Abstract
The granular structure and physicochemical properties of starches isolated from grain amaranth cultivar K112 (Amaranthus cruentus L.) were studied in this study. Detailed physical and chemical analyses were performed by determining the granular morphology, crystallinity, particle size, thermal characteristics, blue value, enzyme susceptibility, and pasting properties. Results showed polygon-shaped A. cruentus L. K112 starch granules. The average diameter was 1.38 μm, in which half of the diameter was <2.91 μm. An A-type X-ray diffraction pattern was revealed with intense peaks of 15.2°, 17.5°, and 23.2°. The peak viscosity was 181 BU and the breakdown value was 2 BU. Amaranth starch obtained the highest pasting temperature (70.7°C) and enzymatic digestibility (absorbance value = 0.41 ± 0.013) compared with corn, cassava, and sweet potato starches.
INTRODUCTION
Studies have focused on amaranth grain mainly obtained from Amaranthus hypochondriacus, A. cruentus, and A. caudatus. A. hypochondriacus and A. cruentus are native to Mexico and Guatemala. A. caudatus is native to Peru and other Andean countries. Cultivated species are currently distributed in Mexico, Central America, South America, Asia, and Africa.[Citation1] This type of grain exhibits various characteristics, such as resistance to drought, hot climate, and pests, as well as high biomass production and nutritional value.[Citation2] Amaranth grain contains higher levels of fiber, calcium, and iron than other commonly used grains. In addition to fiber and minerals, squalene is present in amaranth grain and exhibits cholesterol-lowering properties.[Citation3] High protein and lysine contents also provide an improved nutritional balance.[Citation4] As such, amaranth grain shows a high level of edibility.
Starch is the most abundant carbohydrate found in amaranth grain and accounts for 48 to 69% depending on the species.[Citation1,Citation5] Amaranth starch granules are small, exhibiting an A-type X-ray pattern with low levels of amylase.[Citation6] The small-sized granules of amaranth starches with spherical and polygonal shapes may exhibit unique functional properties, such as high enzymatic digestibility and good thermal stability. These characteristics indicate that amaranth starch can be widely used in numerous industrial and food applications, including paper coatings, food thickeners, and laundry starch.[Citation3,Citation7]
The applications, isolation, and properties of amaranth starch have been discussed in several studies.[Citation5−Citation15] Many studies have also focused on the extraction and degeneration of amaranth starch, but only a few researches were focus on its basic properties. For instance, Baker and Rayas-Duarte[Citation13] investigated the freeze-thaw stability and thermal properties of amaranth, corn, wheat, and rice starches. Kong et al.[Citation14] analyzed the physicochemical and functional properties of starches isolated from 15 grain amaranth cultivars (Amaranthus spp.). However, the comprehensive properties of A. cruentus L. K112 starch are seldom reported. A. cruentus L. K112 is distributed in many regions, mainly popularized in China. Further studies should be conducted to investigate the granular structure and properties of K112 starch.
This study was conducted to examine the granular structure (granular morphology, crystallinity, and particle size) and physicochemical properties (thermal characteristics, enzyme susceptibility, and pasting properties) of starches isolated from grain amaranth cultivar K112. The results can be used as a basis to develop various applications of A. cruentus L. K112 starch.
MATERIALS AND METHODS
Materials
The amaranth seeds of cultivar K112 (A. cruentus L.) were harvested from Chongqing, China. Corn, cassava, and sweet potato starches were purchased from a local market. α-Amylase (>3700 U/g of protein) from Bacillus was purchased from Abxing Biological Technology Co. (Beijing, China). All of the chemicals and solvents were certified to be of analytical reagent grade.
