Abstract
In Turkish kitchen, Cyclotrichium niveum, which is consumed as a dietary supplement, have been extensively used in soup and food for their odor and flavor. The present study examines the possible antioxidant, antimicrobial and radical scavenging capacity of Cyclotrichium niveum (Boiss.) Manden and Scheng. In order to evaluate antioxidant and radical scavenging activity different in vitro methodologies such as 2,2´-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging activity, 1,1-diphenyl-2-picryl-hydrazyl (DPPH·) free radical scavenging, total antioxidant activity by ferric thiocyanate, total reducing power by potassium ferricyanide reduction method, superoxide anion radical scavenging, hydrogen peroxide scavenging, and ferrous ions chelating activities were used. In addition, antimicrobial and antifungal activity of the both extracts tested against twenty five microorganisms. Total phenolic compounds and total flavonoids contents in water extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng (WECN) and ethanol extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng (EECN) were determined.
INTRODUCTION
Oxidation of lipids, which is the main cause of quality deterioration in many food systems, may lead to off-flavours and formation of toxic compounds, and may lower the quality and nutritional value of foods. Furthermore, lipid oxidation is also associated with aging, membrane damage, heart disease and cancer.[Citation1] Lipid peroxidation, which involves a series of free radical mediated chain reaction processes, is also associated with several types of biological damage. Therefore, much attention has been focused on the use of antioxidants, especially natural antioxidants to inhibit lipid peroxidation and to protect from damage due to free radicals. Furthermore, in foods, the development of rancidity is caused by ROS that lead to development of off-flavours and undesirable chemical compounds.[Citation2]
The importance of reactive oxygen species and free radicals has attracted increasing attention over the past decade. Reactive oxygen species (ROS), which include free radicals such as superoxide anion radicals (O2 .−), hydroxyl radicals (OH·) and non free-radical species such as H2O2 and singlet oxygen (1O2), are various forms of activated oxygen. These molecules are exacerbating factors in cellular injury and aging process.[Citation3–5] ROS are continuously produced during normal physiologic events and they can easily initiate the peroxidation of membrane lipids, leading to the accumulation of lipid peroxides. However, they are removed by antioxidant defence mechanisms. There is a balance between the generation of ROS and inactivation of ROS by the antioxidant system in organisms. Oxidative stress occurs when the production of ROS is beyond the protective capability of the antioxidant defences.[Citation6,Citation7] Under pathological conditions, ROS are overproduced and result in oxidative stress. ROS are formed when endogenous antioxidant defences are inadequate. The imbalance between ROS and antioxidant defence mechanisms leads to oxidative modification in cellular membrane or intracellular molecules.[Citation8]
Antioxidants may be defined as compounds that inhibit or delay the oxidation of other molecules by inhibiting the initiation or propagation of oxidizing chain reactions.[Citation9] Its can also protect the human body from free radicals and ROS effects and retard the progress of many chronic diseases as well as lipid peroxidation.[Citation10,Citation11] The most commonly used antioxidants at the present time are BHA, BHT, propylgallate and tert-butylhydroquinone. However, their safety has recently been questioned due to toxicity, liver damage, and possible carcinogenicity.[Citation12–14] Thus, development of safer antioxidants from natural origin make interest.
There is a growing interest of natural products in human diet, both due to the possible negative effects of synthetic food additives on human health and to the increased consumer perception of this problem in recent years. Numerous studies demonstrate that a great number of medicinal and aromatic herbs, as well as fruits and leaves of some berry plants biosynthesize phytochemicals possessing antioxidant activity and may be used as a natural source of free radical scavenging compounds.[Citation15,Citation16] In addition, a great number of spices and aromatic herbs contain chemical compounds exhibiting antioxidant properties. These properties are attributed to a variety of active phytochemicals including vitamins, carotenoids, terpenoids, alkaloids, flavonoids, lignans, simple phenols and phenolic acids, and so on.[Citation17]
Turkey is regarded as an important gene-centre for the Labiatae. The family is represented in Turkey by 45 genera, 546 species and a total of 731 taxa. Cyclotrichium a member of the family Labiatae, is a perennial plant endemic to Turkey. The local name of this plant is ‘dağnanesi’ in Turkish. It is represented in Turkey by five species; Cyclotrichium niveum, Cyclotrichium origanifolium, Cyclotrichium stamineum, Cyclotrichium leucotrichum, Cyclotrichium glabrescens. However, Cyclotrichium. niveum, Cyclotrichium origanifolium are endemic to Turkey. Cyclotrichium niveum is growing in Eastern Anatolia.[Citation18] Chemodiversity in this genus seems to be complementary in taxonomical identification of the species. Extensive research has been carried out into studying the chemical composition and bioactivities of the aromatic plants of Turkey. In recent years, several studies have been performed on the chemical composition of Cyclotrichium niveum. It was reported that Cyclotrichium niveum contains pulegone,[Citation19] isomenthone as main substances.[Citation20,Citation21] Except these, no study on the chemical composition of Cyclotrichium species have previously been reported. The only report on the constituents of Cyclotrichium niveum concerns the isolation of flavonoids and triterpenoids.[Citation22] The essential oil of Cyclotrichium niveum was analyzed the first time by Baser et al.[Citation18] It was found that pulegone was the major component in Cyclotrichium niveum essential oil. The pulegone content was found as 32.5–56.1 g/100 essential oil. Isomenthone content varied between 33.8–35.4 g/100 essential oil. In addition aerial parts of this plants, especially leaves, was commonly used as herbal tea.[Citation23]
As far as our literature survey could ascertain, no information was available on the in vitro total antioxidant activity, reducing power, DPPH· free radical scavenging, ABTS radical scavenging, superoxide anion radical scavenging, hydrogen peroxide scavenging, and metal chelating activities of Cyclotrichium niveum extracts given here. Also antibacterial activity of the WECN and EECN against twenty-one clinical isolated bacterial species and four clinical isolated fungal species were presented.
MATERIALS AND METHODS
Chemicals
2,2´-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), riboflavin, methionine, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), nitroblue tetrazolium (NBT), the stable free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH·), 3-(2-Pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine), linoleic acid, α-tocopherol, polyoxyethylenesorbitan monolaurate (Tween-20) and trichloroacetic acid (TCA) were obtained from Sigma-Aldrich GmbH, Sternheim, Germany. Ammonium thiocyanate was purchased from Merck. Muller Hinton agar was also obtained from Oxoid Ltd. (Basingstoke, Hampshire, England, CM337). All other chemicals used were in analytical grade and obtained from either Sigma-Aldrich or Merck.
Plant Material
Air-dried aerial parts of the plant Cyclotrichium niveum (0.1 kg) were collected during the month of September at the flowering stage from plants from the Nemrut Mountain in Kıran-Subası area of Adıyaman province, Turkey. The plants were identified by senior taxonomists, Dr. Ahmet Zafer Tel at the herbarium of Kütahya Dumlupınar University, Science and Arts Faculty, Department of Biology. The aerial parts were dried in the shade at room temperature.
Extraction Procedures
Extraction was carried out as described previously.[Citation24,Citation25] They were shade dried initially. For water extraction, 25 g of air-dried aerial parts of Cyclotrichium niveum ground into a fine powder in a mill and was mixed with 400 ml boiling water by magnetic stirrer during 15 min. Then the extract was filtered over cheese-cloth and Whatman No. 1 paper, respectively. The filtrates were frozen at –84°C in ultra low temperature freezer (Sanyo, Japan) and lyophilized in lyophilizator at 5 mm-Hg pressures at –50°C (Labconco, Freezone, Japan).
