ABSTRACT
The increasing prevalence of overweight and obesity requires new, effective prevention and treatment strategies. One approach to reduce energy intake is by developing novel foods with increased satiating properties, which may be accomplished by slowing down lipolysis to deliver substrates to the ileum, thereby enhancing natural gut-brain signaling pathways of satiety that are normally induced by meal intake.
To develop slow release food additives, their processing in the gastrointestinal tract has to be understood; therefore, we start from a general description of the digestive system and relate that to in vitro modeling, satiety, and lipolytic mechanisms. The effects of physicochemical lipid composition, encapsulation matrix, and interfacial structure on lipolysis are emphasized. We give an overview of techniques and materials used, and discuss partitioning, which may be a key factor for encapsulation performance.
Targeted release capsules that delay lipolysis form a real challenge because of the high efficiency of the digestive system; hardly any proof was found that intact orally ingested lipids can be released in the ileum and thereby induce satiety. We expect that this challenge could be tackled with structured o/w-emulsion-based systems that have some protection against lipase, e.g., by hindering bile salt adsorption and/or delaying lipase diffusion.
1. Introduction
Since 1980, worldwide obesity prevalence has nearly doubled according to World Health Organization statistics (WHO, Citation2014). Two thirds of the world's population live in countries where overweight and obesity results in higher morbidity and mortality compared to being underweight. The increasing number of overweight and obese people indicates that energy intake and energy expenditure are not balanced. Interestingly, the WHO key facts also state that “obesity is preventable.” The intake of energy could be reduced by making products more satiating, and one approach to achieve this is to slow down digestion, which results in the presence of intact nutrients in more distal parts of the gastrointestinal tract. This in turn can activate the so-called ileal brake, which induces satiety via hormonal and neural signaling routes and thereby reduces the food intake (Maljaars et al., Citation2008a; van Avesaat et al., Citation2015). When dietary triacylglycerols reach the ileum without being digested, they are able to activate this natural feedback mechanism to induce satiety. However, it should be taken into account that it is not easy to achieve delivery of intact orally ingested nutrients to more distal parts of the GI tract due to the high efficiency of the digestive processes. Hence, studies to the ileal brake principle in vivo in humans are at present limited mainly to complex intubation studies.
The present review focuses on how to control lipolysis under physiological conditions. Dietary fat digestion is determined by the physicochemical composition of the lipid and the interfacial area between lipid and the surrounding environment. Encapsulation of the lipid material is expected to help controlling lipolysis, hence this review focuses on encapsulates, their interfacial structure, and composition in relation to digestion.
Over the past century, encapsulation systems that can protect certain ingredients (e.g., antioxidants, colorants) during storage were designed to improve the products' shelf-life (Gibbs et al., Citation1999). Over the last decades, the application of food encapsulation systems has shifted towards controlled delivery in the human digestion system, for example by providing protection against the gastric environment for vitamins, or delivering drugs or probiotics to the colon. Thus, beyond stability during storage, the capsule must provide appropriate stability against the disruptive conditions in the human body.
In the medical field, numerous encapsulation systems are available for controlled delivery of drugs via oral administration, or to improve their bioavailability (Lam and Gambari, Citation2014). However, the materials and techniques used to produce such encapsulates in pharmacy are often not suitable for food applications, as they are not food-grade. Besides, they are often too expensive to be used as food ingredients. Yet, the knowledge on technologies and materials from the medical field is a very sound basis to design food-grade systems that need comparable properties.
In depth understanding of the physiological conditions and digestion processes is required to design encapsulation systems for delayed lipolysis in the human GI tract. Reversed engineering can be used to obtain optimal functionality, which focuses on the application, rather than looking for an application after designing an encapsulation system from a technological point of view (Cerqueira et al., Citation2013). A smart design is crucial for success, because the capsule faces different harsh disruptive environments in the GI tract, as the digestion system has evolved over millions of years towards efficient food processing. In order to achieve controlled release of micro-encapsulated ingredients in the GI tract, these digestive factors—such as pH, electrolyte balance, bile salt composition, temperature, and mechanical stress—have to be counteracted when the capsule needs to be stable or can be used as trigger for release. According to Benshitrit et al., the future challenges in developing oral food-grade delivery systems are (i) the structure–function relationships, particularly the fate of delivery systems, (ii) the sensory properties, and (iii) the human variance and complexity of the digestive process (Benshitrit et al., Citation2012).
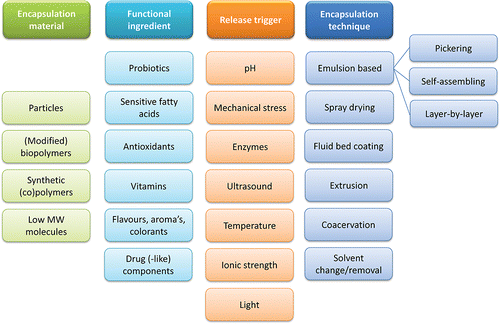
The present review aims at combining physiology and technology insights to highlight the important parameters that should be considered when developing micro-encapsulates for delayed lipolysis in digestive conditions. Relevant physiological and technological insights are first discussed, before we discuss in chapter 6 the available research on ways to delay lipolysis and hence increase feelings of satiety. The physiological conditions in the different parts of the human GI tract will first be described together with existing in vitro models, satiety and digestion of lipids. Next, available micro-encapsulation methods are described, as well as suitable materials for lipid encapsulation, with the focus on food-grade options. The dynamic partitioning of ingredients among the available phases of micro-encapsulation systems will be discussed, since it may strongly influence the performance of encapsulates. A summary table of common encapsulation materials, encapsulated ingredients, release triggers and encapsulation methods/techniques is presented in , and will be discussed into greater detail in the review.
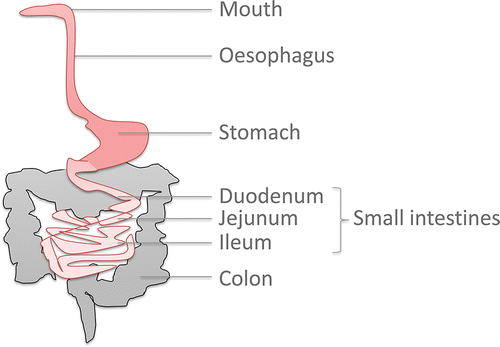
2. The human digestive system
McConnell and co-workers concluded, after critically reviewing the intestinal physiology parameters, that there is no such a thing as an “average person” (McConnell et al., Citation2008). However, gastrointestinal food processing occurs through some general principles (; for dimensions see ). Below we first discuss the physiological conditions in the different compartments, followed by in vitro modeling.
Table 1. Compartments of the human digestive tract and their main characteristics. Adapted from Kenmogne-Domguia (2012), with permission of H. B. Kenmogne-Domguia.
2.1. Mouth and oesophagus
Consumed food will be partially masticated by the teeth, and mixed with saliva in the oral cavity. Saliva contains electrolytes, carbohydrate degrading enzymes (amylase), and biopolymers (e.g., mucin). Mucins are mucosal glycoproteins (polypeptide backbone, with oligosaccharide side chains), present at concentrations of about 30–500 μg/mL saliva (Vingerhoeds et al., Citation2005). Although their solubility in water is limited, the oligosaccharide chains of mucins get readily hydrated and have high water binding capacity, which increases the viscosity and elasticity of saliva, resulting in lubrication of the food bolus (Minekus et al., Citation2014). Mucins can cause aggregation of most food emulsions; the destabilization mechanism depends on the charge of the emulsion droplet. Weakly negatively charged to neutral emulsions most likely undergo depletion flocculation by mucins, while positively charged emulsions show bridging flocculation (van Aken et al., Citation2007). Solid food also undergoes chewing (mastication), which reduces the size of food particles. The food components can interact with the tongue and mouth surface, and the flow in the mouth can be complex, especially for liquid foods.
After swallowing, the formed bolus enters in the oesophagus, with a pH of about 5–6 in the lumen. The bolus is transported through this muscular 25 cm long tube with a diameter approximately 2 cm, by swallowing in a single peristaltic wave of contraction, towards the stomach.
2.2. Stomach
In the stomach, food processing is continued by the action of enzymes and hydrochloric acid. In order to protect the cells of the stomach wall against the destructive lumen content, viscous mucus is secreted by goblet cells that remains on top of the gastric cells where the mucus forms a firmly adherent gel layer, onto which also a looser mucus layer resides (Atuma et al., Citation2001). The stomach is in general acidic due to the secretion of hydrochloric acid by parietal cells, but varies greatly between individuals and within a person over time. Intra-individual variations in stomach acidity is caused by several factors such as dietary intake, seasonal variations, composition of the previous meal, feeding status, health status, age, etc. In general, the stomach in the fasted state—where no food is present —is more acidic than in fed state, but various absolute pH values have been reported. Also the health status can affect this pH. It has been shown that, for example, obese people had a much lower pH than non-obese subjects (1.7 versus 3.7) in fasted state (Vaughan et al., Citation1973).
The presence of nutrients in the stomach induces the release of gastrin by the G-cells in the stomach wall. Gastrin is a hormone that stimulates the production of acid and enzymes, and affects the motility of the stomach. Stomach epithelium produces and secretes several digestive enzymes (Moreau et al., Citation1988); chief cells secrete pepsinogen that is the precursor of pepsin, which breaks down proteins and is active at pH values up to 5, above which it is inactivated. Chief cells can also produce gastric lipase, a lipolytic enzyme that is stable and active at acidic pH (Bakala N'Goma et al., Citation2012).
The stomach generates peristaltic contractions towards the end of the stomach (pylorus), which induces intra-gastric flow, with an average speed of 1.5–3 mm/s for the antral contraction waves, with a frequency of 2.6–3 waves per minute (Bornhorst and Singh, Citation2014). Although there is no consensus about the exact effect of meal composition and the interaction between consumer and food properties, the flow patterns have been shown to depend on certain food properties such as viscosity, density and particle size (Bornhorst and Singh, Citation2014). The overall direction of flow of liquid materials is opposite to the direction of peristalsis and the shear rate profile is described to be hardly affected by the viscosity of liquid phases (Kozu et al., Citation2014). Solid meals require more than just mixing with gastric juice, they have to be broken before leaving the stomach, so antral grinding is an important process (Marciani et al., Citation2001). Softer materials have been shown by Marciani et al. to be broken more rapid and to empty faster than harder materials, like liquids.
In addition to mixing, the stomach functions as a compartment to temporarily store ingested food with a variable residence time [between 5 min (McConnell et al., Citation2008) and 5 h (Lesmes and McClements, Citation2009)]. The gastric emptying rate determines the residence time in the gastric fluid, and controls the speed at which the chyme enters the intestines; fluids and small particles have short residence times, while bigger particles are kept longer to ensure better digestion. The gastric emptying rate directly relates to the caloric content (Calbet and MacLean, Citation1997), and is also dependent on meal composition: for instance fried pasta delayed the emptying as compared to boiled pasta, and a brown rice meal lead to slower emptying than white rice (Bornhorst and Singh, Citation2014). In addition, the order of nutrient ingestion can affect the emptying rate, it being faster when nonfat components are served before fat components (Kunz et al., Citation2005). Besides the texture and composition of the food, the emptying rate is also regulated via several feedback mechanisms (van Aken, Citation2010), for example the presence of residual nutrients in the intestines from the previous meal slows it down, and the gut hormone levels of cholecystokinin (CCK), peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) can also modulate it. Olsson & Holmgren stated that “almost everything seems to affect gastric emptying” (Olsson and Holmgren, Citation2001). (Olsson and Holmgren, Citation2001).
2.3. Small intestine
The small intestine can be considered as a 6–7 meter long tube of which the inside is called the lumen (diameter about 2.5 cm). It can be divided into three zones; the most proximal part is called duodenum, the middle region the jejunum and most distal part the ileum. The stomach content (chyme) is slowly emptied into the lumen of the duodenum, and consists then of a blend of partly digested food and secreted digestion fluid. The overall residence time in the small intestine is 0.5 to 9.5 h, depending on the nutrient composition, and on the physical and chemical structure of the chyme (Coupe et al., Citation1991); the variation within individual subjects was even significant when repetitively providing the same meal.
During the duodenal phase, s secretions are added from the pancreas, gallbladder, and intestinal wall. The pancreas secretes a range of digestive enzymes, including proteases (trypsin and chymotrypsin), esterase, lipase, phospholipase, and amylase. Most of these enzymes are secreted as inactive pro-enzymes, and are activated after being secreted into the lumen of the small intestine (van Aken, Citation2010). The gallbladder secretes bicarbonate and bile. Bicarbonate neutralizes the acidic chyme to a pH of about 5.5 in the duodenum, and to about 7.5 when reaching the ileum. Large variations in pH profiles between individuals have been measured, and also within people under the same feeding conditions (Ibekwe et al., Citation2008). Bile contains bile salts and phospholipids, which are essential for the digestion and absorption of components with low water-solubility; below their role is described in more detail.
The intestinal wall is structured with villi, finger-shaped outgrowths, to provide a large surface area containing the epithelial cells that absorb the digestion products into the blood stream. The epithelial cells, or enterocytes, form a single cell layer covering the small intestine. The enterocytes express microvilli, small protrusions into the intestinal lumen. They increase the cells surface area in the lumen and form the brush border, or glycocalyx, that composes of glycoproteins and digestive enzymes. The villi form the innermost mucosa layer, which is positioned onto the submucosa that contains blood vessels, lymphatic vessels and nerves, that all work together to allow for uptake of the digestion products.
Peristaltic movements are generated by circular and longitudinal muscle layers that surround the mucosa—submucosa. As a result of this peristalsis, the food and digestive components are mixed and moved through the small intestine. In between the epithelial cells, some enteroendocrine and goblet cells are present. Enteroendocrine cells release peptide hormones based on the present nutrients during and after a meal, including cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide YY (PYY) which are described into more detail in section 3.1. Goblet cells secrete a mucous layer that protects the epithelia. Mucus consists mainly of water, with some salts, proteins and the glycoprotein mucin. In contrast to the stomach, only a thin mucus layer is firmly adherent here, and also the loosely adhered layer on top is thinner (Atuma et al., Citation2001). This allows permeation of nutrients and facilitates absorption (van Aken, Citation2010). Food polymers can attach to the mucosa on the intestinal wall, called mucoadhesion, when they are capable of taking up water from the mucus via hydration. The adhesion strength has been shown to depend on the properties of the polymer, such as molecular composition and the actual pH (Grabovac et al., Citation2005).
2.4. Colon
After the ileum, the food reaches the colon, which is approximately 1.5 meter long, and also called the large intestine because of its larger diameter of about 7.5 cm. The main function of the colon is to allow microbial fermentation of unabsorbed nutrients. Accordingly, one gram of intestinal content will hold about 1011–1012 anaerobic microorganisms (Simon and Gorbach, Citation1986). A thick mucus layer protects the mucosal cells of the colon wall against the lumen content. This layer consists of 120 μm thick, firmly adherent mucus to the wall, and about 700 μm thick loosely adhered mucus on top (Atuma et al., Citation2001). Peristaltic contractions and segmentation induce movement through the colon, and finally excretion as feces. The colon does not play an important role in lipolysis. It is worth mentioning that targeted delivery in the colon has been reported, for example of probiotics (Dong et al., Citation2013), which requires the use of encapsulation systems that resist digestion and absorption in earlier stages (Situ et al., Citation2014).
2.5. In vitro modeling
In model systems, theoretically it would be possible to reproduce all conditions involved in human digestion; however, their relative effects and importance are not completely unraveled yet. Moreover, in practice it is not possible to take all factors into account in one model. Therefore, simplified models are used that only include those factors that are assumed to have a major influence on the investigated system.
It should be pointed out that a broad range of in vitro digestion models have been used, with just one factor being the same over all studies: a temperature of 37°C. Due to these nonharmonized in vitro digestion models, the comparability across research teams was not assured (Porter and Charman, Citation2001; McClements and Li, Citation2010a; Hur et al., Citation2011; Li et al., Citation2011; Marze et al., Citation2012). Recently, Minekus et al. published an international consensus on a standardized method for static in vitro digestion for food (Minekus et al., Citation2014). It is a promising initiative since the article gives detailed guidelines for the protocol, such as the concentrations of the stock solutions and the dilution factor to be used, and the authors are recognized scientists in the field. But, the described method is a static model, so does not include the mechanical forces and dynamic processes in the human body.
