Abstract
Structural changes in capsanthin in the presence of exogenous reactive oxygen species such as , H2O2, and ·OH were analyzed by UV-visible spectroscopy, infrared spectroscopy, and mass spectrometry, which were used to explore the fading reaction mechanism of capsanthin. The results showed that reactive oxygen species could induce a blue shift in the maximum UV-visible absorption peak, as well as breakage of conjugated double bonds in capsanthin. Due to the direct oxidation or addition reactions in the presence of reactive oxygen species, the double bonds and carbonyl groups in capsanthin reacted with reactive oxygen species, generating alcohols such as C13H22O2, C15H24O2, and C10H18O in the presence of
, C13H22O3, and C10H18O2 in the presence of H2O2 and C14H30O4 and C10H18O2 in the presence of ·OH. The rest of the products were small molecule compounds. These results suggested that reactive oxygen species could destroy the conjugated double bonds in capsanthin, which resulted in the color fading.
INTRODUCTION
Capsanthin is a ubiquitous carotenoid pigment. It is also the most valuable component during the deep processing of chili. Due to the presence of conjugated double bonds or polyene chain in this chromophore, capsanthin has obvious light-absorbing capabilities and presents as a bright red color under visible light.[Citation1] During the drying process of postharvest peppers, capsanthin fades, which is observed as yellowish white patches known as the “flower shell” or “flower skin phenomenon,” causing a significant decrease in its nutritional value.[Citation2,Citation3] Previous studies have confirmed that microbial infection is the basic reason for producing the “flower shell” phenomenon in dried peppers.[Citation4−Citation6] Pathogen infection can cause anaphylaxis in plants, resulting in the production of a large amount of reactive oxygen species (ROS). ROS such as , H2O2, and ·OH, which are generated during an infection, could react with carotenoids. Among these reactions, the oxidation is closely correlated with the molecular structures of carotenoids.[Citation7−Citation9] However, the effect of ROS on the molecular structure and the fading mechanism of capsanthin remain elusive. In this study, UV-visible (UV-Vis) spectroscopy, infrared spectroscopy (IR), and electrospray ionization-mass spectrometry (ESI-MS) were used to explore the effect of ROS on the molecular structure and the fading mechanism of capsanthin. This report provides a theoretical reference for the development and utilization of pigments in peppers.
MATERIALS AND METHODS
Materials and Reagents
Capsanthin (C40H56O3, purity ≥ 98%) was provided by Guizhou Gall Development Company. Sodium dithionite, 30% H2O2, acetone, and ferrous sulfate were of analytical grade. An electronic balance was provided by Hauser Company in Shanghai. Vector22-type Fourier transform IR was purchased from Germany Brucker Company; the Finnigan MAT (LCQ-Advantage) electrospray ionization mass spectrometer was from Las Hian, Ltd.
Experimental Methods
Preparation of ROS
Superoxide anion () was generated from an alkaline sodium dithionite solution.[Citation10,Citation11] Hydrogen peroxide (H2O2) was prepared from 30% H2O2. Hydroxyl radical (·OH) was produced using a Fenton reaction system containing Fe2+ and H2O2 at the ratio of 10:1 and a reaction temperature of 40°C.[Citation12,Citation13]
Sample handling
Approximately 0.1 g of capsanthin was added to a 250-mL distillation flask and subsequently dissolved in 50 mL of 99.5% acetone with gentle shaking. Following the addition of 5 mL of 30 mmol/L alkaline sodium dithionite, the mixture was incubated in a 40°C water bath for 18 h. The carotenoid-acetone suspension was changed from a red solution into a pale yellow transparent solution. Lyophilized power was produced after sequential cool down, condensation under reduced pressure, pre-freezing at −20°C for 2 h, and vacuum freeze-drying for 24 h. The treatments using H2O2 at a concentration of 50 mmol/L and Fenton’s reagent including Fe2+ and H2O2 at concentrations of 1 mmol/L and 10 mmol/L were conducted according to the previously described procedures.
Characteristic analysis of IR
KBr power was mixed with the capsanthin standard and the treated pigment at a ratio of 100:1. The mixture was grounded and tableted. The infrared spectra of the mixtures were measured within the range of 400–4000 cm−1 and the information on the functional groups was analyzed.