In order to determine of ethanol extraction, 25 g sample of air-dried aerial parts of Cyclotrichium niveum ground into a fine powder in a mill and was mixed with 500 ml ethanol. The residue was re-extracted under same condition until extraction solvents became colourless. The obtained extracts were filtered over Whatman No. 1 paper and the filtrate was collected, then ethanol was removed using a rotary evaporator (RE 100 Bibby, Stone Staffordshire England, ST15 OSA) at 40°C to obtain dry extract. The both extracts were placed in a plastic bottle and then stored at −20°C until used.[Citation26]
Determination of Total Phenolics Content
The content of total phenolics was determined according to a modified version of the procedure described by Slinkard and Singleton[Citation27] with slight modification. Folin-Ciocalteu is a method used for the determination of total phenolic compounds. Gallic acid was used as a standard phenolic compound. Briefly, 1 mg of WECN or EECN in a volumetric flask diluted with 23 ml of distilled water. Then, 0.5 mL of Folin-Ciocalteu reagent was added and the content of the flask mixed thoroughly. After 3 min, 1.5 ml of Na2CO3 (2%) was added and then the mixture was allowed to stand for 2 h with intermittent shaking. The absorbance was measured at 760 nm in a spectrophotometer. The amount of total phenolic compounds in the black pepper extracts determined as microgram of gallic acid equivalent using an equation that was obtained from standard gallic acid graph (r 2: 0.9944):
The content of total phenolics in each extract was calculated by employing a standard above curve prepared using gallic acid and expressed as micrograms of gallic acid equivalents (GAE).
Determination of Total Flavonoids Concentration
Total flavonoids amount in the both extracts was determined as follows: WECN and EECN solution (1 mg) was diluted with 4.3 mL of 80% aqueous ethanol containing 0.1 mL of 10% aluminium nitrate and 0.1 mL of 1 M aqueous potassium acetate. After 40 min incubation at room temperature, the absorbance was determined spectrophotometrically at 415 nm. Total flavonoids concentration was calculated using quercetin as standard:[Citation28]
The content of total flavonoids in WECN and EECN was calculated from a standard above curve prepared using quercetin and expressed as micrograms of quercetin equivalents (QE).
Total Antioxidant Activity Determination by Ferric Thiocyanate Method
The antioxidant activity of WECN, EECN and standards was determined according to the ferric thiocyanate method.[Citation29] For stock solutions, WECN and EECN were diluted with the same solvent, used for extraction to a suitable concentration (1 mg/mL) for analysis. Then, the solution which contains the same concentration of stock WECN and EECN solution or standard samples (from 15 μg/mL to 45 μg/mL) in 2.5 mL of sodium phosphate buffer (0.04 M, pH 7.0) was added to 2.5 mL of linoleic acid emulsion in sodium phosphate buffer (0.04 M, pH 7.0). Therefore, 10 mL of the linoleic acid emulsion was prepared by mixing and homogenising 31 μL of linoleic acid, 1 mg/mL of tween-20 as emulsifier, and 10 mL phosphate buffer (pH 7.0). On the other hand, the same volume of control solution was composed of 5 mL of linoleic acid emulsion and 5 mL, 0.04 M sodium phosphate buffer (pH 7.0). Five mL of these mixed solution was incubated at 37°C in polyethylene flask. The peroxide level was determined by reading the absorbance at 500 nm in a spectrophotometer (Shimadzu, UV-1208 UV-VIS Spectrophotometer, Japan, Cat No: 2006-67603-93, Serial No: A1012 3300010YS) after reaction with FeCl2 and thiocyanate at intervals during incubation. During the linoleic acid oxidation, peroxides are formed and that leads to oxidation of Fe2+ to Fe3+. The latter ions form a complex with ammonium thiocyanate and this complex has a maximum absorbance at 500 nm. This step was repeated every 5 h until the control reached its maximum absorbance value. The percentage inhibition values were calculated at this point (30 h). High absorbance indicates high linoleic acid emulsion peroxidation. The solutions without WECN and EECN were used as blank samples. Total antioxidant activity determination was performed triplicate. All data on total antioxidant activity are the average of duplicate experiments. The inhibition percentage of lipid peroxidation in linoleic acid emulsion was calculated by following equation:
where AControl is the absorbance of control reaction which contains only linoleic acid emulsion and sodium phosphate buffer and ASample is the absorbance in the presence of sample WECN and EECN or standard compounds.[Citation63]
Total Reduction Capability
The samples prepared for ferric thiocyanate method were used for this and the other assays. The reducing power of WECN and EECN was determined by the method of Oyaizu.[Citation30] Different concentrations of WECN and EECN (15–45 μg/mL) in 1 mL of distilled water were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. Aliquots (2.5 mL) of trichloroacetic acid (10%) were added to the mixture. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm in a spectrophotometer. Increased absorbance of the reaction mixture indicates an increase of reduction capability.
Ferrous Ions (Fe2+) Chelating Activity
The chelating of ferrous ions by WECN, EECN, and standards was estimated by the method of Dinis and co-workers.[Citation31] The reaction was performed in an aqueous medium. Briefly, WECN and EECN (15–60 μg/mL) in 0.4 mL was added to a solution of 2 mM FeCl2 (0.2 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.4 mL) and total volume was adjusted to 4 mL of ethanol. Then, the mixture was shaken vigorously and left at room temperature for ten min. Absorbance of the solution was then measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated by using the formula given below:
where AControl is the absorbance of control and ASample is the absorbance in the presence of WECN and EECN or standards. The control contains FeCl2 and ferrozine, complex formation molecules.[Citation14]
Hydrogen Peroxide Scavenging Activity
The hydrogen peroxide scavenging assay of WECN and EECN was carried out following the procedure of Ruch et al.[Citation32] For this aim, a solution of H2O2 (43 mM) was prepared in phosphate buffer (0.1 M, pH 7.4). WECN and EECN at the 15 μg/mL concentration in 3.4 mL phosphate buffer were added to 0.6 mL of H2O2 solution (0.6 mL, 43 mM). The absorbance value of the reaction mixture was recorded at 230 nm. Blank solution was containing the phosphate buffer without H2O2. The concentration of hydrogen peroxide in the assay medium was determined using a standard curve:
The percentage of H2O2 scavenging of WECN, EECN and standard compounds was calculated using the following equation:
where AControl is the absorbance of the control and ASample is the absorbance in the presence of the sample of WECN, EECN or standards.[Citation33]
Radical Scavenging Activity
The total free radical scavenging capacity of WECN and EECN was determined and compared to that of BHA, BHT, α-tocopherol, and trolox by using the DPPH, ABTS, and superoxide anion radical scavenging methods.