In vitro model conditions that can be used to represent these above described compartments are concurrently presented and discussed below.
Oral phase
For liquid meals, the in vitro digestion mostly excludes the oral and esophagus steps, but when starch is present a simulated salivary fluid (SSF) is sometimes used. Conversely, for solid meals the oral phase cannot be excluded from the in vitro digestion model, and in addition to the SSF, it needs to induce a mincing step. Commercial mincers are available, that mimic the mechanical forces of chewing. The saliva simulation includes two minutes mixing liquid food with a volume ratio of 1:1 to SFF (pH of 7, fixed electrolyte concentrations (18.8 mmol/L K+; 13.6 mmol/L Na+; 19.5 mmol/L Cl−; 3.7 mmol/L H2PO4−; 13.7 mmol/L HCO3−,CO32-; 0.15 mmol/L Mg2+; 0.12 mmol/L NH4+; 1.5 mmol/L Ca2+), amylase (75 U/mL) (Minekus et al., Citation2014). The effect of mucin is hard to mimic in vitro, due to large variations between and within individuals (Vingerhoeds et al., Citation2005); therefore, Minekus et al. Citation(2014) also advice not to use it in standardized in vitro digestion.
Gastric phase
As described in the paragraph 2.2, the gastric digestion is a complex and variable system, which is hard to study and, therefore, to model (Bornhorst and Singh, Citation2014). Hence, it is often unclear which factors have to be included. In the widely used static models, the gastric motility, gastric secretions over time and gastric emptying rate are not included, and the sample is simply incubated in a stirred simulated gastric fluid (SGF) at 37°C, generally for two hours). The advised composition includes a pH of 3, pepsin (2,000 U/mL), fixed electrolyte concentrations (7.8 mmol/L K+; 72.2 mmol/L Na+; 70.2 mmol/L Cl−; 0.9 mmol/L H2PO4−; 25.5 mmol/L HCO3−,CO32-; 0.1 mmol/L Mg2+; 1.0 mmol/L NH4+; 0.15 mmol/L Ca2+) and in case the sample does not contain low molecular weight surfactants also 0.17 mM of the phospholipid egg lecithin should be included (Minekus et al., Citation2014).
Gastric lipolysis has mostly been omitted in in vitro digestion so far, due to the absence of commercial gastric lipase substitute for acceptable costs and besides, the experts are not fully convinced about the exact role of human gastric lipase (Bakala N'Goma et al., Citation2012; Minekus et al., Citation2014). However, the use of human gastric fluid for in vitro digestion showed greater lipid hydrolysis than in a simulated fluid with artificial enzymes, although the proteolytic and lipolytic activity was at the same level (Malinauskytė et al., Citation2014). The translation of results obtained in model systems to physiological conditions may be hampered because of this.
Obviously, the most relevant processes need to be included in model systems; however this is not as straightforward as it may sound. In static models, the impact of mechanical force, liquid flow, shear stress, dilutions by gastric secretions over time and gastric emptying is not taken into account. For some research questions this is not problematic, but for others it cannot be neglected. Especially when structural aspects of the solid food matrixes are involved, it is crucial to include the dynamics in the model. Gastric motility has been studied by techniques such as Magnetic Resonance Imaging (Kunz et al., Citation2005), manometry and with the use of wireless capsules, and based on that, model systems were build. Examples of dynamic gastric in vitro models are the TNO Gastro-Intestinal Model (TIM-1) (Minekus, Citation2015), the “Dynamic Gastric Model” (Wickham et al., Citation2012), the “Human Gastric Simulator” (Kong and Singh, Citation2010), and the U-stomacher from our own research group (Luo et al., Citation2015).
Small intestine
In order to mimic the digestion in the small intestine in vitro, simulated small intestinal fluid (SSIF) must be prepared with, at least, a neutral pH, relevant enzymes (i.e., proteases for protein hydrolysis and lipase for lipolysis), biological surface-active components (bile salts and phospholipids), and a proper concentration of calcium and other minerals (7.6 mmol/L K+; 123.4 mmol/L Na+; 55.5 mmol/L Cl−; 0.8 mmol/L H2PO4−; 85 mmol/L HCO3−,CO32-; 0.33 mmol/L Mg2+; 0.6 mmol/L Ca2+) (Minekus et al., Citation2014). It is important but difficult to get the composition of the SSIF right, since the composition of human digestive juice is complex, dynamic and fluctuating, as previously explained (McConnell et al., Citation2008), and it can greatly influence the outcome of simulated digestion experiments (Li et al., Citation2011). Static intestinal models are used mostly, since it is challenging to include the dynamics of intestinal digestion processes in vitro. However, some dynamic models are available, such as the TIM-1 system (TNO, The Netherlands).
Colon
The main role of the colon is to allow bacterial fermentation, hence related in vitro models focus on reproducing the action of the involved microbiota. To investigate ingredient release in the colon using in vitro models, the microbes of the whole gastro-intestinal tract must be taken into account, in addition to all other above-described factors. Example thereof are the TIM-2 system (TNO, The Netherlands) and the Simulator of Human Intestinal Microbial Ecosystem (SHIME), which contains a microbial spectrum (de Boever et al., Citation2000; Molly et al., Citation1994). Such models can be used to investigate the degradation pattern of a food matrix, while also taking the microbial ecology into account, instead of just bacteria enumeration. Specifically for the release of probiotic bacteria, it should also be studied whether the cells are still viable when reaching the colon (Cook et al., Citation2012).
3. Satiety
To define two situations of inhibition of food intake, the terms “satiation” and “satiety” are introduced: satiation denotes the inhibitory processes that start during meal intake and cause people to bring an eating episode to an end; satiety denotes the postmeal inhibitory processes that supress the motivation to eat until the next eating episode (Blundell and Bellisle, Citation2013).
Feelings of hunger and satiety experienced by a person fluctuate over the day, and depend on factors such as earlier meal volume and composition, time since the last meal, energy expenditure and so on. Dietary components are sensed throughout the GI tract, resulting in signaling to regulate hunger, satiety and food intake. Maljaars et al. reviewed the regulation mechanisms of such signaling, which origins in the stomach and small intestine and include humoral and neural pathways (Maljaars et al., Citation2007). The involved mediators include CCK, ghrelin, PYY, GLP-1 and oxyntomodulin (OXM). CCK is a hormone that apart from stimulating gallbladder contraction and pancreatic enzyme secretion, also induces satiety and thereby reduces the food intake (Gibbs and Smith, Citation1982). It is released by intestinal I-cells primarily in the proximal small intestine, when nutrients are present in the duodenum and jejunum. GLP-1 is a peptide that also inhibits food intake, and acts via receptors in the hypothalamus (Turton et al., Citation1996). It is mainly released from entero-endocrine L-cells in the distal intestine, as is the case for PYY. Batterham and co-workers reported that infusing PYY at the concentration that is normal after a meal (0.8 pmol per kg per min for 90 min) reduced the energy intake in the 24 h after the infusion by 33%, compared to a control infusion with saline (Batterham et al., Citation2002). After a meal, the plasma concentrations of PYY are dependent on the caloric meal composition (Pedersen-Bjergaard et al., Citation1996). Oxyntomodulin (OXM) is co-secreted with PYY and GLP-1 by L-cells in the distal intestine and colon, and reduces gastrointestinal motility, induces satiety and decreases food intake (Maljaars et al., Citation2008a). In contrast to these satiety-inducing gut peptides, the hormone ghrelin stimulates appetite and food intake (Inui et al., Citation2004). Ghrelin is mainly secreted in the stomach by special gastric cells, also called X/A like cells.
3.1. Satiety signals induced by undigested nutrients in the small intestine
Transposing a small segment of distal ileum to the jejunum lowered the food consumption of rats, and caused more weight loss than in the control rats (Strader et al., Citation2005). This was associated with a higher release of GLP-1 and PYY in the test rats, and not due to malabsorption, indicating that the distal part of the small intestine is more responsive to nutrients than the more proximal parts, with respect to induction of satiety signals. The presence of unabsorbed nutrients in the ileum is thought to be able to induce satiety, via the so-called ileal brake and thereby reduce food intake (Maljaars et al., Citation2008a). Several human intubation studies have been done to investigate this mechanism (Maljaars et al., Citation2008b, Citation2009, Citation2011; van Avesaat et al., Citation2015). In 2008, Maljaars et al. investigated the effect of infusion of fat (low or high dose, respectively 3 g and 9 g emulsified safflower oil) into the ileum on satiety as compared to a control treatment (oral fat and a saline infusion). The authors found the PYY secretion to be only increased with high fat perfusion in the ileum and not with the lower dose, and CCK was secreted dose-dependent correlating with the levels of satiety (Maljaars et al., Citation2008b). After that, Maljaars and co-workers investigated the effect of ileal perfusion of different oil types—including shea oil (mainly stearic acid, C18:0), canola oil (mainly oleic acid, C18:1), and safflower oil (mainly linoleic acid, C18:2)- on satiety and food intake, as compared to a saline infusion (Maljaars et al., Citation2009). No direct effect on food intake was observed and PYY was not affected; however, the authors did find significantly increased satiety feelings, reduced hunger and increased CCK secretion by C18:2 and C18:1 fatty acids (FAs) compared to the control and compared to shea oil (Maljaars et al., Citation2009). Van Avesaat et al. (van Avesaat et al., Citation2015) recently reported that protein and carbohydrate infusion in the ileum can also reduce the energy intake, compared to the control treatment (saline infusion). The CCK and PYY secretion was increased for all types of macronutrients, the gastric emptying rate and intestinal transit time seemed to be delayed, but GLP-1 secretion was not affected (van Avesaat et al., Citation2015). Thus, the ileal brake can also be activated by other macronutrients than lipids.
The intestinal area exposed to fat may also influence the feelings of satiety. Maljaars et al. investigated this by infusing 2 g of fat each in the duodenum, jejunum and ileum, or 6 g of fat into the ileum, and measuring the effect on satiety compared to a control treatment (6 g fat in liquid meal and saline infusion) (Maljaars et al., Citation2011). None of the treatments resulted in differences in the amount of secreted PYY, CCK nor GLP-1 compared to the control treatment, but the feelings of hunger were reduced by infusion of 6 g fat simultaneously in the three segments (2 g per segment) or in the ileum. The ileal infusion also reduced food intake compared to oral administration of a liquid meal containing 6 g of fat, so the duration and site of exposure influences hunger and food intake (Maljaars et al., Citation2011). Besides, also duodenal infusion of glucose (Lavin et al., Citation1998; Pilichiewicz et al., Citation2007) or protein (Geraedts et al., Citation2011; Ryan et al., Citation2012) decreased energy intake when compared to placebo and increased plasma levels of satiety inducing hormones GLP-1 and, for protein infusion, CCK.
4. Lipolysis
As mentioned in the previous sections, fats and oil play an important role in our metabolism, and may influence satiety to a large extent. In this section we focus on the process of lipolysis, and we will discuss in chapter 6 how lipolysis can be prevented, or at least delayed.
In the human GI tract, the digestion of lipophilic materials starts in the stomach, but mainly takes place in the small intestine, where the digestion products are also absorbed. Dietary oils and fats are mainly triacylglycerols (TAGs), which are digested by region-specific triacylglycerol hydrolases (lipases) that cut the FAs esterified to the sn-1 and sn-3 positions on the glycerol backbone. Lipases are known to act on an interface, where the water-soluble lipase and the lipid substrate meet.
In the human digestive system, the two main types of lipolytic enzymes secreted are human gastric lipase (HGL) in the stomach, and human pancreatic lipase (HPL) in the small intestine. Both have a size of about 50 kDa. Most digestion studies use only pancreatic lipase as a lipolytic enzyme, even though gastric lipase, pancreatic carboxyl-ester hydrolase and pancreatic lipase-related protein 2 have also been mentioned to have an important role in digestion of acylglycerols (Bakala N'Goma et al., Citation2012). HPL needs a co-lipase (a protein cofactor, 5 times smaller than the lipase itself) that enables hydrolysis of TAGs, via avoiding the effect of bile salts that remove HPL from the interface (Bakala N'Goma et al., Citation2012). After 3 h digestion, a 4-time higher amount of HPL is secreted than HGL, but HGL is active for a longer time (in both the stomach and small intestine), so over the whole digestion trajectory HPL hydrolysis contributes only three times more than HGL to the overall conversion of triacylglycerols (Carriere et al., Citation1993).
The amount of interfacial area between lipids and the surrounding aqueous medium, as well as the interfacial structure, also affect the rate and extent of lipolysis. The amount of interfacial area is defined by the size of the lipid droplets, and increases when the droplet size decreases, due to a higher surface to volume ratio. Depending on the ratio between the amount of enzyme and the available surface area, either the amount of enzyme, or the available surface area is rate limiting. For example, Li et al. Citation(2011) found that the initial lipolysis rate increased with increasing lipase concentration up to 4.8 mg/mL, and this indicates that the amount of lipase is rate limiting (Li et al., Citation2011). Surface saturation has been reported, but outside the area of investigation that we report on here, e.g., in biotechnology it was found that the reactor conversion rate increase linearly upon addition of enzyme (at low concentration) but did not increase anymore upon further addition of enzyme.
Decreasing the droplet size of Tween 80-stabilized emulsions from 2.3 μm to 0.2 μm increased the initial rate of in vitro digestion by a 4-fold factor; when just looking at the size (and total surface) ratio this should have been a factor of 11, but probably the droplet size distribution played a role here, or the available amount of lipase was not sufficient to cover all the interface related to the small droplets. Also the amount of FFAs released after 2 h increased slightly from about 57 to 67%, as well as the in vivo bioavailability of coenzyme Q10 increased (Cho et al., Citation2014). Comparable results for in vitro digestion were obtained with β-lactoglobulin-stabilized emulsion droplets: the lipolysis in the first three minutes decreased from about 54%FFA release for small droplets (178 nm) to 48% for 1.4 times bigger droplets and to only 19% FFA release for 4.3-time bigger droplets (758 nm) (McClements and Li, Citation2010b). When testing even smaller droplets, contradictory results were obtained; β-lactoglobulin-stabilized emulsions with a droplet size of 60 nm were digested slower compared to 200 nm droplets (McClements and Li, Citation2010b). The authors have related this finding to the interfacial structure that may be different due to another preparation method. This explanation is in line with the findings in a recent study by Garcia and co-workers, on native and homogenized milk fat globules: When native milk fat globules were subjected to digestion, the lipolysis rate increased when the average globule size decreased (from 6.6 μm to 1.7 μm), due to more available interfacial area. When the globule size was further reduced by high pressure homogenization (down to 0.3 μm), no increase in the lipolysis rate was observed. This may be related to the composition and physical structure of the interfacial layer surrounding the globules (Garcia et al., Citation2014); at the same time, it could also indicate that there was not sufficient lipase to cover the available area. These examples show that different effects of the droplet size have been observed, and they could be very well related to a change in limitation (so either amount of enzyme, or amount of surface area, as previously stated). It should also be pointed out that mostly the initial lipid droplet size is measured, and effects are related to it, but the actual droplet size often varies throughout the digestive tract, which makes it a complex factor to study.
Besides, it can be expected that the total amount of interfacial area is not the only factor controlling lipolysis, but the physical organization of the interface and the lipid composition also matter. The effect of different interfacial structures on lipolysis reduction is discussed in section 6 on “delayed lipolysis to induce satiety.” The lipolysis rate is determined by the composition of the lipid, since the affinity of the enzyme depends on the substrate composition, and the same would be true for the solubility of the formed digestion products (Marze et al., Citation2014). The degree of saturation of the fatty acids (FAs) corresponds to the solid fat content (SFC); at high degree of saturation, the SFC of a lipid will be higher at body temperature (for the same alkyl chain length). The molecules associate in a crystalline lattice, which is more dense, less mobile and more ordered than liquid oil. This could lead to a lower accessibility of TAG molecules to lipase, which may explain the lower digestibility (Bonnaire et al., Citation2008). In addition, the position of the fatty acids on the glycerol backbone influences the lipolysis as lipase has a region-specific affinity (McClements et al., Citation2009). Besides, the length of the FAs affects the rate and extent of lipolysis, the latter being higher for shorter FAs (Marze et al., Citation2014). This was illustrated by Giang et al. Citation(2015) who monitored intestinal in vitro digestion of TAGs with medium chain FAs (MCT), against TAGs with long chain FAs (LCT), and found that MCTs were digested in 15 minutes of, whereas LCF remained undigested after 5 hours. The latter effect is caused by coalescence of the oil resulting in a reduced interfacial area, and consequently less lipase activity.
