Figures & data
Table 1 Reaction conditions and their levels studied by the response surface methodology
Figure 1 ACE-inhibitory activity and degree of hydrolysis (DH) of casein hydrolysate prepared over a hydrolysis period of 8 h. The column chart is for ACE-inhibitory activity and the graph chart is for DH. The final peptide concentration used in the assay system was fixed at 50 μg/mL. Different capital letters above the columns (or below the graph) indicate that one-way ANOVA of the data is significantly different (P < 0.01). (Color figure available online.)
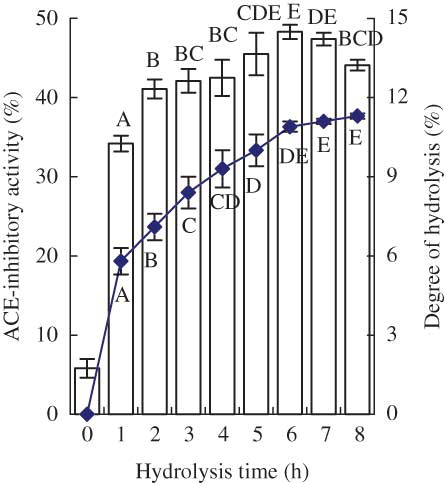
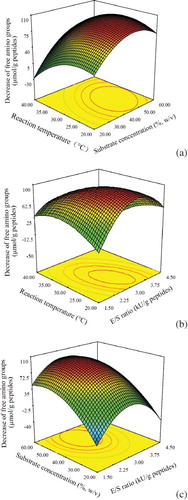
Figure 2 Response surface graphs for the decreased amount of free amino groups of the modified casein hydrolysate as a function of: (a) substrate concentrate and reaction temperature (E/S ratio at the central of its level), (b) E/S ratio and reaction temperature (substrate concentrate at the central of its level), and (c) substrate concentrate and E/S ratio (reaction temperature at the central of its level). (Color figure available online.)