DPPH Free Radical Scavenging Activity
The radical scavenging activity of the WECN and EECN was determined spectrophotometrically by monitoring the disappearance of DPPH· at 517 nm, according to the methodology of Blois[Citation34] previously described by Gulcin.[Citation35] Wherein the bleaching rate of a stable free radical, DPPH· is monitored at a characteristic wavelength in the presence of the sample. In its radical form, DPPH· absorbs at 517 nm, but upon reduction by an antioxidant or a radical species its absorption decreases. Briefly, 0.1 mM solution of DPPH· (10−3 M) was prepared in ethanol and 0.5 ml of this solution was added 1.5 mL of WECN and EECN solution in ethanol at different concentrations (15–60 μg/mL). These solutions were vortexed thoroughly and incubated in dark. A half hour later, the absorbance was measured at 517 nm against blank samples. Lower absorbance of the reaction mixture indicates higher DPPH· free radical scavenging activity. A standard curve was prepared using different concentrations of DPPH·. The DPPH· concentration scavenging capacity was expressed as μM in the reaction medium and calculated from the calibration curve determined by linear regression (r2 :0.9845):
The capability to scavenge the DPPH· radical was calculated using the following equation:
where AControl is the absorbance of the control which contains 0.5 mL DPPH· solution and ASample is the absorbance in the presence of WECN or EECN.[Citation25,Citation36]
ABTS Radical Cation Decolorization Assay
The spectrophotometric analysis of ABTS•+ radical scavenging activity of WECN and EECN was determined according to method of Re and co-workers.[Citation37] This method is based on the ability of antioxidants to quench the long-lived ABTS radical cation, a blue/green chromophore with characteristic absorption at 734 nm, in comparison to that of α-tocopherol, trolox, a water-soluble α-tocopherol analogue, BHA and BHT. The ABTS•+ cation radical was produced by the reaction between 2 mM ABTS in H2O and 2.45 mM potassium persulfate, stored in the dark at room temperature for 4 h. Before usage, the ABTS•+ solution was diluted to get an absorbance of 0.750 ± 0.025 at 734 nm with phosphate buffer (0.1 M, pH 7.4) before use. Then, 1 ml of ABTS•+ solution was added 3 mL of WECN or EECN solution in ethanol at different concentrations (15–60 μg/mL). After thirty min, the percentage inhibition of samples at 734 nm was calculated for each concentration relative to a blank absorbance. Solvent blanks were run in each assay. The extent of decolorization is calculated as percentage reduction of absorbance. For preparation of a standard curve, different concentrations of ABTS•+ was used. The ABTS•+ concentration (mM) in the reaction medium was calculated from the following calibration curve, determined by linear regression (r 2:0.9841):
The scavenging capability of ABTS•+ radical was calculated using the following equation:
where in AControl is the initial concentration of the ABTS•+ and ASample is absorbance of the remaining concentration of ABTS•+ in the presence of WECN or EECN.[Citation38]
Superoxide Anion Radical Scavenging Activity
Superoxide radicals were generated by method of Beauchamp and Fridovich[Citation39] described by Zhishen et al.[Citation40] with slight modification. Superoxide radicals are generated in riboflavin, methionine, illuminate and assayed by the reduction of NBT to form blue formazan. All solutions were prepared in 0.05 M phosphate buffer (pH 7.8). The photo-induced reactions were performed using fluorescent lamps (20 W). The concentration of WECN or EECN in the reaction mixture was 15 μg/mL. The total volume of the reactant mixture was 5 ml and the concentrations of the riboflavin, methionine and nitro blue tetrazolium (NBT) was 1.33 × 10–5, 4.46 × 10–5, and 8.15 × 10–8 M mol/L, respectively. The reactant was illuminated at 25°C for 40 min. The photochemically reduced riboflavins generated O2 •, which reduced NBT to form blue formazan. The unilluminated reaction mixture was used as a blank. The absorbance was measured at 560 nm. WECN or EECN were added to the reaction mixture, in which O2 •− was scavenged, thereby inhibiting the NBT reduction. Decreased absorbance of the reaction mixture indicates increased superoxide anion scavenging activity. The inhibition percentage of superoxide anion generation was calculated by using the following formula:
where ASample is the absorbance of WECN, EECN, or standards.[Citation41]
Preparation of Test Microorganisms
Gram-positive bacteria (Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus saprophyticus, Staphylococcus warneri, Staphylococcus xylosus, Micrococcus luteus, Bacillus cereus, Bacillus subtilis and Streptococcus pneumoniae), gram-negative bacteria (Escherichia coli, Citrobacter freundii, Citrobacter koseri, Enterobacter aerogenes, Neisseria lactamica, Neisseria sicca, Proteus vulgaris, Proteus mirabilis, Pseudomonas aeroginosa, Pseudomonas fluorescens, Klebsiella pneumonia and Klebsiella oxyttoca) and fungi (Candida albicans, Candida tropicalis, Candida parapsilosis and Candida glabrata) were employed for determination of antimicrobial and antifungal activity. Bacteria and fungi were obtained from the stock cultures of Microbiology Laboratory, Department of Microbiology, Veterinary Faculty, Atatürk University, Erzurum. The bacterial and fungal stock cultures were maintained on Muller Hinton Agar (Oxoid CM 337, Basingstoke, Hampshire, UK) slants, respectively, which were stored at 4°C. For the purpose of antimicrobial evaluation, 21 microorganisms were used. These bacteria were maintained on Blood agar base (Oxoid CM55, Basingstoke, Hampshire, UK). The fungus was maintained on Sabouraud-dextrose agar (Oxoid CM41, Basingstoke, Hampshire, UK), which is often used with antifungal for the isolation of the pathogenic fungi.
Antimicrobial Activity Determination
The antibacterial activity of the WECN and EECN was carried out by disc-diffusion test. Agar cultures of the test microorganisms were prepared as described by.[Citation42–44] For this purpose, three to five similar colonies were selected and transferred with loop into 5 ml of tryptone soya broth (Oxoid CM129, Basingstoke, Hampshire, UK). Tryptone soya broth is a highly nutritious versatile medium, which is recommended for general laboratory use and used for the cultivation of aerobes and facultative anaerobes, including some fungi. The broth cultures were incubated for 24 h at 37°C. For screening, sterile, 6-mm diameter filter paper disc were impregnated with 45 μg/mL of the water or ethanol extracts. The both WECN and EECN dissolved in sterile water for the assay by magnetic stirrer. Then the paper discs placed onto Mueller Hinton agar (Oxoid CM337, Basingstoke, Hampshire, UK). The inoculum for each organism was prepared from broth cultures. The concentration of cultures was to 108 colony forming units (1 × 108 CFU/mL). The results were recorded by measuring the zones of growth inhibition surrounding the disc. Clear inhibition zones around the discs indicate the presence of antimicrobial activity.[Citation11,Citation43] All data on antimicrobial activity are the average of triplicate analyses. Ampicillin (10 μg/disc), amoxicillin (25 μg/disc), Sefuroksim (30 μg/disc) and antifungal micanozale nitrate (40 μg/disc, DRG International) were used as reference standards, which recommended by the National Committee for Clinical Laboratory Standards.[Citation45]
Statistical Analysis
The experimental results are expressed as mean ± standard deviation (S.D.) of triplicate measurements and analysed by SPSS (version 11.5 for Windows 2000, SPSS Inc.). One-way analysis of variance was performed by ANOVA procedures. Significant differences between means were determined by Duncan's Multiple Range tests. P < 0.05 was regarded as significant and p < 0.01 was very significant.