In order to ensure optimal lipolysis in the GI tract, several natural mechanisms are involved in increasing the interfacial area, and preventing inhibition due to reaction products. For one TAG molecule, lipolysis results in two free FAs and a monoacylglycerol (MAG) with a fatty acid at the sn-2 position. The monoacylglycerol itself is surface active, but also highly surface-active components are secreted into the duodenum, such as bile salts and phospholipids. These components stabilize newly created oil-water interface to facilitate lipase action (Maldonado-Valderrama et al., Citation2011). Bile salts are also able to displace certain molecules from the oil-water interface, via competitive adsorption. This phenomenon has been referred to as orogenic displacement, and has been found to start with adsorption of the competing species at defects in the interfacial network (). After this, the domains of the competing species grow from this nucleation site (), compress the initially adsorbed material, and thereby force it to desorb (Mackie et al., Citation1999), which lines the surface up for lipolysis () (Maldonado-Valderrama et al., Citation2011).
Figure 3. Schematic representation of the effect of the components of the GI tract on an emulsion and its interface.
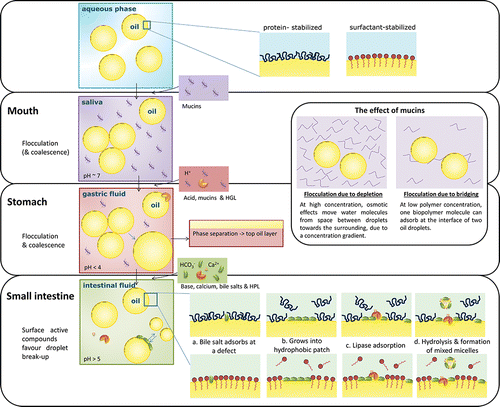
The formed MAGs are surface-active enough to remove lipase from the interface at a certain surface coverage, and are part of a self-regulatory mechanism (Reis et al., Citation2008). On the other hand, bile salts are able to remove MAG's from the interface and thereby promote further lipolysis (). This is done through mixed MAG-bile salt micelles that transport the digestion products through the mucus layer, towards the epithelial cells (Maldonado-Valderrama et al., Citation2011). Calcium plays a multiple role in lipolysis, as it is a co-factor in the activation of HPL, and additionally, it may bind long-chain FAs, precipitate them as soaps, and thereby prevent inhibition of the enzyme at the interface by the digestion products (Hu et al., Citation2010a). Besides, as mentioned previously, calcium may affect droplet flocculation, and also have an effect on biopolymer aggregation and/or bile salt precipitation.
Other factors, such as the feeding status and food matrix composition, can also impact lipolysis (Christophersen et al., Citation2014). Food matrix components—especially dietary fibers can directly interact with lipase, bind bile salts, form a protective membrane around lipid droplets, or enhance the viscosity and thereby change the behavior in the GI tract, as has been reviewed by McClements (McClements et al., Citation2009), and therefore considered outside the scope of this paper.
The release of lipophilic drugs/functional ingredients loaded into lipid-based carriers is often lipolysis rate-dependent (Bakala N'Goma et al., Citation2012; Garti et al., Citation2012). These latter authors showed that drug release from a reversed hexagonal lipid increased from < 5% after 460 min to almost 55% release when lipase was present (33 U/g) (Garti et al., Citation2012). In another example, the in vivo bioavailability of a lipophilic nutraceutical in rats was higher when dissolved in digestible corn oil compared to indigestible mineral oil (and higher when the droplets were smaller) (Cho et al., Citation2014). However, Ahmed et al. Citation(2012) showed the bioaccessibility of curcumin to decrease in the order medium>long>short chain triacylglycerols, while the initial digestion rate decreased in the order SCT > MCT > LCT, and the final digestion decreased in the order MCT > SCT > LCT. This indicates that bio-accessibility of components is much more complex than just related to the rate of lipolysis of the carrier material.
5. Encapsulation
Encapsulation technology is in use to carry a functional ingredient, for instance in food and pharmaceutical applications, which needs to be protected from the influences of the GI tract, to a location at which the content should be released. Various options are available, such as the previously mentioned filled hydrogel particles (Tan et al., Citation2009) or organogel particles (Duffy et al., Citation2009), while other capsules contain a protective shell that controls the release. This shell may be made through e.g., layer-by-layer adsorption or spray coating. Recent trends in encapsulation techniques and materials that are suitable for micro-encapsulation of lipophilic materials that ultimately may be used to reduce induce satiety are summarized below. This is followed by examples where controlled digestion or release was achieved via responsiveness of the systems to external stimuli as present in the GI tract. Finally, the role of dynamic partitioning of molecules in encapsulation system is discussed.
5.1. Micro-encapsulationl techniques
The main methods to create encapsulates include layer-by-layer adsorption, spray-drying, fluidized bed coating, extrusion, coacervation, and solvent change or removal. In the food industry, hydrophobic ingredients are often encapsulated based on an oil-in-water emulsion system, of which the interface layer is engineered in order to control the release and/or digestion. The production method defines the size of the capsules, which can be on micron-scale (several hundreds of nanometers to some hundreds of micrometers) that are called microcapsules (Neubauer et al., Citation2014) or even smaller as is the case in nanodelivery systems (<200 nm) as classified in the review of Borel and Sabliov Citation(2014). The size of the capsules determines the volume of encapsulated material, and the interfacial area, which increases reciprocally with size. Both aspects are important for digestion and/or release-related aspects. Besides, capsule shell structure (thickness, porosity, homogeneity) will be of influence on the final functionality of the capsule.
Emulsion-based systems
Encapsulates are frequently produced starting from emulsions (McClements et al., Citation2007; Li et al., Citation2010; Mantovani et al., Citation2013; Day et al., Citation2014), and if well-defined capsules are required, the emulsification method should be chosen with care. For high throughput processes, traditional devices, such as high pressure homogenisers, colloid mills, etc. are used, but these are not known for their control of droplet size. In that respect, the microstructured devices that are becoming of age nowadays, may be interesting alternatives, although it should be mentioned that throughput may still be an issue (Yuan and Williams, Citation2014; Schroën et al., Citation2015).
As mentioned in the previous section, various options exist to stabilized the oil-water interface, and we already discussed low molecular weight surfactants and amphiphilic biopolymers, solid (lipid) particles, and filled hydrogel particles, (McClements and Li, Citation2010b). Now we take interface structure one step further, and relate that to methods that can be used to make capsules.
5.1.1. Layer-by-layer
To alter the structure and properties of the surface of an emulsion droplet, additional layers can be added on top of the primary emulsifier. The use of layer-by-layer approaches has even been postulated by Neubauer et al. Citation(2014) as one of the best ways to control the capsule's mechanical performance, compared to other strategies. In literature it has been stated that multilayered systems could be useful to obtain improved stability against environmental stresses (pH, salt, thermal, lipid oxidation, dehydration) and controlled release/triggered release (Guzey and McClements, Citation2006).
The physical principles governing multilayer formation is extensively reviewed by Schönhoff Citation(2003). The layers can be added by adsorption based on electrostatic attraction of oppositely charged molecules, and this technique is used to build multi-layered capsules (Hu et al., Citation2010b; Antipina and Sukhorukov, Citation2011; Rossier-Miranda et al., Citation2012; Luo et al., Citation2013; Sakr and Borchard, Citation2013; Deligöz and Tieke, Citation2014; Zeeb et al., Citation2014). As was already clear from the emulsion section, environmental factors and choice of components greatly decides on the result that will be obtained. We will not go into details here, but focus on the charge differences between components that are needed to form consecutive layers, and are the basis for the formation of these capsules.
Depending on the dissociation constant of a component it will carry more or less charge as function of pH. In order to adsorb an additional layer onto an emulsion droplet, the charge of the next component needs to be opposite to the surface charge of the droplet. The distance over which the droplet charge is notable is called the Debye length κ−1 and is expressed as presented in Equation (Equation1(1) ) (Russel et al., Citation1989).
(1) where ε0 is the permittivity of free space, εr is the dielectric constant, kB is the Boltzmann constant, T is the absolute temperature, NA is the Avogadro number, e is the elementary charge and I is the ionic strength of the electrolyte.
From this equation it can be seen that the Debye length is larger when the ionic strength is lower, and this influences how densely molecules can arrange in and between layers. The permeability of a capsule with a fixed composition, can be tuned by changing the preparation parameters, and thereby the porosity of the shell (Klitzing, Citation2006). It is good to mention that the ionic strength and pH also affect emulsion stability against flocculation, but we consider that outside the scope of the current review.
A nice example of mechanically very strong multi-layered capsules can be found in the work of Rossier-Miranda and co-workers (2010), who also used the charge interactions between components, but did not just use molecules but also protein fibrils to fortify the shells. As a result, these capsules that were produced at low pH, were found to be very stable at that pH, while they disintegrate at high pH, because of the loss of the charge interactions. Also an effect of the number of layers was reported; at 8 and more layers, the complete capsule was neatly covered with a coat, while at lower number of layers, holes were detected that also lead to premature disintegration of the capsules, compared to those that had 8 or more layers, and that were shown to be stable at pH 2 and pH 7 for more than 4 h (Rossier-Miranda et al., Citation2010).
5.1.2. Fluidized-bed coating
Alternatively, a shell can be applied by spray-coating in a fluidized bed, and in this case particles need to be used in the range of 5–5000 μm (Zuidam and Shimoni, Citation2010). To get a gradual and equally distributed coverage, the particles are suspended in an air flow at an appropriate temperature and coated in the spray zone by atomized coating material, which can be the material as such or dissolved in an appropriate solvent that is subsequently removed. The rate of drying must be fast enough to prevent agglomeration of the capsules, and slow enough to allow for homogenous coating formation (Santivarangkna et al., Citation2007). In some cases, an additional drying step needs to be carried out in the fluidized bed (Situ et al., Citation2014).
The preferred thickness of shell depends on the application, and defines the amount of coating material to be sprayed per unit of interfacial area. The relative amount of coating is typically between 5 and 50%; most used materials are cellulose derivatives, dextrins, proteins, gums and/or starch derivatives (Zuidam and Shimoni, Citation2010). Multilayered capsules can also be produced, by repeated exposure to different materials (Vitaglione et al., Citation2012).
5.1.3. Extrusion
The typical capsule size that can be achieved by classic extrusion is 300–5000 μm, and this is also the case for particles; both emulsions and particles are rather polydisperse in size (Zuidam and Shimoni, Citation2010). For this, the core material is added to a hot biopolymer solution before extrusion. Upon exiting the machine, the product enters a hardening bath, and a glassy coating is produced, resulting in a good barrier for volatile components such as flavors (Madene et al., Citation2006). Since the extruder is shear and temperature intensive, the components and process conditions need to be chosen with care (Emin et al., Citation2012).
In syringe extrusion, a viscous solution is extruded drop-wise into a hardening solution, without requirement of high temperatures. It is typically used to produce alginate (-chitosan) beads with active cores, by extruding them into a calcium chloride solution where the droplets gel (Tan et al. Citation2009; Yan et al., Citation2014). Alternatively, a spinning disk can be used, and also actuated nozzles have been reported for the large scale production of biocatalyst beads that generally are in the same size range as stated for extrusion, but uniform in size.
Also premix membrane emulsification is sometimes termed extrusion, since it involves passing an emulsion through a membrane, which leads to reduction of the droplet size, to values that are —two to five times the size of the pore applied. Mostly the particles are in the 0.2 to 2 micron range, and not completely monodisperse, but the size distribution is much better as found in regular extrusion. Besides for emulsion formation, the same technique can also be used to make both solid and hollow particle (the latter can e.g., be used as ultrasound contrast agents (Sawalha et al., Citation2008, Citation2009, Citation2011).
5.1.4. Methods using phase separation
The quality of a solvent determines whether a component stays in solution or is more likely to form another phase. First, the solvent, or co-solvent can be removed, therewith inducing a phase transition, and that phase transition may take place on a template emulsion droplet as nicely illustrated by Sawalha and co-workers for ultrasound contrast agents (Sawalha et al., Citation2008, Citation2009, Citation2011). They made tetradecane filled polylactic acid (PLA) microcapsules, starting from PLA dissolved in dichloromethane (DCM) and with tetradecane added, which was brought into contact with a water phase and premix-emulsified. The DCM dissolves in water, leading to precipitation of the PLA on the tetradecane droplets. Also polycaprolactone or polystyrene (Shahidan et al., Citation2013) and poly(lactic-coglycolic acid)-based microparticle loaded with a drug (Krishnamachari et al., Citation2007; Wu et al., Citation2012) have been reported in literature to be produced in this way. Alternatively, an anti-solvent can be added which may also lead to solute precipitation (Joye and McClements, Citation2013).
Also emulsion coacervation can be termed a method that uses phase separation. In this case, liquid-liquid phase separation results in a polymer-rich and a polymer-lean phase. When starting from and emulsion, the polymer-rich phase deposits at the emulsion interface, due to interaction between both biopolymer solutions at the oil in water interface (Butstraen and Salaün, Citation2014). The interface can be firmed by a cooling step below the gelling temperature of the polymer phase, or by cross-linking (Xiao et al., Citation2014), after which the coacervates can be separated, and dried if needed.
At least two polymers with opposite charges are needed for so-called complex coacervate formation. This can be modulated through pH, temperature, or ionic strength. Gelatin and gum arabic are the most used polymers to form the shell, but a wide range of materials can be used: proteins extracted from animal-derived products (gelatin, whey proteins, silk fibroin) and from vegetables (soy proteins, pea proteins), and polysaccharides such as gum arabic, pectin, agar, alginate, carrageenan, and carboxymethyl cellulose (Xiao et al., Citation2014). Chitosan also gets more and more attention, as it is one of the few polycationic biopolymers available and is suitable for the production of coacervated capsules for controlled release (Butstraen and Salaün Citation2014; Yang et al., Citation2014b).
5.1.5. Spray drying of emulsions
Spray drying is widely used to improved shelf life, and stability of ingredients, e.g., in capsules that contain flavors, lipids, or carotenoids (Gharsallaoui et al., Citation2007). The feed stream is atomized into hot air to rapidly evaporate the solvent, and in that respect could also be termed a method that uses phase transition. The feed contains all ingredients for example, canola oil emulsions (50% oil on dry basis, O/W) were made with different structuring materials in the aqueous phase [WPI alone, or protein (NaCas, WPI, HWP, SPI) in combination with one or more carbohydrates (processed Hylon VII, oligofructose, dried glucose syrup or pectin)] and all these mixes were successfully spray dried into micro-encapsulated oil powders (Augustin et al., Citation2014). Turchiuli et al., succeeded to encapsulate tocopherol by spray drying an initial emulsion containing different carrier materials (combinations of maltodextrin, acacia gum and agave inulin) (Turchiuli et al., Citation2014). They used 40% w/w dry mass of which 8% olive oil and 2% tocopherol, and found the initial emulsion structure to be maintained after re-dispersing in water.
A hydrophobic functional ingredient would be added to the oil phase of the emulsion, while a hydrophilic component mostly adheres to the shell material, or is incorporated into a particle that does not contain oil. The feed emulsion should not be too viscous in order to allow spraying. Typical matrix materials are gum arabic, milk proteins, soy proteins, modified starch and maltodextrins (Augustin and Hemar, Citation2009); point of attention is that the shell should not become glassy when encapsulation of low molecular weight components is targeted (Augustin and Hemar, Citation2009). Spray drying will result in a high quality product when the heat and mass transfer processes are well controlled, which in general is very well feasible. Alternatively spray chilling can be used that operates at low outlet temperature.
5.2. Encapsulation materials
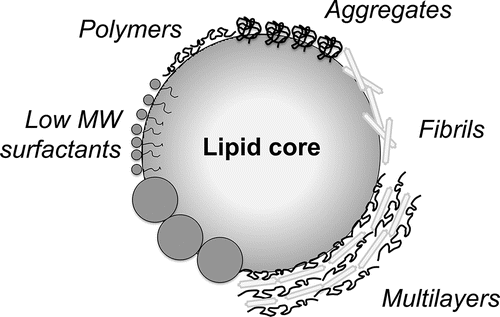
A wide range of materials has been used to construct the shell of encapsulation systems, including lipid-, protein-, and carbohydrate- based ingredients, and frequently combinations thereof, and here we present them based on the following categories: polymers (including proteins), particles and low molecular weight components. The materials at an oil-water interface are visualized in ; digestibility, and bioaccessibility all depend on the properties of the formed layer (Cilla et al. Citation2012; Marze, Citation2013). For example, the digestion of protein molecules will be different compared to aggregated proteins (Barbé et al., Citation2013), but also the subsequent presence of proteins and polysaccharides in multilayered structures is expected to influence the action of enzymes involved in digestion.
