerobic mesophilic bacteria (TVMC) and total aerobic psychrophilic bacteria (TCPC) were determined after growing on plate count agar (HiMedia) and incubating at 30°C for 72 h and 7°C for 7 days, respectively (Garcia-Soto et al., Citation2015). All growing colonies were considered as target bacteria and counted. Enterobacteriaceae were grown on red bile glucose agar (HiMedia). The plates were incubated at 37°C for 24 h, and Enterobacteriaceae were identified as red colonies and counted. Pseudomonas sp. were determined by surface plating on Pseudomonas F agar (HiMedia) and incubated at 35°C for 48 h. Colonies that were cream, fluorescent, or green in color were counted. BAF was determined by surface plating on modified Niven’s medium (Mavromatis & Quantick, Citation2002) and subsequent incubation at 37°C for 72 h. The plates were examined every 24 h for the presence of purple colonies surrounded by purple halos on a yellowish background.
BA analysis
The BA analysis was carried out using the method of Chen, Lee, Lin, Hwang, and Tsai (Citation2010), with minor modifications. Tryptamine hydrochloride (83.22 mg), putrescine dihydrochloride (113.32 mg), cadaverine dihydrochloride (107.52 mg), spermine trihydrochloride (109.52 mg), spermidine tetrahydrochloride (109.52 mg), histamine dihydrochloride (104.62 mg), and tyramine hydrochloride (85.22 mg) (Sigma-Aldrich, St. Louis, MI, USA) were used to prepare the BA solutions. Standards were dissolved in 50 ml of 0.1 M HCl and used as the working solution. The final concentration for each amine in solution was 1 mg/ml.
Each S. brevimana sample was ground in a Waring blender for 3 min. The ground samples (5 g) were transferred to 50 ml centrifuge tubes and homogenized with 20 ml of 6% TCA for 3 min. The homogenates were centrifuged using a REMI CPR-30 plus centrifuge (Vasai, India) at 10,000 g at 4°C for 10 min and filtered through Whatman no. 2 filter paper (Whatman, Maidstone, UK). The filtrates were then placed in volumetric flasks, and TCA was added to bring the final volume to 50 ml. Aliquots (1 ml) of S. brevimana extract and the standard amine solution were derivatized with dansyl chloride (Sigma-Aldrich, USA) using 1 ml of each free base amine solution containing 1 mg/ml. Next, 0.2 ml of 2 M sodium hydroxide and 0.3 ml of saturated sodium bicarbonate were added, followed by 2 ml of 1% dansyl chloride dissolved in acetone. The solution was then vortexed for 1 min. The mixture was allowed to stand at 40°C for 45 min, after which 100 µl of ammonia was added, and the solution was allowed to stand for 30 min. Acetonitrile was added to a final volume of 5 ml, and the solution was centrifuged (10,000 g at 4°C for 5 min). The supernatant was filtered through a 0.45 µm filter and then analyzed by high performance liquid chromatography (HPLC).
Chromatographic conditions included an HPLC instrument (Hitachi, Tokyo, Japan) consisting of a model L-7100 pump, a Rheodyne model 7125 syringe loading sample injector, a model L-4000 UV-vis detector (set at 254 nm), and a model D-2500 chromatointegrator. A LiChrospher 100 RP-18 reversed phase column (5 µm, 125 × 4.6 mm, Merck, Darmstadt, Germany) was used for chromatographic separation. The flow rate was 1 ml/min, and the gradient elution program began with 50:50 (v/v) acetonitrile:water for 19 min, followed by a linear increase to 90:10 and then a decrease to 50:50 during the next 1 min, where it was held for 10 min. The total separation took 30 min, and the gradient was run for 25 min to ensure full separation. The injection volume was 5 µl for standards and 20 µl for test samples.
Statistical analysis
All of the analyses were performed in triplicate. Chemical parameters, BA concentrations, and microbial analysis were calculated from the data obtained from the treated and control samples at each storage time. Analysis of variance was carried out using OriginPro version 8 software (OriginLab Corporation, Northampton, MA, USA). Duncan’s multiple range test was used to determine any statistically significant differences between mean values of the experimental data of the treatments at the significance level p < 0.05.