ESI-MS analysis
Mass spectrum analysis was conducted by Finnigan MAT (LCQ-Advantage) ESI-MS.[Citation14] Samples were dissolved in methanol and analyzed on Finnigan MAT (LCQ-Advantage) ESI-MS including ESO source, with a spray voltage of 4.8 kV, metal-heated capillary temperature of 230°C, capillary voltage of ±4.0 V, tube lens voltage of ±55 V, and O2 as the shell gas. The sampling was conducted through flow injection pump injector, the injection rate was 0.2 μL/min, and the mass-scanning range was 0–1000 m/z. The mass-to-charge ratio (m/z) was detected using a positive ion spectrum mode.
RESULTS AND ANALYSIS
UV–Vis Spectrum Analysis of Capsanthin Treated by ROS
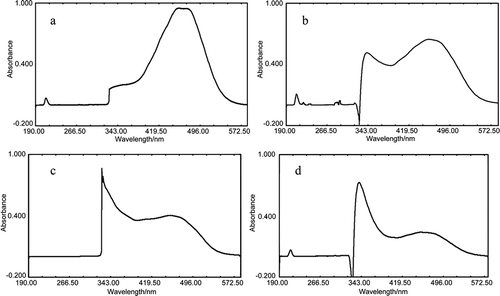
Capsanthin was treated by exogenous ROS such as , H2O2, and ·OH, and the UV-Vis spectra of the products are shown in . The maximum absorption of
-treated capsanthin was detected at 341 nm and 448–463 nm, and exhibited the blue shift when compared to the control. The treatment of H2O2 could have resulted in the blue shift of 8–9 nm of capsanthin and a new absorption peak at 326 nm. In addition, the absorption bands of hydroxyl radical-treated capsanthin were detected at 339 nm and 450–460 nm (blue shift). The absorption band of capsanthin without any treatments was in the spectrum of visible light (400–800 nm), and exhibited a red color. The maximum absorption peaks of capsanthin after treatments were detected within UV spectrum (200–400 nm), and the capsanthin subjected to ROS was colorless. A correlation between the maximum absorption wavelength of capsanthin and the length of conjugated double bonds was observed. The loss and reduction in the number of conjugated double bonds might have induced the blue shift of the maximum absorption peaks, namely, the maximum absorption wavelength (λmax) could have moved toward the longer wavelength.[Citation15] The experimental results indicated that ROS treatments caused the change in absorption maxima and the decrease in absorption intensity, which might be attributable to the decrease in the number of conjugated double bonds.
IR Spectrum Analysis of Capsanthin Treated by ROS
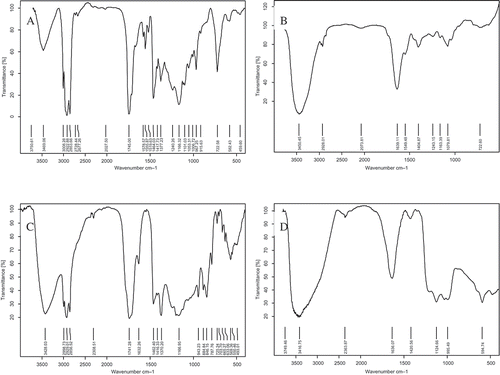
The IR spectral change and structural characteristics of capsanthin subjected to the treatments of , H2O2, and ·OH were further explored by IR spectral technology. shows that capsanthin contains more unsaturated carbon-hydrogen bonds and hydroxyl groups, whereas the conjugated keto group is the special group in capsanthin. Capsanthin is an oxidized xanthophyll. Except for the multiple C5-isoprene groups, the ring located at the end of the molecule was replaced by several oxygen-containing groups, which is well in agreement with previous reports.[Citation15,Citation16]
The IR spectrum of capsanthin after treatment is shown in . The round and blunt absorption peak with the strongest absorption intensity was detected at 3450.5 cm−1, and the stretching vibration of the C-O and O-H bonds at 1079.8 cm−1 with middle intensity readings revealed alcohol properties. A wide absorption peak at 2928.0 cm−1 indicated the existence of a -CH3- group, and the absorption peak at 1639.1 cm−1 showed the bending vibration of C=C, suggesting that capsanthin is a six-ring olefin. Subjected to the treatment of
, the pigment product does not have double bonds of C=O, which is related to the oxidative addition reaction of carbonyl and alkenyl groups to generate the alkyl and hydroxyl groups,[Citation17] thus resulting in the disappearance of conjugated double bonds. Compared with the control group (), the capsanthin subjected to
treatment revealed an obvious decrease in the number of absorption peaks, weak peak intensity, and broad peaks in its IR spectrum, indicating that the oxidation induced by
could damage the chromophore that is composed of double bonds of C=C and carbonyl groups, and finally generate colorless alcohols.
