?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Various stresses are commonly encountered by probiotics in their ecological niches. Effects of adaptation treatments on the heat tolerance of Lactobacillus plantarum KLDS 1.0628 were evaluated. Cells pre-adapted to heat, cold, oxidative, acid, bile salt, and osmotic stresses were evaluated for tolerance and cross-protection against subsequent challenge. Except for cold, pre-adaptation to sublethal levels of stress significantly improved the cell viability after heat challenge compared to the control. Heat-adapted cells showed the highest heat stress tolerance, with their tolerance factor against heat challenge reaching 31.38-fold after pre-adaptation at 45°C for 1 h. Moreover, adaptation treatments improved tolerance to the same stress and evoked cross-protection against heterologous challenges, inducing a significantly lower ratio of saturated to unsaturated fatty acid content in the cell membrane of L. plantarum KLDS 1.0628. The results indicated that adaptation treatments are potential strategies for enhancing the survival rate of probiotics and exerting their functional properties.
RESUMEN
Los probióticos suelen estar expuestos a diversos tipos de estrés en sus nichos ecológicos. Este estudio se propuso evaluar los efectos que conllevan distintos tratamientos de adaptación en la tolerancia al calor de Lactobacillus plantarum KLDS 1.0628. Para ello se valoraron células preadaptadas al calor, el frío, la oxidación, el ácido, la sal biliar y el estrés osmótico, con el fin de determinar la tolerancia y la protección cruzada contra el desafío posterior. Se constató que, excepto en el caso del frío, en comparación con el control la preadaptación a niveles subletales de estrés mejoró significativamente la viabilidad de la célula después del desafío con calor. Tras la preadaptación a 45°C durante 1 hora, las células adaptadas al calor mostraron la mayor tolerancia al estrés térmico, registrando un factor de tolerancia contra el desafío de calor que alcanzó 31.38 veces. Además, los tratamientos de adaptación mejoraron la tolerancia al propio estrés y evocaron una protección cruzada contra desafíos heterólogos, induciendo una ratio significativamente menor de contenido de ácidos grasos saturados a insaturados en la membrana celular de L. plantarum KLDS 1.0628. En conclusión, los resultados indican que los tratamientos de adaptación son estrategias potenciales para aumentar la tasa de supervivencia de los probióticos y potenciar el ejercicio de sus propiedades funcionales.
1. Introduction
Lactobacillus plantarum is a Gram-positive and facultative anaerobic lactic acid bacteria (LAB) species that is a commensal microorganism in the human gastrointestinal tract and widely exists in the food environment (Wang et al., Citation2018). L. plantarum not only can tolerate the simulated digestive tract environment but also has good probiotic characteristics (Cao et al., Citation2019). Many bacteriocins are synthesized by food-grade L. plantarum, which offers the possibility of deliberately manipulating the microbial ecosystem (Cotter et al., Citation2005). Extensive studies have shown that L. plantarum contributes to inhibiting pathogenic bacterial infection, regulating the host immune system, and ameliorating lipid metabolism (Zhao et al., Citation2018). Hence, L. plantarum is widely used as a starter culture and is usually marketed commercially as a probiotic (Papadopoulou et al., Citation2018; Salar-Behzadi et al., Citation2013).
In recent years, there have been numerous studies on the functional properties of probiotic LAB given the growing market of probiotics (Aoudia et al., Citation2016; Ma, Yu et al., Citation2020). Nevertheless, probiotics are inevitably subjected to multiple harsh conditions during production, processing, storage, and passage through the gastrointestinal tract (Marcial-Coba et al., Citation2018). In particular, there are some high-temperature technological processes in the industrial application of Lactobacillus, including spray drying, pasteurization, etc. Heat stress is a common difficulty for survival and requires retention of a sufficient amount of viable probiotic LAB amidst these environmental challenges (Chen et al., Citation2017). Furthermore, heat stress will seriously affect the growth, metabolism and functional properties of probiotics.
To confer health benefits, stress tolerance is a prerequisite for probiotic LAB to survive and maintain high viability throughout processing, storage and consumption (Ma, Xu et al., Citation2020). Therefore, many recent studies have focused on the development of different approaches to improve the survival of probiotics, such as screening resistant strains, adding extra nutrients, microencapsulation and inducing the cellular stress response. Among these approaches, adaptive tolerance responses have been observed in many Lactobacillus, which help to ameliorate the adverse effects and improve the viability of the cells in subsequent lethal challenge (Ricciardi et al., Citation2012). Lactobacillus has multiple defence mechanisms to cope with various stress conditions, including preserving cell energy and changing cell membrane compositions (Papadimitriou et al., Citation2016). The role of pre-adaptation with sublethal thermal shock in heat-challenged cells has been reported (Kang et al., Citation2015). Furthermore, previous research also indicated that a variety of adaptations could induce homologous tolerance as well as cross-protection against other types of stress challenges (Papadimitriou et al., Citation2016).
The adaptive response and cross-protection of Lactobacillus have attracted wide attention in recent years. However, to the best of our knowledge, few studies have examined the stress response and tolerance of L. plantarum to multiple environmental stresses. The aim of the present work was to evaluate the effect of adaptation treatments on the viability of L. plantarum KLDS 1.0628 after heat challenge. The cross-protection of heat-, cold-, H2O2-, acid-, bile salt- and NaCl-adapted cells against stress challenges was evaluated. Additionally, changes in the cell membrane fatty acid composition were examined.
2. Materials and methods
2.1. Bacterial strain and culture conditions
Lactobacillus plantarum KLDS 1.0628 was previously isolated from traditional fermented pickles in Heilongjiang Province, China, and kept at the Key Laboratory of Dairy Science (KLDS), Ministry of Education, Northeast Agricultural University. The strain was identified according to morphological, biochemical and physiological characteristics and then identified by 16S rDNA gene sequencing, followed by BLAST homology searching using the NCBI database (Du et al., Citation2018). L. plantarum KLDS 1.0628 was stored at −80°C, inoculated (1%, v/v) into De Man, Rogosa, and Sharpe (MRS) broth and then cultured at 37°C for 18–22 h. The activated cells were used in further experiments after three consecutive transfers in sterile MRS broth.
