ABSTRACT
Brewing conditions influence the composition and properties of herbal infusions. Acantholippia deserticola (Phil.) (RR) is a native and medicinal, Chilean Altiplano herb. This study assessed the effect of the section plant used, mass:water ratio and brewing time, on the infusions RR characteristics. RR leaves had higher total polyphenols (TPP) and antioxidant capacity (A.C.) than the stem. Polyphenol, chlorophyll and pheophytin extraction was maximized after 5 min brewing. Infusion prepared with 1 g has lower extraction ratio, per herb mass used, than those with 0.5 g. The infusion contained 10.1 µmol/L TPP, 5.5 µg/mL chlorophyll and 4.3 µg/mL pheophytins. Its A.C. was 69.2 µmol Trolox eq./L (FRAP), its IC50 was µg/mL 337 (DPPH) and 438 µg/ml (ABTS), and a dose of 300 mg/kg had an anti-inflammatory effect. The section of the plant, the herb : water ratio and the brewing time affect the characteristics of the RR infusions.
RESUMEN
Las condiciones de preparación inciden en la composición y las propiedades de las infusiones herbales. Acantholippia deserticola (Phil.) (RR) es una hierba medicinal nativa del altiplano chileno. El presente estudio evaluó el efecto provocado por la parte de la planta utilizada, la ratio masa:agua y el tiempo de preparación en las características de las infusiones elaboradas con RR. Las hojas de RR mostraron un contenido total de polifenoles y una capacidad antioxidante más elevados que el tallo de la planta. La extracción de polifenoles, clorofila y feofitina se maximizó tras cinco minutos de preparación. La infusión preparada con 1 g tiene una ratio de extracción más baja por masa de hierba utilizada que las que utilizan 0.5 g. Se comprobó que la infusión contiene 10.1 µmol/L de polifenoles totales, 5.5 µg/mL de clorofila y 4.3 µg/mL de feofitina. Asimismo, se constató que su capacidad antioxidante fue 69.2µmol Trolox eq./L (FRAP), su IC50 fue µg/mL 337 (DPPH) y 438 µg/ml (ABTS), y que una dosis de 300 mg/kg tiene efecto antiinflamatorio. Así, se concluye que la parte de la planta utilizada, la ratio hierba:agua y el tiempo de preparación afectan las características de las infusiones de RR.
1. Introduction
Aromatic herbs contain bioactive compounds with useful industrial applications. Many of these herbs are traditionally used as medicines and for the treatment of diverse ailments, through the consumption of leaves, seeds, fruits and roots (Martins et al., Citation2015). Aqueous herbal extracts can be prepared by decoction or infusion. In decoction, the plant material is boiled, or kept at high temperatures, in water for a determined time. An infusion is obtained by adding hot water to a specific amount of plant material and leaving the mix to brew, similar to the preparation of tea (Martins et al., Citation2015; Rodrigues et al., Citation2017). The different extraction methods have a direct impact on the composition of the final extract and, thus, on its bioactivity (Guimarães et al., Citation2013; Quesille-Villalobos, Saavedra, & Gálvez, Citation2013; Riehle, Vollmer, & Rohn, Citation2013).
The literature indicates many ways to prepare an infusion. For example, the plant mass used in 100 mL of water can vary between 0.4 and 25 g, the water temperatures can vary between 50 and 100ºC, the infusion time can range from 3–60 min, and the infusions can be prepared with or without stirring (Da Silva, Campos, Teixeira, & Prado, Citation2013; Guimarães et al., Citation2013; Marete, Jacquier, & O’Riordan, Citation2013; Martins et al., Citation2015; Oh, Jo, Cho, Kim, & Han, Citation2013; Quesille-Villalobos et al., Citation2013; Rodrigues et al., Citation2017; Simirgiotis, Silva, Becerra, & Schmeda-Hirschmann, Citation2012). Finally, factors linked to the season and method of plant collection have also been evaluated in the literature, and while some authors report a clear effect (Coelho et al., Citation2016), others do not find any notable differences in the final composition (Dincer et al., Citation2017).
Of plant species described in Chile, 46.8% are endemic, and many have played an important part in the life of Native American populations (Simirgiotis et al., Citation2012). Acantholippia deserticola (Phil. ex F. Phil.) Moldenke (Rica-rica, RR) is a small shrub 30–60 cm high, which grows on the Chilean Altiplano at altitudes of 2500–3000 m above sea level (Morales, Paredes, Sierra, & Loyola, Citation2008; Rojo et al., Citation2009). RR has traditionally been used as a medicinal herb by Native Americans, who prepared infusions, decoctions and wound dressings from it (Rojo et al., Citation2009, Citation2006). It is registered as herbal medicine by the Chilean Ministry of Health, which recognizes the use of the leaves and stems as an antispasmodic and antiseptic (Parada, Citation2012). It has been reported that hydroalcoholic extracts and essential oils of RR contain molecules with antioxidant capacity (A.C.) (Morales et al., Citation2008), and potential health benefits due to their antimicrobial activity (Rojo et al., Citation2006), anxiolytic and antidepressant effect (Benites et al., Citation2013). A.C. and polyphenols contents were examined by Rojo et al. (Citation2009) in aqueous extracts, obtained by a method more similar to a short decoction than a typical infusion.