The IR spectrum of the pigment product of capsanthin subjected to H2O2 treatment is presented in . The absorption peaks at 2999 cm−1 and 1632 cm−1 revealed the bending vibration of C=C; the absorption peak at 1741 cm−1 induced the product as a carbonyl-containing compound; the absorption peaks at 2998.8, 2929, 2857, and 1462.4 cm−1 suggested the existence of -CH3- and -CH2- groups; the strong absorption at 1370 cm−1 and 1460 cm−1 indicated the presence of dimethyl group; strong absorption and the round and blunt peak shape at 3428 cm−1 revealed the stretching vibration of ·OH; combined with the absorption at 1167 cm−1, this product could be inferred as an alcohol; the absorption peaks at 1245.3, 1205.7, 943.2, 884.8, 846.2, and 787.8 cm−1 indicated the presence of an epoxy ether group (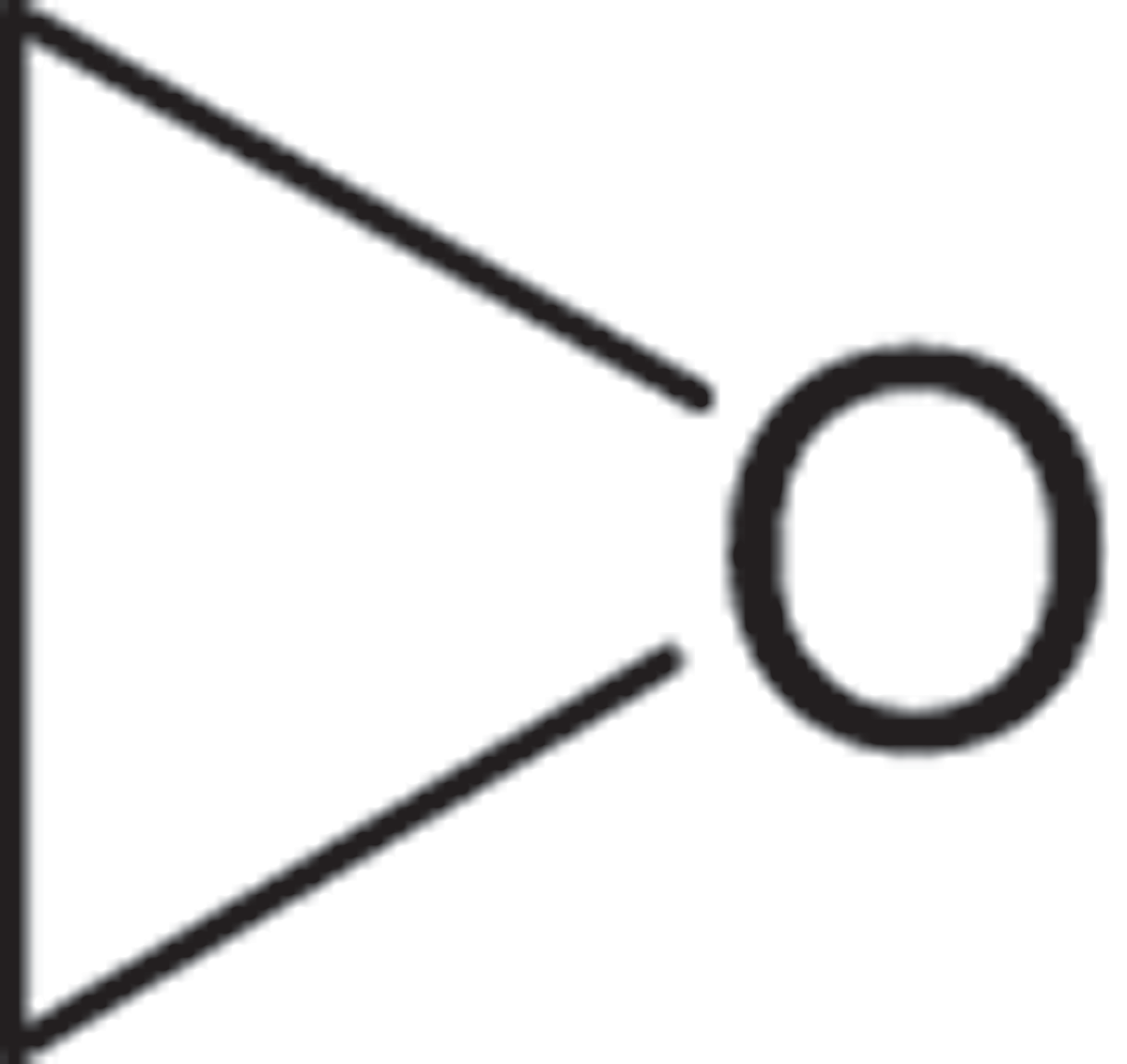 ) in the product, which may be associated with the epoxidation reaction of C=C group by H2O2 to produce epoxy ether groups.[Citation18,Citation19] Compared to the control (), the products of H2O2-treated capsanthin showed characteristic peaks and a blue shift of the peaks with strong absorption.
) in the product, which may be associated with the epoxidation reaction of C=C group by H2O2 to produce epoxy ether groups.[Citation18,Citation19] Compared to the control (), the products of H2O2-treated capsanthin showed characteristic peaks and a blue shift of the peaks with strong absorption.
The IR spectrum of the product of capsanthin subjected to ·OH treatment is shown in . The round and blunt absorption peak at 3416.8 cm−1 revealed the stretching vibration of OH, and the absorption at 1124.7 cm−1 revealed the stretching vibration of CO, suggesting that the product is an alcohol. The strong absorption peak at 995.5 cm−1 indicated the presence of γC-H vibration and a -CH2- group. Therefore, ·OH treatment can result in the loss of carbonyl groups and a reduced number of absorption peaks in the IR spectrum of capsanthin. Due to the strong oxidative capability of ·OH generated from the Fenton reaction, it can oxidize C=O groups to generate