Abstract
Casein was hydrolyzed by alcalase to a degree of hydrolysis of 10.9% to obtain a hydrolysate having ACE-inhibition in vitro with an IC50 value of 52.6 μg/mL. The prepared hydrolysate was modified by alcalase-catalyzed plastein reaction with extrinsic proline added at 0.4 mol/mol free amino groups (on the basis of the hydrolysate), and fractionated by ethanol- or methanol-water solvents in proportions of 3:7, 5:5, or 7:3 (v/v), respectively. With the decrease of free amino groups of the modified hydrolysate as the response, the optimized plastein reaction conditions were alcalase addition of 3.1 kU/g peptides, substrate concentration of 50% (w/v), and reaction temperature of 25°C. Four modified hydrolysates prepared with different reaction times exhibited higher ACE-inhibitory activities than the original hydrolysate. The evaluation results showed that solvent fractionation of the modified hydrolysate with the maximum activity (IC50 = 13.0 μg/mL) yielded the separated soluble fraction's higher activity but the precipitate fraction's lower one. Further enzymatic digestion of the modified hydrolysate with the maximum activity and its two fractionated products by four proteases in vitro caused damage to the activities, but the residual activities of the final digests were higher than that of the original hydrolysate, indicating that the plastein reaction could confer casein hydrolysate protease resistance.
INTRODUCTION
Extensive scientific evidence has been provided for the existence of biologically active peptides derived from food proteins that have beneficial effects on human health. Among these bioactive peptides, angiotensin converting enzyme (ACE)−inhibitory peptides are receiving special attention as hypertension becomes more prevalent and important in many countries.[Citation1,Citation2] ACE−inhibitory peptides have been found in many food protein sources,[Citation3,Citation4] among which milk proteins are a particularly good source of bioactive peptides.[Citation5] By in vitro digestion, casein hydrolysates with ACE−inhibitory activity were obtained from casein preparations[Citation6,Citation7] or individual components of casein,[Citation8 Citation–10] showing that casein was a good source of ACE−inhibitory peptides. Pripp et al.[Citation11] studied the structure–activity relationship of ACE−inhibitory peptides derived from milk proteins and concluded that increased hydrophobicity of the amino acid and absence of positive charge in the C−terminal position of the peptides enhanced the activity. Proline, tryptophane, tyrosine, and phenylalanine were found to be the most effective at the ultimate C-terminal position.[Citation12] Higher ACE−inhibitory activities of the peptides, such as IPP, VPP,[Citation13] LPYPP,[Citation14] VYPFPGPIPNSLPQNIPP, and LVYPFPGPIPNSLPQNIPP,[Citation15] indicated the importance of proline in these peptides.
The plastein reaction is a classic enzyme reaction and has been used for many purposes. For example, Yamashita et al.[Citation16] found that, when the mixture of soybean protein hydrolysate and estered L−methionine was incubated with papain, the methionine was incorporated into the peptides. The plastein reaction was also used to remove the bitterness of protein hydrolysates,[Citation17] reduce the content of some amino acids (e.g., phenylalanine) for dietetic applications,[Citation18] or modify functional properties of the proteins (e.g., solubility).[Citation19] In recent years, the plastein reaction has been used to modify some protein hydrolysates to improve their antioxidant,[Citation20] ACE−inhibitory,[Citation21 Citation–23] or radical scavenging[Citation24] activities in vitro.
Although ACE-inhibitory peptides are generated from enzymatic hydrolysis of protein substrates, further hydrolysis might cause damage to structures and activities. Some peptides were found to be susceptible to enzymatic digestion.[Citation25,Citation26] Whether a protein hydrolysate modified by the plastein reaction is also sensitive to proteases is unknown. In other reported works, organic solvents were used to enhance immunological activity of egg yolk extract[Citation27] or ACE-inhibitory activity of a fermented soybean food extract.[Citation28] The two results showed the potential of an organic solvent to separate protein hydrolysates with higher activity. Based on these mentioned facts, a casein hydrolysate was prepared by alcalase-catalyzed hydrolysis of casein and modified by alcalase-catalyzed plastein reaction in the presence of extrinsic proline in the present work. The modified hydrolysate was then fractionated by organic solvents or digested by some proteases. The ACE-inhibitory activities in vitro of the resulted hydrolysates were evaluated and compared. The objective of the present study was to investigate the impacts of the plastein reaction and solvent fractionation on the activity and enzymatic resistance of the prepared casein hydrolysate.