Results and discussion
Sensory evaluation
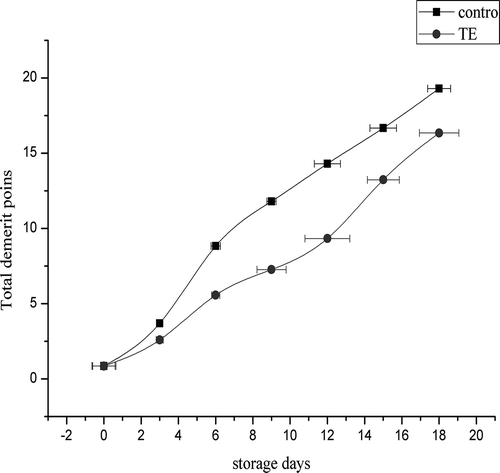
The QIM scores for turmeric-treated and control S. brevimana samples are shown in . Sensory deterioration was rapid in the control samples, and their overall sensory assessment reached the rejection QIM score (14.1) on day 12 due to the presence of unpleasant odors and severe ocular, muscular, and skin deterioration. Contrariwise, S. brevimana treated with turmeric extract received good quality scores, showed good characteristics, and reached the rejection QIM score (13.4) on day 15. The results indicated that treatment with turmeric extract was highly effective in delaying the spoilage indices and extending the shelf life of S. brevimana by 3 days compared to the control samples. These results are better than Vaz-Pires et al. (Citation2008), who analyzed the sensory score of cuttlefish (S. officinalis) and broadtail shortfin squid (Illex coindetii) stored at 2°C for 13 days. S. officinalis and I. coindetii flesh maintained good quality for 7–8 days, and spoilage was noticed at day 10 for S. officinalis and day 9 for I. coindetii. Previous studies reported that the preservative action of turmeric extract could be due to the presence of essential oils, curcumins, curcuminoids, turmeric oil, turmerol, and valeric acid, which are the major phenolic compounds in turmeric (Basniwal, Butter, Jan, & Jain, Citation2011), with potential antibacterial and antioxidant activity (Gul & Bakht, Citation2015; Sathishkumar et al., Citation2015).
Figure 1. Changes in the overall acceptability score of turmeric-treated and control samples of S. brevimana during ice storage (average ± SD).
Figura 1. Cambios en la aceptabilidad general en las muestras de S. brevimana controles y tratados con extracto de cúrcuma durante el almacenamiento refrigerado (media ± SD).
Biochemical determinations
The initial TVB-N content of the controls and turmeric-treated samples was 11.2 mg/100 g, and subsequent changes in TVB-N content in the samples stored at 4°C are shown in . The TVB-N content of S. brevimana muscle in control and turmeric-treated samples increased significantly during storage to reach final values of 43.68 and 33.6 mg/100 g, respectively, on their maximum rejection days (day 12 in control samples and day 15 in treated counterparts). Starting from day 9, the control samples had a significantly higher TVB-N content than treated samples. This could be attributed to the preservative effect of the turmeric extract, inhibiting bacterial growth and thus volatile base formation during spoilage. A previous report stated that a TVB-N of 30–35 mg/100 g fish is generally considered the maximum limit of acceptability for ice-stored marine fish (Soltanian, Behnam, Anvari, Rezaei, & Safari, Citation2015), which mainly depended on the fish species, specific treatments, and processing conditions.
Table 1. Total volatile base nitrogen (TVB-N), trimethylamine-nitrogen (TMA-N), and free fatty acid (FFA) values of turmeric-treated and control samples of cuttlefish during 18 days of ice storage.
Tabla 1. Nitrógeno básico volatil total (TVB-N), nitrógeno de trimetilamina (TMA-N) y ácidos grasos libres (FFA) en muestras controles y tratadas con extracto de cúrcuma almacenados en refrigeración durante 18 días.
TMA is derived from trimethylamine oxide (TMAO) and is essential for osmoregulation in marine fish. During spoilage, TMAO is reduced to TMA by enzymatic activity (Cai et al., Citation2014; Viji et al., Citation2015). During storage (), the TMA-N value increased significantly from an initial 0.6 mg/100 g to final values of 16.8 and 14.7 mg/100 g, respectively, for controls and turmeric-treated samples – significantly higher for the control samples. Thus, the results indicated that the turmeric extracts were effective at inhibiting the decarboxylation of TMAO to TMA by the action of bacteria. According to Vaz-Pires et al. (Citation2008), a low TMA content of 0.2–10 mg/100 g was found in cuttlefish, while 0.1–8.4 mg/100 g was detected in shortfin squid after 18 days of ice (2°C) storage. The limit of TMA in marine foods for human consumption is 10–15 mg/100 g (Ozogul, Kuley, Kenar, Citation2011).