This research aimed to determine the effect of the infusion preparation conditions, section of plant, herb mass:water ratio and brewing times on the pigments and polyphenols contents and biological activity of RR infusion
2. Material and methods
2.1. Plant material
Whole RR plants were collected in San Pedro de Atacama, Antofagasta, Chile (23°11ʹ14.2656” S, 68°0ʹ16.9596” W). The plants were authenticated and registered by Dr. Roberto Rodríguez, Department of Botany, School of Natural Sciences and Oceanography, Universidad de Concepción, Chile, under specimen number CONC 182473. Stems and leaves were manually separated, weighed and vacuum packed. In addition, chamomile and mint packaged herbal infusions were acquired for comparison with RR.
2.2. Plant mass and brewing times
RR infusions were prepared from the whole herb (stem and leaves), using different brewing times (0, 5, 1, 2, 5, 30 and 60 min) and herbal masses (0.5 and 1 g), in 250 mL water at 100°C. In addition, infusions of a commercial preparation of chamomile and infusions of a commercial preparation of mint were prepared following the manufacturer’s instructions (1 g in 250 mL water at 100°C, 5 min). All infusions were left to cool at room temperature and tested for total polyphenol (TPP) content, A.C., and chlorophyll and pheophytin content on the same day. All measurements were performed in triplicate.
2.3. TPP content and A.C.
TPP were quantified in a spectrophotometer, using the Folin–Ciocolteu method, with 50–500 μg/mL gallic acid as a standard. The A.C. was measured as FRAP (Ferric ion Reducing Antioxidant Power) against a standard curve of trolox (10–90 µM). Both TPP and A.C., were expressed as umol gallic acid or trolox equivalent by liter of infusion, respectively. A.C. was also assessed using DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), as described by Morales and Paredes (Citation2014) with some modifications. The values of the value of radical-scavenging activity were expressed as IC50, which corresponds to the concentration of trolox or infusion in ug/mL needed to inhibit 50% of the radical present in solution.
2.4. Chlorophyll and pheophytin
Chlorophyll (a, b and total) and pheophytins (a, b and total) content was measured using the spectrophotometric methods (Maiocchi and Avanza (Citation2004).
2.5. Anti-inflammatory capacity
The anti-inflammatory capacity of RR infusions was assessed through a rat paw edema assay (Morales, Paredes, Olivares, & Bravo, Citation2014), using physiological serum (negative control), 10 mg/kg indomethacin (positive control) and 300 mg/kg lyophilized infusion of RR. The procedure followed the norms of the Ethics Committee of the Universidad de Antofagasta.
2.6. Statistical methods
Data represent the mean of triplicate analysis, using one-way ANOVA, with software Statgraphics Centurion.
3. Results and discussion
3.1. Characteristics of the dry herb
3.1.1. Analysis of the pigments in RR stems and leaves
The analysis of chlorophylls and pheophytins gave similar results for RR stems and leaves (), indicating that the visually observable and measurable color differences between them are probably due to other types of compounds (Itoh et al., Citation2008; Thongphasuk, Suttisri, Bavovada, & Verpoorte, Citation2004). Pigments other than those considered here, such as β-carotene, xanthophyll and pheophorbide, which have been reported for green tea (Kusmita, Puspitaningrum, & Limantara, Citation2015), might be present in the analyzed herbs and influence their color. Kusmita et al. (Citation2015) found a chlorophyll a:b ratio of 3:1. In RR, this ratio was close to one, potentially explaining the greenish yellow of its stems and leaves. Koca, Karadeniz, and Burdurlu (Citation2007) found a significant correlation between chlorophyll (a and b) content and some color parameters measured instrumentally, as the values of the coordenate a* (related with red color), the index a*/b* (related with the angle, in the color sphere), and the hº value, related with the hue, according the CIELab notation system (Francis & Clydesdale, Citation1975). In the case of RR (results not shown), the leaf was significantly darker (lower Luminosity, L*) and greener (lower value of a *) than the stem. No correlations were found between the content of pigments and the CIELab color parameters.
Table 1. Total polyphenol content (TPC), antioxidant capacity, chlorophyll and pheophytin content of dry RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke,) stems and leaves, and commercially available mint and chamomile.
Tabla 1. Contenido total de polifenoles (TPC), capacidad antioxidante, clorofila y contenido de feofitina de tallos y hojas deshidratados de (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke), de menta y manzanilla disponibles comercialmente.
Different plant parts differ in the composition of their pool of secondary metabolites. This has been reported for lignans in the stems of Strychnos vanprukii (Thongphasuk et al., Citation2004), for phenolic and iridoid glycosides in the wood and bark of Strychnos axillaris (Itoh et al., Citation2008) and for organic acids, such as quinic and loganic acid, and their esters in the bark and wood of Strychnos lucida. The quantity of these and other unidentified compounds can differ between different parts of the plant and thus directly affect the color of these parts. This would explain the observed differences in color coordinates between RR leaves and stems in the presence of similar chlorophyll and pheophytin contents. It would be interesting to identify the type of phenolic compounds and quantify the tannins content of each of the different parts of the RR plant in future studies.