RESULTS AND DISCUSSION
In the past two decades, research in nutrition and food science has focused on plant products with potential antioxidant and antimicrobial activities. Such products are also rich in fibre, have no cholesterol and contain antioxidants such as carotenoids and flavonoids and others phenolic compounds.[Citation46] Phenolic compounds are called high-level antioxidants because of their ability to scavenge free radicals and ROS such as singlet oxygen, superoxide free radicals and hydroxyl radicals and metal ion chelators.[Citation47] The yield of crude extracts, total phenolics content of WECN or EECN are shown in . No significant differences in scavenging potential could be determined among different amount WECN and EECN (p < 0.05). For determining total phenolic contents, calibration curves were obtained using known quantities of standard gallic acid. The phenolic compounds of 0.5 mg of WECN and EECN ranged from 32.21 ± 1.8 to 37.21 ± 2.2 μg GAE, respectively. In both extracts, EECN possessed the highest the phenolic compounds. It was reported that phenolic compounds were associated with antioxidant activity and play an important role in stabilizing lipid peroxidation.[Citation26,Citation48] According to the recent reports, a highly positive relationship between total phenols and antioxidant activity was found in many plant species. These results indicate that there is correlation between antioxidant activity and total phenolic content. Different results were reported on this aspect; some authors found correlation between phenolic content and antioxidant activity.[Citation49]
Table 1 The yield of crude extracts, total phenolics content and total flavonoids content of Cyclotrichium niveum extracts [WECN: Water extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng, EECN: Ethanol extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng]
Flavonoids are an important group of natural compounds, which can prevent coronary heart disease and have antioxidant properties. The content of total flavonoids in WECN and EECN was determined spectrophotometrically found to be 1.98 ± 0.15 and 3.92 ± 0.17 quercetin equivalent, respectively. These results clearly demonstrated that there is a positive correlation between total flavonoids content in WECN and EECN and antioxidant activities.
It was reported in many studies, natural antioxidants were closely related to their biofunctionalities, such as the reduction of chronic diseases like DNA damage, mutagenesis, carcinogenesis and inhibition of pathogenic bacteria growth which is often associated with the termination of free radical propagation in biological systems.[Citation50] Thus, antioxidant capacity is widely used as a parameter for food, pharmaceutical and medicinal extracts. In this study, the antioxidant activity of the WECN and EECN was compared to α-tocopherol and its water-soluble analogue trolox. α-Tocopherol is a biological lipid antioxidant that prevents the formation of free radicals from lipid peroxidation.[Citation51] The antioxidant activity of the WECN, EECN, BHA, BHT, α-tocopherol, and trolox has been evaluated in a series of in vitro tests: DPPH free radical scavenging, ABTS•+ radical scavenging, scavenging of superoxide anion radical-generated non-enzymatic system, total antioxidant activity by ferric thiocyanate method, reducing power by Fe3+-Fe2+ transformation, hydrogen peroxide scavenging, and metal chelating activities.
Total Antioxidant Activity Determination in Linoleic Acid Emulsion by Ferric Thiocyanate Method
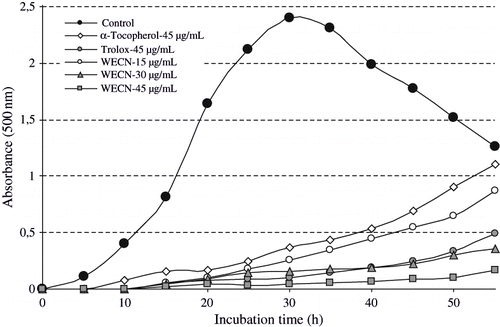
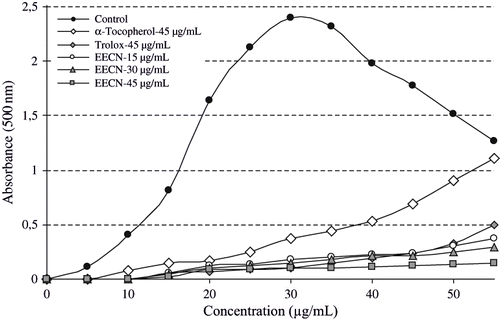
Lipid peroxidation involves a series of free radical-mediated chain reaction processes and is also associated with several types of biological damage. The role of free radicals and ROS is becoming increasingly recognized in the pathogenesis of many human diseases, including cancer, aging, and atherosclerosis.[Citation52] The ferric thiocyanate method measures the amount of peroxide produced during the initial stages of oxidation which is the primary product of lipid oxidation. Total antioxidant activity of WECN, EECN, α-tocopherol, and trolox was determined by the ferric thiocyanate method in the linoleic acid system WECN, EECN and standard compounds exhibited effective antioxidant activity in this system. The effects of different concentrations (15–45 μg/mL) of WECN and EECN on lipid peroxidation of linoleic acid emulsion are shown in and . The percentage inhibition of lipid peroxidation of 45 μg/mL of WECN and EECN was found to be 98.0 and 95.9%, respectively, and their activities are greater than that of α-tocopherol (84.6%) and similar to trolox (95.6%) at the same concentration.
Total Reductive Capability Using the Potassium Ferricyanide Reduction Method
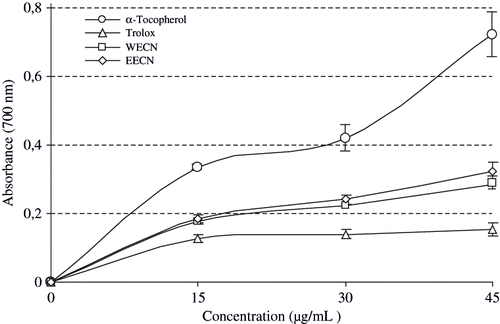
In this assay, the yellow colour of the test solution changes to various shades of green and blue depending on the reducing power of antioxidant samples. The presence of reductants such as antioxidant substances in the antioxidant samples causes the reduction of the Fe3+/ferricyanide complex to the ferrous form. Therefore, Fe2+ can be monitored by measuring the formation of Perl's Prussian blue at 700 nm.[Citation53]
shows the reducing power of the WECN and EECN and standards (α-tocopherol and trolox) using the potassium ferricyanide reduction method. Fe3+-Fe2+ transformation was investigated for the measurements of the reductive ability of WECN and EECN using the method of Oyaizu.[Citation30] The reducing power of WECN, EECN, α-tocopherol and trolox increased with increasing concentration of samples (from 15 to 45 μg/mL). At these different concentrations, WECN and EECN showed an effective reducing power (). When these activities compared to the control values statistically significant differences was found (p < 0.05). Reducing power of WECN, EECN and standard compounds exhibited the following order: α-tocopherol > EECN > WECN > trolox. The results on reducing power demonstrate the electron donor properties of WECN, EECN, thereby neutralizing free radicals by forming stable products. The outcome of the reducing reaction is to terminate the radical chain reactions that may otherwise be very damaging.
Ferrous Ions (Fe2+) Chelating Capacity
Ferrous ions (Fe2+) chelation may render important antioxidative effects by retarding metal-catalyzed oxidation. Ferrous ion chelating activities of WECN, EECN, α-tocopherol, and trolox are shown in . Among the transition metals, iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. The effective ferrous ions chelators may also afford protection against oxidative damage by removing iron (Fe2+) that may otherwise participate in HO• generating Fenton reaction.