The FFAs significantly increased as storage progressed (), from an initial 0.96% to 5.76% and 5.20% in the control and treated samples, respectively, at day 18 of storage. Thus, the rate of lipid hydrolysis was higher in the treated samples than in the controls. Previously, Viji et al. (Citation2015) observed that the increase in FFA amount during storage might be attributed to the hydrolysis of triglycerides and phospholipids, mediated by endogenous and/or microbial lipolytic enzymes. Other preservative agents, such as citric and lactic acids, had an inhibitory effect on lipid hydrolysis during chilled storage of megrim (Lepidorhombus whiffiagonis), according to Aubourg, Garcia-Soto, Bohme, and Barros-Velazquez (Citation2014).
The variations in PV during storage of the control and turmeric extract-treated samples are shown in . The initial PV was 0.56 meq O2/kg, and there was no significant difference between the control and treated samples until day 9. Similar to these findings, Sathishkumar et al. (Citation2015) observed that turmeric-containing curcuminoids are natural antioxidants, which effectively control PV development.
Table 2. Peroxide values (PV) and thiobarbituric acid index (TBA) content of turmeric-treated and control samples of cuttlefish during 18 days of ice storage.
Tabla 2. Índice de peróxidos (PV), y prueba del ácido tiobarbitúrico (TBA) en muestras controles y tratadas con extracto de cúrcuma almacenados en refrigeración durante 18 días.
TBA-i is widely used to indicate the extent of secondary lipid oxidation in seafood. The TBA-i values of the control and treated samples during chilled storage are shown in . The results indicate that the TBA-i values of the control and treated samples increased gradually from 0.46 MA/kg in fresh S. brevimana to 2.16 MA/kg at day 12 and 2.78 MA/kg at day 15 of storage, respectively, and showed a decreasing trend thereafter. Compared to the present results, Ozyurt, Kuley, Ozkutuk, and Ozogul (Citation2009) reported higher initial TBA-i values of 0.51 and 0.57 mg MA/kg in red mullet (Mullus barbatus) and goldband goatfish (Upeneus moluccensis), respectively.
Microbiological quality
shows the microbial counts, including TVMC, TVPC, Enterobacteriaceae, BAF, and Pseudomonas count during the chilled storage of control and turmeric-treated S. brevimana muscle. The overall microbial changes increased more significantly in the controls than treated samples during chilled storage for all parameters after day 3. The control samples reached >7 log CFU/g for all parameters, except Pseudomonas, at day 15 of storage, whereas the treated samples reached significantly lower counts. Hu et al. (Citation2012) demonstrated that TVMC are the dominant bacteria found in octopus after 10 days of refrigerated storage and after 24 h storage at 25°C. Kim et al. (Citation2009) reported that Enterobacteriaceae were the dominant microbial group found throughout refrigerated storage and that BAF were found during storage at 0, 4, 10, 15, and 20°C but not at 25°C. In the current study, the turmeric-treated S. brevimana muscle possessed antimicrobial effects against the tested bacteria and enhanced the shelf life by up to 10 days compared to the control samples. BAF increased rapidly in S. brevimana muscle after day 12 of storage (), with more than 6.65 log CFU/g BAF in the control samples. Houicher, Kuley, Ozogul, and Bendeddouche (Citation2015) made a similar observation, whereby histamine content increased suddenly in fish muscle after 14 days of storage when the BAF number reached 6 log CFU/g in the controls and tested samples of sardine. The results of the present study show that the turmeric extract treatment decreased the microbial growth and formation of BAs under chilled storage, probably due to compounds in the turmeric extract that could delay BAF growth and effectively control the activity of decarboxylase enzymes in S. brevimana muscle during storage (Sathishkumar et al., Citation2015).
Table 3. Bacterial content (log10 CFU/g) of control and turmeric extract-treated samples of S. brevimana during storage at 4°C for 18 days.
Tabla 3. Recuentos bacterianos (log10 CFU/g) de muestras de S. brevimana controles y tratadas con extracto de cúrcuma almacenados a 4°C durante 18 dias.
Table 4. Content of biogenic amines (mg/100 g) in control and turmeric extract-treated S. brevimana samples for 18 days in chilled storage at 4°C.