3.1.2. TPP and A.C. of RR stems and leaves
Chlorophylls and pheophytins have been attributed an anti-inflammatory (Higashi-Okai, Yamazaki, Namagori, & Okai, Citation2001; Park et al., Citation2014) and antioxidant effect. Fernandes et al. (Citation2017) report that the A.C. of chlorophyll is 200 times that of α-tocopherol. According to Higashi-Okai et al. (Citation2001), pigment A.C. varies in the following order: chlorophyll a > lutein > pheophytin a > chlorophyll b > β-carotene > pheophytin b, while Lanfer-Marquez, Barros, and Sinnecker (Citation2005) find a higher antioxidant activity in pheophytin b than chlorophyll a. Importantly, the antioxidant activity of the extracts is higher than that of the purified compounds, potentially due to a synergistic effect of compounds, even those present in low quantities (Khattab, Goldberg, Lin, & Thiyam, Citation2010; Kusmita et al., Citation2015).
Polyphenol quantification revealed a significantly higher TPP content (p < 0.05) in mint than in the other herbs. Nevertheless, all values fell into the range reported in the literature of 6.76–307.72 µmol GAE/g for medicinal plants (Li et al., Citation2013), 12.9–2956.7 µmol GAE/g for traditional Chinese plants (Cai, Luo, Sun, & Corke, Citation2004), and 10.98–144.52 mg GAE/g for herbal tea (Oh et al., Citation2013). FRAP A.C. varied significantly between samples (p < 0.05). From highest to lowest FRAP, samples appeared in the following order: RR leaves > RR stem > mint > chamomile, with values higher than those reported for Indian medicinal plants (0.17–140.45 µmol Trolox equivalents/g; Surveswaran, Cai, Corke, & Sun, Citation2007) and traditional Chinese plants (0.17–0.47 µmol Trolox equivalents/g; Cai et al., Citation2004). In this context, it is of interest that RR leaves had a significantly higher TPP content and A.C. than stems, in line with results from laurel (Tapia-Torres et al., Citation2014).
Rojo et al. (Citation2009) evaluated the TPP and the A.C. of the aqueous decoction extracts of 12 Altiplano herb species including RR. They measured the TPP content of RR as 72 mg/L, one of the lowest among herbs. This was in stark contrast with RR antioxidant activity, which was measured as 56,200 µmol/L, one of the highest values of the study. The differences between this study and that of Rojo et al. (Citation2009) may be due to the different sites and times of plant collection (Jordan et al., Citation2013) as well as, to an important extent, the different methods of aqueous extraction. The plant mass:water ratio used by Rojo et al. (Citation2009) was 10 times higher than ratio used here, and in addition, extraction was carried out under continuous heating for 5 min (the authors do not specify the temperature). This procedure might lead to the increased extraction of some compounds, while potentially resulting in the degradation of other thermolabile compounds (Guimarães et al., Citation2013).
3.2. Preparation of the RR infusion
3.2.1. Pigments in RR infusions
Chlorophyll and pheophytin content was monitored in RR infusions prepared with 0.5 g and 1.0 g plant material and applying the different brewing times (see ). Similar trends were observed for the different herbal masses used, the greatest extraction being achieved with brewing times between 2 and 5 min (p < 0.05), in agreement with the changes seen for the chromatic coordinates and color parameters. Beyond that time, pigment levels returned to their initial values, possibly due to the degradation caused by temperatures and oxygen exposure (Lima, Pereira, Baraldi, & Malheiro, Citation2017; Pumilia et al., Citation2014). Chlorophyll levels were higher than pheophytin levels, thus offering a plausible explanation for the greenish-yellow coloration of the infusions.
Figure 1. Pigment content of RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke) infusions prepared with 0.5 g herbal material (panel A) and 1.0 g herbal material (panel B) and different brewing times. Chl, chlorophyll; Phe, pheophytin.
Figura 1. Contenido de pigmentos de infusiones de RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke) preparadas con 0.5 g de material herbal (panel A) y 1.0 g material herbal (panel B) y distintos tiempos de preparación. Chl, clorofila; Phe, feofitina.
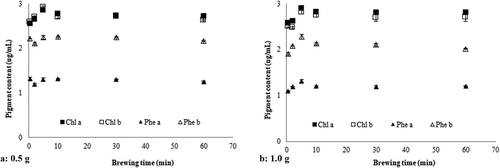
In the infusion prepared from 0.5 g RR, chlorophyll b levels were slightly higher than chlorophyll a levels after short brewing times; however, with increasing brewing times, chlorophyll a levels slightly surpassed those of chlorophyll b (). This might be due to an increased extraction over chlorophyll a over time, or to an increase in chlorophyll b degradation over time. Lima et al. (Citation2017) report that the thermostability of chlorophyll a is greater than that of chlorophyll b. This is however, in contrast with findings by Koca et al. (Citation2007), who found chlorophyll b to be more stable than chlorophyll a. In the infusion prepared with 1.0 g RR, the ratio of chlorophyll a to chlorophyll b was close to 1. The concentration of pheophytin b, on the other hand, was twice that of pheophytin a (). In both infusions, pigment extraction was maximized at a brewing time of 5 min, with diminishing levels beyond that time. At the time of maximum pigment extraction, the C parameter, which was related to color intensity, also took on its maximum value.