Table 2 Comparison of hydrogen peroxide (H2O2) scavenging activity, ferrous ions (Fe2 +) chelating activity, and superoxide anion radicals (O2 • -) scavenging activity of WECN, EECN and standard antioxidant compounds such as BHA, BHT, α-tocopherol, and trolox at the concentration of 15 μg/mL [BHA: Butylated hydroxyanisole, BHT: Butylated hydroxytoluene, WECN: Water extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng, EECN: Ethanol extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng]
Ferric (Fe3+) ions also produce radicals from peroxides although the rate is tenfold less than that of ferrous (Fe2+) ions.[Citation54] Fe2+ ion is the most powerful pro-oxidant among the various species of metal ions.[Citation55] Fe2+ is able to generate free radicals from peroxides by Fenton reactions and may be involved in the progression of human cardiovascular disease. Thus, antioxidants capable of chelating with Fe2+ will minimize the ion's concentration and inhibit its capacity to catalyze free radical formation, which will results in protection against oxidative damage. Minimizing ferrous (Fe2+) ions may afford protection against oxidative damage by inhibiting production of ROS and lipid peroxidation. Ferrozine can quantitatively form complexes with Fe2+. In the presence of chelating agents, the complex formation is disrupted, resulting in a decrease in the red colour of the complex. Measurement of colour reduction, therefore, allows estimating the metal chelating activity of the coexisting chelator. In this assay, WECN and EECN are interfered with the formation of ferrous and ferrozine complex, suggesting that they have chelating activity and are able to capture ferrous ion before ferrozine.
In regard to , the absorbance of Fe2+-ferrozine complex was decreased at 15 μg/mL concentration of WECN or EECN. The difference between the 15 μg/mL concentration of WECN, EECN and control was found statistically significant (p < 0.01). At above mentioned concentration, WECN and EECN exhibited 62.5 ± 5.3 and 55.6% ± 2.1 chelation of ferrous ion at 15 μg/mL concentration. On the other hand, the percentages of metal chelating capacity of same concentration of BHA, BHT, α-tocopherol, and trolox were found as 69.9 ± 7.5, 60.0 ± 9.3, 31.3 ± 5.5, and 45.2% ± 6.2, respectively. The metal chelating effect of those samples decreased in the order of BHA > WECN > BHT > EECN > trolox > α-tocopherol.
Metal chelating capacity was significant since it reduced the concentration of the catalysing transition metal in lipid peroxidation. Chelating agents are effective as secondary antioxidants because they reduce the redox potential thereby stabilizing the oxidized form of the metal ion. The data obtained from reveal that WECN and EECN demonstrates a marked capacity for iron binding, suggesting that their main action as peroxidation protector may be related to its iron binding capacity.
Hydrogen Peroxide Scavenging Activity
Hydrogen peroxide can be formed in vivo by many oxidizing enzymes such as superoxide dismutase. It can cross membranes and may slowly oxidize a number of compounds. The ability of WECN and EECN to scavenge hydrogen peroxide was determined according to the method of Ruch and co-workers[Citation32] as shown in and compared with that of BHA, BHT, α-tocopherol, and trolox as standards. WECN and EECN were capable of scavenging H2O2. At 15 μg/mL concentration, WECN and EECN exhibited 88.0 ± 4.8 and 76.1% ± 3.1 scavenging activity. On the other hand, BHA, BHT, α-tocopherol and trolox exhibited 36.4 ± 3.5, 34.3 ± 4.1, 39.3 ± 2.9, and 25.5% ± 3.3 hydrogen peroxide scavenging activity at the same concentration, respectively. These results showed that WECN and EECN had an effective hydrogen peroxide scavenging activity. At 15 μg/mL concentration, the hydrogen peroxide scavenging effect of WECN, EECN and four standard compounds decreased in the order of WECN > EECN > α-tocopherol > BHA > BHT > trolox. Hydrogen peroxide is not very reactive; however it can sometimes be toxic to cell because it may give rise to hydroxyl radical in the cells. Addition of hydrogen peroxide to cells in culture can lead to transition metal ion-dependent OH radicals mediated oxidative DNA damage. Levels of hydrogen peroxide at or below about 20–50 mg seem to have limited cytotoxicity to many cell types. Thus, removing hydrogen peroxide, as well as superoxide anion is very important for protection of pharmaceutical and food systems.
Radical Scavenging Activity
Antioxidant properties, especially radical scavenging activities, are very important due to the deleterious role of free radicals in foods and in biological systems. Excessive formation of free radicals accelerates the oxidation of lipids in foods and decreases food quality and consumer acceptance.[Citation56] A lot of methods are currently used to assess the antioxidant activity of plant extracts. Chemical assays are based on the ability to scavenge synthetic free radicals, using a variety of radical-generating systems and methods for detection of the oxidation end-point. ABTS•+ or DPPH radical scavenging methods are common spectrophotometric procedures for determining the antioxidant capacities of plant components. These chromogens (the violet DPPH radical and the blue green ABTS radical cation) are easy to use, have a high sensitivity, and allow for rapid analysis of the antioxidant activity of a large number of samples. These assays have been applied to determine the antioxidant activity of food, wine, plant extracts and pure components.[Citation56–58]
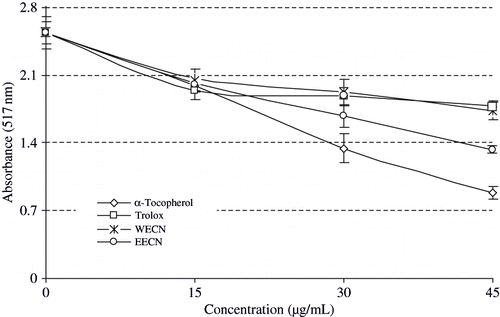
DPPH is long-lived nitrogen radical. Many antioxidants that react quickly with transient radicals such as peroxyl radical may react slowly or may be even inert to DPPH•.[Citation59] DPPH• has also been widely used for evaluation of free radical scavenging effectiveness of various antioxidant substances.[Citation60] In the DPPH• assay, the antioxidants were able to reduce the stable radical DPPH• to the yellow coloured diphenyl-picrylhydrazine. The method is based on the reduction of alcoholic DPPH• solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H by the reaction. DPPH• is usually used as a reagent to evaluate free radical scavenging activity of antioxidants.[Citation30] DPPH• is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule.[Citation61] The reduction capability of DPPH radical is determined by the decrease in absorbance at 517 nm induced by antioxidants.
With this method it was possible to determine the antiradical power of an antioxidant activity. Resulting a colour change from purple to yellow, the absorbance decreased when the DPPH· was scavenged by an antioxidant through donation of hydrogen to form a stable DPPH· molecule. In the radical form, this molecule had an absorbance at 517 nm, which disappeared after acceptance of an electron or hydrogen radical from an antioxidant compound to become a stable diamagnetic molecule.[Citation62] illustrates a significant decrease (p < 0.01) in the concentration of DPPH radical due to the scavenging ability of WECN, EECN, and standards. BHA, BHT, α-tocopherol, and trolox were used as references for radical scavengers. The scavenging effect of WECN, EECN and standards on the DPPH radical decreased in the order of BHA > α-tocopherol > BHT > EECN > WECN > trolox, which were 67.8, 64.9, 62.5, 51.9, 31.8, and 29.4%, at the concentration of 15 μg/mL, respectively. Free radical scavenging activity of these samples also increased with an increasing concentration.