2.2. Growth characteristics of Lactobacillus plantarum KLDS 1.0628 under different stress conditions
One hundred millilitres of fresh MRS broth was inoculated with 1% (v/v) of an overnight culture of L. plantarum KLDS 1.0628 and further incubated at 37°C for 4 h until the optical density at 600 nm reached 0.4–0.5. Cultures were heated to 37 (control), 40, 45, 50, 55, and 60°C for 1 h and then restored to 37°C. For cold stress, the cultures were incubated at 4, 10, 15, 20, and 25°C for 1 h. Furthermore, bacterial cells were harvested by centrifugation at 5000 × g for 10 min at 4°C, washed twice with sterilized phosphate-buffered saline at pH 7.1, and then resuspended in fresh MRS broth supplemented with 0 (control), 0.2, 0.5, 1, 2, and 5 mmol/L H2O2 for 1 h at 37°C. For acid stress, the washed cells were subcultured in fresh MRS broth adjusted to pH values of 2.5, 3, 4, 5, and 6 with 2 M HCl and incubated for 1 h at 37°C. MRS broth supplemented with 0 (control), 1%, 2%, 4%, 6%, and 8% NaCl was prepared to evaluate the osmotic tolerance response of L. plantarum KLDS 1.0628. For bile salt stress, the washed cells were exposed to varying concentrations of oxgall bile (0.1%, 0.2%, 0.3%, 0.4%, and 0.5%). Samples (10 mL) were collected every 2 h for 12 h, and changes in optical density were routinely monitored at 600 nm.
2.3. Stress adaptation and challenge assays
After incubation overnight in MRS broth at 37°C, L. plantarum KLDS 1.0628 was inoculated in 100 mL of fresh MRS broth and incubated at 37°C for 4 h until it reached an optical density of 0.4–0.5 at 600 nm. Bacterial cells were harvested by centrifugation (5000 × g, 10 min, 4°C), washed twice with PBS, and then resuspended in the same volume of MRS broth for stress adaptation and stress challenge experiments. The tolerance of L. plantarum KLDS 1.0628 to sublethal levels of heat (45°C, 1 h), cold (15°C, 1 h), oxidative (1 mmol/L, 1 h), acid (pH 4.0, 1 h), bile salt (0.2%, 1 h), and osmotic stresses (2%, 1 h) was evaluated. Cell viability was enumerated after serial dilution of each sample in sterile 0.85% (w/v) sodium chloride solution and spread on MRS agar plates. The cell plates were incubated at 37°C anaerobically for 24–48 h, and the number of colony forming units per millilitre (CFU/mL) was determined. For heat challenge, 1 mL of each of these non-adapted and pre-adapted cells was then transferred to the same volume of MRS broth and subjected to the lethal level of heat challenge (60°C for 1 h). Cells grown in MRS broth at 37°C without any stress treatment were used as controls. Cell viability was also determined after heat challenge.
2.4. Cross-protection assays
Based on the results of preliminary growth tests of L. plantarum KLDS 1.0628 under different conditions, stress adaptations were carried out under the following experimental conditions: 45°C for 1 h, 15°C for 1 h, 1 mmol/L H2O2 for 1 h at 37°C, pH 4.0 (adjusted by 2 M HCl) for 1 h at 37°C, 0.2% bile salt for 1 h at 37°C, and 2% NaCl for 1 h at 37°C. Non-adapted cells grown in fresh MRS broth at 37°C for 1 h were used as controls. Then, 1 mL of each of these non-adapted and pre-adapted cells was transferred to the same volume of MRS broth and subjected to the following stress challenge conditions: 60°C for 1 h, −20°C for 14 d, 5 mmol/L H2O2 for 1 h at 37°C, pH 2.5 (adjusted by 2 M HCl) for 1 h at 37°C, 0.5% bile salt for 1 d at 37°C, and 8% NaCl for 1 d at 37°C. The cell viability of L. plantarum KLDS 1.0628 affected by various stress challenges was estimated. The survival rate (%) was calculated by applying EquationEquation (1)(1)
(1) :
In addition, the tolerance factor against the stress challenge was evaluated as defined in Equationequation (2)(2)
(2) :
2.5. Membrane fatty acid analysis
Cell membrane fatty acids of L. plantarum KLDS 1.0628 were separated and identified as described earlier (Ma, Wang et al., Citation2020). Samples were analysed using a 6890 N gas chromatograph (Agilent, USA) equipped with an HP-5 elastic quartz capillary column (30 m × 0.25 mm × 0.25 μm, Agilent, USA). Chromatography was carried out with an injection volume of 1 μL. High-purity helium was used as the carrier gas at a flow rate of 1 mL/min. The column front pressure and interface temperature were controlled at 73.0 kPa and 250°C, respectively. The program temperature rose from 130°C to 230°C. The relative contents of cell membrane fatty acids were expressed as molar percentages (mol %).
2.6. Statistical analysis
All experiments were performed independently in triplicate, and the data were collected. One-way analysis of variance (ANOVA) was performed using SPSS software 17.0, and means were separated by using Tukey’s range test with statistical significance when P < .05.