Pumilia et al. (Citation2014) report a strong correlation between changing pigment levels (especially of chlorophylls and pheophytins) and color parameters in Pistacia vera L. On the other hand, it should be noted that the plant material content of the infusion is not reflected proportionally in pigment concentrations. Jovanović et al. (Citation2017) report a similar effect of herbal masses on extraction in Thymus serpyllum, where extraction yields increase with an increased herbal mass, but not in a proportional way, such that a better proportional yield was achieved using a lower plant mass.
3.2.2. TPP and A.C. of RR infusions
The content and speed of extraction of TPP, and the A.C. of RR infusions were assessed as a function of herbal mass and brewing times (). Independent of the herbal mass used, TPP increased with increasing brewing times, until 5 min. Beyond this, levels went back down. These results can be contrasted with those obtained by Santos et al. (Citation2016), who obtained the highest extraction yield of TPP in rooibos at a brewing time of 10 min.
Figure 2. Extraction yield (A) and speed (B) of total polyphenols and antioxidant capacity in RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke) infusions as a function of herbal mass and brewing times.
Figura 2. Rendimiento de extracción (A) y velocidad (B) de polifenoles totales y capacidad antioxidante de infusiones de RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke) en función de la masa herbal y los tiempos de preparación.
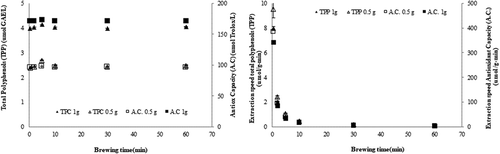
A.C. was constant, independent of brewing times, in line with a previous report (Perez-Ramirez et al., Citation2015). The speed of extraction, both of TPP and of antioxidants, was elevated during the first 5 min of brewing; beyond that, it was reduced to almost zero. For Turkish black tea, Kelebek (Citation2016) reports the greatest extraction of compounds with an antioxidant activity after 10 min brewing; however, the extraction speed was highest at 3 min, where it was double that seen at 6 min. Similar results have been obtained by Jovanović et al. (Citation2017) in T. serpyllum, and by Riehle et al. (Citation2013) in Cistus incanus; in both of these studies, the polyphenol content increased with extraction times, and extraction speed was higher at shorter extraction times. Extraction efficiency seems to be the highest at a brewing time of 5 min, in accordance with the recommendations of the manufacturers of commercially available infusions. For brewing times over 5 min, a reduction in TPP could be observed, possibly due to the thermolability of the compounds. Izquierdo, Lopez, and Gonzalez (Citation2011) observed a significant negative effect of increasing extraction times on the yield of extracted solids for a time frame of 0.5–1.5 h. Perez-Ramirez et al. (2015) report that the extraction yield of pigments, TPP and antioxidants results in a bell-shaped curve against time, with an initial increase in the level of extracted compounds driven by a high rate of diffusion; beyond the maximum, the levels of pigments and polyphenols are reduced by the degradation resulting from these compounds’ thermolabililty. This is an important factor to consider when determining a preparation time and method to maximize the extraction of relevant compounds in a minimum of time.
It should be stressed that an infusion made with more herbal mass (e.g. 1.0 g) will contain higher levels of polyphenols and present a higher A.C., but that the relationship between the plant mass and the levels of these compounds will be more advantageous in an infusion made with less herbal mass (e.g. 0.5 g). Soto-García and Rosales-Castro (Citation2016) obtained similar results for the bark of Pinus durangensis and Quercus sideroxyla: a higher initial plant mass resulted in a greater extraction yield and a higher antioxidant potential, but the yield per mass was greater at a lower initial plant mass. Jovanović et al. (Citation2017) report that at a higher mass:solvent ratio, the extraction yield of polyphenols decreased in T. serpyllum. Together, these findings indicate that the extraction process is more efficient when the herbal mass:water ratio is low.