MATERIALS AND METHODS
Materials and Chemicals
Rabbit lung acetone powder, caseinate, trypsin (40 kU/g), and N-(3-[2-furyl]acryloyl)-L-phenylalanylglycylglycine (FAPGG) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Alcalase (100 kU/mL) was purchased from Novozymes Biotechnology Co., Ltd. (Tianjin, China). Pepsin (30 kU/g) was purchased from Hui Shi Biochem. Reagent Co. (Shanghai, China). Papain (28 kU/g) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Other reagents used were of analytical grade. Highly purified water prepared with Milli-Q PLUS (Millipore Corporation, New York, NY, USA) was used for the preparation of all buffers and solutions.
Preparation and Modification of Casein Hydrolysate
Casein hydrolysate was prepared with the conditions of Zhao and Li[Citation21] but with an alcalase addition of 1 kU/g proteins. Caseinate solution of 10% (w/v) was hydrolyzed for 1–8 h, heated at 90°C for 15 min, cooled to room temperature, and centrifuged at 3000 × g for 15 min. The supernatant (casein hydrolysate) was collected, lyophilized, stored at −20°C, and evaluated for its degree of hydrolysis and ACE-inhibitory activity in vitro. The hydrolysate with the maximum activity was bulk prepared, lyophilized, and used as the substrate of the plastein reaction.
Optimal plastein reaction conditions were accomplished by employing the response surface methodology with a central composite design. An experimental design consisting of 20 runs and three independent variables, including alcalase addition (i.e., E/S ratio, kU/g peptides), reaction temperature (°C), and substrate concentration (%, w/v) at five levels, was applied. The detailed design is listed in . The lyophilized hydrolysate was dissolved in water (containing different amounts of alcalase to give different E/S ratios) to give the selected substrate concentration. Then, some proline was added to give a fixed level of 0.4 mol/mol free amino groups (on the basis of the hydrolysate). The mixture was kept at the designed temperature for 6 h with continuous stirring, heated at 90°C for 15 min, cooled to room temperature, and analyzed for the content of free amino groups. The decrease of free amino groups of the modified hydrolysate was calculated by subtracting the content of free amino groups of the modified hydrolysate from that of the original hydrolysate, expressed as μmol/g peptides and taken as the dependent variable (i.e., the response) to select suitable conditions.
Table 1 Reaction conditions and their levels studied by the response surface methodology
With the selected conditions, four modified hydrolysates were prepared by applying reaction time of 1, 3, 6, and 9 h, respectively, and evaluated for ACE-inhibitory activities and content of free amino groups. The modified hydrolysate with the maximum activity was bulk prepared, lyophilized, and subjected to solvent fractionation and further enzymatic hydrolysis.
Solvent Fractionation of Casein Hydrolysate or Modified Hydrolysate
The modified hydrolysate with the maximum activity or the original hydrolysate was mixed well with either of the ethanol- or methanol-water solvents (in volume proportions of 3:7, 5:5, or 7:3) at room temperature to give a ratio of 1 g peptides/mL. The mixture was centrifuged at 9000× g for 30 min to obtain supernatant and precipitate fractions (the fractionated hydrolysates). The fractionated hydrolysates were lyophilized, reconstituted in water, and analyzed for peptide recovery, content of free amino groups, and ACE-inhibitory activities. Pure solvent water was also used to fractionate the modified hydrolysate with the same conditions, and the obtained two fractions were subjected to the same assaying as above.