3. Results and discussion
3.1. Heat tolerance response of heat-adapted L. plantarum KLDS 1.0628 cells
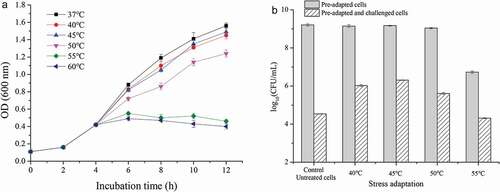
It was observed that the growth rate of L. plantarum KLDS 1.0628 decreased slightly when the heat treatment temperature was 40 and 45°C ()). When the temperature was higher than 50°C, the cell growth rate was significantly inhibited. The viable count of differentially heat-adapted and non-adapted cells of L. plantarum KLDS 1.0628 after heat challenge at 60°C for 1 h is shown in ). The heat adaptations at 40°C and 45°C for 1 h exhibited no significant effect on the viable counts of L. plantarum KLDS 1.0628 compared with that of untreated cells (P > .05). The survival rate of L. plantarum KLDS 1.0628 decreased by more than 95% after 1 h of exposure at 55°C (6.73 log10 CFU/mL) compared with that of non-adapted cells (9.21 log10 CFU/mL). After heat challenge at 60°C for 1 h, the survival rate of L. plantarum KLDS 1.0628 without heat adaptation was 0.08%. It should be noted that L. plantarum cells subjected to heat adaptation at 45°C for 1 h exhibited higher heat tolerance than other heat adaptation treatments (P < .05). In our study, the heat tolerance of L. plantarum 1.0628 could be enhanced by a sublethal level of heat stress, which was similar to the results reported by Kang et al. (Citation2015). Moreover, the results suggested that a heat tolerance response could be induced when exposed to a wide range of sublethal temperatures. This effect is mainly due to heat stress resulting in significant protein denaturation, cell wall, and nucleic acid molecule damage (Salar-Behzadi et al., Citation2013). Additionally, LAB can produce a series of heat-induced proteins called heat shock proteins (HSPs) when exposed to high temperatures, which contribute to stabilizing the cytoskeleton and repairing damaged proteins (Sottile & Nadin, Citation2018).
Figure 1. Heat tolerance of L. plantarum KLDS 1.0628. (a) Growth of L. plantarum KLDS 1.0628 under heat stress; (b) Effects of different heat adaptation treatments on the viable counts of L. plantarum KLDS 1.0628 after heat challenge at 60°C.
Figura 1. Tolerancia al calor de L. plantarum KLDS 1.0628. (a) Crecimiento de L. plantarum KLDS 1.0628 bajo estrés térmico; (b) Efectos de diferentes tratamientos de adaptación al calor en los recuentos viables de L. plantarum KLDS 1.0628, después del desafío térmico a 60°C
3.2. Heat tolerance response of cold-adapted L. plantarum KLDS 1.0628 cells
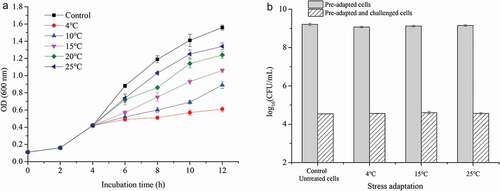
The growth of L. plantarum KLDS 1.0628 during various cold stress treatments was investigated ()). When exposed to cold stress, the growth of L. plantarum KLDS 1.0628 was inhibited to different degrees. When the incubation temperature decreased from 37°C to 4°C, the growth rate significantly decreased (P < .05), and the lowest growth rate of L. plantarum KLDS 1.0628 was observed when the temperature was 4°C. Based on the results presented in ), the cold adaptation at 4°C for 1 h showed significantly lower viability of L. plantarum KLDS 1.0628 than that of the control (P < .05), but no significant difference was observed when cells were subjected to cold stress at 4°C and 15°C for 1 h (P > .05). Additionally, after heat challenge at 60°C, the viability of cold-adapted cells did not significantly differ from that of control untreated cells (P > .05). Previous studies have also shown that cold adaptation can enhance the stress tolerance of L. plantarum K25, and isobaric tags for relative and absolute quantification proteomic analysis confirmed that this phenomenon is mainly due to changes in stress tolerance gene expression and proteins related to DNA repair, transcription and translation (Liu et al., Citation2020). Moreover, the two-component system and quorum sensing are also involved in the cell cold-adaptation process.
Figure 2. Effects of different cold stresses on the heat tolerance of L. plantarum KLDS 1.0628. (a) Growth of L. plantarum KLDS 1.0628 under cold stress; (b) Effects of different cold adaptation treatments on the viable counts of L. plantarum KLDS 1.0628 before and after heat challenge at 60°C.
Figura 2. Efectos de diferentes tipos de estrés por frío sobre la tolerancia al calor de L. plantarum KLDS 1.0628. (a) Crecimiento de L. plantarum KLDS 1.0628 bajo estrés por frío; (b) Efectos de diferentes tratamientos de adaptación al frío en los recuentos viables de L. plantarum KLDS 1.0628, antes y después del desafío de calor a 60°C
3.3. Heat tolerance response of oxidative-adapted L. plantarum KLDS 1.0628 cells
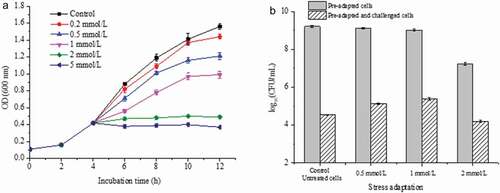
The impact of different H2O2 concentrations on the growth of L. plantarum KLDS 1.0628 is presented in ). The growth rate of L. plantarum KLDS 1.0628 decreased slowly with increasing H2O2 concentration when the concentration of H2O2 ranged from 0.2 mmol/L to 1 mmol/L. A detrimental effect on cell growth was also observed when the cultures were treated with 2 or 5 mmol/L H2O2. Compared to that of untreated cells, the survival rate of L. plantarum KLDS 1.0628 decreased by 90.10% after 2 mmol/L oxygen adaptation for 1 h ()). Biological macromolecules such as proteins, nucleic acids, and lipids may be damaged by ROS, resulting in cell senescence and death (Papadimitriou et al., Citation2016). Generally, ROS are scavenged to protect cells from oxidative stress through antioxidant defence systems (Zhang et al., Citation2018). Among all heat-challenged cells, L. plantarum KLDS 1.0628 cells that were pre-treated with 1 mmol/L showed the highest heat tolerance to 60°C for 1 h. However, all of the oxidative-adapted cells suffered a more than 3.05 log10 CFU/mL decrease in viable counts after heat challenge at 60°C. These results revealed that pre-adaptation to oxygen stress at lower concentrations could enhance the heat tolerance of L. plantarum KLDS 1.0628. This idea was confirmed by Zhang et al. (Citation2020), who found that an adaptive response of Lactobacillus to one stress could lead to cross-protection against another stress by changing the physiological characteristics and basic metabolic pathways.