alcohols. In contrast, ·OH can execute an addition reaction on C=C groups,[Citation20] leading to the reduction in the number of double bonds and increase in hydroxyl groups in capsanthin. Above experimental results indicated that the treatments using ROS such as , H2O2, and ·OH can result in an obvious change in the IR spectrum, a loss or reduction in the number of double bonds, and damages to conjugated system in capsanthin, thus leading to the fading of capsanthin.
MS Spectral Analysis of Capsanthin Subjected to ROS Treatments
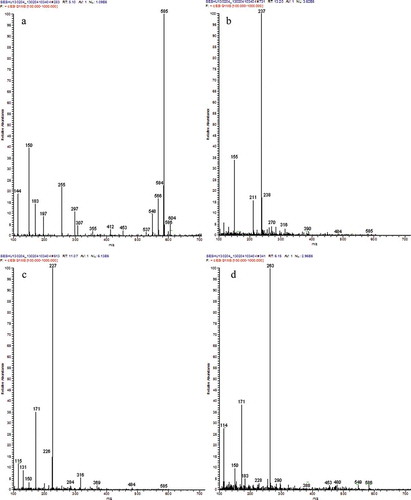
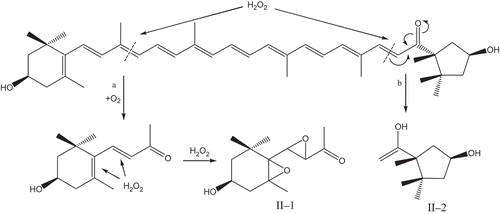
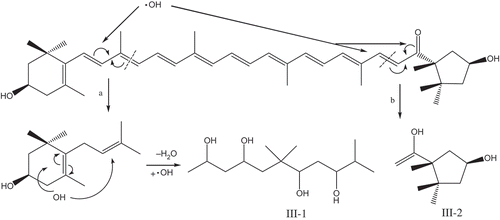
The MS spectra of capsanthin after the treatments using , H2O2, and ·OH are shown in . Capsanthin, which has a molecular weight of 584 () after treatment using
, produced three products with the molecular weights of 236, 210, and 154, respectively (). After H2O2 treatment, the molecular weights of the major products were 226 and 170 (); subjected to the treatment by ·OH, the molecular weights of major products were 262 and 170 (). In addition, a greater reduction in the number of carbon atoms after the fading of capsanthin was observed. Combined with the IR spectra, the loss of linear C=C groups, and damage to the conjugated system in capsanthin could result in the generation of H2, CO2, and H2O, as well as a small amount of short hydrocarbons and alcohols after the oxidation reaction or degradation of capsanthin.[Citation21,Citation22]
Mechanism of Capsanthin Oxidation by ROS
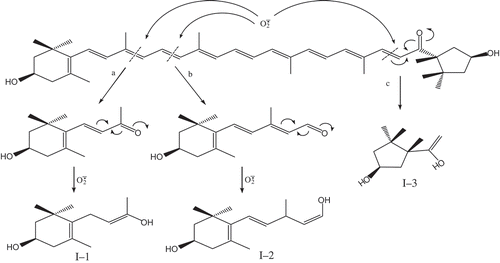
The oxidative cleavage reactions of carotenoids generally occur at the double bonds of C7-C8, C9-C10, C11-C12, and C5ʹ-C6ʹ, and C7ʹ-C8ʹ.[Citation15,Citation22−Citation24] Carbonyl groups (C=O) impart an electron-attracting effect. However, the interaction between carbonyl carbon and hydrogen atom is weak. Because the single hydrogen atom has only one electron, the electron density between carbonyl carbon and hydrogen atom is low and easy to break. Therefore, the aldehyde easily loses H and is involved in oxidation reaction.