TPP and A.C. have been found to be highly correlated, with reported correlation coefficients of 0.9378 (Surveswaran et al., Citation2007) and 0.964 (Cai et al., Citation2004). Oh et al. (Citation2013) found that this correlation was stronger for ethanolic than aqueous extracts; they suggest that certain non-hydrophilic compounds can have antioxidant activity. Here, a higher correlation between TPP and A.C. was found for infusions prepared with 0.5 g (0.8547) that with 1.0 g (0.6807). On the other hand, since the infusions’ A.C. was constant across brewing times, while TPP increased initially and decreased at longer brewing times, it could not be assumed that the A.C. of RR infusions is determined exclusively by the TPP. The variation of polyphenol levels is similar to that of pheophytin a levels; the latter is also known to have antioxidant activity (Kusmita et al., Citation2015) and, together with other compounds, it probably contributes to the infusions’ observed A.C.. Rojo et al. (Citation2009) note that it is important to consider that the FRAP method assesses the totality of reducing substances in a matrix, not just the phenolic compounds, and that other compounds, such as vitamin C, might thus contribute to the antioxidant effect. Katalinic, Milos, Kulisic, and Jukic (Citation2004) classify herbs by their FRAP A.C. into very low (<1 mmol/L), low (1–5 mM/L), good (5–10 mM/L), high (10–20 mM/L), and very high (>20 mM/L). Within this classification, RR infusions fall into the category with very low A.C.. The TPP content of herbal infusions has variously been reported as 9 and 2218 mg/L (Katalinic et al., Citation2004) and 12–62 mg GAE/g dry weight (Da Silva et al., Citation2013); however, these values can only serve as a reference, since they were obtained using different herbs, grown and collected elsewhere, and extracted using a different protocols.
Rojo et al. (Citation2009) determined the TPP content and A.C. of aqueous RR extracts obtained by decoction as part of a wider study of 12 medicinal herbs. The polyphenol content of these herbs ranged from 218–1781 µmol/L. At 423 µmol/L, the value for RR was considerably higher than the value found here (~2–4 µmol/L). The A.C. of RR was among the highest of the herbs evaluated by Rojo et al. (Citation2009); at 56,200 µm Trolox equivalents/L, it was far greater than the value obtained here (~2–4 µmol Trolox equivalents/L). Even for the same herbal species, collection site and season, as well as extraction methods differ between studies. In addition, the concentration of the herbs used by Rojo et al. (Citation2009) was 20 g in 80 ml water (1:4), whereas we used 0.5 g and 1 g in 250 ml (1:500 and 1:250, respectively), proportions that are much closer to the real conditions under which an infusion for direct human consumption is prepared, and which reflect the mass of a typical commercial tea bag. Likewise, the extraction method reported by Rojo et al. (Citation2009) includes a 5 min heating step, meaning it resembles a decoction more than an infusion. Such a heating step promotes the leaching of compounds into the infusion, even though this might affect the stability of these compounds. Guimarães et al. (Citation2013) found a greater extraction yield in decoction than infusion; however, the decoction did not present the antitumor effects found in the infusion, possibly due to the degradation of the phenolic compounds responsible for the antitumor activity during decoction. Martins et al. (Citation2015) also found a higher antioxidant activity and TPP content in decoction than infusion, while Marete et al. (Citation2013) found that higher extraction temperature increased both TPP and tannins, which are associated with a darker infusion color.
Based on the results obtained here, the following experiments were performed with a RR infusion of 1.0 g herbal mass brewed in 250 water for 5 min.
3.3. Comparison of the RR infusion with commercially available mint and chamomile infusions
The TPP content of the commercial mint infusion was double that of the RR infusion (). While its A.C. was also higher than that of RR, the difference was smaller. The pigment and TPP content, but not the A.C., of the RR infusion was higher than that of the chamomile infusion. Considering that antioxidants are produced as part of the plant’s defense against environmental aggression, it might be expected that RR, which grows on the Chilean Altiplano under extreme agroclimatic conditions including high solar irradiation, high salinity and water scarcity, should have a higher polyphenol content and A.C. than commercial herbs grown in managed cultures under more benign conditions (Martins et al., Citation2015; Rojo et al., Citation2009). However, there are considerable differences in the bioactive potential of different species of aromatic and medicinal plants (Katalinic et al., Citation2004; Martins et al., Citation2015). Following the classification of Katalinic et al. (Citation2004), all infusions evaluated here are low in antioxidant activity.
Table 2. Comparison of the RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke,) infusion and commercially available mint and chamomile infusions.
Tabla 2. Comparación de las infusiones de RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke), de menta y manzanilla disponibles comercialmente.
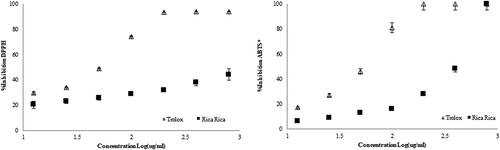
Based on our results, a greater leaf:stem ratio, together with an optimization of the season of collection, could help achieve an infusion with more beneficial properties. shows that, at higher concentrations, the A.C. of RR infusions, as measured by analytic methods other than FRAP, might equal the inhibitory effect of Trolox. The IC50 obtained was 337 µmol/mL for DPPH and 438 µmol/mL for ABTS.
Figure 3. Antioxidant capacity of the RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke) infusion measured by (A) DPPH and (B) ABTS* methods.
Figura 3. Capacidad antioxidante de la infusión de RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke), medida con los métodos (A) DPPH y (B) ABTS*.