Figure 3. Effects of different oxidative stresses on the heat tolerance of L. plantarum KLDS 1.0628. (a) Growth of L. plantarum KLDS 1.0628 under oxidative stress; (b) Effects of different oxidative adaptation treatments on the viable counts of L. plantarum KLDS 1.0628 before and after heat challenge at 60°C.
Figura 3. Efectos de diferentes tipos de estrés oxidativo sobre la tolerancia al calor de L. plantarum KLDS 1.0628. (a) Crecimiento de L. plantarum KLDS 1.0628 bajo estrés oxidativo; (b) Efectos de diferentes tratamientos de adaptación oxidativa en los recuentos viables de L. plantarum KLDS 1.0628, antes y después del desafío térmico a 60°C
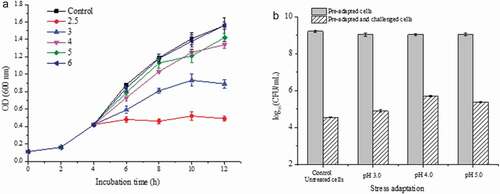
3.4. Heat tolerance response of acid-adapted L. plantarum KLDS 1.0628 cells
The growth of L. plantarum KLDS 1.0628 subjected to acid stresses was recorded as shown in . There were no remarkable differences in the growth of L. plantarum KLDS 1.0628 when the pH was between 6.0 and the control (6.5) (). When the pH was decreased from 6.0 to 5.0 or 4.0, the cell growth of L. plantarum KLDS 1.0628 became slow. The growth rate of L. plantarum cells declined markedly when the initial pH value was adjusted to 3.0, and L. plantarum KLDS 1.0628 incubated at pH 2.5 showed growth arrest. In addition, the pre-adapted cells at different pH values (3.0, 4.0, and 5.0) for 1 h exhibited lower viability than that of untreated cells (). After heat challenge at 60°C for 1 h, L. plantarum KLDS 1.0628 pre-adapted to acid (pH 4.0) achieved the highest viable count of 5.69 log10 CFU/mL. The number of heat-challenged cells at 60°C after pre-adaptation at pH 3.0, 4.0, and 5.0 was significantly higher than that of the control (P < .05), suggesting that an adaptive acid tolerance response (ATR) was observed in L. plantarum KLDS 1.0628. The mechanism of LAB against acid stress can be summarized as follows: increasing the activity of proton pump H+-ATPase activity, production of alkali (arginine deiminase pathway), the expression of stress protein, and the change of membrane fatty acid composition (Huang et al., Citation2016), which may have a correlation with the enhancement of heat tolerance of the strain.
Figure 4. Effects of different acid stresses on the heat tolerance of L. plantarum KLDS 1.0628. (a) Growth of L. plantarum KLDS 1.0628 under acid stress; (b) Effects of different acid adaptation treatments on the viable counts of L. plantarum KLDS 1.0628 before and after heat challenge at 60°C.
Figura 4. Efectos de diferentes tipos de estrés por ácidos sobre la tolerancia al calor de L. plantarum KLDS 1.0628. (a) Crecimiento de L. plantarum KLDS 1.0628 bajo estrés ácido; (b) Efectos de diferentes tratamientos de adaptación al ácido en los recuentos viables de L. plantarum KLDS 1.0628, antes y después del desafío térmico a 60°C
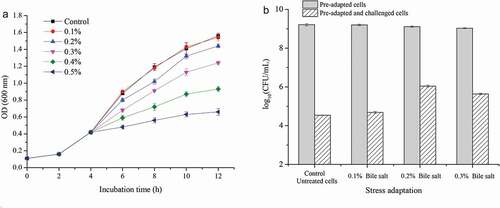
3.5. Heat tolerance response of bile salt-adapted L. plantarum KLDS 1.0628 cells
The growth of L. plantarum KLDS 1.0628 was not noticeably affected by the addition of 0.1% bile salt within the incubation time ()). Along with an increased concentration of bile salt, a significant reduction in the growth rate of L. plantarum KLDS 1.0628 was observed (P > .05). Furthermore, L. plantarum KLDS 1.0628 can also survive and grow when the concentration of bile salt was up to 0.5%. Moreover, 0.1% bile salt adaptation for 1 h exhibited no significant difference in the viability of L. plantarum KLDS 1.0628 compared to that of the control (P < .05). However, a significant decrease in cell count was detected when pre-adapted for 1 h in the presence of 0.2% or 0.3% bile salt for 1 h (). In addition, the 0.1% bile salt stress treatment did little to enhance the heat tolerance of L. plantarum KLDS 1.0628. Remarkably, L. plantarum KLDS 1.0628 pre-adapted to 0.2% bile salt had significantly higher viability than that of other 0.3% bile salt adaptation and control untreated cells after heat challenge at 60°C for 1 h. This phenomenon may be due to the differentially expressed proteins, including those involved in stress response, DNA repair, and peptidoglycan biosynthesis, providing tolerance towards environmental challenges (Kaur et al., Citation2017).
Figure 5. Effects of different bile salt stresses on the heat tolerance of L. plantarum KLDS 1.0628. (a) Growth of L. plantarum KLDS 1.0628 under bile salt stress; (b) Effects of different bile salt adaptation treatments on the viable counts of L. plantarum KLDS 1.0628 before and after heat challenge at 60°C.