Enzymatic Hydrolysis of the Modified or Fractionated Casein Hydrolysate
The modified hydrolysate of the maximum activity and its two fractionated hydrolysates by ethanol-water of 7:3 (v/v) were reconstituted in water to a peptide content of 10% (w/v). A portion of the prepared solutions was adjusted to either of the selected pHs by adding a few drops of 2 mol/L NaOH or HCl solution. The further hydrolysis of these hydrolysates was triggered by adding papain (2 kU/g peptides, pH 6.5, 45°C), alcalase (1 kU/g peptides, pH 8.0, 55°C), trypsin (2 kU/g peptides, pH 8.0, 37°C), or pepsin (4 kU/g peptides, pH 2, 37°C), and lasted for 10 or 30 min, respectively. For the blank sample (zero time), the protease added was preheated at 90°C for 15 min. After the hydrolysis, all 24 samples were heated at 90°C for 15 min and assayed for the content of free amino groups and the ACE-inhibitory activities.
Chemical Analyses or Activity Assays
Protease activity was assayed with the protocol described by Sarath et al.[Citation29] Protein or peptide content of all samples was determined by multiplying the nitrogen content from the Kjeldahl procedure[Citation30] by a factor of 6.38. The content of free amino groups of the hydrolysate was assayed by the method of Church et al.[Citation31] and used to calculate the degree of hydrolysis (DH) with the method suggested by Adler-Nissen.[Citation32] ACE-inhibitory activity in vitro of all samples at different concentrations was assayed by the method of Murray et al.[Citation33] with FAPGG as the substrate, while the extract of rabbit lung acetone powder was used as the ACE source. IC50 value of the selected hydrolysate was estimated as the reported method of Shalaby et al.[Citation34]
Statistical Analysis
All trials were carried out in triplicate. All data were expressed as means and standard deviations. Differences between the means of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan's multiple range tests. Design Expert 7.0 software (Stat-Ease Inc., Minneapolis, MN, USA) was used in the central composite design. Microsoft Excel 2003 software (Microsoft Corporation, Redmond, WA, USA) and SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) were used to analyze or report the data.
RESULTS AND DISCUSSION
Preparation of Casein Hydrolysate
ACE-inhibitory activities of eight casein hydrolysates prepared over a hydrolysis period of 8 h were evaluated at a final peptide content of 50 μg/mL and are shown in , together with the hydrolysis extent (DH) of the hydrolysates. As the hydrolysis progressed from 0 to 6 h, the DH and activity of the prepared hydrolysate was enhanced from 0 and 5.8% to 10.9 and 48.3%, respectively. Hydrolysis time longer than 6 h led to the hydrolysate little increased DH but lower activity. The hydrolysate with a DH of 10.9% had the maximum activity (IC50 = 52.6 μg/mL) and was selected as the substrate of the plastein reaction.
Figure 1 ACE-inhibitory activity and degree of hydrolysis (DH) of casein hydrolysate prepared over a hydrolysis period of 8 h. The column chart is for ACE-inhibitory activity and the graph chart is for DH. The final peptide concentration used in the assay system was fixed at 50 μg/mL. Different capital letters above the columns (or below the graph) indicate that one-way ANOVA of the data is significantly different (P < 0.01). (Color figure available online.)
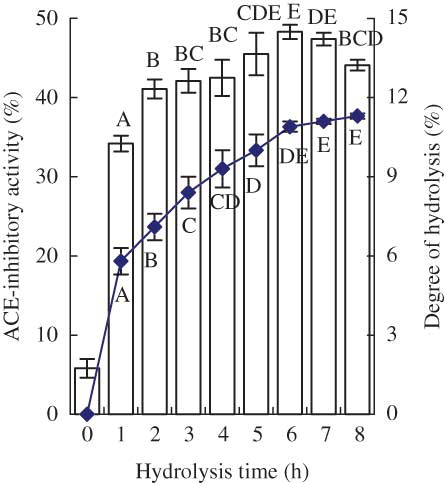
When rapeseed proteins were hydrolyzed by alcalase for 30 min, the hydrolysate showed higher ACE-inhibitory activity.[Citation35] Wu and Ding[Citation36] had prepared a soybean protein hydrolysate by alcalase with an IC50 value of 65 μg/mL. The casein hydrolysate prepared in the present study showed an increasing trend in the activity when hydrolysis time increased from 0 to 6 h; after that, a decreasing trend in the activity was observed. This result shared similarity to the findings of Mao et al.,[Citation37] who hydrolyzed yak (Bos grunniens) casein by alcalase.