Tabla 4. Contenido en aminas biógenas (mg/100 g) en muestras de S. brevimana controles y tratadas con extracto de cúrcuma almacenados a 4°C durante 18 dias. Los valores se representan como media de tres muestras ± desviación estándar.
Determination of BA compounds
The concentrations of BAs in the control and turmeric-treated samples stored for 0–11 days at 4°C are given in . At day 0, no differences were found in the BAs between control and treated samples, with tryptamine the only exception, which was significantly higher in the control samples (0.78 ± 0.00 mg/100 g). This difference was also observed at day 3 and later, with the tryptamine content always higher in the control than in the turmeric extract-treated samples. For the other BAs investigated, higher contents were found in the controls than in the treated samples, significantly so after day 3 for putrescine, after day 9 for cadaverine, after day 12 for histamine and tyramine, and after day 18 for spermine.
Histamine content in control samples gradually increased to 7.08 ± 0.08 mg/100 g at the end of storage, while it reached only 2.10 ± 0.08 mg/100 g in treated samples. Overall, significant differences in histamine levels were noticed between the control and turmeric-treated samples (p < 0.05) after day 12 of storage. Additionally, spermidine was not found in S. brevimana muscles during storage, and spermine was present in control samples at day 15 and reached 7.63 ± 0.01 mg/100 g at day 18. No spermine or spermidine was produced in the turmeric-treated S. brevimana muscle samples between 0 and 18 days of storage.
The results obtained in the present work align with those previously reported by other authors for BAs in cephalopods. Prester et al. (Citation2011) reported that putrescine and other BAs were found at <2.0 mg/100 g during squid decomposition. Hu et al. (Citation2012) reported BA contents ranging from 0.12 mg/100 g (spermidine) to 2.55 mg/100 g (cadaverine) in octopus during storage at 4°C for 10 days. Similarly, they found BAs from 0.1 mg/100 g (spermidine) to 0.96 mg/100 g (putrescine) in squid after storage at 4°C for 10 days. Compared to the present findings, Kim et al. (Citation2009) also reported higher BA contents, ranging from 0.56 mg/100 g (tyramine) to 4.51 mg/100 g (cadaverine) in Sepia officinalis. Prester (Citation2011) noted that histamine and putrescine were found in European common squid at 1.53 and 0.83 mg/100 g, respectively, at 22ºC prior to storage. These values increased after 12 h storage, to 1.97 mg/100 g for histamine, 2.93 mg/100 g for putrescine, and 1.53 mg/100 g for tyramine.
The toxicity and defect histamine levels established for tuna (Thunnus alalunga), mahi-mahi (Coryphaena hippurus), and related marine fish are 50 and 5 mg/100 g, respectively (FDA, Citation2011). Thus, the combined effects of ice and turmeric extract showed powerful effects against BAF and decreased BAs, particularly histamine (Basniwal et al., Citation2011). In addition to histamine poisoning, tyramine can cause high levels of intoxication from seafood and fishery products. Tyramine was initially detected in the control samples at day 12 at 3.37 ± 0.02 mg/100 g and reached a final level of 6.8 ± 0.08 mg/100 g on day 20. In contrast, the treated samples contained 3.76 ± 0.03 mg tyramine/100 g at the end of storage. The allowable limit of tyramine in marine products is 10 mg/100 g (Ten-BrinK, Damirik, Joosten, & Huis In’t Veld, Citation1990). Ozogul et al. (Citation2011) stated that rosemary and sage tea extract prevented fish spoilage and decreased tyramine formation in sardines during storage. In the current study, the antimicrobial properties of curcumin and curcuminoids present in turmeric possibly played a major role in preventing tyramine formation in turmeric-treated S. brevimana muscle samples during storage (Basniwal et al., Citation2011).
Conclusions
The combination of turmeric and low temperature controlled the mesophilic, psychrophilic, BAF and Pseudomonas bacterial growth; delayed the formation of negative biochemical parameters and BAs; and extended the shelf life of S. brevimana. Based on the sensory evaluation, the turmeric coating enhanced the shelf life of S. brevimana muscle without changing its appearance, flavor, and texture up to day 15 of storage at 4°C. This study confirmed the biopreservative effect of turmeric extract against spoilage bacteria and suggests its potential use in the fish-processing industries.