Figura 5. Efectos de diferentes tipos de estrés por sales biliares sobre la tolerancia al calor de L. plantarum KLDS 1.0628. (a) Crecimiento de L. plantarum KLDS 1.0628 bajo estrés por sales biliares; (b) Efectos de diferentes tratamientos de adaptación a sales biliares en los recuentos viables de L. plantarum KLDS 1.0628, antes y después del desafío térmico a 60°C
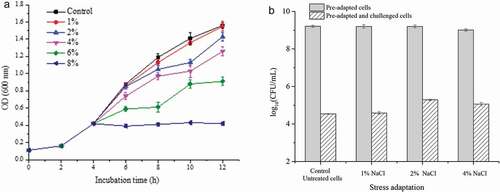
3.6. Heat tolerance response of osmotic-adapted L. plantarum KLDS 1.0628 cells
There was hardly any significant impact on the growth of L. plantarum KLDS 1.0628 when the addition of NaCl was 1% (). However, it is obvious that the proliferation of L. plantarum KLDS 1.0628 was inhibited in the presence of 2%, 4%, or 6% NaCl, and L. plantarum KLDS 1.0628 could not grow when exposed to NaCl at a higher concentration of 8% (P < .05). There were no significant differences in the viable counts of L. plantarum KLDS 1.0628 between the treatment with low osmotic concentrations (1% and 2%) for 1 h and non-adapted cells (). Cells pre-adapted to 4% NaCl osmotic stress for 1 h had significantly lower viable counts than the control (P < .05). After 1 h of exposure at 60°C, the pre-adaptation of 2% NaCl for 1 h resulted in a strong prolongation of cell viability, followed by the pre-adaptation of 4% NaCl. Previous research indicated that the downregulation of carbamoyl phosphate synthase in carbohydrate metabolism of L. plantarum induced a bacterial regulation mechanism to save energy (Wang et al., Citation2016). Nevertheless, the osmotic adaptation of 1% NaCl showed no marked influence on the bacterial number compared to the control.
Figure 6. Effects of different osmotic stresses on the heat tolerance of L. plantarum KLDS 1.0628. (a) Growth of L. plantarum KLDS 1.0628 under osmotic stress; (b) Effects of different osmotic adaptation treatments on the viable counts of L. plantarum KLDS 1.0628 before and after heat challenge at 60°C.
Figura 6. Efectos de diferentes tipos de estrés osmótico sobre la tolerancia al calor de L. plantarum KLDS 1.0628. (a) Crecimiento de L. plantarum KLDS 1.0628 bajo estrés osmótico; (b) Efectos de diferentes tratamientos de adaptación osmótica en los recuentos viables de L. plantarum KLDS 1.0628, antes y después del desafío de calor a 60°C
3.7. Effects of cross-protection on the stress tolerance L. plantarum KLDS 1.0628
The survival rates (%) and tolerance factors of non-adapted and pre-adapted cells of L. plantarum KLDS 1.0628 under different stress conditions were summarized and are shown in and , respectively. Except for cold adaptation, the other five pre-adaptation treatments used in this study can all activate the defence mechanism of L. plantarum KLDS 1.0628 against thermal lethal conditions compared with non-adapted cells (). The highest survival rate was observed in heat-adapted cells (2.51%). Prior bile salt and acid adaptation also significantly improved the survival rate of L. plantarum KLDS 1.0628 cells from 0.08% to 1.63% and 0.99%, respectively (P < .05). The tolerance factors of heat-, bile salt- and acid-adapted cells against heat challenge increased 31.38-, 20.41-, and 12.34-fold, respectively (). Pre-exposure to other types of stresses also induced cross-adaptation against heat challenge, which indicates that the adaptive mechanism of lactic acid bacteria under different types of stress has a certain degree of overlap (Zhang et al., Citation2018). However, homologous pre-adaptation appears to be more effective. Studies have indicated that cell characteristics are altered to cope with heat stress, such as cell morphology, membrane fatty acid composition, surface charge, and adhesion ability (Haddaji et al., Citation2017).
Table 1. The survival rates (%) of non-adapted and pre-adapted cells of L. plantarum after stress conditions.
Tabla 1. Tasas de supervivencia (%) de las células no adaptadas y preadaptadas de L. plantarum después de condiciones de estrés
Figure 7. Stress tolerance and cross-protection of L. plantarum KLDS 1.0628 against (a) heat; (b) cold; (c) peroxide; (d) acid; (e) bile salt; (f) osmotic challenge after different preadaptation treatments.
Figura 7. Tolerancia al estrés y protección cruzada de L. plantarum KLDS 1.0628 contra (a) el calor; (b) el frío; (c) el peróxido; (d) el ácido; (e) la sal biliar; (f) el desafío osmótico después de diferentes tratamientos de preadaptación
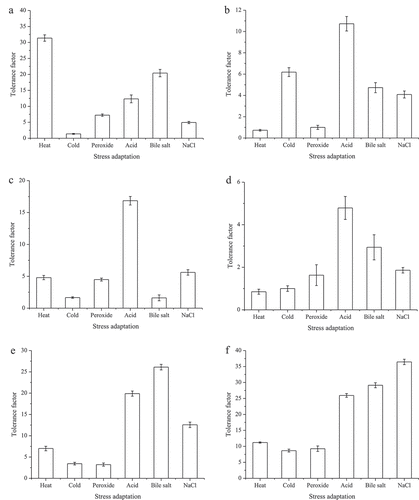
It was observed that both heat and peroxide adaptation had no effect on cold tolerance (). Adaptation to cold, acid, bile salt, and osmotic stress resulted in significant cross-protection against lethal levels of cold stress, and the cold tolerance increased 6.19-, 4.73-, and 4.09-fold, respectively ()). It has been confirmed that cold shock proteins (CSPs) are able to participate in transcription, translation, and protein folding and can bind to single-stranded nucleic acids and break down secondary structures formed at low temperatures, thereby increasing the cold tolerance of cells (Papadimitriou et al., Citation2016). CSPs can also be induced when exposed to stresses other than cold stress. Our results are in agreement with the report by Streit et al. (Citation2008), who found that pre-adaptation to acid improved the cold tolerance of L. delbrueckii and L. acidophilus subsp. bulgaricus.