[Citation25] is an anion radical with strong oxidative or reducing properties, thus leading to the redistribution molecular rearrangement of carbonyl electrons and the addition reaction to form double bonds and hydroxyl groups.[Citation17] Accordingly, UV-Vis, IR, and ESI-MS analysis of capsanthin revealed that the oxidation of capsanthin by
was located at C9-C10, C11-C12, and C7ʹ-C8ʹ, generating three major products and small molecule products due to the formation of carbonyl groups after the breakage of double bonds, a decrease in C=C number, and the shortening of the conjugation system. Moreover, due to the role of strong reducing function from
, the carbonyl groups of the products could further generate alcohols through addition reaction, resulting in the redistribution of more electrons and translocation of double bonds. Combined with the molecular structure of capsanthin, we deduced the possible mechanism for oxidative degradation of capsanthin, as shown in .
H2O2 has a strong oxidative capability and possesses weak acidity. It can spontaneously decompose to form water and oxygen due to its weak stability. Based on the analysis of UV-Vis, IR, and ESI-MS results, the oxidative process of capsanthin by H2O2 is shown in . The strong oxidative effect of H2O2 on C9-C10 and C7ʹ-C8ʹ of capsanthin could result in the breakage of conjugated C=C bonds, generating two major products and some small molecule products. The mechanism for the oxidation of cyclic olefin by H2O2[Citation26] revealed that the direct oxidative cleavage of C=C bonds in the ring by H2O2 is more difficult in the absence of catalysts such as tungstate or H+. Therefore, the epoxidation of the olefin by H2O2 in the absence of catalysts and acidic conditions can result in the formation of cyclic olefin oxides through a direct reaction between H2O2 and double bonds, rather than the breakage of the ring in the cyclic enol structure of capsanthin. Epoxy groups in the products exhibited enhanced absorption peak intensity, which was correlated with the degree of cyclization reactions.