5.2.1. Polymer-based materials
Both biopolymers and synthetic polymers are discussed below. Commonly, more than one polymer is used, which can be applied as a blend or subsequently; in both cases, aiming at enhanced functionality.
Biopolymers
The two main biopolymers that occur in nature are proteins (amino acids as building blocks) and polysaccharides (sugar units). In drug micro-encapsulation, the most used biopolymers are gelatin (a protein), chitosan, alginate and cellulose (polysaccharides) (Lam and Gambari, Citation2014). Gelatin is derived from collagen, which forms a viscous liquid above 30–40°C and a gel upon cooling. Chitosan is a linear cationic polysaccharide obtained from crustacean shells, which has low digestibility and weak short-lasting mucoadhesion (Grabovac et al., Citation2005). Alginate is a cell wall component of brown algae, and often used for its gelling behavior with calcium ions (Paques et al., Citation2014). Cellulose is an insoluble linear polymer obtained from the primary cell wall of green plants, and some algae and bacteria. Chitosan, alginate and cellulose have different charges, alginate being anionic, cellulose nonionic and chitosan cationic.
In the food industry, other components maybe be used for instance, dairy proteins (caseins, whey proteins), egg, and plant proteins (e.g., soy, pea), starch, dextran, agar, galactomannans, pectin, xanthan, carrageenan, gum arabic, gellan (McClements et al., Citation2007). In general, the costs allowed for encapsulation are much less in food as compared to pharma, therefore in general, less expensive ingredients are used, which have a lower purity. Still chemical modification may be used to enhance the functionality of any of the previously mentioned ingredients (Gibbs et al., Citation1999). The length is adjusted, or side groups can be added, removed or modified seemingly at will, mostly leading to improved solubility, interfacial activity, or reduced viscosity, which is essential in the production of capsules. Modification can also be used to affect the interaction within in the GI tract, such as increasing the mucoadhesion by thiolation, which is favorable for the release of specific drugs (Grabovac et al., Citation2005). However, it is difficult to make ingredients that are satisfactory, both from a production and release point of view. Another disadvantage of such ingredients is, that they are not always allowed to use in food, for example the use of covalently modified starch with octenyl succinic anhydride (OSA) is approved as food additive (E1450) up to a 3% degree of modification based on the dry weight of starch (Rayner et al., Citation2012).
Besides protein molecules, also protein fibrils have attracted attention as building blocks for capsules. These can be made by heating an acidic protein or peptide solution under some stirring, which leads to both hydrolysis and fibril assembly at the same time, albeit at a different rate. The fibril length depends on reaction-time and shear rate (Akkermans et al., Citation2008), and they have been used to reinforce microcapsules (Rossier-Miranda et al., Citation2010; Kroes-Nijboer et al., Citation2012). Recently, β-LG fibrils were found not only to provide a more elastic interface compared to native β-LG, but also a better oxidative stability to encapsulated fish oil core (Serfert et al., Citation2014). At the same time it should be mentioned that some concerns exist regarding the potential toxicity of protein fibrils because these nanostructures might relate to protein misfolding diseases (e.g., CJD, Alzheimer's, Parkinson's, and Huntington's diseases) (Raynes et al., Citation2014).
As mentioned previously, also supra-molecular structures such as coacervates can be used to stabilize capsules. Protein-polysaccharide complexes may be formed under conditions that favor both hydrophobic and electrostatic interactions. Four pH-regions can be distinguished (Li et al., Citation2013); above a certain pH, the polymers are dissociated. Upon acidification, soluble complexes will form till a certain pH is reached at which they start to form insoluble complexes due to aggregation. At an even lower pH, the complexes disassociate again into separate polymers. These regions also depend on the protein/polysaccharide ratio as shown by Li et al. Citation(2013).
Protein-polysaccharide conjugates also consist of proteins and polysaccharides, but these are covalently coupled, through heating of a dry mixture of proteins and polysaccharides, leading to Maillard reaction products (Xu et al., Citation2012). Although conjugated WPI and pectin are able to improve the physical stability of emulsions compared to unconjugated WPI-pectin mixture (Xu et al., Citation2012), lipid digestion in these emulsions was not affected (Xu et al., Citation2014).
Synthetic (co-)polymers
A limited amount of synthetic polymers can be applied in pharma (and to some extent in food). Examples are acrylic polymers (Eudragit) (Ibekwe et al., Citation2006; Krishnamachari et al., Citation2007) and polylactide (Sawalha et al., Citation2009). Safety of components can be found on the websites of the European Food Safety Authorisation (EFSA, Citation2014), for example Eudragit (EFSA, Citation2010), and in the United States on the website of the Food and Drug Administration (FDA, Citation2014).
5.2.2. Colloidal particles
Pickering emulsions are known to be very stable in solution due to the presence of colloidal particles in their interface (Pickering, Citation1907). These particles may be added through the continuous phase, or the dispersed phase; and need to have dual affinity for oil and water, which gives them the potential to practically irreversibly adsorb at the interface and thereby physically stabilize the droplets. The adsorption/desorption energy of a particle is generally estimated by the relationship in Equation (Equation2(2) ) (Binks, Citation2002; Leal-Calderon et al., Citation2007).
(2) with γint being the interfacial tension between oil and water phases, R the particle radius and θ the contact angle between the particle tangent at contact and the interface.
From this equation it can be seen that a contact angle of 90° (resulting in a cos θ of zero) gives the highest desorption energy E, and the most irreversible adsorption. The amount of energy involved in binding very small particles is much larger as that generated in Brownian motion, therewith making desorption practically impossible, while the Brownian motion of low molecular weight surfactants is still such that they may leave the interface.
The use of particles for food emulsion stabilization has recently been reviewed by our group (Berton-carabin and Schroën, Citation2014). The particles used for Pickering food applications are mostly protein- or carbohydrate-based, such as chitin nanocrystals (Tzoumaki et al., Citation2013), colloidal lactoferrin particles (Meshulam and Lesmes, Citation2013), and coacervated WPI-pectin particles (Salminen and Weiss, Citation2014). Also colloidal solid lipid particles and surfactant-based crystals were reported (Gupta and Rousseau, Citation2012; Rousseau, Citation2013), that can be prepared by cooling from the liquid state. Alternatively, lipid ingredients can be dissolved in a solvent, such as ethanol or acetone, and dispersed into an aqueous phase containing emulsifier, after which phase separation sets in; e.g., phytosterol particles were produced in this way (Liu and Tang, Citation2014). Recently, micron-sized microorganisms were also described as food-grade Pickering stabilizers, in combination with maltodextrin-gelatin systems (Firoozmand and Rousseau, Citation2014). Besides also silica particles were used in a multilayered shell (Rossier-Miranda et al., Citation2012) or in a W/O/W emulsion (Chen et al., Citation2014).
5.2.3. Low molecular weight components
Typical components that fall in this category have a molar weight between about 250 and 1200 g/mol (Berton-carabin and Schroën, Citation2014), with a polar head and an apolar fatty acid tail. Some surfactants are natural polar lipids (e.g., lecithin), but most components are produced by synthetic esterification of fatty acids (10–20 C atoms long) and polar molecules. The polar headgroup may be just one or more glycerol units, or glycerol esterified with acid as is the case in CITREM, DATEM, and LACTEM, or with a sugar group, as is the case in sucrose esters, galactolipids; or the headgroup may be sorbitan (Span) or sorbitan with polyoxyethylene groups (Tweens). The length and degree of saturation of the FAs mostly determines the melting point of the surfactant.
Depending on their so-called hydrophilic/lipophilic balance (HLB), surfactants are more likely to form oil in water (high HLB) or water in oil emulsions (low HLB); this is known as Bancroft's rule. To make a double emulsion (W/O/W or O/W/O), both are needed (Park et al., Citation2014). Besides the charge of the head group is relevant. Anionic surfactants have a negatively charge (e.g., SSL, CITREM, DATEM, and LACTEM) and are more commonly encountered in foods than cationic surfactants. An example of a zwitterionic surfactant is lecithin, and nonionic surfactants are e.g., polysorbates and sorbitan esters. Depending on their charge, surfactants will be more or less closely packed, and may result in repulsion between emulsion droplets. The overall geometry of the surfactant determines their preferential way of assembling in supra-molecular structures. Cone-shaped surfactants will form micelles or reversed micelles, cylindrical-shaped surfactants are likely self-assembled into lamellar layers and the “truncated cone” surfactants will form vesicles. An example of this is the so-called liposome, a double layer of phospholipids surrounding a water phase that is typically in the range of 0.1--1 μm. Also nano-vesicles have been reported, consisting of hydrophobin, an amphipathic protein with high cysteine content. These vehicles (235 nm) effectively encapsulated vitamin D without loss after 3 weeks of storage (Israeli-Lev and Livney, Citation2014).
Nonpolar lipids. High melting point lipids (HML) can be sprayed in liquid state onto capsules and when cooled they crystallize and form a solid coating that provides barrier properties. For example, curcumin encapsulated in a hydrogenated oil coating was found to improve bioavailability in bread (Vitaglione et al., Citation2012), and NaCl encapsulated in a coating of stearic/palmitic acid blend, candelilla wax or carnauba wax, was found to reduce the Maillard reaction (Fiore et al., Citation2012). HML is also used in drug encapsulation systems such as lipospheres that have a solid lipid core and an embedded phospholipid shell (Elgart et al., Citation2012).
5.3. Responsiveness
Apart from the design of the microcapsule and its stability during preparation and storage, obviously also an appropriate trigger needs to be present that allows release at the required position. These triggers can be biological, chemical or physical. The use of biological stimuli, like enzymes (Park et al., Citation2014) and receptors, is less common than chemical and physical triggers as those are more difficult to control and to investigate.
The most used trigger for disrupting edible microcapsules is pH (Ibekwe et al., Citation2006, Citation2008; Rossier-Miranda et al., Citation2010; Abbaspourrad et al., Citation2013; Gun and Routh, Citation2013; Laouini et al., Citation2013; Patel et al., Citation2013); other chemical triggers relate to ionic strength, solvent removal, and electrochemical effects.
Physical stimuli that can be used to induce release of encapsulated molecules include light, electric or magnetic signals, ultrasound (Wrenn et al., Citation2012), mechanical forces, or temperature (Choi et al., Citation2010). For medical applications, microcapsules have been made light sensitive through the use of specific polymers, functional dyes and metal nanoparticles (Bédard et al., Citation2010), although it should be mentioned that it is not expected to be of great use for food applications.
The design of capsules for controlled digestion is more challenging than inducing responsiveness to a single stimulus due to the complexity of the GI tract. In that respect, multilayered microcapsules may need repeated triggers prior to disintegration, and that may be beneficial from a residence time point of view, that me be extended because of this. This type of research is still very early on in its development, but some examples can be found (Antipina and Sukhorukov, Citation2011). Besides, interindividual variability has to be considered, and this may require personalized production of capsules. In order to achieve this, reversed engineering may be used to lead to better insights in delivery, unlike the current approach that seems to be more formulation driven (Cerqueira et al., Citation2013).
5.4. Partitioning
One of the factors that has hardly been touched in literature is partitioning of (active) components. Encapsulation systems for delayed lipolysis, such as O/W emulsions, contain three regions: a lipid core, the surrounding aqueous phase and an interfacial region, as schematically shown in . In such systems, encapsulated molecules generally partition and may dynamically exchange between the available phases. In the absence of driving forces, (temperature, chemical potential), an equilibrium is reached where no net mass transfer occurs between the phases. The molecular structure and -related to that- affinity for oil and water, determine how components distribute over the phases. The region where the solubility is highest is the energetically preferred one, and the majority of the molecules will be located there, and in the absence of kinetic barriers, polar components will mostly be in the aqueous region, and nonpolar ones in the oil, although small amounts with be present in the other phase. Amphiphilic molecules partition in all phases, but tend to locate at the interface, and when present in excess start forming micelles when a critical concentration is exceeded in solution.
Figure 5. Schematic representation of the equilibria between the aqueous phase (w), interface region (i), and lipid droplets (o) for an encapsulate, along with the digestion by enzyme E. The reaction constant ke describes the rate of digestion by that enzyme. Cx is the concentration of component C in the region x. Knm is the partition coefficient of C over the regions n and m, and represents Cn/Cm (Equation (Equation3(3) )).
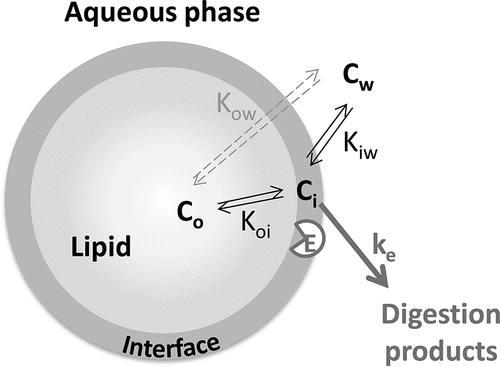
To describe the distribution, or partitioning, of components between two phases x and y the partition coefficient Kow, is used, which is based on the concentration ratio cx/cy in dilute systems (EquationEquation (3)(3) ).
(3) with co being the concentration in oil and cw the concentration in water. Molecules that are non-polar have a KOW value of above 1, and the opposite is true for polar components.
As a reference, often the logP value is used to describe the relative polarity of a molecule in a water-octanol mixture; with P, the octanol–water partition coefficient at a specified temperature. This P value can be measured or calculated based on the molecular structure of a component (Moriguchi et al., Citation1992; Wang et al., Citation1997). In real systems, partitioning is much more complex, because also components come into play, that may bind part of the component, but in general the logP value is a first indication of a component's behavior.
Accumulation at the interface
Even though the volume of the interface region in general is small compared to the volumes of the oil and aqueous phases, it can have a significant influence on the partitioning of encapsulated ingredients (McClements, Citation2005). In fact, three different partition coefficients have to be defined: oil-water, oil-interface, and interface-water. The logP only describes the overall affinity between oil and water, and does not give information about the propensity of molecules to accumulate at the oil-water interface. In order to predict the interfacial fraction of certain components in emulsions (mainly in equilibrated systems) some techniques have been developed:
Front-surface fluorescence spectroscopy: This technique can be used to assess the partitioning of proteins in emulsions, to evaluate the modifications upon aging or the displacement of proteins by surfactants (Rampon et al., Citation2001, Citation2003; Granger et al., Citation2005).
The pseudophase kinetic model has been developed to assess partitioning of antioxidants. All components are assumed to be in dynamic equilibrium between the oil, interface and water. The antioxidant distribution can then be described by the partition constant between the interface and water, and the partition constant between the interface and oil, which can be estimated by using two kinetic data sets (Gunaseelan et al., Citation2006; Losada-Barreiro et al., Citation2013).
Fluorescence microscopy: This technique can be used to visually assess the partitioning of fluorescent probes in multiphase systems (Tikekar and Nitin, Citation2011).
Electron paramagnetic resonance: This technique can be used to assess the partitioning of spin probes (model ingredients) between environments of various polarities (Berton-Carabin et al., Citation2013; Yucel et al., Citation2013).
Short timescale dynamics
Multiphase systems (such as emulsions and capsules) are not static but continuously exchange molecules between the available regions, even at equilibrium. This implies that any transport from one compartment to another has to be counterbalanced by the reverse transport. The rate of exchange depends on the mass transfer of molecules through the different regions, implying that this can greatly contribute to reactions occurring in the systems (or in the prevention thereof) particularly when the diffusivity of (small) molecules is much faster than the chemical reactions they are involved in (Chaprenet et al., Citation2014). Chaprenet et al. reported that a small hydrophobic spin probe partitioned mostly in the oil droplet core (∼ 75%), but diffused very fast between the oil droplets and the surrounding aqueous phase—much faster than its rate of reduction by ascorbate. Varying the composition and structure of the oil-water interface did not have any effect on the probe reduction rate (Chaprenet et al., Citation2014). This could also explain why, in some studies, interfacial layers designed to reduce lipolysis have not been functional in that respect. Since lipase converts the lipid substrate (triacylglycerols) into more polar products (free fatty acids, mono- and diacylglycerols); these components may partition into the aqueous phase, and without a diffusion barrier thermodynamics will drive mass transfer towards the aqueous phase, and lipolysis at the interface continues.