After 1 h of exposure to 5 mmol/L H2O2, the survival of acid-adapted cells was the highest (0.51%), followed by osmotic-, heat-, and oxidative-adapted cells, which may be due to the transcription of genes related to oxidative stress tolerance being regulated in response to sublethal stress. Furthermore, it has been revealed that antioxidant-related genes contribute to coping with oxidative stress (Zhao et al., Citation2018). In addition, the oxidative stress tolerance of L. plantarum KLDS 1.0628 was increased 16.85- and 5.61-fold, respectively, after pre-adaptation to acid and sodium chloride ().
The acid-adapted cells displayed a higher survival of 0.10% and acid tolerance (4.79-fold) compared to that of other pre-treatments after exposure to acid challenge. In addition, H2O2, bile salt and NaCl pre-treatment also enhanced the acid tolerance of L. plantarum KLDS 1.0628. However, prior heat and cold adaptation did little to enhance the acid tolerance, which is similar to the results reported by Chen et al. (Citation2017) and is different from Kulkarni et al. (Citation2018). The results illustrated that acid adaptation can be used as a potential strategy to improve cell survival in response to multiple lethal stresses. Prior bile salt adaptation of L. plantarum KLDS 1.0628 also contributed to inducing a 2.94-fold increase in acid tolerance ().
Bile stress tolerance is important for probiotic LAB to survive in the gastrointestinal tract and confer beneficial impacts on human health. After a 1 d exposure to 0.5% bile salts, the survival of all pre-adapted L. plantarum KLDS 1.0628 was significantly higher than that of the non-adapted control (P > .05) (). The results observed were comparable with previous studies in which the adaptive response induced by bile salt stress contributed to ameliorating the negative impacts of various lethal challenges (Noriega et al., Citation2004). As shown in , the highest bile salt tolerance factor induced by stress adaptation treatments was observed in the following decreasing order: bile salt > acid > osmotic > cold = peroxide. The deconjugation of bile salts by bile salt hydrolases (BSHs) could be an effective molecular mechanism against bile salt stress (Bustos et al., Citation2018). The crucial role of membrane phospholipid cardiolipin and F1F0-ATPase in bile acid adaptation by LAB is well established (Bi et al., Citation2016). These results could also be explained by the changes in gene expression in different biological processes, including carbohydrate, amino acid, peptide metabolism, transcription factors, and transmembrane transport (Ma et al., Citation2018).
LAB often encounter the challenge of high salt concentrations when applied to the processing of foods such as cheese fermentation. Osmotic stress results in intracellular water outflow and cytoplasmic separation, causing cells to stop growing or even die (Gandhi & Shah, Citation2015). Studies have shown that the expression of proteins involved in carbohydrate metabolism, amino acid metabolism, nucleotide metabolism, and ATP-binding cassette (ABC) transporter was changed when exposed to salt stress (Li et al., Citation2019). To maintain cellular osmotic equilibrium, compatible solutes (osmolytes) such as betaine, carnitine, choline, and proline were accumulated in LAB using self-synthesis or absorption from the external environment (Papadimitriou et al., Citation2016). It was also shown that all the various pre-adaptations had significantly higher (P < .05) survival rates than those of non-adapted cells (0.06%) after exposure to sodium chloride (8%) for 1 d. Furthermore, the osmotic stress tolerance (36.44-fold) and highest survival rate (2.19%) were obtained following pre-adaptation with sodium chloride, followed by bile salt-, acid-, and heat-adapted cells ().
3.8. Cell membrane fatty acid analysis
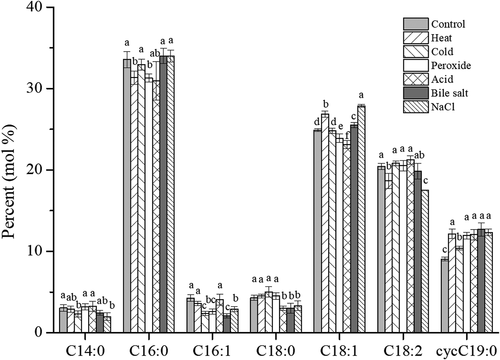
Gas chromatography was carried out to explore the effect of different stress adaptations on the cell membrane fatty acid composition, including saturated fatty acids (SFAs) and unsaturated fatty acids (USFAs). In this study, myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2) and cyclopropane fatty acid (cyc C19:0) were the main fatty acids in the cell membrane of L. plantarum KLDS 1.0628, accounting for more than 97% of the total cell membrane fatty acids (). The results showed that USFAs accounted for 58.63% (control), 61.26% (heat group), 58.38% (cold group), 58.96% (peroxide group), 60.45% (acid group) and 60.11% (bile salt group) and 60.64% (NaCl group) of the total cell membrane fatty acids. Moreover, the ratio of SFA to USFA varied with different stress adaptation treatments. A similar trend was observed for the SFA/USFA ratio of Lactobacillus casei under stress conditions (Wu et al., Citation2012). In general, the ratio of SFA/USFA was lower when L. plantarum KLDS 1.0628 was subjected to all types of pre-adaptation treatments. Previous studies have shown that bacterial membrane fluidity is reduced by changing the composition of fatty acids to protect proton flow and maintain pH homeostasis (Huang et al., Citation2016). Furthermore, the content of cyc C19:0 increased remarkably in all stress adaptation groups compared to that for the control cells, which may be because L. plantarum KLDS 1.0628 had the ability to regulate the elasticity, fluidity and flexibility of its cell membrane to resist various environmental stresses (Wang et al., Citation2018). Before evaluation of their functional properties in a complex food matrix or host with a harsh environment, it is crucial to investigate the stress responses of L. plantarum in less complex laboratory media with limited interfering factors. Some reports have shown the physiological response of L. plantarum to stress (Aoudia et al., Citation2016; Hlaing et al., Citation2018). Moreover, exposure to environmental stresses may alter the physiological characteristics of L. plantarum. These results further demonstrated the possible existence of stress response mechanisms regulated by the distribution of SFAs and USFAs in cell membranes.