OH generated by the Fenton reaction system showed a higher oxidation potential (·OH + H+ = H2O2; Eφ = 2.80 V)[Citation27,Citation28] that is only followed by fluorine and has a strong oxidization capacity and strong electrophilic addition property.[Citation13] Hydroxyl radicals can oxidize organic compounds through the addition reaction on C-O or C=C bonds.[Citation20] Meanwhile, hydroxyl radicals can promote the cleavage of double bonds, generating CO2, H2O, and other small molecule products. Due to the high electronegativity and electrophilicity of ·OH, it can cleave the structure of benzene rings in aromatic compounds that could not be completed by other general oxidants.[Citation29,Citation30] Hydroxyl radicals can also cause electronic redistribution and molecular rearrangement.[Citation17] According to UV-Vis, IR, and ESI-MS results, the major reaction of capsanthin in the presence of ·OH is presented in . The ·OH reacts with C9-C10, and C7ʹ-C8ʹ initially bonds with capsanthin to result in the breakage of conjugated C=C bonds and generate two major macromolecular products and other small molecule products. Under the action of ·OH coupled with a redistribution of electrons and translocation of double bonds, C=O bond can be oxidized to form a hydroxyl group, and then the ring of cyclohexenol is cleaved. After connecting to both ends of the double bonds, the addition reaction results in the formation of polyhydroxyl alcohols.
DISCUSSION AND CONCLUSIONS
The process of capsanthin fading in the presence of , H2O2, and ·OH produced different colorless alcohols, including C13H22O2, C15H24O2, and C10H18O after
treatment, C13H22O3 and C10H18O2 after H2O2 treatment, and C14H30O4 and C10H18O2 after ·OH treatment. Capsanthin contains several unsaturated conjugated double bonds as its chromophore. The red color of capsanthin is the characterized by the number of conjugated double bonds.[Citation22] The maximum absorption wavelength (λmax) was reduced as the number of conjugated double bonds decreased,[Citation14] and the color of capsanthin was attenuated due to the decrease in the number of conjugated double bonds. The treatments of capsanthin by ROS such as
, H2O2, and ·OH can result in the breakage of long-ene-chain at different locations. Due to the oxidative and reducing capacity of ROS, these can cause the electron redistribution between double bonds and carbonyl groups, structural rearrangement, redox reactions, or even the cleavage of olefin ring to generate hydroxyl alcohols or other colorless small molecule products. These reactions finally result in the fading of capsanthin.
The pathogen infection of pepper fruits can result in the accumulation of a large amount of ROS through anaphylactic reactions, and finally lead to damages to capsanthin structure due to the loss of pepper pigments. Correspondingly, the marketable value of peppers is reduced. Therefore, the prevention of pathogen infection on post-harvest pepper fruits has important significance in preventing the occurrence of fading or spot shells in peppers.
FUNDING
This work was supported by the National Natural Science Foundation of China (No. 31060228).
Additional information
Funding
REFERENCES
- Goodwin, T.W. Chemistry and Biochemistry of Plant Pigments, Academic Press: London & New York, 1965; 80–105.
- Qiao, C.J.; Li, H.M. Cause and prevention measures for pericarp-browned disease of pepper. China Fruit & Vegetable 2010, 11, 34.
- Li, H.; Pordesimo, L.O.; Igathinathane, C.; Vinyard, B. Physical property effects on drying of chile peppers. International Journal of Food Properties 2009, 12 (2), 316–330.
- Snowdon, A.L. A Colour Atlas of Post-Harvest Diseases and Disorders of Fruits and Vegetables, Vol. 1. General Introduction & Fruits; Manson Publishing: London, 2010; 30–53.
- Koffi-Nevry, R.; Kouassi, K.C.; Nanga, Z.Y.; Koussémon, M.; Loukou, G.Y. Antibacterial activity of two bell pepper extracts: Capsicum annuum L. and Capsicum frutescens. International Journal of Food Properties 2012, 15 (5), 961–971.
- Liu, H.; Ding, Z.H.; Zheng, W.Y.; Xiao, Z.R.; Deng, C.; Yang, Q. Isolation and identification of bacteria causing discoloration in red pepper fruits (Capsicum annuum L.). Food Science 2013, 34 (1), 160–165.
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology 1997, 48, 251–275.
- Song, Y.; Lu, C.Q.; Chen, J.S. Factors of antioxidant and prooxidant activities of carotenoids. Journal of Hygiene Research 2003, 32 (4), 417–419.