Figure 4 DPPH free radical scavenging activity of WECN, EECN, α-tocopherol and trolox (DPPH: 1,1-diphenyl-2-picryl-hydrazyl; WECN: Water extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng, EECN: Ethanol extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng).
Generation of the ABTS radical cation forms the basis of one of the spectrophotometric methods that have been applied to the measurement of the total antioxidant activity of solutions of pure substances, aqueous mixtures and beverages.[Citation38,Citation54] A more appropriate format for the assay is a decolorization technique in that the radical is generated directly in a stable form prior to reaction with putative antioxidants. The improved technique for the generation of ABTS•+ described here involves the direct production of the blue/green ABTS•+ chromophore through the reaction between ABTS and potassium persulfate.[Citation63]
WECN and EECN exhibited effectual radical cation scavenging activity. As seen in , the both extracts had effective ABTS•+ radical scavenging activity in a concentration-dependent manner (15–60 μg/mL). There is a significant decrease (p < 0.01) in the concentration of ABTS•+ due to the scavenging capacity of all WECN and EECN concentrations. Also, the scavenging effect of WECN and EECN and standards on the ABTS•+ decreased in that order: BHA > BHT > α-tocopherol > trolox > WECN > EECN, which were 100, 95.7, 79.2, 53.8, 45.7, and 28.8%, at the concentration of 60 μg/mL, respectively.
Figure 5 ABTS•+ radical scavenging activity of WECN, EECN, α-tocopherol and trolox (ABTS: 2,2´-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), WECN: Water extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng, EECN: Ethanol extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng).
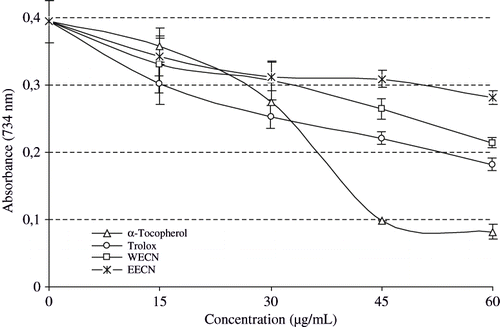
Superoxide, an oxygen-centred radical with selective reactivity, has been observed to directly initiate lipid peroxidation. Also, they are free radicals that have potential of reacting with biological macromolecules and thereby inducing tissue damage.[Citation55] Additionally, it has been implicated in several pathophysiological processes due to its transformation into more reactive species such as hydroxyl radical that initiate lipid peroxidation.[Citation64] It has also been reported that antioxidant properties of some flavonoids are effective mainly via scavenging of superoxide anion radical.[Citation65] Superoxide anion plays an important role in the formation of other ROS such as hydrogen peroxide, hydroxyl radical, and singlet oxygen, which induce oxidative damage in lipids, proteins, and DNA.[Citation66] These species are produced by a number of enzyme systems in autoxidation reactions and by nonenzymatic electron transfers that univalently reduce molecular oxygen. It can also reduce certain iron complex such as cytochrome c.
Superoxide radical is normally formed first, and its effects can be magnified because it produces other kinds of free radicals and oxidizing agents.[Citation67] Superoxide anion derived from dissolved oxygen by riboflavin/methionine/illuminate system. In this method, superoxide anion reduces the yellow dye (NBT2+) to produce the blue formazan which is measured spectrophotometrically at 560 nm. Antioxidants are able to inhibit the blue NBT formation.[Citation68] The decrease of absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion in the reaction mixture. shows the inhibition percentage of superoxide radical generation by 15 μg/mL concentration of WECN, EECN and standards. As can be see from , the percentage inhibition of superoxide anion radical generation by 15 μg/mL concentration of WECN, EECN was found as 57.6 ± 3.2 and 58.1 ± 4.2%. On the other hand, at the same concentration BHA, BHT, α-tocopherol and trolox exhibited 75.3 ± 6.5, 70.2 ± 7.1, 22.2 ± 3.3, and 16.0% ± 1.9 superoxide anion radical scavenging activity, respectively.
Antimicrobial Activity
Herbs and spices with antimicrobial activity have been widely used both traditionally and commercially to increase the shelf-life and safety of foods.[Citation69,Citation70] Many naturally occurring extracts from edible and medicinal plants, herbs, and spices have been shown to possess antimicrobial functions and could serve as a source for antimicrobial agents against food spoilage and pathogens.[Citation71] Antimicrobial and antifungal activities of WECN and EECN were made according to disc-diffusion method. This method is extensively used for investigation of antibacterial activity of natural substances and plant extracts. These assays are based on the use of discs as reservoirs containing solutions of substances to be examined. In the case of solutions with a low activity, however, a large concentration or volume is needed. The limited capacity of discs means that holes or cylinders are preferably used.[Citation72]
In this study, twenty-one different microbial and four fungi species were used to screen the possible antimicrobial activities of both WECN and EECN. summarizes the microbial growth inhibition by WECN and EECN. As can be seen from , most of the gram-positive and gram-negative bacterial species and the fungi species were inhibited by WECN and EECN. However, the antimicrobial activity of WECN was not detected against Staphylococcus aureus, Escherichia coli and Enterobacter aerogenes. Besides that EECN had effective antibacterial activity against Escherichia coli which is a gram-negative bacterium, belonging to the normal flora of humans. However, an enterohemmoragic strain of Escherichia coli has caused serious food poisoning, and preservatives to eliminate its growth are needed. Therefore, the both extracts had inhibition effects on the generation Candida albicans which is the microbe responsible for most clinical yeast infections, e.g., in mouth infections (See ). Ampicillin (10 μg/disc), Amoxicillin (25 μg/disc), and Sefuroksim (30 μg/disc) were used as positive controls for bacteria. Micanozale nitrate (40 μg/disc) was used as positive controls for antifungal activity.
Table 3 Antibacterial activity of WECN and EECN against the bacteria strains based on disc diffusion assay [WECN: Water extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng, EECN: Ethanol extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng]
Table 4 Antifungal activity of WECN and EECN against the fungal based on disc diffusion and microdilution assay [WECN: Water extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng, EECN: Ethanol extract of Cyclotrichium niveum (Boiss.) Manden. & Scheng]
CONCLUSION
According to the data of the present study, WECN and EECN was found to be an effective antioxidant and antiradical in different in vitro assay including reducing power, DPPH radical ABTS radical and superoxide anion radical scavenging, hydrogen peroxide scavenging, and metal chelating activities, when it is compared to standard antioxidant compounds such as α-BHA, BHT, tocopherol, a natural antioxidant, and trolox, which is a water-soluble analogue of tocopherol. This study showed that WECN and EECN are a good source of antioxidants for food, medicinal, and pharmaceuticals. Based on the previous discussion, both of these extracts can be used for minimizing or preventing lipid oxidation in food products, retarding the formation of toxic oxidation products, maintaining nutritional quality, and prolonging the shelf life of foods and pharmaceuticals. Besides these properties, WECN and EECN could be used as a preservative in food products to protect them from microbial spoilage.
REFERENCES
- Liyana-Pathirana , C.M. and Shahidi , F. 2006 . Antioxidant properties of commercial soft and hard winter wheats (Triticum aestivum L.) and their milling fractions . Journal of the Science of Food and Agriculture , 86 : 477 – 485 .