Modification of Casein Hydrolysate in the Presence of Extrinsic Proline
Response surface methodology was applied to select the optimal plastein reaction conditions. shows the practical results and the effects of substrate concentrate, reaction temperature, and alcalase addition (i.e., E/S ratio) on the decrease of free amino groups of the modified hydrolysate (i.e., the response). A detailed discussion for is not given here. Besides the fixed proline addition (0.4 mol/mol free amino groups) optimized from previous work (not discussed here), the suitable conditions were optimized as follows: substrate concentration of 50% (w/v), E/S ratio of 3.1 kU/g peptides, and reaction temperature of 25°C. With these conditions, the predicted response was 117.0 μmol/g peptides but the actual one was 112.1 μmol/g peptides. Plastein reaction usually takes place at a higher substrate concentration,[Citation38,Citation39] and lower reaction temperature is beneficial as the reaction is exothermic.[Citation17] The substrate concentration and reaction temperature selected in the present study were consistent to the mentioned conclusions.
Figure 2 Response surface graphs for the decreased amount of free amino groups of the modified casein hydrolysate as a function of: (a) substrate concentrate and reaction temperature (E/S ratio at the central of its level), (b) E/S ratio and reaction temperature (substrate concentrate at the central of its level), and (c) substrate concentrate and E/S ratio (reaction temperature at the central of its level). (Color figure available online.)
Four modified hydrolysates were prepared with a reaction time of 1, 3, 6, or 9 h, respectively. The evaluation results () show that the four modified hydrolysates were different in reaction extent (i.e., contents of free amino groups), and had higher activities than the original hydrolysates (45.8–76.4% versus 27.8%), particularly for MCH6 (IC50 = 13.0 μg/mL). This result indicates that the alcalase-catalyzed plastein reaction in the presence of extrinsic proline was capable of improving ACE inhibition of the casein hydrolysate. Therefore, MCH6 with the maximum activity was subjected to solvent fractionation and further hydrolysis in vitro.
Table 2 Content of free amino groups and ACE-inhibitory activities of four modified casein hydrolysates with different plastein reaction times
Influence of Solvent Fractionation on ACE-Inhibitory Activity
Three ethanol-water (E-W) or methanol-water (M-W) solvents were applied to fractionate the prepared MCH6 and obtain corresponding fractionated products (supernatant or precipitate fractions). The data in show that the solvent fractionation led to the two fractions different in the content of free amino groups and activities. Fractionation solvents with higher ethanol or methanol content (i.e., 7:3 E-W or M-E, v/v) had lower polarity and extracted less soluble fractions from the MCH6. The supernatant fractions obtained had higher activity than the precipitate fractions (81.2% versus 69.4% or 79.3% versus 68.8%). When less ethanol or methanol was added into water, the applied solvents (i.e., 3:7 E-W or M-E, v/v) had higher polarity and extracted more soluble fractions. The supernatant fractions obtained had slightly higher activity than the precipitate fractions (76.8% versus 74.6% or 76.8% versus 75.4%). Fractionation of the original hydrolysate by the two solvents of lower polarity (7:3 E-W or M-W, v/v) also led to the supernatant (or precipitate) fractions higher (or lower) activity. If pure solvent water of the highest polarity was used to fractionate the MCH6, the result was opposite to the result mentioned above. The supernatant fractions of the MCH6 by water exhibited lower activity than the precipitate fractions. These results indicate that an organic solvent-water-based system with suitable polarity might be applicable to separate physically the modified or original hydrolysate with higher activity, and ethanol was more effective than methanol.
Table 3 Effects of ethanol- and methanol-water solvent fractionation on ACE-inhibitory activity (ACEI) of a modified casein hydrolysate (MCH) and casein hydrolysate (CH)Footnote a
ACE-inhibitory peptides are usually rich in Pro, Leu, Ile, and aromatic amino acids,[Citation12,Citation40,Citation41] such as PLPLL and VPP from milk proteins[Citation37,Citation42] and DLP from soybean proteins.[Citation43] Zhang et al.[Citation44] used resins to separate the peptides with a higher content of hydrophobic amino acids, which led to the peptides’ higher ACE-inhibitory activity. Ethanol or methanol has lower polarity than water. In the present work, the applied fractionation solvents with lower polarity than water could extract the peptides with lower polarity (i.e., more hydrophobic amino acids), or precipitate the peptides with higher polarity (i.e., more hydrophilic amino acids) from the MCH6. Thus, the soluble fractions obtained had higher activity while the precipitate factions had lower activity. The formed peptides with lower polarity during plastein reaction were insoluble in water (a classic result of plastein reaction); therefore, the precipitate fractions of MCH6 by water exhibited logically higher activity.