Isolation and Chemical Composition
Starch was extracted according to the method described by Teli et al.[Citation16] with slight modifications. In brief, the grains were immersed in 0.25% aqueous NaOH solution for 24 h and washed thoroughly until NaOH was completely removed. The slurry was subsequently blended in a GWJ blender (Zhejiang, China) for approximately 3 min and subjected to a stepwise filtration through 60- (250 μm), 80- (180 μm), and 300-mesh (48 μm) sieves. The filtrate was then centrifuged at 4000 rpm for 10 min. The supernatant and the top yellow protein layer were removed. The lower starch layer was resuspended in double deionized water and centrifuged at 4000 rpm for 10 min. This procedure was repeated until no protein was present. The isolated starch was dried in an oven at 45°C and ground to pass through an 80-mesh sieve. The amylose content and main components were determined according to Resio et al.[Citation17]
Scanning Electron Microscopy
Starch granule morphology was obtained by using a Hitachi S-4800 environmental scanning electron microscope (ESEM, Japan) according to the method described by Ritika et al.[Citation15]
X-ray Diffraction (XRD)
X-ray diffraction analysis was performed using an XD-3 X-ray diffractometer (PERSEE, Beijing, China) equipped with Cu–Ka (1.54° A) radiation. An accelerating voltage of 40 kV and a current of 30 mA with a scan rate of 10°/min were used. The diffractograms were recorded in a 2θ range of 4–70°.[Citation18]
Particle Size Distribution
The particle size of the starches was analyzed using a Mastersizer 2000 laser light scattering particle size analyzer (Malvern, England) according to the method described by Sogi et al.[Citation19] with slight modifications. Starch (1.0 g) was combined with distilled water in a small glass vial. The refractive indexes of distilled water and the particles are 1.330 and 1.530, respectively. The sample was added to the sample port until the instrument read 15 to 20% obscuration.
Differential Scanning Calorimetry (DSC)
The thermal characteristics of isolated starches were measured by thermal analysis using a DSC 200 F3 Maia differential scanning calorimeter (NETZSCH, Germany) equipped with a thermal analysis data station. This procedure was conducted as described by Pietrzyk et al.[Citation20] Before the experiment, DSC was calibrated with indium. Dry starch (2.5 mg) was weighed directly in an aluminum DSC pan. Deionized water was added using a microsyringe to obtain a starch-water suspension containing 70 g/100 g water. The samples were hermetically sealed and allowed to stand for 1 h at room temperature before they were heated in the DSC. The pan containing the sample was placed in the calorimeter and heated at a rate of 10°C/min from 25 to 140°C; an empty pan was used as reference. Parameters such as onset (To), peak (Tp), and conclusion (Tc) temperatures, width at half the peak height (ΔT1/2), and enthalpy (ΔH) of gelatinization were calculated automatically.
Blue Value
The sample (1.00 g, dry basis) was weighed accurately and placed in a 50-mL standard volumetric flask. Dimethyl sulfoxide was then added to obtain a constant volume and induce dispersal. Next, 5 mL of the sample was transferred quantitatively into a 100-mL standard volumetric flask, and distilled water was added to obtain a final volume of 100 mL. Subsequently, 4 mL of the solution was poured into a 100-mL volumetric flask and 8 mL iodine solution (0.1 g iodine and 1.0 g potassium iodide in 100 mL aqueous solution) was added. The solution was diluted with distilled water to obtain a final volume of 100 mL and then left for 30 min to allow the complete development of the color. Absorbsence was then measured at 680 nm by using a 2450 ultraviolet and visible (UV-Vis) spectrophotometer (Shimadzu, Japan).
Enzymatic Digestibility
Enzyme susceptibility studies on amaranth starches were conducted according to the procedure described by Hoover et al.[Citation1] with slight modifications. Starch (0.2 g) was suspended in distilled water (20 mL) and 2 mL of α-amylase (5%) was added. Starch was then incubated with agitation at 39°C for 90 min before 1 mL of HCL (1 mol/L) was added. The precipitate was filtered and diluted six times. Approximately 1 mL of DNS solution (18.2 g/100 mL C4H4KNaO6-4H2O, 0.03 g/100 mL C7H4N2O7, 2.1 g/100 mL NaOH, and 0.5 g/100 mL C6H6O) was added. The container with the resulting mixture was immersed in boiling water for 5 min and immediately cooled in an ice water bath. Afterward, 10 mL of distilled water was added and the absorbance was determined at 540 nm in a 2450 UV-vis spectrophotometer (Shimadzu).