Longer-term evolution of the system
Emulsion systems are also subjected to changes over longer timescales than described in the previous paragraph, due to slow migration mechanisms and gradual reorganization in the system, such as Oswald ripening and compositional ripening. This typically takes minutes to days or even longer, and it is safe to say that many (food) systems are actually metastable (Walstra, Citation2003), and changes in interfacial composition will occur as function of time. The extent of this depends on the components that are used and the processing conditions they were subjected to. For example, homogenization pressure and temperature were shown to affect protein partitioning, and with that the oxidative stability of emulsions in time (Horn et al., Citation2013). The sequence of ingredient addition can also affect their long-term localization within emulsions, as shown for proteins and phospholipids of which the composition at the oil-water interface (after 48 h storage) was different when proteins were added before homogenization, and phospholipids added afterwards; or vice versa (Waninge et al., Citation2005). Besides, chemical degradation of certain components, e.g., by lipid oxidation, may lead to surface-active molecules that cause a change in interfacial organization (Berton-Carabin et al., Citation2014).
It has been postulated that multilayered interfaces can act as physical barriers; however, after reviewing the reduction of lipolysis, it can be concluded that most structures do not sufficiently protect. Probably, this can be explained by the same mechanism discussed above for low molecular weight molecules for which the short-timescale diffusion is faster compared to the digestive reactions (Chaprenet et al., Citation2014). Lipase converts the substrate into more polar low molecular weight products, and they most likely partition more into the aqueous phase in the form of micelles. Without barrier against diffusion across the interface, thermodynamics will drive mass transfer, and lipolysis continues. Only if lipase action is completely prevented (by physical exclusion from the interface) capsules may be not affected by the above mentioned effect.
From all the above, it is clear that partitioning is a factor that can explain some of the findings reported in literature; however, since it was hardly ever investigated as such, it is hard to pinpoint the results to this phenomenon, also because this is a very complex factor that changes dramatically during passage in the GI tract.
6. Delayed lipolysis to induce satiety
Considering the factors that affect lipolysis, various strategies may be applied to formulate lipolysis-resistant edible matrices. Here we go beyond the basic, well-established trends that we previously described, namely, that lipolysis is slower for longer FAs (Joyce et al. Citation2014; Marze et al. Citation2014; Giang et al. Citation2015) and for higher degree of saturation (McClements et al., Citation2009); and that the rate of lipolysis is related to the available amount of interfacial area, and hence droplet size (Singh et al., Citation2009). Systems that aimed at delayed lipolysis are described in this section. We believe that delayed lipolysis can allow targeted delivery of unabsorbed lipids in the small intestine. As described in section 3, sensing of unabsorbed nutrients in the ileum might induce distal to proximal negative feedback, including prolonged gastric retention, which induces satiety.
Matrix large-scale structure
Reducing lipolysis has been achieved both in vitro and in vivo with the use of filled hydrogels (Li et al., Citation2012). In this study, the authors used whey protein-stabilized oil droplets that were trapped within alginate hydrogel beads (D43 = 510 μm), which led to a delayed lipolysis (7% FFA release after 30 min) compared to the nontrapped emulsion (D43 = 0.36 μm) or emulsion droplets with alginate bilayer (D43 = 4.66 μm) that both released about 68% FFA after 30 minutes. By entrapping the emulsion into the gel, the interfacial area of the emulsion remains constant for a longer time, and the effective diffusion distance of lipase is increased. This would also explain that Li et al. Citation(2012) measured a lower lipolysis (19% FFA release after 30 minutes) after making large clusters (D43 = 200 μm) of bilayer emulsion droplets. Besides, it has been reported that alginate can inhibit lipase, via direct interaction with the enzyme or with the substrate at the oil-water interface to directly inhibit the enzyme, or indirectly via interaction with mucin forming a gel, especially when the guluronic acid/manuronic acid ratio is high (Wilcox et al., Citation2014). Recently, the same research group investigated filled hydrogels in more detail: they improved the preparation method (Matalanis and McClements, Citation2013), reported pH-dependent hydrogel particles that are only stable at pH 4–5, so could be applied to release in the mouth (Zhang et al., Citation2015) and they studied the effect on the lipid digestion of such hydrogels again (Mun et al., Citation2015). Mun and co-workers found a 13–53% lower initial digestion rate of mung bean-based filled hydrogel and a 16–20% higher final lipolysis compared to the corresponding emulsion, but these effects were not found with rice-starch-based filled hydrogels.
Organogels are liquid oils structured in the form of gels by phytosterols, waxes, fibers, phospholipids, etc. Phytosterol-structured oil was found to resist in vitro lipolysis more as compared to the corresponding nonstructured vegetable oil (Duffy et al., Citation2009). The authors gave three possible explanations for this: oil structuring would slow down substrate diffusion to the interface; micellar tubes would incorporate TAGs; or sterol would inhibit lipase action. Besides, oil structuring might prevent droplet size reduction that does take place under the action of bile salts in liquid fat, resulting in a constant area for lipolysis instead of an increased surface area. Which of these effects is ruling is not fully understood, but it is clear that lipolysis can also be affected by strategies other than interface structuring.
Oil-water interface
A number of components that can locate at the oil-water interface, or alter its composition and structure may be used to influence lipolysis. The effects we describe go beyond the effect of available surface that has been previously touched upon.
Emulsifiers
As discussed previously, components that are present in an interface, such as monoacylglycerols and phospholipids, can influence lipase activity, and this is also true for other emulsifiers. Emulsifiers are defined here as amphiphilic compounds that tend to adsorb at the oil-water interface, lower the interfacial tension, and thereby kinetically stabilize the emulsion. In in the appendix, we have compiled a number of studies for O/W emulsions. The galactolipid digalactosyldiacylglycerol (DGDG) can inhibit bile salt adsorption via steric hindrance, which delayed lipolysis and reduced the reaction rate compared to lecithin from egg yolk (Chu et al., Citation2009). This effect was not observed with monogalactosyldiacylglycerol (MGDG). The same research group also compared DGDG to dipalmitoylphosphatidylcholine (DPPC), and reported that DGDG-stabilized interfaces were more resistant against adsorption of bile salts, colipase, and lipase (Chu et al., Citation2010). They hypothesize that a certain amount of headgroup-free interfacial area has to be filled with bile salts, and thereby create large enough patches to allow colipase to adsorb, and subsequent lipase action. The larger headgroup of DGDG would prevent the creation of large patches of bile salts via steric hindrance, and would result in more scattered, smaller, and discontinuous patches that are considered less effective (Chu et al., Citation2010).
Similar effects were found when the interfacial behavior of a nonionic, brush-like emulsifier (poloxamer Pluronic F68) was compared with an anionic, more compact one (phospholipid Epikuron 145 V) at different bile salt concentrations, using interfacial tension and dilatational rheology studies (Torcello-Gómez et al., Citation2012). The authors found that when Pluronic was used, the rate and extent of bile salt adsorption was lower. This is in agreement with previous findings of the same research group on emulsion stability; the bile salt concentration at which destabilization (reversible phase separation, most likely due to flocculation) occurred is higher for Pluronic, and destabilization proceeded slower, as compared to emulsions made with Epikuron (Jódar-Reyes et al., Citation2010).
Surfactant charge can also influence lipolysis, as can be concluded from a study in which nine different surfactants were tested. It showed that nonionic surfactant-stabilized emulsions have a lower in vitro lipolysis rate than anionic surfactant-stabilized ones (Speranza et al., Citation2013). The hydrophilic-lipophilic balance (HLB) and alkyl chain length of surfactants were also shown to affect the rate of lipolysis and the bioaccessibility of the core oil (measured as the amount of FAs generated in the jejunum, ileum and ileum efflux). The authors found that the rate of lipolysis in the jejunum was lower when higher HLB-surfactants were used, but the bioaccessibility of the FAs from the emulsified oil was higher due to a longer induction time (Speranza et al., Citation2013). The relation between HLB and lipolysis rate can be explained as follows: surfactants with a high HLB have a large headgroup located on the aqueous phase side, where they may give protection against adsorption of bile salt and lipase. Such high HLB surfactants form micelles more easily, accounting for improved bioaccessibility.
The addition of a low amount of co-surfactant (0.25 wt% monoacylglycerol) to a protein-stabilized emulsion (1 wt% caseinate, 20 wt% canola oil) resulted in a 2-fold reduction in lipolysis of emulsified liquid oil, which was explained by easier coalescence of droplets due to reduced charge effects, therewith reducing the available oil-water interfacial area (Day et al., Citation2014). Addition of more monoacylglycerol (0.5 wt% or 0.75 wt%) did increase lipolysis compared to emulsions stabilized with caseinate only, due to increased access of lipase to the surface (Day et al., Citation2014). The addition of Tween 20 as co-surfactant has also been described to facilitate in vitro lipid digestion of WPI stabilized emulsions rather than reduce it, but in this case also the 4-time smaller droplet size and consequently higher interfacial area, may have played a role (Li and McClements, Citation2014a). The presence co-surfactant in the primary emulsion also affected the lipolysis of emulsions with additional layers of alginate (Li and McClements, Citation2014a), or alginate and chitosan (Li and McClements, Citation2014b). Clearly, various effects can be expected when working with co-surfactants.
Interfacial structures extracted from nature have also been investigated with respect to lipolysis. Thylakoids (membranes of chloroplasts from green leaves) were found to inhibit hydrolysis of emulsified oil by lipase/colipase, also in the presence of bile salts (Albertsson et al., Citation2007). To relate this to either binding of the membrane to the oil-water interface, reducing the access of the lipase/colipase complex or by binding of the membranes to the lipase/colipase complex, blocking the active site of the enzyme. Also in vivo, Albertsson et al. found a suppressed food intake when such membranes were added to rat food.
Another natural interfacial structure that has been investigated for its behavior during gastrointestinal lipolysis is that of oil bodies (Beindorff et al., Citation2007; Gallier and Singh, Citation2012; Gallier et al., Citation2013; Makkhun et al., Citation2015). Oil bodies are fat storage structures in seeds, composing of a TAG core in a shell of phospholipids and proteins (e.g., oleosin) (Makkhun et al., Citation2015). A natural emulsion of oil bodies can be obtained by an aqueous extraction from several raw materials, such as sunflower seeds, walnut, almond, and maize. Such oil bodies have been described to induce a feeling of satiety, especially when the proteins are cross-linked, by providing resistance against human digestion (Beindorff et al., Citation2007). In washed sunflower seed oil bodies, the oleosin has been described to be easily replaced by bile salts, but, the presence of a (natural) protein layer provided protection (Makkhun et al., Citation2015). Also almond proteins of the oil body membrane have been described provide some protection under in vitro digestion, as they are pepsin-resistant (Gallier and Singh, Citation2012), whereas the almond oil body emulsion behaved comparable under in vitro digestion to conventional protein-stabilized emulsions. Such pepsin-resistant peptides were also described to be present on walnut oil bodies, and suggested to play a major role in the lipid digestion (Gallier et al., Citation2013).
Colloidal particles
Instead of conventional emulsifiers, also particles can be used to stabilize the oil-water interface, forming so-called Pickering stabilized interfaces, of which the effect on lipolysis is listed in in the appendix. It has been mentioned, that due to a stronger irreversible adsorption of the particles at the oil droplet surface, they are able to reduce the extent and rate of lipolysis more as compared to conventional emulsifier-stabilization (Tzoumaki et al., Citation2013; Yang et al., Citation2014a). Chitin nanocrystal-stabilized emulsions showed slower and lower lipolysis compared to conventional emulsions stabilized with whey protein isolate (WPI) and caseinate (Tzoumaki et al., Citation2013), although the comparison should be treated with care, since different components were used. Emulsions stabilized by particles of Ginkgo biloba extracts or their flavonoid glycosides fraction also showed a lower rate and extent of lipolysis, compared to conventional Tween 20-stabilized emulsions (Yang et al., Citation2014a). For lactoferrin (LF) particles mixed results were obtained. Lactoferrin nanoparticles (NP) did not affect lipolysis markedly compared to native LF-stabilized emulsions (Meshulam and Lesmes, Citation2013), but when combined alginate this resulted in a 14% reduced lipolysis, while with carrageenan an increased lipolysis by 10% was found (Meshulam and Lesmes, Citation2013). This may however have been a direct effect of the alginate that can inhibit lipase directly (Wilcox et al., Citation2014). In conclusion, there is a possible effect of particles, but before drawing any conclusions, this would need to be investigated in much more detail.
Multi-layers
In in the appendix various studies are listed that use more than one layer to influence lipolysis. However, before going into detail, it should be mentioned, that trying to apply multiple layers can be rather tricky, also in view of the previously mentioned competitive adsorption effects. The results are in general rather difficult to interpret as no clear trends were observed, but we still try to discuss them shortly.
Addition of beet pectin to a WPI-stabilized emulsion was reported to reduce lipolysis (Xu et al., Citation2014), both when WPI and pectin were conjugated first or adsorbed subsequently, and this could indicate that an interlinked layer is formed that has synergistic effects, or as the authors prefer to think that unadsorbed pectin may bind to lipase or bile salts therewith reducing lipolysis. In another study, the addition of a chitosan layer on top of a β-lactoglobulin (BLG)-stabilized emulsion did reduce lipolysis, and a third layer of either alginate or pectin reduced lipolysis slightly further (Li et al., Citation2010). In a third study, when using a primary caseinate-stabilized emulsion at low pH and adsorbing a chitosan layer by increasing the pH to 5, the lipolysis was also reduced (from 90% to 75% after 15 min of digestion) (Hu et al., Citation2010b). However, when adding a third pectin layer, the authors found the lipolysis much less reduced as compared to the bilayered emulsion. Besides, only minor reduction in lipolysis was observed when adsorbing a pectin secondary layer by lowering the pH from 7 to 4.5, and adding a third chitosan and fourth pectin layer, which even enhanced lipolysis (Hu et al., Citation2010b). The addition of a chitosan third layer onto a secondary emulsion of alginate on top of BLG also increased the lipolysis rather than reducing it (Li and McClements, Citation2014b).
It is obvious that the choice of components and the conditions under which they are used are critical in order to be successful in reduction in lipolysis. More precisely, the ultrastructure, and in particular the porosity of the interfacial layer, which defines the accessibility of the core lipid to lipase, need to be considered. Joyce and co-workers investigated various porous structures, and found that the diameter of the pore relative to the size of lipase is important (Joyce et al., Citation2014). Digestion will be inhibited when the pores are small enough to hinder lipase to enter, and a pore size just larger than the diameter of lipase has been described to enhance its action (Joyce et al., Citation2014). Also some effects were dedicated to the hydrophobicity/hydrophilicity of the porous structure in this work, but it is not clear whether these effects can be solely contributed to that, or that the pore size also influenced these findings.
The barrier properties for lipase can be improved when a more cohesive layer at the interface is formed; heating starch particle-stabilized emulsions decreased lipase activity up to 60% at the temperature where starch gelatinized enough to create a dense layer but not swelled further into a more loose structure more accessible for lipase to penetrate (Timgren et al., Citation2011; Sjöö et al., Citation2015). Another way to improve the barrier properties of the interface is cross-linking interfacial biopolymers; cross-linking interfacial casein with the functional ingredient genipin enhanced acid stability and delayed in vitro digestibility (Hu et al., Citation2015). But, cross-linking does not essentially improve the barrier properties, as in vitro lipid digestion was not greatly affected by cross-linking an interfacial pectin layer with laccase (Zeeb et al., Citation2015).
All effects described above in relation to interface structure, suggest that characterizing the ultrastructure and morphology of oil-water interfaces, at scales relevant to the dimensions of a lipase molecule, could be a viable strategy to assess ab initio how fast and to which extent lipolysis is likely to occur in O/W emulsions.