Influence of Further Enzymatic Hydrolysis on ACE-Inhibitory Activity
The sensitivity of the MCH6 and its fractionated products (FMCH6) to enzymatic hydrolysis was examined in vitro. After hydrolysis of the MCH6 or the FMCH6 by one of the selected four proteases, the activities and the hydrolysis extent of the resulted 24 digests were evaluated and are given in . Each resulting digest had higher content of free amino groups than the corresponding substrate, showing that the MCH6 and the FMCH6 were hydrolyzed. The residual activities of the digests were from 22.0 to 54.0%, less than that of the MCH6 (76.4%) or the FMCH6 (81.2% for supernatant, 69.4% for precipitates), and were clearly related to the protease and hydrolysis time applied. The result indicates that further hydrolysis of the hydrolysates led to the activities impaired. Among the 24 digests, 2 were from the FMCH6 hydrolyzed by alcalase for 30 min. Their residual activities were 22.0 and 24.7%, less than the original hydrolysate (27.8%). Other digests had residual activities from 33.8 to 54.0%, higher than the original hydrolysate, indicating that the alcalase-catalyzed plastein reaction might be capable of enhancing enzymatic resistance of casein hydrolysate in ACE inhibition.
Table 4 Effect of further hydrolysis in vitro on ACE-inhibitory activities of a modified casein hydrolysate or its two fractionated products
Among the four proteases studied, alcalase caused the strongest damage to the activities of the MCH6 and the FMCH6, as the resulted digests by alcalase had the lowest activities. It is difficult to distinguish the different influences of other proteases. In comparison with the FMCH6, the MCH6 was more resistant to enzymatic hydrolysis, as the resulted digests from it had higher residual activities. This fact reveals that solvent fractionation could not improve the enzymatic resistance of the modified hydrolysate, although such treatment might enhance the activity.
Gómez-Ruiz et al.[Citation25] found that the ACE-inhibitory activities of two peptides isolated from Manchego cheese were decreased after enzymatic digestion. Three peptides, YAEERYPIL, RADHPFL,[Citation45] and LVYPFPGPIPNSLPQNIPP,[Citation26] showed susceptibility to pepsin or pancreatic extract with decreased activities. In the work of Tavares et al.,[Citation46] two peptides, DKVGINY and KGYGGVSL, also exhibited decreased activities upon enzymatic digestion. The mentioned results supported the present result that enzymatic hydrolysis of the modified or fractionated casein hydrolysate had a negative effect on the activity. Alcalase has higher specificity mainly for hydrophobic amino acids,[Citation47] thus inducing more hydrophobic amino acids released from the MCH6 or the FMCH6 (together with higher hydrolysis extent, as shown in ); consequently, the obtained digests had lower activities.
Resistance of ACE-inhibitory peptides to physiological digestion is an important and interesting scientific issue. The present study shows that the plastein reaction could give casein hydrolysate an improved ACE-inhibition and better enzymatic resistance. More works are needed to reveal the details about peptide composition, relationship between residual ACE-inhibition, and protease or hydrolysis profiles.
CONCLUSION
In vitro ACE-inhibitory activity of a casein hydrolysate prepared by alcalase was enhanced from 27.8 to 76.4% by applying an alcalase-catalyzed plastein reaction in the presence of proline addition, leading to the IC50 value decreasing from 52.6 to 13.0 μg/mL. Solvent fractionation of the modified hydrolysate by ethanol- or methanol-water solvent with a ratio of 7:3 (v/v) led to the soluble (or precipitate) fractions higher (lower) activity. The modified or fractionated hydrolysate showed sensitivity in ACE inhibition to the enzymatic hydrolysis by four proteases, but 22 out of 24 resulted digests had residual activities higher than the original casein hydrolysate. It was the plastein reaction that conferred casein hydrolysate enzymatic resistance in ACE inhibition.