Pasting Properties
Pasting properties were determined using a Viskograph-E Brabender viscometer (Brabender, Germany). Six parts of starch per 100 parts (460 mL) of distilled water were heated from 30 to 95°C at 1.5°C/min, maintained at the same temperature for 45 min, cooled to 55°C at the same rate, and held constant for 45 min. A 700 cmg sensitivity cartridge was operated at a bowl speed of 75 r/min.
Statistical Analysis
Numerical data were the average of triplicate experiments. ANOVA and Tukey’s test were conducted, with a confidence level of 95% (p ≤ 0.05), using the software Microcal Origin 7.5 (Microcal Software, Inc., Northampton, USA) to determine significant differences.
RESULTS AND DISCUSSION
Composition
The following contents and their corresponding levels were found in A. cruentus L. K112 starch: starch, 85.94%; protein, 0.29%; fat, 0.27%; ash, 0.48%; moisture, 12.13%; and amylose, 6.12%. The starch of K112 contained lower amounts of protein and fat than that from other amaranth cultivars.[Citation5,Citation13] Previous studies reported that the moisture content of different amaranth starches ranges from 11.6 to 13.9%; the amylose content ranges from 4.7 to 12.5%.[Citation8,Citation14] Basing from these values, we could suggest that K112 exhibited normal moisture and lower amylose contents. Amylose is an important factor affecting the functional properties of amaranth starch; thus, A. cruentus L. K112 produced in Chongqing should exhibit specific properties.
Scanning Electron Microscopy
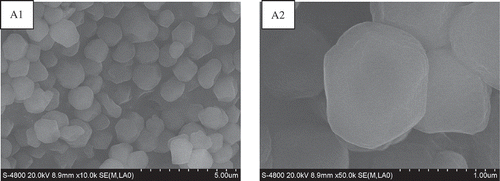
shows the starch granules magnified at different times (A1 = 10,000 times, A2 = 50,000 times) using a scanning electron microscope. The diameter of starch granules ranged from 0.65 to 1.92 μm with an average diameter of 1.38 μm, which is slightly higher than that in previous reports.[Citation13,Citation14] This difference may be due to various growth conditions. Polygon-shaped granules were observed in the micrographs and are similar to those of other amaranth cultivars. The surfaces appeared smooth without fissures, suggesting that amaranth starch is classified as small grain. Amaranth starch is also characterized as a small-grain starch according to freeze-thaw stability, solubility, and swelling power.[Citation1] Morphological characteristics were consistent with those found in a previous study.[Citation14]
X-Ray Diffraction
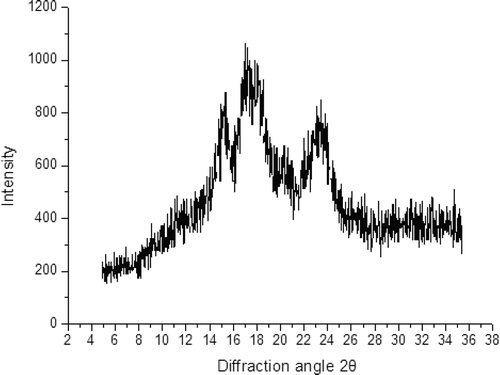
The X-ray diffraction pattern of starches () shows intense peaks at 2θ values of 15.2°, 17.5°, and 23.2°. This observation indicates that the diffraction pattern of A. cruentus L. K112 starch was characteristically an A-type structure common among amaranth starches.[Citation14,Citation21] The crystallinity of this starch is 64.67%, which is generally higher than that of other Amaranthus species, such as Rajgira,[Citation16] Hy030 (A. hypochondriacus L.), and Tibet Yellow (A. paniculatus L.).[Citation21] This finding is consistent with that in previous reports, in which the degree of crystallinity decreases as amylose content increases.[Citation22] The crystalline pattern of native starch is mainly divided into A, B, C, Vh, Va, and Eh types; among these types, A, B, and C are the most common.[Citation23] For instance, cereal starches are mostly A-type high-crystallization starches. Root, tuber, stem, and high-amylose starches exhibit B-type crystallinity with low crystallization.[Citation24] The C-type starch shows the polymorphs of both A and B types commonly found in bean starch.[Citation25]
Particle Size Distribution
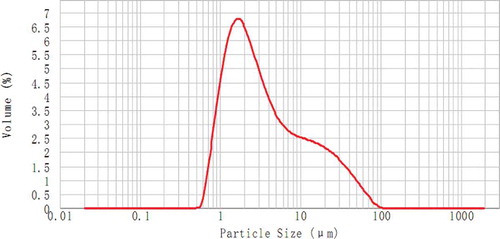
A curve representing the granule diameter of amaranth grain seed starches is shown in . In particular, two peaks were observed: the significant peak ranged between 1.26 and 8.71 μm and the weaker peak ranged between 8.71 and 39.81 μm. Approximately 50 and 90% of the starches exhibited a diameter of <2.91 and <22.93 μm, respectively (). Hence, the amaranth seed starches consisted mainly of small granules. Previous studies reported that the average diameter of starch granules from different amaranth cultivars range from 1.05 to 1.32 μm.[Citation14] This range is slightly smaller than that of starch granules observed in the present study. This difference may be attributed to the biological origin, amyloplast biochemistry, and plant physiology.[Citation26]