4. Conclusions
The present study suggested that pre-adaptation to sublethal levels of heat, oxidative, acid, bile salt, and osmotic stresses enhances the heat tolerance of L. plantarum KLDS 1.0628. Furthermore, different types of adaptation could induce cross-protection of L. plantarum KLDS 1.0628 against homologous and heterologous challenges. Exposure to these stresses significantly decreased the SFA/USFA ratio in the cell membrane of L. plantarum KLDS 1.0628. The above results indicated that pre-adaptation strategies have the potential to enhance the stress tolerance of L. plantarum KLDS 1.0628 against multiple stresses. Further research is needed to clarify the molecular mechanism of the adaptation response and cross-protection of L. plantarum KLDS 1.0628 to various stresses.
Disclosure statement
The authors declare no conflict of interests.
Additional information
Funding
References
- Aoudia, N., Rieu, A., Briandet, R., Deschamps, J., Chluba, J., Jego, G., Garrido, C., & Guzzo, J. (2016). Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: Effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiology, 53(Pt A), 51–59. https://doi.org/https://doi.org/10.1016/j.fm.2015.04.009
- Bi, J., Liu, S., Du, G., & Chen, J. (2016). Bile salt tolerance of Lactococcus lactis is enhanced by expression of bile salt hydrolase thereby producing less bile acid in the cells. Biotechnology Letters, 38(4), 659–665. https://doi.org/https://doi.org/10.1007/s10529-015-2018-7
- Bustos, A. Y., de Valdez, G., Fadda, S., & Taranto, M. P. (2018). New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Research International, 112, 250–262. https://doi.org/https://doi.org/10.1016/j.foodres.2018.06.035
- Cao, P., Wu, L., Wu, Z., Pan, D., Zeng, X., Guo, Y., & Lian, L. (2019). Effects of oligosaccharides on the fermentation properties of Lactobacillus plantarum. Journal of Dairy Science, 102(4), 2863–2872. https://doi.org/https://doi.org/10.3168/jds.2018-15410
- Chen, M.-J., Tang, H.-Y., & Chiang, M.-L. (2017). Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiology, 66, 20–27. https://doi.org/https://doi.org/10.1016/j.fm.2017.03.020
- Cotter, P. D., Hill, C., & Ross, R. P. (2005). Bacteriocins: Developing innate immunity for food. Nature Reviews. Microbiology, 3(10), 777–788. https://doi.org/https://doi.org/10.1038/nrmicro1273
- Du, H., Yang, J., Lu, X., Lu, Z., Bie, X., Zhao, H., Lu, X., Lu, Z., Zhang, C., & Lu, F. (2018). Purification, characterization, and mode of action of plantaricin GZ1-27, a novel Bacteriocin against Bacillus cereus. Journal of Agricultural and Food Chemistry, 66(18), 4716–4724. https://doi.org/https://doi.org/10.1021/acs.jafc.8b01124
- Gandhi, A., & Shah, N. P. (2015). Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microbiology, 49, 197–202. https://doi.org/https://doi.org/10.1016/j.fm.2015.02.003
- Haddaji, N., Mahdhi, A. K., Ismaiil, M. B., & Bakhrouf, A. (2017). Effect of environmental stress on cell surface and membrane fatty acids of Lactobacillus plantarum. Archives of Microbiology, 199(9), 1243–1250. https://doi.org/https://doi.org/10.1007/s00203-017-1395-9
- Hlaing, M. M., Wood, B. R., McNaughton, D., Rood, J. I., Fox, E. M., & Augustin, M. A. (2018). Vibrational spectroscopy combined with transcriptomic analysis for investigation of bacterial responses towards acid stress. Applied Microbiology and Biotechnology, 102(1), 333–343. https://doi.org/https://doi.org/10.1007/s00253-017-8561-5
- Huang, R., Pan, M., Wan, C., Shah, N. P., Tao, X., & Wei, H. (2016). Physiological and transcriptional responses and cross protection of Lactobacillus plantarum ZDY2013 under acid stress. Journal of Dairy Science, 99(2), 1002–1010. https://doi.org/https://doi.org/10.3168/jds.2015-9993
- Kang, C.-H., Jeon, H., Shin, Y., Kwon, Y.-J., & So, J.-S. (2015). Heat adaptation improves viability of Lactococcus lactis subsp. lactis HE-1 after heat stress. Food Science and Biotechnology, 24(5), 1823–1827. https://doi.org/https://doi.org/10.1007/s10068-015-0238-1
- Kaur, G., Ali, S. A., Kumar, S., Mohanty, A. K., & Behare, P. (2017). Label-free quantitative proteomic analysis of Lactobacillus fermentum NCDC 400 during bile salt exposure. Journal of Proteomics, 167, 36–45. https://doi.org/https://doi.org/10.1016/j.jprot.2017.08.008
- Kulkarni, S., Haq, S. F., Samant, S., & Sukumaran, S. (2018). Adaptation of Lactobacillus acidophilus to thermal stress yields a thermotolerant variant which also exhibits improved survival at pH 2. Probiotics and Antimicrobial Proteins, 10(4), 717–727. https://doi.org/https://doi.org/10.1007/s12602-017-9321-7
- Li, M., Wang, Q., Song, X., Guo, J., Wu, J., & Wu, R. (2019). iTRAQ-based proteomic analysis of responses of Lactobacillus plantarum FS5-5 to salt tolerance. Annals of Microbiology, 69(4), 377–394. https://doi.org/https://doi.org/10.1007/s13213-018-1425-0
- Liu, S., Ma, Y., Zheng, Y., Zhao, W., Zhao, X., Luo, T., Zhang, J., & Yang, Z. (2020). Cold-stress response of probiotic Lactobacillus plantarum K25 by iTRAQ proteomic analysis. Journal of Microbiology and Biotechnology, 30(2), 187–195. https://doi.org/https://doi.org/10.4014/jmb.1909.09021