3.4. In vivo anti-inflammatory activity of RR infusions
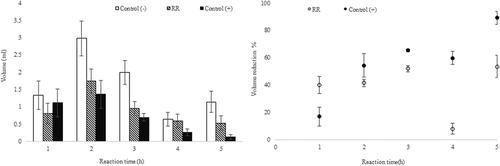
The anti-inflammatory activity of a lyophilized RR infusion was assessed using a rat paw edema assay. The RR extract showed anti-inflammatory activity at a concentration of 300 mg/kg (). Until three hours after the induced lesion, the effect of the RR extract was comparable to that of the positive control indomethacin. This anti-inflammatory effect could be due to several of the compounds present in the infusion, such as pigments and phenolic compounds (Higashi-Okai et al., Citation2001; Park et al., Citation2014). Anti-inflammatory activity has been described for various herbs and plant extracts, like as extracts of M. calabura (Preethi, Premasudha, & Keerthana, Citation2012), of Cymbopogon citratus (DC) (Rojas, Ronceros, Palacios, & Sevilla, Citation2012), and of flowers from Limonium algarvense, obtained by infusions and decoctions (Rodrigues et al., Citation2017). Tadic et al. (Citation2017) report that yarrow (Achillea millefolium L.) oil has anti-inflammatory properties and Boeris et al. (Citation2002) described greater anti-inflammatory effect from hydroalcoholic extracts than aqueous and chloroformic extracts.
Figure 4. Anti-inflammatory activity of the lyophilized RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke,) infusion, measured in a rat paw edema assay. Panel (A) shows the volume, panel (B) the percentage volume reduction at different reaction times after the injection of lyophilized RR (300 mg/kg), indomethacin (10 mg/kg, positive control) or physiological serum (negative control).
Figura 4. Actividad antiinflamatoria de la infusión liofilizada de RR (Acantholippia deserticola (Phil. ex F. Phil.) Moldenke), medida a través de una prueba de edema en una pata de rata. El panel (A) muestra el volumen, el panel (B) el porcentaje de reducción de volumen en distintos momentos de la reacción, después de la inyección de RR liofilizada (300 mg/kg), indometacina (10 mg/kg, control positivo) o suero fisiológico (control negativo).
4. Conclusion
RR leaves had a higher TPP content and A.C. than stems. The herbal mass and brewing times used to prepare RR infusions had a significant effect on the drink’s color, pigment and TPP content and A.C.. Biological activity, antioxidant ant anti-inflammatory, was demonstrated for an infusion of 0.5 g of RR brewed for 5 min in 250 mL water, thus providing an interesting opportunity to promote the development and commercialization of natural drinks prepared from RR infusions.
Acknowledgments
This work was supported by the University of Antofagasta under Grant [number 5310] “Sustainable agriculture in the desert.” This work was possible thanks to the scientific writing workshops organized by the Research Direction of the Universidad de Antofagasta.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Benites, J., Bustos, L., Rios, D., Bravo, F., López, J., Gajardo, S., … Buc-Calderon, P. (2013). Antidepressant and anxiolytic-like effects of essential oil from Acantholippia deserticola Phil in female rats. Boletín Latinoamericano Y Del Caribe De Plantas Medicinales Y Aromaticas, 12, 413–419. Retrieved from http://www.redalyc.org/articulo.oa?id=85628141009
- Boeris, M. A., Toso, R. E., Ochoa, G. J., Manso, D. A., Cuccolo, M. E., & Skliar, M. I. (2002). Estudio de las Propiedades Antiinflamatorias de Marrubium vulgare. Ciencia Veterinaria. Facultad De Ciencias Veterinarias. U.N.L. Pam, 4, 1–6. Retrieved from http://www.biblioteca.unlpam.edu.ar/pubpdf/revet/n04a01boeris.pdf
- Cai, Y., Luo, Q., Sun, M., & Corke, H. (2004). Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences, 74, 2157–2184.
- Coelho, M., Rocha, C., Cunha, L. M., Cardoso, L., Alves, L., Lima, R. C., … Pintado, M. (2016). Influence of harvesting factors on sensory attributes and phenolic and aroma compounds composition of Cymbopogon citratus leaves infusions. Food Research International, 89, 1029–1037.
- da Silva, P., Campos, R., Teixeira, H., & Prado, M. (2013). The phenolic compounds and the antioxidant potential of infusion of herbs from the Brazilian Amazonian region. Food Research International, 53, 875–881.
- Dincer, C., Torun, M., Tontul, I., Topuz, A., Sahin-Nadeem, H., Gokturk, R. S., … Ozdemir, F. (2017). Phenolic composition and antioxidant activity of Sideritis lycia and Sideritis libanotica subsp. linearis: Effects of cultivation, year and storage. Journal of Applied Research on Medicinal and Aromatic Plants, 5, 26–32.
- Fernandes, A. S., Nogara, G. P., Menezes, C. R., Cichoski, A. J., Mercadante, A. Z., Jacob-Lopes, E., & Zepka, L. Q. (2017). Identification of chlorophyll molecules with peroxyl radical scavenger capacity in microalgae Phormidium autumnale using ultrasound-assisted extraction. Food Research International. doi:10.1016/j.foodres.2016.11.011
- Francis, F. J., & Clydesdale, F. M. (1975). Food colorimetry: Theory and applications. Westport, CT: AVI Publishing Co. Inc.