TABLE 1 Particle size distribution and differential scanning calorimetry results of amaranth starch
DSC
DSC is conducted to monitor the changes in the physical and chemical properties of starches during gelatinization.[Citation6] The internal granule structure is disrupted when the whole granule disintegrates, accompanied by various changes. The gelatinization enthalpy of starches is affected by granule percentage and shape, amylose content, size, shape, distribution, and water-binding capacity.[Citation27] The gelatinization characteristics of starch in a DSC thermogram can be presented at various temperatures: To, 66.5°C; Tp, 72.2°C; and Tc, 78.4°C. These temperatures and the heat of gelatinization are summarized in . Furthermore, these temperatures are lower than those of other A. cruentus grain cultivars, such as Mexican, African, and A200D cultivars.[Citation1] These results may be attributed to the differences in growth conditions. Previous studies reported that ΔH of the amaranth cultivar K112 is 15.1 J/g,[Citation14] which is lower than that in the present study (22.93 J/g); such differences are partly attributed to various analysis environments.
Blue Value
The blue value indicates the iodine-binding capacity and the free starch content of amaranth starch. The chain length and the molecular degree of polymerization mainly correspond to the blue value. For instance, a straight-chain polymer exhibits strong affinity to iodine. Thus, amylose shows a higher blue value than amylopectin because amylose consists of an unbranched chain, whereas amylopectin consists of a branched chain.[Citation28] The blue values of different starches are shown in . Amaranth starch showed significantly (p < 0.05) weaker starch-iodine absorption (0.085), which is consistent with the low amylose content (6.12%). The starch-iodine absorption characteristic of A. cruentus L. K112 starch was not different from that of Amaranthus (Rajgeera).[Citation16]
TABLE 2 Blue value, enzymatic digestibility, and key parameters of Brabender viscosity of different starches
Enzymatic Digestibility
A greater absorbance value indicates higher enzymatic digestibility. The enzymatic digestibility capacity of starches is in the following order: amaranth > cassava > corn > sweet potato starches (). The capacity of amaranth was significantly higher than others (p < 0.05). Different analysis methods were used, and thus, comparing our results with the A. cruentus Mexican, African, and A200D cultivars reported by Hoover et al.[Citation1] is difficult. Several reports showed that small starch granules are digested more quickly than larger ones because large starch granules have a smaller surface area compared with smaller ones. A smaller surface area of the substrate in larger starch granules reduces the chance for amylase absorption.[Citation29] Differences in starch digestibility among and within species were also attributed to the interplay of various factors, such as amylose/amylopectin ratio, granule size, and starch source.[Citation11]
Pasting Properties
Key parameters of Brabender viscosity of the starch samples () were different from those presented by Stone et al.[Citation5] partly because of the different analysis methods used. The gelatinization temperatures were higher than the DSC results () probably because the Brabender viscometer begins to record the temperature only when the interaction between starch and water reaches a certain degree.[Citation30] Cassava starch had the lowest paste temperature (61.4°C, p < 0.05) and the shortest gelling time (1.86 min, p < 0.05), which agreed with previous reports.[Citation31] The peak viscosity of amaranth starches was 181 BU (Point B), which was smaller than the other three starches, and reflected its poor water absorbency and thickening property. The breakdown value was only 2 BU (B–D) and indicated good thermal stability, which may be due to the lower peak viscosity and strong intermolecular forces within the granules.[Citation5] The amaranth starches used in this experiment contained less amylose. Hence, the setback value was lowest (E–D, 129 BU).