Clinical trials
One available product that is claimed to induce satiety by reducing lipolysis is Olibra® (Lipid Technologies Provider AB, Sweden). This is an emulsion of fractionated palm/oat oil that naturally contains galactolipids. Diepvens et al. Citation(2007) provided Olibra® twice daily for 26 weeks in a yoghurt (per serving 5 g fat, of which 40% from the vegetable oils) and found an improved weight management after an initial weight loss period in overweight women, as compared to providing a placebo (5 g milk fat per serving). This indicates the absence of compensation behavior during long-term use. In another human intervention study, the commercially available yoghurt Fabuless® (DSM Food Specialities, Netherlands, also containing palm and oat oil, previously called Olibra® and Reducal®, with 28.3 g total fat) resulted in a 2-fold higher amount of undigested lipids in the jejunum compared to the control (with 28.3 g milk fat) (Knutson et al., Citation2010). They observed needle-shaped fat crystals in the jejunal samples from subjects that consumed the test samples, which were not seen in the control group. Knutson et al. suggest that galactolipids from oat oil would play a crucial role in the formation of crystals from palm oil. These crystals are proposed to be digested slower, so the ileum is longer exposed to fatty acids.
The effectiveness of this type of product to induce satiety via activation of the ‘ileal brake’ is still not completely confirmed. The intervention study of Smith et al. Citation(2011) did not show an effect of Fabuless° on appetite, and only a weak effect on food intake. Nevertheless, the industry does see a potential in this concept as several patents are based on satiety emulsions (Herslof et al. Citation2003; Bialek et al., Citation2006; Golding et al., Citation2009).
7. Conclusions
Dietary lipids have been encapsulated using many different processes and construction materials, but the performance regarding reduction of lipolysis is overall poor and varies considerably. The fact that the results show only minor effects and are not consistent indicates that some key factors in the process of GI lipolysis are not yet fully understood and hence cannot be sufficiently controlled to induce satiety. The nonconsistency in results also stems from the in vitro models that varied greatly; hopefully the standardized method that is now agreed upon will help in that respect. Still, this method can only give a limited impression of the effects that are generated since it does not include biological feedback routes that determine lipolysis and its effect on satiety.
Until present, only one type of product has been commercialized to induce satiety via lipid signaling in the distal small intestine, but its effectiveness is controversial. Therefore we conclude that it is challenging to delay lipolysis; and that would be even more the case for release of lipids in the more distal parts of the small intestine where they can induce satiety.
In our view, the most promising route to achieve delayed lipolysis is by designing the structure and morphology of the oil-water interface, at scales relevant to the dimensions of a lipase molecule. In that way, lipase would get controlled access to the oil/water interface. This is far from trivial as stated in this review, and most probably multidisciplinary teams are needed to link medical and technical challenges, but at the same time the advantages will be great since such capsules could provide a strategy for noninvasive long-term weight management.
References
- Abbaspourrad, A., Datta, S. S. and Weitz, D. A. (2013). Controlling release from pH-responsive microcapsules. Langmuir. 29(41):12697–12702.
- Ahmed, K., Li, Y., McClements, D. J. and Xiao, H. (2012). Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 132(2):799–807.
- Akkermans, C., van der Goot, A. J., Venema, P., van der Linden, E. and Boom, R. M. (2008). Formation of fibrillar whey protein aggregates: Influence of heat and shear treatment, and resulting rheology. Food Hydrocolloid. 22(7):1315–1325.
- Albertsson, P.-A., Köhnke, R., Emek, S. C., Mei, J., Rehfeld, J. F., Akerlund, H.-E. and Erlanson-Albertsson, C. (2007). Chloroplast membranes retard fat digestion and induce satiety: Effect of biological membranes on pancreatic lipase/co-lipase. The Biochem. J. 401(3):727–733.
- Antipina, M. N. and Sukhorukov, G. B. (2011). Remote control over guidance and release properties of composite polyelectrolyte based capsules. Adv. Drug Deliv. Rev. 63(9):716–729.
- Atuma, C., Strugala, V., Allen, A. and Holm, L. (2001). The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280(5):922–929.
- Augustin, M. A. and Hemar, Y. (2009). Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 38(4):902–912.
- Augustin, M. A., Sanguansri, L., Rusli, J. K., Shen, Z., Cheng, L. J., Keogh, J. and Clifton, P. (2014). Digestion of microencapsulated oil powders: In vitro lipolysis and in vivo absorption from a food matrix. Food Funct. 5(11):2905–2912.
- Bakala N'Goma, J.-C., Amara, S., Dridi, K., Jannin, V. and Carrière, F. (2012). Understanding the lipid-digestion processes in the GI tract before designing lipid-based drug-delivery systems. Ther. Deliv. 3(1):105–124.
- Barbé, F., Ménard, O., Le Gouar, Y., Buffière, C., Famelart, M.-H., Laroche, B., Le Feunteun, S., et al. (2013). The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chem. 136:1203–1212.
- Batterham, R. L., Cowley M a., Small, C. J., Herzog, H., Cohen M. a., Dakin, C. L., Wren, A. M., et al. (2002). Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 418:650–654.
- Bédard, M. F., De Geest, B. G., Skirtach, A. G., Möhwald, H. and Sukhorukov, G. B. (2010). Polymeric microcapsules with light responsive properties for encapsulation and release. Adv. Colloid Interface Sci. 158:2–14.
- Beindorff, C. M., Ghislain, C. Y., Sergey, L., Melnikov, M. and Winter, I. (2007). Satiety enhancing food products. Patent WO 2007115899 A1. 1–23.
- Benshitrit, R. C., Levi, C. S., Tal, S. L., Shimoni, E. and Lesmes, U. (2012). Development of oral food-grade delivery systems: Current knowledge and future challenges. Food Funct. 3(1):10–21.
- Berton-Carabin, C. C., Coupland, J. N. and Elias, R. J. (2013). Effect of the lipophilicity of model ingredients on their location and reactivity in emulsions and solid lipid nanoparticles. Colloids Surf. A. 431:9–17.
- Berton-Carabin, C. C., Ropers, M.-H. and Genot, C. (2014). Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 13(5):945–977.
- Berton-carabin, C. C. and Schroën, K. (2014). Pickering emulsions for food applications: Background, trends and challenges. Ann. Rev. Food Sci. Technol. 31(0):1–55.
- Bialek, J. M., Melnikov, S. M. and Winter, I. (2006). Satiety emulsions and food compositions. Patent US 2006/0105093 A1.
- Binks, B. P. (2002). Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 7:21–41.
- Blundell, J. E. and Bellisle, F. (2013). Satiation, Satiety and the Control of Food Intake. Woodhead Publishing Limited, Cambridge.
- Bonnaire, L., Sandra, S., Helgason, T., Decker, E. A., Weiss, J. and McClements, D. J. (2008). Influence of lipid physical state on the in vitro digestibility of emulsified lipids. J. Agric. Food Chem. 56(10):3791–3797.
- Borel, T. and Sabliov, C. M. (2014). Nanodelivery of bioactive components for food applications: Types of delivery systems, properties, and their effect on ADME profiles and toxicity of nanoparticles. Ann. Rev.Food Sci. Technol. 5:1–17.
- Bornhorst, G. M. and Singh, R. P. (2014). Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestive process. Ann. Rev. Food Sci. Technol. 5:1–22.
- Burton, D. D., Kim, H. J., Camilleri, M., Stephens, D. A., Mullan, B. P., O’Connor, M. K. and Talley, N. J. (2005). Relationship of gastric emptying and volume changes after a solid meal in humans. Am J Physiol Gastrointest Liver Physiol, 289(2):261–266.
- Butstraen, C. and Salaün, F. (2014). Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 99:608–616.
- Calbet, J. A. L. and MacLean, D. A. (1997). Role of caloric content on gastric emptying in humans. J. Physiol. 498(2):553–559.
- Carriere, F., Barrowman, J. A., Verger, R. and Laugier, R. (1993). Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 105(3):876–888.
- Cerqueira, M. a., Pinheiro, A. C., Silva, H. D., Ramos, P. E., Azevedo M. a., Flores-López, M. L., Rivera, M. C., et al. (2013). Design of bio-nanosystems for oral delivery of functional compounds. Food Eng. Rev. 6(1–2):1–19.
- Chaprenet, J., Berton-Carabin, C. C., Elias, R. J. and Coupland, J. N. (2014). Effect of interfacial properties on the reactivity of a lipophilic ingredient in multilayered emulsions. Food Hydrocolloid. 42:56–65.
- Chen, P. W., Cadisch, G. and Studart, A. R. (2014). Encapsulation of aliphatic amines using micro fluidics. Langmuir. 30:2346–2350.
- Cho, H. T., Salvia-Trujillo, L., Kim, J., Park, Y., Xiao, H. and McClements, D. J. (2014). Droplet size and composition of nutraceutical nanoemulsions influences bioavailability of long chain fatty acids and Coenzyme Q10. Food Chem. 156:117–122.
- Choi, S.-W., Zhang, Y. and Xia, Y. (2010). A temperature-sensitive drug release system based on phase-change materials. Angew. Chem. Int. Edit. 49(43):7904–7908.
- Christophersen, P. C., Christiansen, M. L., Holm, R., Kristensen, J., Jacobsen, J., Abrahamsson, B. and Müllertz, A. (2014). Fed and fasted state gastro-intestinal in vitro lipolysis: In vitro in vivo relations of a conventional tablet, a SNEDDS and a solidified SNEDDS. Eur. J. Pharm. Sci. 57:232–239.
- Chu, B.-S., Gunning, a. P., Rich, G. T., Ridout, M. J., Faulks, R. M., Wickham, M. S. J., Morris, V. J., et al. (2010). Adsorption of bile salts and pancreatic colipase and lipase onto digalactosyldiacylglycerol and dipalmitoylphosphatidylcholine monolayers. Langmuir. 26(12):9782–9793.
- Chu, B.-S., Rich, G. T., Ridout, M. J., Faulks, R. M., Wickham, M. S. J. and Wilde, P. J. (2009). Modulating pancreatic lipase activity with galactolipids: Effects of emulsion interfacial composition. Langmuir. 25(16):9352–9360.
- Cilla, A., Alegría, A., De Ancos, B., Sánchez-Moreno, C., Cano, M. P., Plaza, L., Clemente, G., et al. (2012). Bioaccessibility of tocopherols, carotenoids, and ascorbic acid from milk- and soy-based fruit beverages: Influence of food matrix and processing. J. Agric. Food Chem. 60:7282–7290.
- Cook, M. T., Tzortzis, G., Charalampopoulos, D. and Khutoryanskiy, V. V. (2012). Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release. 162(1):56–67.
- Coupe, A. J., Davis, S. S. and Wilding, I. R. (1991). Variation in gastrointestinal transit of pharmaceutical dosage forms in healthy subjects. Pharm. Res. 8(3):360.
- Day, L., Golding, M., Xu, M., Keogh, J., Clifton, P. and Wooster, T. J. (2014). Tailoring the digestion of structured emulsions using mixed monoglyceride–caseinate interfaces. Food Hydrocolloid. 36:151–161.
- De Boever, P., Deplancke, B. and Verstraete, W. (2000). Fermentation by gut microbiota cultured in a simulator of the human intestinal microbial ecosystem is improved by supplementing a soygerm powder. J. Nutr. 130(10):2599–2606.
- Deligöz, H. and Tieke, B. (2014). QCM-D study of layer-by-layer assembly of polyelectrolyte blend films and their drug loading-release behavior. Colloids Surf. A. 441:725–736.
- Diepvens, K., Soenen, S., Steijns, J., Arnold, M., Westerterp-Plantenga, M. (2007). Long-term effects of consumption of a novel fat emulsion in relation to body-weight management. Int. J. Obes. 31(6):942–949.
- Dong, Q.-Y., Chen, M.-Y., Xin, Y., Qin, X.-Y., Cheng, Z., Shi, L.-E., Tang, Z.-X. (2013). Alginate-based and protein-based materials for probiotics encapsulation: A review. Int. J. Food Sci. Technol. 48(7):1339–1351.
- Duffy, N., Blonk, H. C. G., Beindorff, C. M., Cazade, M., Bot, A., Duchateau, G. S. M. J. E. (2009). Organogel-based emulsion systems, micro-structural features and impact on in vitro digestion. J. Am. Oil Chem. Soc. 86(8):733–741.
- EFSA. (2010). Scientific Opinion on the safety of anionic methacrylate copolymer for the proposed uses as a food additive. EFSA J. 8(7):1–1656.
- EFSA. (2014). Regulated Food Ingredients Applications. European Food Safety Authority. Available from http://www.efsa.europa.eu/en/applicationshelpdesk/foodingredients.htm
- Elgart, A., Cherniakov, I., Aldouby, Y., Domb, A. J. and Hoffman, A. (2012). Lipospheres and pro-nano lipospheres for delivery of poorly water soluble compounds. Chem. Phys. Lipids. 165(4):438–453.
- Emin, M. A., Mayer-Miebach, E. and Schuchmann, H. P. (2012). Retention of β-carotene as a model substance for lipophilic phytochemicals during extrusion cooking. LWT - Food Sci. Technol. 48(2):302–307.
- FDA. (2014). Ingredients, Packaging & Labeling. U.S. Food and Drug Administration. Available from http://www.fda.gov/Food/IngredientsPackagingLabeling/
- Fiore, A., Troise, A. D., Mogol, A., Roullier, V. and Gourdon, A. (2012). Controlling the maillard reaction by reactant encapsulation: Sodium chloride in cookies. J. Agric. Food Chem. 60:10808–10814.
- Firoozmand, H. and Rousseau, D. (2014). Tailoring the morphology and rheology of phase-separated biopolymer gels using microbial cells as structure modifiers. Food Hydrocolloid. 42:204–214.
- Gallier, S. and Singh, H. (2012). Behavior of almond oil bodies during in vitro gastric and intestinal digestion. Food Funct. 3(5):547–555.
- Gallier, S., Tate, H. and Singh, H. (2013). In vitro gastric and intestinal digestion of a walnut oil body dispersion. J. Agric. Food Chem. 61(2):410–417.
- Garcia, C., Antona, C., Robert, B., Lopez, C. and Armand, M. (2014). The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion. Food Hydrocolloids. 35:494–504.
- Garti, N., Hoshen, G. and Aserin, A. (2012). Lipolysis and structure controlled drug release from reversed hexagonal mesophase. Colloids Surf. B.. 94:36–43.
- Geraedts, M. C. P., Troost, F. J., Munsters, M. J. M., Stegen JHCH., de Ridder, R. J., Conchillo, J. M., Kruimel, J. W., et al. (2011). Intraduodenal administration of intact pea protein effectively reduces food intake in both lean and obese male subjects. PLoS ONE. 6(9):1–7.
- Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A. and Saurel, R. (2007). Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 40(9):1107–1121.
- Giang, T. M., Le Feunteun, S., Gaucel, S., Brestaz, P., Anton, M., Meynier, a. and Trelea, I. C. (2015). Dynamic modeling highlights the major impact of droplet coalescence on the in vitro digestion kinetics of a whey protein stabilized submicron emulsion. Food Hydrocolloid. 43:66–72.
- Gibbs, B. F., Kermasha, S., Alli, I. and Mulligan, C. N. (1999). Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 50(3):213–224.
- Gibbs, J. and Smith, G. P. (1982). Gut peptides and food in the gut produce similar satiety effects. Peptides. 3(3):553–557.
- Golding, M., Wooster, T. J., Day, L., Xu, M., Lundin, L., Keogh, J. and Clifton, P. (2011). Impact of gastric structuring on the lipolysis of emulsified lipids. Soft Matter. 7(7):3513
- Golding, M. D., de Groot, P. W. N., Koppert, R. J., Melnikov, S. M. and Pelan, E. G. (2009). Satiety emulsions and food compositions. Patent US 2009/0317509 A1:1–7.
- Grabovac, V., Guggi, D., Bernkop-Schnürch, A. (2005). Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 57(11):1713–1723.
- Granger, C., Barey, P., Toutain, J. and Cansell, M. (2005). Direct quantification of protein partitioning in oil-in-water emulsion by front-face fluorescence: Avoiding the need for centrifugation. Colloids Surf. B.. 43(3-4):158–162.
- Gun, W. J. and Routh, A. F. (2013). Formation and characterization of pH-responsive liquid core microcapsules. Langmuir. 29(40):12541–12548.
- Gunaseelan, K., Romsted, L. S., Gallego, M.-J. P., González-Romero, E., Bravo-Díaz, C. (2006). Determining alpha-tocopherol distributions between the oil, water, and interfacial regions of macroemulsions: Novel applications of electroanalytical chemistry and the pseudophase kinetic model. Adv. Colloid Interface Sci. 123–126:303–311.