ACKNOWLEDGMENTS
This work was funded by the Innovative Research Team of Higher Education of Heilongjiang Province (Project No. 2010td11) and the National Natural Science Foundation of China (Project Nos. 30972132 and 31140009). The authors wish to thank the anonymous reviewers and the editors for their valuable work and suggestions to this article.
REFERENCES
- López-Fandiño , R. , Otte , J. and van Camp , J. Physiological . 2006 . chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity . International Dairy Journal , 16 ( 11 ) : 1277 – 1293 .
- Murray , B.A. and FitzGerald , R.J. 2007 . Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production . Current Pharmaceutical Design , 13 ( 8 ) : 773 – 791 .
- Jang , A. and Lee , M. 2005 . Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates . Meat Science , 69 ( 4 ) : 653 – 661 .
- Li , G.H. , Le , G.W. , Shi , Y.H. and Shrestha , S. 2004 . Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects . Nutrition Research , 24 ( 7 ) : 469 – 486 .
- Meisel , H. 1998 . Overview on milk protein derived peptides . International Dairy Journal , 8 ( 5 ) : 363 – 373 .
- Karaki , H. , Doi , K. , Sugano , S. , Uchiwa , H. , Sugai , R. , Murakami , U. and Takemoto , S. 1990 . Antihypertensive effect of tryptic hydrolysate of milk casein in spontaneously hypertensive rats . Comparative Biochemistry and Physiology , 96 ( 2 ) : 367 – 371 .
- Maeno , M. , Yamamoto , N. and Takano , T. 1996 . Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP790 . Journal of Dairy Science , 79 ( 8 ) : 1316 – 1321 .
- Kim , Y.K. , Yoon , S. , Yu , D.Y. , Lönnerdal , B. and Chung , B.H. 1999 . Novel angiotensin-I-converting enzyme peptides derived from recombinant human αS1-casein expressed in Escherichia coli . Journal of Dairy Research , 66 ( 3 ) : 431 – 439 .
- Maruyama , S. , Mitachi , H. , Awaya , J. , Kurono , M. , Tomizuka , N. and Suzuki , H. 1987 . Angiotensin I-converting enzyme inhibitory activity of the C-terminal hexapeptide of αS1-casein . Agricultural and Biological Chemistry , 51 ( 9 ) : 2557 – 2561 .
- Tauzin , J. , Miclo , L. and Gaillard , J.L. 2002 . Angiotensin-I-converting enzyme inhibitory peptides from tryptic hydrolysate of bovine αS2-casein . FEBS Letters , 531 ( 2 ) : 369 – 374 .
- Pripp , A.H. , Isaksson , T. , Stepaniak , L. and Sørhaug , T. 2004 . Quantitative structure-activity relationship modeling of ACE-inhibitory peptides derived from milk proteins . European Food Research Technology , 219 ( 6 ) : 579 – 583 .
- Cheung , H.S. , Wang , F.L. , Ondetti , M.A. , Sabo , E.F. and Cushman , D.W. 1980 . Binding of peptide substrates and inhibitors of angiotensin converting enzyme . Journal of Biological Chemistry , 255 ( 2 ) : 401 – 407 .
- Nakamura , Y. , Yamamoto , N. , Sakai , K. , Okubo , A. , Yamazaki , S. and Takano , T. 1995 . Purification and characterization of angiotensin-I converting enzyme inhibitors from a sour milk . Journal of Dairy Science , 78 ( 4 ) : 777 – 783 .
- Jiang , J. , Chen , S. , Ren , F. , Luo , Z. and Zeng , S.S. 2007 . Yak milk casein as a functional ingredient: preparation and identification of angiotensin-I-converting enzyme inhibitory peptides . Journal of Dairy Research , 74 ( 1 ) : 18 – 25 .
- Otte , J. , Shalaby , S.M. , Zakora , M. , Pripp , A.H. and El-Shabrawy , S.A. 2007 . Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: Effect of substrate, enzyme and time of hydrolysis . International Dairy Journal , 17 ( 5 ) : 488 – 503 .