CONCLUSIONS
Lower amylose content and small uniform granules of A. cruentus L. K112 starches produce many unique functional properties, including high pasting temperature, high enzymatic digestibility, poor iodine-binding capacity, and good thermal stability. Considering these properties, we found that amaranth starch can be used for diverse applications. Further studies should be conducted to investigate the nutritional properties, explain the relationship between structure and function, and broaden its application in functional products.
FUNDING
This work was supported by the Fundamental Research Funds for the Central Universities (XDJK2013D021).
REFERENCES
- Hoover, R.; Sinnott, A.W.; Perera, C. Physicochemical characterization of starches from Amaranthus cruentus grains. Starch/Stärke 1998, 50, 456–463.
- Teutonico, R.A.; Knorr, D. Amaranth: Composition, properties and applications of a rediscovered food crop. Food Technology 1985, 39, 49–60.
- Resioa, A.C.; Aguerreb, R.J.; Suarez, C. Hydration kinetics of amaranth grain. Journal of Food Engineering 2006, 72, 247–253.
- Bunzel, M.; Ralph, J.; Steinhart, H. Association of non-starch polysaccharides and ferulic acid in grain amaranth (Amaranthus caudatus L.) dietary fiber. Molecular Nutrition and Food Research 2003, 49, 551–559.
- Stone, L.A.; Lorenz, K.; Collins, F. The starch of Amaranthus—Physico-chemical properties and functional characteristics. Starch/Stärke 1984, 36, 232–237.
- Loubes, M.A.; Resio, A.N.C.; Tolaba, M.P.; Suarez, C. Mechanical and thermal characteristics of amaranth starch isolated by acid wet-milling procedure. Food Science and Technology 2012, 46, 519–524.
- Teli, M.D.; Shanbag, V.; Dhande, S.S.; Singha, R.S. Chemical processing—Storage stability of Amaranthus paniculates (Rajgeera) starch thickener in textile printing. Journal of the Textile Association 2006, 3.
- Choi, H.; Kim, W.; Shin, M. Properties of Korean amaranth starch compared to waxy millet and waxy sorghum starches. Starch/Stärke 2004, 56, 469–477.
- Tapia-Blácido, D.; Mauri, A.N.; Menegalli, F.C.; Sobral, P.J.A.; Añón, M.C. Contribution of the starch, protein, and lipid fractions to the physical, thermal, and structural properties of amaranth (Amaranthus caudatus) flour films. Journal of Food Science 2007, 72, E293–E300.
- Villarreal, M.E.; Ribotta, P.D.; Iturriaga, L.B. Comparing methods for extracting amaranthus starch and the properties of the isolated starches. LWT–Food Science and Technology 2013, 51, 441–447.
- Wankhede, D.B.; Gunjal, B.B.; Sawate, R.A.; Patil, H.B.; Bhosale, M.B.; Gahilod, A.T.; Walde, S.G. Studies on isolation and characterization of starch from rajgeera grains (Amaranthus paniculatus Lin). Starch/Stärke 1989, 41, 167–171.
- Park, Y.J.; Nishikawa, T. Characterization and expression analysis of the starch synthase gene family in grain amaranth (Amaranthus cruentus L.). Genes & Genetic Systems 2012, 87, 281–289.
- Baker, L.A.; Rayas-Duarte, P. Freeze-thaw stability of amaranth starch and the effects of salt and sugars. Cereal Chemistry 1998, 75, 301–307.
- Kong, X.L.; Bao, J.S.; Corke, H. Physical properties of Amaranthus starch. Food Chemistry 2009, 113, 371–376.