- Gupta, R. and Rousseau, D. (2012). Surface-active solid lipid nanoparticles as Pickering stabilizers for oil-in-water emulsions. Food Funct. 3(3):302–311.
- Guzey, D. and McClements, D. J. (2006). Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 128(130):227–248.
- Herslof, B., Lindmark, L., Bohlinder, K. and Carlsson, A. (2003). Satiety Products. Patent US 6,517,883 B1
- Hoebler, C., Guillon, F., Fardet, A., Cherbut, C. and Barry, J. (1998). Gastrointestinal or Simulated In Vitro Digestion Changes Dietary Fibre Properties and their Fermentation. Journal of the Science of Food and Agriculture, 77:327–333.
- Horn, A. F., Barouh, N., Nielsen, N. S., Baron, C. P. and Jacobsen, C. (2013). Homogenization pressure and temperature affect protein partitioning and oxidative stability of emulsions. J. Am. Oil Chem. Soc. 90(10):1541–1550.
- Hu, M., Li, Y., Decker, E. A., McClements, D. J. (2010a). Role of calcium and calcium-binding agents on the lipase digestibility of emulsified lipids using an in vitro digestion model. Food Hydrocolloid. 24(8):719–725.
- Hu, M., Li, Y., Decker, E. A., Xiao, H., McClements, D. J. (2010b). Impact of layer structure on physical stability and lipase digestibility of lipid droplets coated by biopolymer nanolaminated coatings. Food Biophys. 6:37–48.
- Hu, B., Zhang, L., Liang, R., Chen, F., He, L., Hu, B. and Zeng, X. (2015). Cross-linking of interfacial casein layer with genipin prevented ph induced structural instability and lipase digestibility of the fat droplets. J. Agric. Food Chem. 63(7):2033–2040.
- Hur, S. J., Lim, B. O., Decker, E. a. and McClements, D. J. (2011). In vitro human digestion models for food applications. Food Chem. 125:1–12.
- Hur, S. J., Decker, E. A and McClements, D. J. (2009). Influence of initial emulsifier type on microstructural changes occurring in emulsified lipids during in vitro digestion. Food Chemistry. 114:253–262.
- Ibekwe, V. C., Fadda, H. M., McConnell, E. L., Khela, M. K., Evans, D. F. and Basit, A. W. (2008). Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm. Res. 25(8):1828–1835.
- Ibekwe, V. C., Fadda, H. M., Parsons, G. E. and Basit, A. W. (2006). A comparative in vitro assessment of the drug release performance of pH-responsive polymers for ileo-colonic delivery. Int. J. Pharm. 308(1–2):52–60.
- Inui, A., Asakawa, A., Bowers, C. Y., Mantovani, G., Laviano, A., Meguid, M. M. and Fujimiya, M. (2004). Ghrelin, appetite, and gastric motility: The emerging role of the stomach as an endocrine organ. FASEB J. 18(3):439–456.
- Israeli-Lev, G. and Livney, Y. D. (2014). Self-assembly of hydrophobin and its co-assembly with hydrophobic nutraceuticals in aqueous solutions: Towards application as delivery systems. Food Hydrocolloid. 35:28–35.
- Jódar-Reyes, a. B., Torcello-Gómez, a., Wulff-Pérez, M., Gálvez-Ruiz, M. J., Martín-Rodríguez, a. (2010). Different stability regimes of oil-in-water emulsions in the presence of bile salts. Food Res. Int. 43(6):1634–1641.
- Joyce, P., Tan, A., Whitby, C. P. and Prestidge, C. a. (2014). The role of porous nanostructure in controlling lipase-mediated digestion of lipid loaded into silica particles. Langmuir. 30:2779–2788.
- Joye, I. J. and McClements, D. J. (2013). Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 34(2):109–123.
- Keogh, J. B., Wooster, T. J., Golding, M., Day, L. and Clifton, P. M. (2011). Slowly and Rapidly Digested Fat Emulsions Are Equally Satiating but Their Triglycerides Are Differentially Absorbed and Metabolized. The Journal of Nutrition. 141(5):809–815.
- van Klitzing, R. (2006). Internal structure of polyelectrolyte multilayer assemblies. Phys. Chem. Chem. Phys. 8(43):5012–5033.
- Knutson, L., Fridblom, H., Viberg, A., Sein, A. and Lennerna, H. (2010). Gastrointestinal metabolism of a vegetable-oil emulsion in healthy. Am. J. Clin. Nutr. 92:515–524.
- Klinkesorn, U. and McClements, D. J. (2010). Impact of Lipase, Bile Salts, and Polysaccharides on Properties and Digestibility of Tuna Oil Multilayer Emulsions Stabilized by Lecithin–Chitosan. Food Biophysics. 5(2):73–81.
- Kong, F. and Singh, R. P. (2010). A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 75(9):E627–E635.
- Kozu, H., Kobayashi, I., Neves, M. a., Nakajima, M., Uemura, K., Sato, S. and Ichikawa, S. (2014). PIV and CFD studies on analyzing intragastric flow phenomena induced by peristalsis using a human gastric flow simulator. Food Funct. 5(8):1839–1847.
- Krishnamachari, Y., Madan, P. and Lin, S. (2007). Development of pH- and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. Int. J. Pharm. 338:238–247.
- Kroes-Nijboer, A., Venema, P., van der Linden, E. (2012). Fibrillar structures in food. Food Funct. 3(3):221–227.
- Kunz, P., Feinle-Bisset, C., Faas, H., Boesiger, P., Fried, M., Steingötter, A. and Schwizer, W. (2005). Effect of ingestion order of the fat component of a solid meal on intragastric fat distribution and gastric emptying assessed by MRI. J. Magn. Reson. Imaging. 21(4):383–390.
- Lam, P. L. and Gambari, R. (2014). Advanced progress of microencapsulation technologies: In vivo and in vitro models for studying oral and transdermal drug deliveries. J. Control. Release. 178:25–45.
- Laouini, A., Koutroumanis, K. P., Charcosset, C., Georgiadou, S., Fessi, H., Holdich, R. G. and Vladisavljević, G. T. (2013). pH-sensitive micelles for targeted drug delivery prepared using a novel membrane contactor method. Appl. Mater. Interfaces. 5(18):8939–8947.
- Lavin, J. H., Wittert, G. A., Andrews, J., Yeap, B., Wishart, J. M., Morris, H. A., Morley, J. E., et al. (1998). Interaction of insulin, glucagon-like peptide 1, gastric inhibitory polypeptide, and appetite in response to intraduodenal carbohydrate. Am. J. Clin. Nutr. 68:591–598.
- Leal-Calderon, F., Schmitt, V. and Bibette, J. (2007). Emulsion Science, Basic Principles. Springer Science, NY.
- Lesmes, U., Baudot, P. and McClements, D. J. (2010). Impact of interfacial composition on physical stability and in vitro lipase digestibility of triacylglycerol oil droplets coated with lactoferrin and/or caseinate. Journal of Agricultural and Food Chemistry. 58(13):7962–7969
- Lesmes, U. and McClements, D. J. (2009). Structure–function relationships to guide rational design and fabrication of particulate food delivery systems. Trends Food Sci. Technol. 20(10):448–457.
- Li, X., Fang, Y., Phillips, G. O., Al-Assaf, S. (2013). Improved Sugar Beet Pectin-Stabilized Emulsions through Complexation with Sodium Caseinate. J. Agric. Food Chem. 61(6):1388–1396.
- Li, Y., Hu, M. and McClements, D. J. (2011). Factors affecting lipase digestibility of emulsified lipids using an in vitro digestion model: Proposal for a standardised pH-stat method. Food Chem. 126(2):498–505.
- Li, Y., Hu, M., Xiao, H., Du, Y., Decker, E. A. and McClements, D. J. (2010). Controlling the functional performance of emulsion-based delivery systems using multi-component biopolymer coatings. Eur. J. Pharm. Biopharm. 76(1):38–47.
- Li, Y., Kim, J., Park, Y. and McClements, D. J. (2012). Modulation of lipid digestibility using structured emulsion-based delivery systems: Comparison of in vivo and in vitro measurements. Food Funct. 3(5):528–536.
- Li, Y. and McClements, D. J. (2014a). Modulating lipid droplet intestinal lipolysis by electrostatic complexation with anionic polysaccharides: Influence of cosurfactants. Food Hydrocolloid. 35:367–374.
- Li, Y. and McClements, D. J. (2014b). Influence of cosurfactant on the behavior of structured emulsions under simulated intestinal lipolysis conditions. Food Hydrocolloid. 40:96–103.
- Liu, F. and Tang, C.-H. (2014). Phytosterol colloidal particles as Pickering stabilizers for emulsions. J. Agric. Food Chem. 62(22):5133–5141.
- Losada-Barreiro, S., Bravo-Diaz, C., Paiva-Martins, F. and Romsted, L. S. (2013). Maxima in antioxidant distributions and efficiencies with increasing hydrophobicity of gallic acid and its alkyl esters. The pseudophase model interpretation of the “cut off effect.” J. Agric. Food Chem. 61:6533–6543.
- Luo, Q., Boom, R. M. and Janssen, A. E. M. (2015). Digestion of protein and protein gels in simulated gastric environment. LWT - Food Sci. Technol. 63(1):161–168.
- Luo, R., Venkatraman, S. S. and Neu, B. (2013). Layer-by-layer polyelectrolyte—polyester hybrid microcapsules for encapsulation and delivery of hydrophobic drugs. Biomacromolecules. 14:2262–2271.
- Mackie, A., Gunning, A., Wilde, P. and Morris, V. (1999). Orogenic displacement of protein from the air/water interface by competitive adsorption. J. Colloid Interface Sci. 210(1):157–166.
- Madene, A., Jacquot, M., Scher, J. and Desobry, S. (2006). Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 41(1):1–21.
- Makkhun, S., Khosla, A., Foster, T., McClements, D. J., Grundy, M. M. L. and Gray, D. a. (2015). Impact of extraneous proteins on the gastrointestinal fate of sunflower seed (Helianthus annuus) oil bodies: A simulated gastrointestinal tract study. Food Funct. 6(1):124–133.
- Maldonado-Valderrama, J., Wilde, P., Macierzanka, A. and Mackie, A. (2011). The role of bile salts in digestion. Adv Colloid Interface Sci. 165:36–46.
- Malinauskytė, E., Ramanauskaitė, J., Leskauskaitė, D., Devold, T. G., Schüller, R. B. and Vegarud, G. E. (2014). Effect of human and simulated gastric juices on the digestion of whey proteins and carboxymethylcellulose-stabilised O/W emulsions. Food Chem. 165:104–112.
- Maljaars, P. W. J., Peters, H. P. F., Kodde, A., Geraedts, M., Troost, F. J., Haddeman, E. and Masclee, A. A. M. (2011). Length and site of the small intestine exposed to fat influences hunger and food intake. Br. J. Nutr. 106(10):1609–1615.
- Maljaars, J., Peters, H. P. F. and Masclee, A. M. (2007). Review article: The gastrointestinal tract: Neuroendocrine regulation of satiety and food intake. Aliment. Pharmacol. Ther. 26(2):241–250.
- Maljaars, P. W. J., Peters, H. P. F., Mela, D. J. and Masclee, A. A. M. (2008a). Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 95(3):271–281.
- Maljaars, J., Romeyn, E. A., Haddeman, E., Peters, H. P. F. and Masclee, A. A. M. (2009). Effect of fat saturation on satiety, hormone release, and food intake. Am. J. Clin. Nutr. 89:1019–1024.
- Maljaars, P. W. J., Symersky, T., Kee, B. C., Haddeman, E., Peters, H. P. F. and Masclee, A. A. M. (2008b). Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int. J. Obes. 32(11):1633–1639.
- Mantovani, R. A., Cavallieri, A. L. F., Netto, F. M. and Cunha, R. L. (2013). Stability and in vitro digestibility of emulsions containing lecithin and whey proteins. Food Funct. 4(9):1322–1331.
- Marciani, L., Gowland, P. a., Fillery-Travis, a., Manoj, P., Wright, J., Smith, a., Young, P., et al. (2001). Assessment of antral grinding of a model solid meal with echo-planar imaging. Am. J. Physiol. Gastrointest. Liver Physiol. 280(5):G844–G849.
- Marze, S. (2013). Bioaccessibility of nutrients and micronutrients from dispersed food systems: Impact of the multiscale bulk and interfacial structures. Crit. Rev. Food Sci. Nutr. 53(1):76–108.
- Marze, S., Algaba, H. and Marquis, M. (2014). A microfluidic device to study the digestion of trapped lipid droplets. Food Funct. 5(7):1481–1488.
- Marze, S., Choimet, M. and Foucat, L. (2012). In vitro digestion of emulsions: Mechanistic and experimental models. Soft. Matter. 8(42):10982–10993.
- Matalanis, A. and McClements, D. J. (2013). Hydrogel microspheres for encapsulation of lipophilic components: Optimization of fabrication & performance. Food Hydrocolloid. 31(1):15–25.
- McClements, D. J. (2005). Food Emulsions: Principles, Practices, and Techniques. Second ed., CRC Press.
- McClements, D. J. (2010). Design of nano-laminated coatings to control bioavailability of lipophilic food components. Journal of Food Science. 75(1):R30–42.
- McClements, D. J., Decker, E. A. and Park, Y. (2009). Controlling lipid bioavailability through physicochemical and structural approaches. Crit. Rev. Food Sci. Nutr. 49(1):48–67.
- McClements, D. J., Decker, E. a. and Weiss, J. (2007). Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 72(8):109–124.
- McClements, D. J. and Li, Y. (2010a). Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 1(1):32–59.
- McClements, D. J. and Li, Y. (2010b). Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 159(2):213–228.
- McConnell, E. L., Fadda, H. M. and Basit, A. W. (2008). Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J Pharm. 364(2):213–226.
- Meshulam, D. and Lesmes, U. (2013). Responsiveness of emulsions stabilized by lactoferrin nano-particles to simulated intestinal conditions. Food Funct. 5(1):65–73.
- Minekus, M. (2015). The TNO Gastro-Intestinal Model (TIM). In: Verhoeckx K, Cotter P, López-Expósito I et al (eds) The impact of food bioactives on health: in vitro and ex vivo models. Springer International Publishing, New York, pp. 37–46.
- Minekus, M., Alminger, M., Alvito, P., Ballance, S., Bohn, T., Bourlieu, C., Carrière, F., et al. (2014). A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 5:1113–1124.
- Molly, K., van de Woestyne, M., de Smet, I. and Verstraete, W. (1994). Validation of the simulator of the human intestinal microbial ecosystems (SHIME) reaction using microorganism-associated activities. Microb. Ecol. Health Dis. 7:191–200.
- Moreau, H., Sauniere, J. F., Gargouri, Y., Pieroni, G., Verger, R. and Sarles, H. (1988). Human gastric lipase: variations induced by gastrointestinal hormones and by pathology. Scand. J. Gastroenterol. 23(9):1044–1048.
- Moriguchi, I., Hirono, S., Liu, Q., Nakagome, I. and Matsushita, Y. (1992). Simple method of calculating octanol/water partition coefficient. Chem. Pharm. Bull. 40(17):127–130.
- Mun, S., Decker, E. A. and McClements, D. J. (2007). Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Research International. 40(6):770–781.
- Mun, S., Decker, E. A., Park, Y., Weiss, J. and McClements, D. J. (2006). Influence of Interfacial Composition on in vitro Digestibility of Emulsified Lipids: Potential Mechanism for Chitosan's Ability to Inhibit Fat Digestion. Food Biophysics. 1(1):21–29.
- Mun, S., Kim, Y.-R., Shin, M. and McClements, D. J. (2015). Control of lipid digestion and nutraceutical bioaccessibility using starch-based filled hydrogels: Influence of starch and surfactant type. Food Hydrocolloid. 44:380–389.
- Neubauer, M. P., Poehlmann, M. and Fery, A. (2014). Microcapsule mechanics: From stability to function. Adv. Colloid Interface Sci. 207:65–80.
- Marieb, E.N. (1999). Anatomie et physiologie humaines. Québec, Canada: De Boeck, pp. 1–1194.
- Malaki Nik, A., Wright, A. J. and Corredig, M. (2011). Impact of interfacial composition on emulsion digestion and rate of lipid hydrolysis using different in vitro digestion models. Colloids and Surfaces B: Biointerfaces. 83(2):321–330.