- Horton , A.A. and Fairhurst , S. 1987 . Lipid peroxidation and mechanism of toxicity . Critical Reviews in Toxicology , 18 : 27 – 29 .
- Halliwell , B. and Gutteridge , J.M.C. 1989 . Free radicals in biology and medicine , 23 – 30 . Oxford : Clarendon Press .
- Gülçin , İ , Büyükokuroğlu , M.E. , Oktay , M. and Küfrevioğlu , Ö.İ . 2002 . On the in vitro antioxidant properties of melatonin . Journal of Pineal Research , 33 : 167 – 171 .
- Gülçin , İ , Oktay , M. , Küfrevioğlu , Ö.İ and Aslan , A. 2002 . Determination of antioxidant activity of lichen Cetraria islandica (L) Ach . Journal of Ethnopharmacology , 79 : 325 – 329 .
- Oktay , M. , Gülçin , İ. and Küfrevioğlu , Ö.İ . 2003 . Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts . Lebensmittel Wissenchaft und-Technologie , 36 : 263 – 271 .
- Gülçin , İ , Şat , İ.G. , Beydemir , Ş and Küfrevioğlu , Ö.İ . 2004 . Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.) . Italian Journal of Food Science , 16 : 17 – 30 .
- Büyükokuroğlu , M.E. , Gülçin , İ , Oktay , M. and Küfrevioğlu , Ö.İ. 2001 . In vitro antioxidant properties of dantrolene sodium . Pharmacological Research , 44 : 491 – 495 .
- Velioglu , Y.S. , Mazza , G. , Gao , L. and Oomah , B.D. 1998 . Antioxidant activity and total phenolics in selected fruits, vegetables and grain products . Journal of Agricultural and Food Chemistry , 46 : 4113 – 4117 .
- Gülçin , İ . 2006 . Antioxidant and antiradical activities of L-Carnitine . Life Sciences , 78 : 803 – 811 .
- Gülçin , İ , Oktay , M. , Kireçci , E. and Küfrevioğlu , Ö.İ. 2003 . Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts . Food Chemistry , 83 : 371 – 382 .
- Sherwin , E.R. , Branen , A.L. , Davidson , P.M. and Salminen , S. , eds. 1990 . Food additives , 139 – 193 . New York : Marcel Dekker Inc .
- Sun , B. and Fukuhara , M. 1997 . Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug metabolizing enzymes in mice . Toxicology , 122 : 61 – 72 .
- Gülçin , İ , Mshvildadze , V. , Gepdiremen , A. and Elias , R. 2006 . Screening of antioxidant and antiradical activity of monodesmosides and crude extract from Leontice smirnowii Tuber . Phytomedicine , 13 : 343 – 351 .
- Yu , L.L. , Zhou , K.K. and Parry , J. 2005 . Antioxidant properties of cold pressed black caraway, carrot, cranberry, and hemp seed oils . Food Chemistry , 91 : 723 – 729 .
- Sacchetti , G. , Maietti , S. , Muzzoli , M. , Scaglianti , M. , Manfredini , S. , Radice , M. and Bruni , R. 2005 . Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods . Food Chemistry , 91 : 621 – 632 .
- Liu , F. and Ng , T.B. 2000 . Antioxidative and free radical scavenging activities of selected medicinal herbs . Life Sciences , 66 : 725 – 735 .
- Baser , K.H.C. , SarıkardaŞoglu , S. and Tümen , G. 1994 . The Essential Oil of Cyclotrichium niveum (Boiss.) Manden & Scheng . Journal of Essential Oil Research , 6 : 9 – 12 .
- Tümen , G. , Kırımer , N. , Ermin , N. and Başer , K.H.C. 1998 . The essential oil of Satureja cuneifolia: A substitute of Thyme in Turkey . Planta Medica , 64 : 81 – 83 .
- Başer , K.H.C. , Kırımer , N. , Ermin , N. , Kürkçüoğlu , M. and Tümen , G. 1996 . Essential oils from four chemotypes of Thymus zygioides Griseb. var. lycaonicus (Celak) Ronniger . Journal of Essential Oil Research , 8 : 615 – 618 .
- Başer , K.H.C. 2002 . Aromatic biodiversity among the flowering plant taxa of Turkey . Pure and Applied Chemistry , 74 : 527 – 545 .
- Doğanca , S. , Ulubelen , A. and Tuzlaci , E. 1989 . Flavones from Cyclotrichium niveum . Phytochemistry , 28 : 3561 – 3562 .
- Baytop , T. 1999 . Therapy with medicinal plants in Turkey (Past and Present). No. 3255 , 2nd , 82 – 83 . Istanbul : Publications of the Istanbul University .
- Gülçin , İ , Şat , İ.G. , Beydemir , Ş , Elmastaş , M. and Küfrevioğlu , Ö.İ. 2004 . Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.) . Food Chemistry , 87 : 393 – 400 .
- Gülçin , İ , Beydemir , Ş , Alici , H.A. , Elmastaş , M. and Büyükokuroğlu , M.E. 2004 . In vitro antioxidant properties of morphine . Pharmacological Research , 49 : 59 – 66 .
- Gülçin , , İ . 2005 . The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds . International Journal of Food Sciences and Nutrition , 56 : 491 – 499 .
- Slinkard , K. and Singleton , V.L. 1977 . Total phenol analyses: Automation and comparison with manual methods . American Journal of Enology and Viticulture , 28 : 49 – 55 .
- Park , Y.K. , Koo , M.H. , Ikegaki , M. and Contado , J.L. 1997 . Comparison of the flavonoid aglycone contents of Apis mellifera propolis from various regions of Brazil . Arquivos de Biologiae Technologia , 40 : 97 – 106 .
- Mitsuda , H. , Yuasumoto , K. and Iwami , K. 1996 . Antioxidation action of indole compounds during the autoxidation of linoleic acid . Eiyo to Shokuryo , 19 : 210 – 214 .
- Oyaizu , M. 1986 . Studies on product of browning reaction prepared from glucose amine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Dinis , T.C.P. , Madeira , V.M.C. and Almeida , L.M. 1994 . Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers . Archive of Biochemistry and Biophysics , 315 : 161 – 169 .
- Ruch , R.J. , Cheng , S.J. and Klaunig , J.E. 1989 . Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea . Carcinogenesis , 10 : 1003 – 1008 .
- Elmastaş , M. , Gülçin , , İ , Öztürk , L. and Gökçe , , İ . 2005 . Investigation of antioxidant properties of spearmint (Mentha spicata L.) . Asian Journal of Chemistry , 17 : 137 – 148 .
- Blois , M.S. 1958 . Antioxidant determination by the use of a stable free radical . Nature , 26 : 1199 – 1200 .
- Gülçin , , İ . 2007 . Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa . Amino Acids , 32 : 431 – 438 .
- Elmastaş , M. , Gülçin , İ , Beydemir , Ş , Küfrevioğlu , Ö.İ. and Aboul-Enein , H. Y. 2006 . (A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) seeds extracts . Analytical Letters , 39 : 47 – 65 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Gülçin , , İ , Elias , R. , Gepdiremen , A. and Boyer , L. 2007 . Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.) . European Food Research and Technology , 223 : 759 – 767 .