- Guimarães, R., Barros, L., Dueñas, M., Calhelha, R. C., Carvalho, A. M., Santos-Buelga, C., … Ferreira, I. C. (2013). Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chemistry, 136, 718–725.
- Higashi-Okai, K., Yamazaki, M., Namagori, H., & Okai, Y. (2001). Identification and antioxidant activity of several pigments from the residual green tea (Camellia sinensis) after hot water extraction. Journal of UOEH, 23, 335–344. Retrieved from https://www.jstage.jst.go.jp/article/juoeh/23/4/23_KJ00001658291/_article
- Itoh, A., Tanaka, Y., Nagakura, N., Akita, T., Nishi, T., & Tanahashi, T. (2008). Phenolic and iridoid glycosides from Strychnos axillaris. Phytochemistry, 69, 1208–1214.
- Izquierdo, S. S., Lopez, O. D., & Gonzalez, M. L. (2011). Desarrollo de una tecnología para la obtención de extracto acuoso de Momordica charantia L. Revista Cubana De Plantas Medicinales, 16, 304–312. Retrieved from http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962011000400001
- Jordán, M. J., Lax, V., Rota, M. C., Lorán, S., & Sotomayor, J. A. (2013). Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis l. Food Control, 30, 463–468.
- Jovanović, A. A., Đorđević, V. B., Zdunić, G. M., Pljevljakušić, D. S., Šavikin, K. P., Gođevac, D. M., & Bugarski, B. M. (2017). Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Separation and Purification Technology, 179, 369–380.
- Katalinic, V., Milos, M., Kulisic, T., & Jukic, M. (2004). Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chemistry, 94, 550–557.
- Kelebek, H. (2016). LC-DAD–ESI-MS/MS characterization of phenolic constituents in Turkish black tea: Effect of infusion time and temperature. Food Chemistry, 204, 227–238.
- Khattab, R., Goldberg, E., Lin, L., & Thiyam, U. (2010). Quantitative analysis and free-radical-scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chemistry, 122, 1266–1272.
- Koca, N., Karadeniz, F., & Burdurlu, H. S. (2007). Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chemistry, 100, 609–615.
- Kusmita, L., Puspitaningrum, I., & Limantara, L. (2015). Identification, isolation and antioxidant activity of pheophytin from green tea (Camellia sinensis (L.) Kuntze). Procedia Chemistry, 14, 232–238.
- Lanfer-Marquez, U. M., Barros, R. M. C., & Sinnecker, P. (2005). Antioxidant activity of chlorophylls and their derivatives. Food Research International, 38, 885–891.
- Li, S., Li, S., Gan, R., Song, F., Kuang, L., & Li, H. (2013). Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Industrial Crops and Products, 51, 289–298.
- Lima, A., Pereira, J. A., Baraldi, I., & Malheiro, R. (2017). Cooking impact in color, pigments and volatile composition of grapevine leaves (Vitis vinifera L. var. Malvasia Fina and Touriga Franca). Food Chemistry, 221, 1197–1205.
- Maiocchi, M. G., & Avanza, J. R. (2004). Degradación de clorofilas y feofitinas a diferentes temperaturas en Ilex dumosa e Ilex paraguariensis. Universidad Nacional Del Nordeste, Comunicaciones Científicas y Tecnológicas. Retrieved from http://www.unne.edu.ar/unnevieja/Web/cyt/com2004/8-Exactas/E-085.pdf
- Marete, E. N., Jacquier, J. C., & O’Riordan, D. (2013). Effect of processing temperature on the stability of parthenolide in acidified feverfew infusions. Food Research International, 50, 593–596.
- Martins, N., Barros, L., Santos-Buelga, C., Silva, S., Henriques, M., & Ferreira, I. C. (2015). Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterization. Food Chemistry, 167, 131–137.
- Morales, G., & Paredes, A. (2014). Antioxidant activities of Lampaya medicinalis extracts and their main chemical constituents. BMC Complementary and Alternative Medicine, 14, 259–270.
- Morales, G., Paredes, A., Olivares, A., & Bravo, J. (2014). Acute oral toxicity and anti-inflammatory activity of hydroalcoholic extract from Lampaya medicinalis Phil in rats. Biological Research, 47, 1–7.
- Morales, G., Paredes, A., Sierra, P., & Loyola, L. (2008). Antioxidant activity of 50% aqueous - ethanol extract from Acantholippia deserticola. Biological Research, 41, 151–155.
- Oh, J., Jo, H., Cho, A. R., Kim, S. J., & Han, J. (2013). Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control, 31, 403–409.
- Parada, M. (2012). Legislación en Chile sobre fitofármacos y plantas medicinales. Revista De Farmacología De Chile, 5, 7–11. Retrieved from http://www.sofarchi.cl/otras-publicaciones/legislacionfitofarmacos.html
- Park, S., Choi, J. J., Park, B. K., Yoon, S. J., Choi, J. E., & Jin, M. (2014). Pheophytin a and chlorophyll a suppress neuroinflammatory responses in lipopolysaccharide and interferon-γ-stimulated BV2 microglia. Life Science, 103, 59–67.