- Perera, C.O.; Yen, G.M. Functional properties of carotenoids in human health. International Journal of Food Properties 2007, 10 (3), 201–230.
- Milazzo, G.; Caroli, S. Tables of Standard Electrode Potentials, Wiley Interscience: New York, 1978; 367–383.
- Weng, Y.K.; Huang, S.; Weng, N.Y. Production of superoxide anion radicals in sodium hyposulfite solution. Progress in Biochemistry and Biophysics 1989, 16 (3), 209.
- Kiwi, J.; Pulgarin, C.; Peringer, P. Effect of Fenton and photo-Fenton reactions on the degradation and biodegradability of 2 and 4-nitrophenols in water treatment. Applied Catalysis B: Environmental 1994, 3 (4), 335–350.
- Liu, Y.D.; Xu, S.C. Application of Fenton-like reagents to industrial wastewater treatment. Shanghai Environmental Sciences 1994, 13 (3), 26–28.
- Lian, X.J. Studies on Photostability of Monascus Pigments, Tianjin University of Science and Technology: Tianjin, China, 2004; 21–23.
- Maoka, T.; Fujiwara, Y.; Hashimoto, K.; Akimoto, N. Isolation of a series of apocarotenoids from the fruits of the red Paprika Capsicum annuum L. Journal of Agricultural and Food Chemistry 2001, 49 (3), 1601–1606.
- Holden, M. Chemistry and Biochemistry of Plant Pigments, 2nd Ed.; Academic Press: New York, 1976; 462–488.
- Lian, X.J. The study on mechanism of photobleaching for red monascus pigment. Meat Research 2005, 8, 32–35.
- Lane, B.S.; Burgess, K. A cheap, catalytic, scalable, and environmentally benign method for Alkene epoxidations. Journal of the American Chemical Society 2001, 123 (12), 2933–2934.
- Lane, B.S.; Vogt, M.; DeRose, V.J. Manganese-catalyzed epoxidation of alkenes in bicarbonate solutions. Journal of the American Chemical Society 2002, 124 (40), 11946–11954.
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. Journal of Hazardous Materials 2003, 98 (1–3), 33–50.
- Guo, P.Z. Oxygen Diffusion and Structural Evolution during Stabilization of Polyacrylonitrile Fibers, Shangdong University: Jinan, China, 2008; 8–19.
- Mordi, R.C. Mechanism of β-carotene degradation. Biochemical Journal 1993, 292, 310–312.
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A.D. Characterization of a novel carotenoid cleavage dioxygenase from plants. Journal of Biological Chemistry 2001, 76, 25208–25211.
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. The Plant Journal 2004, 40 (6), 882–892.
- Fei, W. Advanced Organic Chemistry, 1st Ed.; Zhejiang University Press: Hangzhou, China, 2006; 375–400.
- Xu, X.H.; Chen, H.Y.; Deng, J.F.; Jiang, A.R. Preparation of glutaraldehyde by catalytic oxidation of cyclopentene with aqueous solution of hydrogen peroxide. Acta Chimica Sinica 1993, 51 (4), 399–403.
- Bossmann, S.H.; Oliveros, E.; Göb, S; Siegwart, S.; Dahlen, E.P.; Payawan, L. Jr.; … Braun, A.M. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. The Journal of Physical Chemistry A 1998, 102 (28), 5542–5550.
- Bellik, Y.; Benabdesselam, F.; Ayad, A.; Dahmani, Z.; Boukraa, L.; Nemmar, A.; Iguer-Ouada, M. Antioxidant activity of the essential oil and oleoresin of Zingiber Officinale Roscoe as affected by chemical environment. International Journal of Food Properties 2013, 16 (6), 1304–1313.
- Fukushima, M.; Tatsumi, K.; Morimoto, K. The fate of aniline after a photo-Fenton reaction in an Aqueous System Containing Iron (III), humic acid, and hydrogen peroxide. Environmental Science and Technology 2000, 34 (10), 2006–2013.
- Tian, Y.L.; Li, M.Y.; Ma, T.S.; Li, G.M.; Yan, Q.S.; Li, D.L. Mechanism of treatment of aromatic compounds—containing water by Fenton’s reagent. Pollution Control Technology 2003, 16 (1), 12–15.