- Ritika, B.Y.; Khatkar, B.S.; Yadav, B.S. Physicochemical, morphological, thermal and pasting properties of starches isolated from rice cultivars grown in India. International Journal of Food Properties 2010, 13, 1339–1354.
- Teli, M.D.; Rohera, P.; Sheikh, J.; Singhal, R. Use of Amaranthus (Rajgeera) starch vis-à-vis wheat starch in printing of vat dyes. Carbohydrate Polymers 2009, 79, 460–463.
- Resio, A.N.C.; Tolaba, M.P.; Suárez, C. Effect of drying temperature and soaking conditions on wet-milling characteristics of amaranth grain. International Journal of Food Engineering 2010, 6, 1–12.
- Olu-Owolabi, B.I.; Afolabi, T.A.; Adebowale, K.O. Pasting, thermal, hydration, and functional properties of annealed and heat-moisture treated starch of sword bean (Canavalia gladiata). International Journal of Food Properties 2011, 14, 157–174.
- Sogi, D.S.; Oberoi, D.P.S.; Malik, S. Effect of particle size, temperature, and total soluble solids on the rheological properties of watermelon juice: A response surface approach. International Journal of Food Properties 2010, 13, 1207–1214.
- Pietrzyk, S.; Fortuna, T.; Dyrek, K.; Łabanowska, M.; Bidzińska, E.; Orawska, J. Effects of saccharose substitutes on physicochemical properties and free radical generation in oxidized potato starch. International Journal of Food Properties 2011, 14, 1255–1263.
- Kong, X.L.; Kasapis, S.; Bao, J.S.; Corke, H. Effect of gamma irradiation on the thermal and rheological properties of grain amaranth starch. Radiation Physics and Chemistry 2009, 78, 954–960.
- Saikia, J.P.; Konwar, B.K. Physicochemical properties of starch from Aroids of North East India. International Journal of Food Properties 2012, 15, 1247–1261.
- Wang, S.J.; Copeland, L. Effect of alkali treatment on structure and function of pea starch granules. Food Chemistry 2012, 135, 1635–1642.
- Stewart, H.E.; Farkas, B.E.; Blankenship, S.M.; Boyette, M.D. Physical and thermal properties of three sweet potato cultivars (Ipomoea batatas L.). International Journal of Food Properties 2000, 3, 433–446.
- Lawal, O.S.; Adebowale, K.O. Physicochemical characteristics and thermal properties of chemically modified jack bean (Canavalia ensiformis) starch. Carbohydrate Polymers 2005, 60, 331–341.
- Svegmark, K.; Hermansson, A.M. Microstructure and rheological properties of composites of potato starch granules and amylose: A comparison of observed and predicted structure. Food Structure 1993, 12, 181–193.
- Jiang, Q.Q.; Gao, W.Y.; Lia, X.; Xia, Y.Z.; Wang, H.Y.; Wu, S.S.; Huang, L.Q.; Liu, C.X.; Xiao, P.G. Characterizations of starches isolated from five different Dioscorea L. species. Food Hydrocolloids 2012, 29, 35–41.
- Greenwood, C.T.; Rossotti, H. Physicochemical studies on starches. VII. The infrared absorption spectrum of the amylose-iodine complex. Journal of Polymer Science 1958, 27, 481–488.
- Noda, T.; Takigawa, S.; Matsuura-Endo, C.; Suzuki, T.; Hashimoto, N.; Kottearachchi, N.S.; Yamauchi, H.; Zaidul, I.S.M. Factors affecting the digestibility of raw and gelatinized potato starches. Food Chemistry 2008, 110, 465–470.
- Limpisut, P.; Limpisut, V.K. Comparison of rice flour pasting properties using Brabender viscoamylograph and rapid visco analyser for evaluating cooked rice texture. Starch/Stärke 2002, 8, 350–357.
- Onitilo, M.O.; Sanni, L.O.; Oyewole, O.B.; Maziya-Dixon, B. Physicochemical and functional properties of sour starches from different cassava varieties. International Journal of Food Properties 2007, 10, 607–620.