- Ma, J., Wang, W., Sun, C., Gu, L., Liu, Z., Yu, W., Chen, L., Jiang, Z., & Hou, J. (2020). Effects of environmental stresses on the physiological characteristics, adhesion ability and pathogen adhesion inhibition of Lactobacillus plantarum KLDS 1.0328. Process Biochemistry, 92, 426–436. https://doi.org/https://doi.org/10.1016/j.procbio.2020.02.001
- Ma, J., Xu, C., Yu, H., Feng, Z., Yu, W., Gu, L., Liua, Z., Chen, L., Jiang, Z., & Hou, J. (2020). Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology. Food Hydrocolloids, 111, 106381. https://doi.org/https://doi.org/10.1016/j.foodhyd.2020.106381
- Ma, J., Yu, W., Hou, J., Han, X., Shao, H., & Liu, Y. (2020). Characterization and production optimization of a broad-spectrum bacteriocin produced by Lactobacillus casei KLDS 1.0338 and its application in soybean milk biopreservation. International Journal of Food Properties, 23(1), 677–692. https://doi.org/https://doi.org/10.1080/10942912.2020.1751656
- Ma, X., Wang, G., Zhai, Z., Zhou, P., & Hao, Y. (2018). Global transcriptomic analysis and function identification of malolactic enzyme pathway of Lactobacillus paracasei L9 in response to bile stress. Frontiers in Microbiology, 9, 1978. https://doi.org/https://doi.org/10.3389/fmicb.2018.01978
- Marcial-Coba, M. S., Cieplak, T., Cahu, T. B., Blennow, A., Knochel, S., & Nielsen, D. S. (2018). Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food & Function, 9(11), 5868–5879. https://doi.org/https://doi.org/10.1039/c8fo01331d
- Noriega, L., Gueimonde, M., Sanchez, B., Margolles, A., & de Los Reyes-gavilan, C. G. (2004). Effect of the adaptation to high bile salts concentrations on glycosidic activity, survival at low PH and cross-resistance to bile salts in Bifidobacterium. International Journal of Food Microbiology, 94(1), 79–86. https://doi.org/https://doi.org/10.1016/j.ijfoodmicro.2004.01.003
- Papadimitriou, K., Alegria, A., Bron, P. A., de Angelis, M., Gobbetti, M., Kleerebezem, M., … Kok, J. (2016). Stress physiology of lactic acid bacteria. Microbiology and Molecular Biology Reviews, 80(3), 837–890. https://doi.org/https://doi.org/10.1128/MMBR.00076-15
- Papadopoulou, O. S., Argyri, A. A., Varzakis, E. E., Tassou, C. C., & Chorianopoulos, N. G. (2018). Greek functional Feta cheese: Enhancing quality and safety using a Lactobacillus plantarum strain with probiotic potential. Food Microbiology, 74, 21–33. https://doi.org/https://doi.org/10.1016/j.fm.2018.02.005
- Ricciardi, A., Parente, E., Guidone, A., Ianniello, R. G., Zotta, T., Abu Sayem, S. M., & Varcamonti, M. (2012). Genotypic diversity of stress response in Lactobacillus plantarum, Lactobacillus paraplantarum and Lactobacillus pentosus. International Journal of Food Microbiology, 157(2), 278–285. https://doi.org/https://doi.org/10.1016/j.ijfoodmicro.2012.05.018
- Salar-Behzadi, S., Wu, S., Toegel, S., Hofrichter, M., Altenburger, I., Unger, F. M., Wirth, M., & Viernstein, H. (2013). Impact of heat treatment and spray drying on cellular properties and culturability of Bifidobacterium bifidum BB-12. Food Research International, 54(1), 93–101. https://doi.org/https://doi.org/10.1016/j.foodres.2013.05.024
- Sottile, M. L., & Nadin, S. B. (2018). Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress and Chaperones, 23(3), 303–315. https://doi.org/https://doi.org/10.1007/s12192-017-0843-4
- Streit, F., Delettre, J., Corrieu, G., & Beal, C. (2008). Acid adaptation of Lactobacillus delbrueckii subsp. bulgaricus induces physiological responses at membrane and cytosolic levels that improves cryotolerance. Journal of Applied Microbiology, 105(4), 1071–1080. https://doi.org/https://doi.org/10.1111/j.1365-2672.2008.03848.x
- Wang, P., Wu, Z., Wu, J., Pan, D., Zeng, X., & Cheng, K. (2016). Effects of salt stress on carbohydrate metabolism of Lactobacillus plantarum ATCC 14917. Current Microbiology, 73(4), 491–497. https://doi.org/https://doi.org/10.1007/s00284-016-1087-8
- Wang, W., He, J., Pan, D., Wu, Z., Guo, Y., Zeng, X., Lian, L., & Nychas, G.-J. (2018). Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS One, 13(5), e0196231. https://doi.org/https://doi.org/10.1371/journal.pone.0196231
- Wu, C., Zhang, J., Wang, M., Du, G., & Chen, J. (2012). Lactobacillus casei combats acid stress by maintaining cell membrane functionality. Journal of Industrial Microbiology & Biotechnology, 39(7), 1031–1039. https://doi.org/https://doi.org/10.1007/s10295-012-1104-2
- Zhang, C., Lu, J., Yang, D., Chen, X., Huang, Y., & Gu, R. (2018). Stress influenced the aerotolerance of Lactobacillus rhamnosus hsryfm 1301. Biotechnology Letters, 40(4), 729–735. https://doi.org/https://doi.org/10.1007/s10529-018-2523-6
- Zhang, H., Wang, Q., Liu, H., Kong, B., & Chen, Q. (2020). In vitro growth performance, antioxidant activity and cell surface physiological characteristics of Pediococcus pentosaceus R1 and Lactobacillus fermentum R6 stressed at different NaCl concentrations. Food & Function, 11(7), 6376–6386. https://doi.org/https://doi.org/10.1039/c9fo02309g
- Zhao, J., Tian, F., Yan, S., Zhai, Q., Zhang, H., & Chen, W. (2018). Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose-induced aging mice. Food & Function, 9(2), 917–924. https://doi.org/https://doi.org/10.1039/c7fo01574g