Acknowledgments
The authors gratefully acknowledge the Department of Science and Technology (DST), Science and Engineering Research Board (SERB) (Government of India, New Delhi) for their financial support [grant no. SR/FT/LS-22/2010; 2 May 2012] and to European Regional Development Funds (FEDER), grant GRC 2014/004 for covering the costs to publish in open access. The authors also thank Alagappa University for providing the necessary facilities to carry out the research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aubourg, S.P., Garcia-Soto, B., Bohme, K., & Barros-Velazquez, J. (2014). Inhibition of quality loss in chilled megrim (Lepidorhombus whiffiagonis) by employing citric and lactic acid icing. International Journal of Food Science and Technology, 49, 18–26. doi:10.1111/ijfs.12268
- Aubourg, S.P., Losada, V., Gallardo, J.M., Miranda, J.M., & Barros-Velázquez, J. (2006). On-board quality preservation of megrim (Lepidorhombus whiffiagonis) by a novel ozonized-slurry ice system. European Journal of Research and Technology, 223, 232–237. doi:10.1007/s00217-005-0182-z
- Basniwal, R.K., Butter, H.S., Jan, V.K., & Jain, N. (2011). Curcumin nanoparticles: Preparation, characterization and antimicrobial study. Journal of Agricultural and Food Chemistry, 59, 2056–2061. doi:10.1021/jf104402t
- Bligh, E., & Dyer, W. (1959). A rapid method of total extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917. doi:10.1139/o59-099
- Cai, L., Wu, X., Li, X., Zhong, K., Li, Y., & Li, J. (2014). Effects of different freezing treatments on physicochemical responses and microbial characteristic of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT-Food Science and Technology, 59, 122–129. doi:10.1016/j.lwt.2014.04.062
- Chapman, R., & Mckay, J. (1949). The estimation of peroxides in fats and oils by the ferric thiocynate method. Journal of the American Oil Chemists Society, 26, 360–363. doi:10.1007/BF02651444
- Chen, H.C., Lee, Y.C., Lin, C.M., Hwang, D.F., & Tsai, Y.H. (2010). Determination of histamine and bacterial isolation in marlin fillets (Makaira nigricans) implicated in a food borne poisoning. Journal of Food Safety, 30, 699–710.
- Chong, C.Y., Abu Bakar, F., Russly, A.R., Jamilah, B., & Mahyudin, N.A. (2011). The effects of food processing on biogenic amines formation. International Food Research Journal, 18, 867–876.
- Cobb, B.F., Aonaiz, I., & Thompson, C.A. (1973). Biochemical and microbiology studies on shrimp: Volatile nitrogen and amino nitrogen analysis. Journal of Food Science, 38, 431–438. doi:10.1111/j.1365-2621.1973.tb01447.x
- Fernandez-No, I.C., Bohme, K., Gallardo, J.M., Velazquez, J.B., Canas, B., & Calo-Mata, P. (2010). Differential characterization of biogenic amine producing bacteria involved in food poisoning MALDI-TOF Mass fingerprinting. Electrophoresis, 31, 1116–1127.
- Food and Agriculture Organization of the United Nations (FAO). 2007. Fishery statistic. Capture production. In FAO yearbook of fishery captures. Rome (Italy): FAO.
- Food and Drug Administration (FDA). (2011). Fish and fishery products hazards and controls guidance (4th ed.). Washington, DC: Department of Health and Human Services, Food and Drug Administration, Centre for Food Safety and Applied Nutrition.