- Beauchamp , C. and Fridovich , I. 1971 . Superoxide dismutase: improved assays and an assay applicable to acrylamide gels . Analytical Biochemistry , 44 : 276 – 287 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoid contents on mulberry and their scavenging effects on superoxide radical . Food Chemistry , 64 : 555 – 559 .
- Gülçin , , İ , Mshvildadze , V. , Gepdiremen , A. and Elias , R. 2004 . Antioxidant activity of saponins isolated from ivy: α-Hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside F . Planta Medica , 70 : 561 – 563 .
- Mackeen , M.M. , Ali , A.M. , El-Sharkawy , S.H. , Manap , M.Y. , Salleh , K.M. , Lajis , N.H. and Kawazu , K. 1997 . Antimicrobial and cytotoxic properties of same Malaysian traditional vegetables . International Journal of Pharmacognosy , 3 ( 5 ) : 237 – 243 .
- Gülçin , İ , Uğuz , M.T. , Oktay , M. , Beydemir , Ş and Küfrevioğlu , Ö.İ . 2004 . Evaluation of antioxidant and antimicrobial activities of clary sage (Salvia sclarea L.) . Turkish Journal of Agriculture and Forestry , 28 : 25 – 33 .
- Gülçin , İ , Küfrevioğlu , Ö.İ. , Oktay , M. and Büyükokuroğlu , M.E. 2004 . Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) . Journal of Ethnopharmacology , 90 : 205 – 215 .
- National Committee For Clinical Laboratory Standards . 1999 . Performance Standards for Antimicrobial Susceptibility Testing; 9th Informational Supplement , Wayne, PA : NCCLS . (NCCLS document M100-S9).
- Kulkarni , S.D. , Tilak , J.C. , Acharya , R. , Rajurkar , N.S. , Devasagayam , T.P.A. and Reddy , A.V.R. 2006 . Evaluation of the antioxidant activity of wheatgrass (Triticum aestivum L.) as an aunction of growth under different conditions . Phytotherapy Research , 20 : 218 – 227 .
- Yildirim , A. , Mavi , A. and Kara , A.A. 2003 . Antioxidant and antimicrobial activities of Polygonum cognaturm Meissn extracts . Journal of Sciences Food and Agriculture , 83 : 64 – 69 .
- Yang , J.H. , Lin , H.C. and Mau , J.L. 2002 . Antioxidant properties of several commercial mushrooms . Food Chemistry , 77 : 229 – 235 .
- Zhu , Q.Y. , Hackman , R.M. , Ensunsa , J.L. , Holt , R.R. and Keen , C.L. 2002 . Antioxidative activities of oolong tea . Journal of Agricultural and Food Chemistry , 50 : 6929 – 6934 .
- Gülçin , , İ , Mshvildadze , V. , Gepdiremen , A. and Elias , R. 2006 . Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-D-glucopyranosyl)-hederagenin . Phytotherapy Research , 20 : 130 – 134 .
- Tavan , E. , Mazier , S. , Narbonne , J.F. and Cassand , P. 1997 . Effects of vitamin A and E on methylazoxymethanol-induced mutagenesis in Salmonella typhimurim strains TA100 . Mutation Research , 377 : 231 – 237 .
- Perry , G. , Raina , A. K. , Nonomura , A. , Wataya , T. , Sayre , L.M. and Smith , M.A. 2000 . How important is oxidative damage? Lessons from Alzheimer's disease . Free Radical Biology and Medicine , 28 : 831 – 834 .
- Chung , Y.C. , Chang , C.T. , Chao , W.W. , Lin , C.F. and Chou , S.T. 2002 . Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1 . Journal of Agricultural and Food Chemistry , 50 : 2454 – 2458 .
- Miller , D.D. , Mineral and Fennema , O.R. , eds. 1996 . Food chemistry , 618 – 649 . New York : Marcel Deckker .
- Halliwell , B. and Gutteridge , J.M.C. 1984 . Oxygen toxicology, oxygen radicals, transition metals and disease . Biochemical Journal , 219 : 1 – 4 .
- Awika , J.M. , Rooney , L.W. , Wu , X. , Prior , R.L. and Cisneros-Zevallos , L. 2003 . Screening methods to measure antioxidant activity of Sorghum (Sorghum bicolor) and Sorghum product . Journal of Agricultural and Food Chemistry , 51 : 6657 – 6662 .
- van den Berg , R. , Haenen , G.R.M.M. , van den Berg , H. , van den Vijgh , W. and Bast , A. 2000 . The predictive value of the antioxidant capacity of structurally related flavonoids using trolox equivalent antioxidant capacity (TEAC) assay . Food Chemistry , 70 : 391 – 395 .
- Yu , L. , Halley , S. , Perret , J. , Harris , M. , Wilson , J. and Qian , M. 2002 . Free radical scavenging properties of wheat extracts . Journal of Agricultural and Food Chemistry , 50 : 1619 – 1624 .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The chemistry behind antioxidant capacity assays . Journal of Agricultural and Food Chemistry , 53 : 1841 – 1856 .
- Özcelik , B. , Lee , J.H. and Min , D.B. 2003 . Effects of light, oxygen and pH on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method to evaluate antioxidants . Journal of Food Science , 68 : 487 – 490 .
- Soares , J.R. , Dins , T.C.P. , Cunha , A.P. and Ameida , L.M. 1997 . Antioxidant activity of some extracts of Thymus zygis . Free Radical Research , 26 : 469 – 478 .
- Matthäus , B. 2002 . Antioxidant activity of extracts obtained from residues of different oilseeds . Journal of Agricultural and Food Chemistry , 50 : 3444 – 3452 .
- Gülçin , , İ . 2006 . Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) . Toxicology , 217 ( 2–3 ) : 213 – 220 .
- Wickens , A.P. 2001 . Aging and the free radical theory . Respiratory Physiology , 128 : 379 – 391 .
- Yen , G.C and Duh , P.D. 1994 . Scavenging effect of methanolic extract of peanut hulls on free radical and active oxygen species . Journal of Agricultural and Food Chemistry , 42 : 629 – 632 .
- Pietta , P.G. 2000 . Flavonoids as antioxidants . Journal of Natural Products , 63 : 1035 – 1042 .
- Liu , F. , Ooi , V.E.C. and Chang , S.T. 1997 . Free radical scavenging activities of mushroom polysaccharide extracts . Life Sciences , 60 : 763 – 771 .
- Parejo , I. , Viladomat , F. , Bastida , J. , Rosas-Romero , A. , Flerlage , N. , Burillo , J. and Codina , C. 2002 . Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants . Journal of Agricultural and Food Chemistry , 50 : 6882 – 6890 .
- Brul , S. and Coote , P. 1999 . Preservative agents in foods. Mode of action and microbial resistance mechanisms . International Journal of Food Microbiology , 50 : 1 – 17 .
- Dupont , S. , Caffin , N. , Bhandari , B. and Dykes , G.A. 2006 . In vitro antibacterial activity of Australian native herb extracts against food-related bacteria . Food Control , 17 : 929 – 932 .
- Oussalah , M. , Caillet , S. , Saucier , L. and Lacroix , M. 2006 . Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat . Meat Science , 73 : 236 – 244 .
- Bartner , A. , Pfeifer , K.P. and Bartner , H. 1994 . Applicability of disc diffusion methods required by the pharmacopoeias for testing antibacterial activity of natural compounds . Pharmazie , 49 : 512 – 516 .