- Pérez-Ramírez, I. F., Castaño-Tostado, E., Ramírez-de León, J. A., Rocha-Guzmán, N. E., & Reynoso-Camacho, R. (2015). Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa l.) Beverage. Food Chemistry, 172, 885–892.
- Preethi, K., Premasudha, P., & Keerthana, K. (2012). Anti-inflammatory activity of Muntingia calabura fruits. Pharmacognosy Journal, 4, 51–56.
- Pumilia, G., Cichon, M. J., Cooperstone, J. L., Giuffrida, D., Dugo, G., & Schwartz, S. J. (2014). Changes in chlorophylls, chlorophyll degradation products and lutein in pistachio kernels (Pistacia vera L.) during roasting. Food Research International, 65, 193–198.
- Quesille-Villalobos, A. M., Saavedra, T. J., & Gálvez, R. L. (2013). Phenolic compounds, antioxidant capacity, and in vitro α-amylase inhibitory potential of tea infusions (Camellia sinensis) commercialized in Chile. CyTA - Journal of Food, 11, 60–67.
- Riehle, P., Vollmer, M., & Rohn, S. (2013). Phenolic compounds in Cistus incanus herbal infusions — Antioxidant capacity and thermal stability during the brewing process. Food Research International, 53, 891–899.
- Rodrigues, M. J., Neves, V., Martins, A., Rauter, A. P., Neng, N. R., Nogueira, J. M. F., … Custódio, L. (2017). In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chemistry, 200, 322–329.
- Rojas, J., Ronceros, S., Palacios, O., & Sevilla, C. (2012). Efecto anti-Trypanosoma cruzi del aceite esencial de Cymbopogon citratus (DC) Stapf (hierba luisa) en ratones Balb/c. Anales De La Facultad De Medicina, 73, 7–12.
- Rojo, L., Benites, J., Rodrigues, A., Venâncio, F., Ramalho, L., Teixeira, A., … Costa, M. (2006). Composition and antimicrobial screening of the essential oil of Acantholippia deserticola (Phil.ex F. Phil.) Moldenke. Journal of Essential Oil Research, 18(6), 695–697.
- Rojo, L. E., Benites, J., López, J., Rojas, M., Díaz, P., Ordóñez, J., & Pastene, E. (2009). Antioxidant capacity and polyphenolic content of twelve traditionally used herbal medicinal infusions from the South American Andes. Boletín Latinoamericano Y Del Caribe De Plantas Medicinales Y Aromáticas, 8, 498–508. Retrieved from https://www.researchgate.net/profile/Julio_Benites3/publication/279573101_Antioxidant_capacity_and_polyphenolic_content_of_twelve_traditionally_used_herbal_medicinal_infusions_from_the_South_American_Andes/links/57a60efa08aefe6167b690e7.pdf
- Santos, J. S., Deolindo, C. T. P., Esmerino, L. A., Genovese, M. I., Fujita, A., Marques, M. B., & Granato, D. (2016). Effects of time and extraction temperature on phenolic composition and functional properties of red rooibos (Aspalathus linearis). Food Research International, 89, 476–487.
- Simirgiotis, M. J., Silva, M., Becerra, J., & Schmeda-Hirschmann, G. (2012). Direct characterisation of phenolic antioxidants in infusions from four Mapuche medicinal plants by liquid chromatography with diode array detection (HPLC-DAD) and electrospray ionisation tandem mass spectrometry (HPLC-ESI–MS). Food Chemistry, 131, 318–327.
- Soto-García, M., & Rosales-Castro, M. (2016). Efecto del solvente y de la relación masa/solvente, sobre la extracción de compuestos fenólicos y la capacidad antioxidante de extractos de corteza de Pinus durangensis y Quercus sideroxyla. Maderas, Ciencia Y Tecnología, 18, 701–714.
- Surveswaran, S., Cai, Y., Corke, H., & Sun, M. (2007). Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chemistry, 102, 938–953.
- Tadić, V., Arsić, I., Zvezdanović, J., Zugić, A., Cvetković, D., & Pavkov, S. (2017). The estimation of the traditionally used yarrow (Achillea millefolium l. Asteraceae) oil extracts with anti-inflamatory potential in Topical application. Journal of Ethnopharmacology, 199, 138–148.
- Tapia-Torres, N. A., de la Paz-Pérez-Olvera, C., Román-Guerrero, A., Quintanar-Isaías, A., García-Márquez, E., & Cruz-Sosa, F. (2014). Histoquímica, contenido de fenoles totales y actividad antioxidante de hoja y de madera de Litsea glaucescens Kunth (Lauraceae). Madera Bosques, 20, 125–137. Retrieved from http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-04712014000300011
- Thongphasuk, P., Suttisri, R., Bavovada, R., & Verpoorte, R. (2004). Antioxidant lignan glucosides from Strychnos vanprukii. Fitoterapia, 75, 623–628.