- Garcia-Soto, B., Miranda, J.M., De Quiros, A.R.B., Sendon, R., Rodriguez-Martinez, A.V., Barros-Velazquez, J., & Aubourg, S.P. (2015). Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lephidorhombus whiffiagonis). International Journal of Food Sciences and Technology, 50, 1891–1900. doi:10.1111/ijfs.12821
- Gul, P., & Bakht, J. (2015). Antimicrobial activity of turmeric extract and its potential use in food industry. Journal of Food Science and Technology, 52, 2272–2279. doi:10.1007/s13197-013-1195-4
- Houicher, A., Kuley, E., Ozogul, F., & Bendeddouche, B. (2015). Effect of natural extracts (Mentha spicata L and Artemisia campestris) on biogenic amines formation of sardine vacuum-packed and refrigerated (Sardina pilchardus) fillets. Journal of Food Processing and Preservation, 39, 393–2403. doi:10.1111/jfpp.12489
- Hu, Y., Hung, Z., Li, J., & Yang, H. (2012). Concentration of biogenic amines in fish, squid and octopus and their changes during storage. Food Chemistry, 135, 2604–2611. doi:10.1016/j.foodchem.2012.06.121
- Kim, M.K., Mah, J.H., & Hwang, H.J. (2009). Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chemistry, 116, 87–95. doi:10.1016/j.foodchem.2009.02.010
- Lowry, R., & Tinsley, I. (1976). Rapid colorimetric determination of free fatty acids. Journal of the American Oil Chemists´ Society, 53, 470–472. doi:10.1007/BF02636814
- Mavromatis, P., & Quantick, P. (2002). Modification of Nivens medium for the enumeration of histamine forming bacteria and discussion of the parameters associated with its use. Journal of Food Protection, 65, 546–551. doi:10.4315/0362-028X-65.3.546
- Mohamed, G.G., Ei-Hameed, A.K.A., Nezam EI-Din, A.M., & El-Din, L.A. (2010). High performance liquid chromatography, thin layer chromatography and spectrophotometeric studies on the removal of biogenic amines from some Egyptian foods using organic, inorganic and natural compounds. Journal of Toxicological Sciences, 35, 175–187. doi:10.2131/jts.35.175
- Naila, A., Flint, S., Fletcher, G., Bremer, P., & Meerdink, G. (2010). Control of biogenic amines in food existing and emerging approaches. Journal of Food Science, 75, 139–150. doi:10.1111/j.1750-3841.2010.01774.x
- Ozogul, F., Kuley, E., & Kenar, M. (2011). Effects of rosemary and sage tea extract on biogenic amines formation of sardine (Sardine pilchardus) fillets. International Journal of Food Science and Technology, 46, 761–766. doi:10.1111/j.1365-2621.2011.02560.x
- Ozyurt, G., Kuley, S., Ozkutuk, S., & Ozogul, F. (2009). Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbutus) and gold bamd goatfish (Upeneus moluccensis) during storage in ice. Food Chemistry, 114, 505–510. doi:10.1016/j.foodchem.2008.09.078
- Prester, L. (2011). Biogenic amines in fish, fish products and shellfish: A review. Food Additives & Contaminants. Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment., 28, 1547–1560. doi:10.1080/19440049.2011.600728
- Sathishkumar, P., Hemalatha, S., Arulkumar, M., Ravikumar, R., Mohd Yusoff, S.R., Hadibarata, T., & Palvannan, T. (2015). Curcuminoid extraction from turmeric (Curcuma longa L.): Efficacy of bromine-modified curcuminoids against food spoilage flora. Journal of Food Biochemistry, 39, 325–333. doi:10.1111/jfbc.12133
- Soltanian, S., Behnam, S., Anvari, M., Rezaei, M., & Safari, R. (2015). Effect of nisin as a biopreservative agent on quality and self life of vacuum packaged rainbow trout (Oncorhynchus mykiss) stored at 4°C. Journal of Food Science and Technology, 52, 2184–2192. doi:10.1007/s13197-013-1241-2
- Ten-BrinK, B., Damirik, C., Joosten, H.M., & Huis In’t Veld, H.J. (1990). Occurrence and formation of biologically active amines in foods. International Journal of Food Microbiology, 11, 73–84. doi:10.1016/0168-1605(90)90040-C
- Vaz-Pires, P., & Seixas, P. (2006). Development of new Quality Index Method (QIM) schemes for cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii). Food Control, 17, 942–949. doi:10.1016/j.foodcont.2005.07.004
- Vaz-Pires, P., Seixas, P., Mota, M., Lapa-Guimaraes, J., Pickova, J., Lindo, A., & Silva, T. (2008). Sensory, microbiological, physical and chemical properties of cuttlefish (Sepia officinalis) and broadtail shorfin squid (Illex coindetii) stored in ice. LWT-Food Science and Technology, 41, 1655–1664. doi:10.1016/j.lwt.2007.10.003
- Viji, P., Binsi, P.K., Visnuvinayagam, S., Bindu, J., Ravishankar, C.N., & Srinivasa Gopal, T.K. (2015). Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts as natural preservatives for shelf life extension of chill storage Indian mackerel. Journal of Food Science and Technology, 52, 6278–6289. doi:10.1007/s13197-015-1788-1
- Vyncke, W. (1970). Direct determination of the thiobarbituric acid value in trichloacetic acids extracts of the fish as a measure of oxidative rancidity. Chemische Revue Über Die Fett- Und Harz-Industrie, 72, 1084–1087.