- Yamashita , M. , Arai , S. , Tsai , S.J. and Fujimaki , M. 1971 . Plastein reaction as a method for enhancing the sulfur-containing amino acid level of soybean protein . Journal of Agricultural and Food Chemistry , 19 ( 6 ) : 1151 – 1154 .
- Fujimaki , M. , Kato , M. , Aria , S. and Yamashita , M. 1971 . Application of microbial proteinase to soybean and other materials to improve acceptability . Journal of Applied Bacteriology , 34 ( 1 ) : 119 – 131 .
- Ashley , D.V.M. , Temler , R. , Barclay , D. , Dormond , C.A. and Jost , R. 1983 . Amino acid-enriched plasteins: A source of limiting amino acids for the weanling rat . Journal of Nutrition , 113 ( 1 ) : 21 – 27 .
- Andrews , A.T. and Alichanidis , E. 1990 . The plastein reaction revisited: Evidence for a purely aggregation reaction mechanism . Food Chemistry , 35 ( 4 ) : 243 – 261 .
- Ono , S. , Kasai , D. , Sugano , T. , Ohba , K. and Takahashi , K. 2004 . Production of water soluble antioxidative plastein from squid hepatopancreas . Journal of Oleo Science , 53 ( 5 ) : 267 – 273 .
- Zhao , X.H. and Li , Y.Y. 2009 . An approach to improve ACE inhibitory activity of casein hydrolysates with plastein reaction catalyzed by Alcalase . European Food Research and Technology , 229 ( 5 ) : 795 – 805 .
- Gao , B. and Zhao , X.H. Modification of soybean protein hydrolysates by alcalase-catalyzed plastein reaction and the ACE-inhibitory activity of the modified product in vitro. International Journal of Food Properties 2012 15 (5), 982–996.
- Xu , W. , Li , T.J. and Zhao , X.H. Coupled Neutrase-catalyzed plastein reaction mediated the ACE-inhibitory activity in vitro of casein hydrolysates prepared by Alcalase. International Journal of Food Properties 2013 16 (2), 429–443.
- Zhao , X.H. , Wu , D. and Li , T.J. 2010 . Preparation and the radical scavenging activity of papain catalyzed casein plasteins . Dairy Science and Technology , 90 ( 5 ) : 521 – 535 .
- Gómez-Ruiz , J.A. , Ramos , M. and Recio , I. 2004 . Angiotensin-converting enzyme-inhibitory activity of peptides isolated from Manchego cheese . Stability under simulated gastrointestinal digestion. International Dairy Journal , 14 ( 12 ) : 1075 – 1080 .
- Quirós , A. , Contreras , M.M. , Ramos , M. , Amigo , L. and Recio , I. 2009 . Stability to gastrointestinal enzymes and structure-activity relationship of β-casein-peptides with antihypertensive properties . Peptides , 30 ( 10 ) : 1848 – 1853 .
- Kwan , L. , Chan , E.L. , Helbig , N. and Nakai , S. 1991 . Fractionation of water-soluble and insoluble components from egg yolk with minimum use of organic solvents . Journal of Food Science , 56 ( 6 ) : 1537 – 1541 .
- Hang , M. and Zhao , X.H. 2011 . Fermentation time and water/ethanol-based solvent systems impacted in vitro ACE-inhibitory activity of the extracts of a mao-tofu product fermented by Mucor spp . Biotechnology , 10 ( 1 ) : 60 – 69 .
- Sarath , G. , De La Motte , R.S. and Wagner , F.W. 1989 . “ Protease assay methods ” . In Proteolytic Enzymes: A Practical Approach; , Edited by: Beynon , R.J. and Bond , J.D. 25 – 55 . UK : IRL Press: Oxford . InEds.
- 1993 . IDF. Determination of the nitrogen (Kjeldahl method) and calculation of the crude protein content. IDF Standard 20B , Brussels , Belgium : International Dairy Federation .
- Church , F.C. , Swaisgood , H.E. , Porter , D.H. and Catignani , G.L. 1983 . Spectrophotometric assay using ο-phthaldialdehyde for determination of proteolysis in milk and milk proteins . Journal of Dairy Science , 66 ( 6 ) : 1219 – 1277 .