- Olsson, C. and Holmgren, S. (2001). The control of gut motility. Comp. Biochem. Physiol. Part A. 128(3):479–501.
- Paques, J. P., van der Linden, E., van Rijn, C. J. M. and Sagis, L. M. C. (2014). Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 209:163–171.
- Park, K. M., Sung, H., Choi, S. J., Choi, Y. J. and Chang, P.-S. (2014). Double-layered microparticles with enzyme-triggered release for the targeted delivery of water-soluble bioactive compounds to small intestine. Food Chem. 161:53–59.
- Patel, A. R., Drost, E., Seijen ten Hoorn, J. and Velikov, K. P. (2013). Fabrication and characterization of emulsions with pH responsive switchable behavior. Soft. Matter. 9(29):6747–6751.
- Pedersen-Bjergaard, U., Høt, U., Kelbæk, H., Schifter, S., Rehfeld, F. J., Faber, J. and Christensen, N. J. (1996). Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand. J. Clin. Lab. Invest. 56(6):497–503.
- Pickering, S. U. (1907). Emulsions. J. Chem. Soc. 91:2001–2021.
- Pilichiewicz, A. N., Chaikomin, R., Brennan, I. M., Wishart, J. M., Rayner, C. K., Jones, K. L., Smout, A. J. P. M., et al. (2007). Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am. J. Phys. Endocrinol. Metabolism. 293:743–753.
- Porter, C. J. and Charman, W. N. (2001). In vitro assessment of oral lipid based formulations. Adv. Drug Deliv. Rev. 50:S127–S147.
- Rampon, V., Genot, C., Riaublanc, a., Anton, M., Axelos, M. a. V. and McClements, D. J. (2003). Front-face fluorescence spectroscopy study of globular proteins in emulsions: Displacement of BSA by a nonionic surfactant. J. Agric. Food Chem. 51(9):2482–2489.
- Rampon, V., Lethuaut, L., Mouhous-Riou, N. and Genot, C. (2001). Interface characterization and aging of bovine serum albumin stabilized oil-in-water emulsions as revealed by front-surface fluorescence. J. Agric. Food Chem. 49(8):4046–4051.
- Rayner, M., Timgren, A., Sjöö, M. and Dejmek, P. (2012). Quinoa starch granules: A candidate for stabilising food-grade Pickering emulsions. J. Sci. Food Agric. 92(9):1841–1847.
- Raynes, J. K., Carver, J. a., Gras, S. L. and Gerrard, J. a. (2014). Protein nanostructures in food—Should we be worried? Trends Food Sci. Technol. 37(1):42–50.
- Reis, P., Holmberg, K., Miller, R., Krägel, J., Grigoriev, D. O., Leser, M. E. and Watzke, H. J. (2008). Competition between lipases and monoglycerides at interfaces. Langmuir. 24(14):7400–7407.
- Rossier-Miranda, F. J., Schroën, K. and Boom, R. (2010). Mechanical characterization and pH response of fibril-reinforced microcapsules prepared by layer-by-layer adsorption. Langmuir. 26(24):19106–19113.
- Rossier-Miranda, F. J., Schroën, K. and Boom, R. (2012). Microcapsule production by an hybrid colloidosome-layer-by-layer technique. Food Hydrocolloid. 27(1):119–125.
- Rousseau, D. (2013). Trends in structuring edible emulsions with Pickering fat crystals. Curr. Opin. Colloid Interface Sci. 18(4):283–291.
- Ruiz-Rodriguez, P. E., Meshulam, D. and Lesmes, U. (2014). Characterization of Pickering O/W Emulsions Stabilized by Silica Nanoparticles and Their Responsiveness to In vitro Digestion Conditions. Food Biophysics. 4:406–415.
- Russel, W. B., Saville, D. A. and Schowalter, W. R. (1989). Colloidal Dispersions. Cambridge University Press, Cambridge. pp. 1–525.
- Ryan, A. T., Feinle-Bisset, C., Kallas, A., Wishart, J. M., Clifton, P. M., Horowitz, M. and Luscombe-Marsh, N. D. (2012). Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 96(7):474–482.
- Sakr, O. S. and Borchard, G. (2013). Encapsulation of Enzymes in layer-by-layer (LbL) structures: latest advances and applications. Biomacromolecules. 14:2117–2135.
- Salminen, H. and Weiss, J. (2014). Electrostatic adsorption and stability of whey protein–pectin complexes on emulsion interfaces. Food Hydrocolloid. 35:410–419.
- Santivarangkna, C., Kulozik, U. and Foerst, P. (2007). Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol. Prog. 23(2):302–315.
- Sawalha, H., Fan, Y., Schroen, K. and Boom, R. (2008). Preparation of hollow polylactide microcapsules through premix membrane emulsification—Effects of nonsolvent properties. J. Membr. Sci. 325(2):665–671.
- Sawalha, H., Schroën, K. and Boom, R. (2009). Hollow polylactide microcapsules with controlled morphology and thermal and mechanical properties. AIChE J. 55(11):2827–2834.
- Sawalha, H., Schroën, K. and Boom, R. (2011). Biodegradable polymeric microcapsules: Preparation and properties. Chem. Eng. J. 169(1–3):1–10.
- Schönhoff, M. (2003). Layered polyelectrolyte complexes: Physics of formation and molecular properties. J. Phys. Condens. Matter. 15:1781–1808.
- Schroën, K., Bliznyuk, O., Muijlwijk, K., Sahin, S. and Berton-Carabin, C. C. (2015). Microfluidic emulsification devices: From micrometer insights to large-scale food emulsion production. Curr. Opin. Food Sci. 3:33–40.
- Serfert, Y., Lamprecht, C., Tan, C.-P., Keppler, J. K., Appel, E., Rossier-Miranda, F. J., Schroen, K., et al. (2014). Characterisation and use of β-lactoglobulin fibrils for microencapsulation of lipophilic ingredients and oxidative stability thereof. J. Food Eng. 143:53–61.
- Shahidan, N., Liu, R., Thaiboonrod, S., Alexander, C., Shakesheff, K. M. and Saunders, B. R. (2013). Hollow colloidosomes prepared using accelerated solvent evaporation. Langmuir. 29(45):13676–13685.
- Simon, G. L. and Gorbach, S. L. (1986). The human intestinal microflora. Digest. Dis. Sci. 31(S9):147–162.
- Simovic, S., Heard, P., Hui, H., Song, Y., Peddie, F., Davey, A. K., Lewis, A., et al. (2009). Dry Hybrid Lipid - Silica Microcapsules Engineered from Submicron Lipid Droplets and Nanoparticles as a Novel Delivery System for Poorly Soluble Drugs. Molecular pharmaceutics. 6(3):861–872.
- Singh, H., Ye, A. and Horne, D. (2009). Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 48(2):92–100.
- Situ, W., Chen, L., Wang, X. and Li, X. (2014). Resistant starch film-coated microparticles for an oral colon-specific polypeptide delivery system and its release behaviors. J. Agric. Food Chem. 62(16):3599–3609.
- Sjöö, M., Emek, S. C., Hall, T., Rayner, M. and Wahlgren, M. (2015). Barrier properties of heat treated starch Pickering emulsions. J. Colloid Interface Sci. 450:182–188.
- Smit, H. J., Keenan, E., Kovacs, E. M. R., Wiseman, S. a., Peters, H. P. F., Mela, D. J. and Rogers, P. J. (2011). No efficacy of processed Fabuless (Olibra) in suppressing appetite or food intake. Eur. J. Clin. Nutr. 65(1):81–86.
- Speranza, A., Corradini, M. G., Hartman, T. G., Ribnicky, D., Oren, A. and Rogers, M. A. (2013). Influence of emulsifier structure on lipid bioaccessibility in oil—water nanoemulsions. J. Agric. Food Chem. 61:6505–6515.
- Strader, A. D., Vahl, T. P., Jandacek, R. J., Woods, S. C., Alessio, D. A. D., Seeley, R. J., April, D., et al. (2005). Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am. J. Physiol. Endocrinol. Metabolism. 288:447–453.
- Tan, C. S., Jejurikar, A., Rai, B., Bostrom, T., Lawrie, G. and Grøndahl, L. (2009). Encapsulation of a glycosaminoglycan in hydroxyapatite/alginate capsules. J. Biomed. Mater. Res. Part A. 91(3):866–877.
- Tan, A., Colliat-dangus, P., Whitby, C. P. and Prestidge, C. A. (2014). Controlling the Enzymatic Digestion of Lipids Using Hybrid Nanostructured Materials. Applied Materials & Interfaces. 6:15363–15371.
- Tikekar R. V. and Nitin, N. (2011). Effect of physical state (solid vs. liquid) of lipid core on the rate of transport of oxygen and free radicals in solid lipid nanoparticles and emulsion. Soft. Matter. 7(18):8149.
- Timgren, A., Rayner, M., Sjöö, M. and Dejmek, P. (2011). Starch particles for food based Pickering emulsions. Procedia Food Sci. 1:95–103.
- Tokle, T., Mao, Y. and McClements, D. J. (2013). Potential biological fate of emulsion-based delivery systems: lipid particles nanolaminated with lactoferrin and β-lactoglobulin coatings. Pharmaceutical research. 30(12):3200–3213.
- Torcello-Gómez, A., Maldonado-Valderrama, J., Martín-Rodríguez, A. and McClements, D. J. (2011). Physicochemical properties and digestibility of emulsified lipids in simulated intestinal fluids: influence of interfacial characteristics. Soft Matter. 7(13):6167.
- Torcello-Gómez, a., Jódar-Reyes, a. B., Maldonado-Valderrama, J. and Martín-Rodríguez, a. (2012). Effect of emulsifier type against the action of bile salts at oil–water interfaces. Food Res. Int. 48(1):140–147.
- Turchiuli, C., Jimenez Munguia, M. T., Hernandez Sanchez, M., Cortes Ferre, H. and Dumoulin, E. (2014). Use of different supports for oil encapsulation in powder by spray drying. Powder Technol. 255:103–108.
- Turton, M. D., O'shea, D., Gunn, I., Beak, S. A., Edwards, C. M., Meeran, K., Choi, S. J., et al. (1996). A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 379(4):69–72.
- Tzoumaki, M. V., Moschakis, T., Scholten, E. and Biliaderis, C. G. (2013). In vitro lipid digestion of chitin nanocrystal stabilized o/w emulsions. Food Funct. 4(1):121–129.
- Van Aken, G. A. (2010). Relating food emulsion structure and composition to the way it is processed in the gastrointestinal tract and physiological responses: What are the opportunities? Food Biophys. 5(4):258–283.
- Van Aken, G. A., Vingerhoeds, M. H. and de Hoog, E. H. A. (2007). Food colloids under oral conditions. Curr. Opin. Colloid Interface Sci. 12(4–5):251–262.
- Van Avesaat, M., Troost, F. J., Ripken, D., Hendriks, H. F. and Masclee, a. a. M. (2015). Ileal brake activation: Macronutrient specific effects on eating behavior? Int. J. Obes. 39(April):235–243.
- Vaughan, R. W., Bauer, S. and Wise, L. (1973). Volume and pH of gastric juice in obese patients. Anesthesiology. 43(6):686–689.
- Vingerhoeds, M. H., Blijdenstein, T. B. J., Zoet, F. D. and van Aken, G. A. (2005). Emulsion flocculation induced by saliva and mucin. Food Hydrocolloid. 19(5):915–922.
- Vitaglione, P., Barone Lumaga, R., Ferracane, R., Radetsky, I., Mennella, I., Schettino, R., Koder, S., et al. (2012). Curcumin bioavailability from enriched bread: The effect of microencapsulated ingredients. J. Agric. Food Chem. 60(13):3357–3366.
- Walstra, P. (2003). Phys. Chem. Foods. Marcel Dekker, New York, pp. 1–832.
- Wang, R., Fu, Y. and Lai, L. (1997). A new atom-additive method for calculating partition coefficients. J. Chem. Inf. Comput. Sci. 37:615–621.
- Waninge, R., Walstra, P., Bastiaans, J., Nieuwenhuijse, H., Nylander, T., Paulsson, M. and Bergenståhl, B. (2005). Competitive adsorption between beta-casein or beta-lactoglobulin and model milk membrane lipids at oil-water interfaces. J. Agric. Food Chem. 53(3):716–724.
- WHO. (2014). Obesity and Overweight. World Health Organization Media centre, Fact sheet N311. Available from http://www.who.int/mediacentre/factsheets/fs311/en/
- Wickham, M. J. S., Faulks, R. M., Mann, J. and Mandalari, G. (2012). The design, operation, and application of a dynamic gastric model. Dissolut. Technol. 19(3):15–22.
- Wilcox, M. D., Brownlee I. a., Richardson, J. C., Dettmar, P. W. and Pearson, J. P. (2014). The modulation of pancreatic lipase activity by alginates. Food Chem. 146:479–484.
- Wrenn, S. P., Dicker, S. M., Small, E. F., Dan, N. R., Mleczko, M., Schmitz, G. and Lewin, P. a. (2012). Bursting bubbles and bilayers. Theranostics. 2(12):1140–1159.
- Wu, Z. M., Zhou, L., Guo, X. D., Jiang, W., Ling, L., Qian, Y., Luo, K. Q., et al. (2012). HP55-coated capsule containing PLGA/RS nanoparticles for oral delivery of insulin. Int. J. Pharm. 425(1–2):1–8.
- Xiao, Z., Liu, W., Zhu, G., Zhou, R. and Niu, Y. (2014). A review of the preparation and application of flavour and essential oils microcapsules based on complex coacervation technology. J. Sci. Food Agric. 94(8):1482–1494.
- Xu, D., Wang, X., Jiang, J., Yuan, F. and Gao, Y. (2012). Impact of whey protein—Beet pectin conjugation on the physicochemical stability of β-carotene emulsions. Food Hydrocolloid. 28(2):258–266.
- Xu, D., Yuan, F., Gao, Y., Panya, A., McClements, D. J. and Decker, E. A. (2014). Influence of whey protein-beet pectin conjugate on the properties and digestibility of β-carotene emulsion during in vitro digestion. Food Chemistry. 156:374–379.
- Yan, M., Liu, B., Jiao, X. and Qin, S. (2014). Preparation of phycocyanin microcapsules and its properties. Food Bioprod. Process. 92:89–97.
- Yang, Z., Peng, Z., Li, J., Li, S., Kong, L., Li, P. and Wang, Q. (2014b). Development and evaluation of novel flavour microcapsules containing vanilla oil using complex coacervation approach. Food Chem. 145:272–277.
- Yang, D., Wang, X.-Y., Ji, C.-M., Lee, K.-T., Shin, J.-A., Lee, E.-S. and Hong, S.-T. (2014a). Influence of Ginkgo biloba extracts and of their flavonoid glycosides fraction on the in vitro digestibility of emulsion systems. Food Hydrocolloid. 42:196–203.
- Yuan, Q. and Williams, R. a. (2014). Precision emulsification for droplet and capsule production. Adv. Powder Technol. 25:122–135.
- Yucel, U., Elias, R. J. and Coupland, J. N. (2013). Localization and reactivity of a hydrophobic solute in lecithin and caseinate stabilized solid lipid nanoparticles and nanoemulsions. J. Colloid Interface Sci. 394:20–25.
- Zeeb, B., Lopez-Pena, C. L., Weiss, J. and McClements, D. J. (2015). Controlling lipid digestion using enzyme-induced crosslinking of biopolymer interfacial layers in multilayer emulsions. Food Hydrocolloid. 46:125–133.
- Zeeb, B., Thongkaew, C. and Weiss, J. (2014). Theoretical and practical considerations in electrostatic depositioning of charged polymers. J. Appl. Polym. Sci. 131(7):1–11.
- Zhang, Z., Zhang, R., Decker, E. A. and McClements, D. J. (2015). Development of food-grade filled hydrogels for oral delivery of lipophilic active ingredients: pH-triggered release. Food Hydrocolloid. 44:345–352.
- Zuidam, N. J. and Shimoni, E. (2010). Overview of microencapsulates for use in food products or processes and methods to make them. In: Encapsulation Technologies for Active Food Ingredients and Food Processing, Springer Science+Business Media, Dordrecht, pp. 122–135.
Appendix
Table 1A. Overview of studies on the effect of emulsifier interfaces on lipolysis.
Table 2A. Lipolysis of particle-stabilized emulsions.
Table 3A. Effect of layered interfaces on lipolysis.