- Adler-Nissen , J. 1979 . Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid . Journal of Agricultural and Food Chemistry , 27 ( 6 ) : 1256 – 1262 .
- Murray , B.A. and Walsh , D.J. 2004 . FitzGerald . R.J. Modification of the furanacryloyl-L-phenylalanyl-glycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity. Journal of Biochemical and Biophysical Methods , 59 ( 2 ) : 127 – 137 .
- Shalaby , S.M. , Zakora , M. and Otte , J. 2006 . Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates . Journal of Dairy Research , 73 ( 2 ) : 178 – 186 .
- Pedroche , P.J. , María-Yust , M. , Megías , C. , Lqari , H. , Alaiz , M. , Girón-Calle , J. , Millán , F. and Vioque , J. 2004 . Utilisation of rapeseed protein isolates for production of peptides with angiotensin I-converting enzyme (ACE)-inhibitory activity . Grasas y Aceites , 55 ( 4 ) : 354 – 358 .
- Wu , J. and Ding , X. 2002 . Characterization of inhibition and stability of soy protein derived angiotensin I-converting enzyme inhibitory peptides . Food Research International , 35 ( 4 ) : 367 – 375 .
- Mao , X.Y. , Ni , J.R. , Sun , W.L. , Hao , P.P. and Fan , L. 2007 . Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides . Food Chemistry , 103 ( 4 ) : 1282 – 1287 .
- Lozano , P. and Combes , D. 1991 . α-Chymotrypsin in plastein synthesis: influence of substrate concentration on enzyme activity . Biotechnology and Applied Biochemistry , 14 ( 2 ) : 212 – 221 .
- Pallavicini , C. , Finley , J.W. , Stanley , W.L. and Watters , G.G. 1980 . Plastein synthesis with α-chymotrypsin immobilised on chitin . Journal of the Science of Food and Agriculture , 31 ( 3 ) : 273 – 278 .
- Kawakami , A. and Kayahara , H. 1993 . Synthesis of Leu-Lys-Tyr derivatives and their interaction with angiotensin converting enzyme . Journal of the Japanese Society of Nutrition and Food Science , 46 ( 5 ) : 425 – 428 .
- Suetsuna , K. and Nakano , T. 2000 . Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida) . The Journal of Nutritional Biochemistry , 11 ( 9 ) : 450 – 454 .
- Pan , D. , Luo , Y. and Tanokura , M. 2005 . Antihypertensive peptides from skimmed milk hydrolysate digested by cell-free extract of Lactobacillus helveticus JCM1004 . Food Chemistry , 91 ( 1 ) : 123 – 129 .
- Kuba , M. , Tana , C. , Tawata , S. and Yasuda , M. 2005 . Production of angiotensin-I converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase . Process Biochemistry , 40 ( 6 ) : 2191 – 2196 .
- Zhang , F. , Wang , Z. and Xu , S. 2009 . Macroporous resin purification of grass carp fish (Ctenopharyngodon idella) scale peptides with in vitro angiotensin-I converting enzyme (ACE) inhibitory ability . Food Chemistry , 117 ( 3 ) : 387 – 392 .
- Miguel , M. , Aleixandre , M.A. , Ramos , I. and López-Fandiño , R. 2006 . Effect of simulated gastrointestinal digestion on the antihypertensive properties of ACE-inhibitory peptides derived from ovalbumin . Journal of Agricultural and Food Chemistry , 54 ( 3 ) : 726 – 731 .
- Tavares , T. , Contreras , M.D.M. , Amorim , M. , Pintado , M. , Recio , I. and Malcata , F.X. 2011 . Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: In vitro effect and stability to gastrointestinal enzymes . Peptides , 32 ( 5 ) : 1013 – 1019 .
- Kunst , T. , Whitaker , J.R. , Voragen , A.F.J. and Wong , D.W.S. 2003 . “ Protein modification to optimize functionality: protein hydrolysates ” . In Handbook of Food Enzymology , 221 – 236 . New York , NY : Marcel Dekker . InEds.
