Abstract
The aim of this work was to determine antioxidant capacities, neuroprotective, skin care, antidiabetic effects, and fatty acid composition of Anchusa undulata subsp. hybrida. The antioxidant activity was screened by four different test systems including total antioxidant, antiradical, reducing power, and metal chelating activities. Neuroprotective potential was determined by anticholinesterase inhibitor assay. Tyrosinase inhibitory activity was tested to detect skin care effect. Antidiabetic effects were evaluated with α-amylase and α-glucosidase inhibitory assays. Inhibitory activities on acetycholinesterase, butyrylcholinesterase, tyrosinase, α-amylase, and α-glucosidase enzymes were observed as 2.238 and 1.239 μmol GALAEs/g, 0.339 mmol KAEs/g, 0.193, and 0.219 mmol ACEs/g extract, respectively. Amount of total phenolics and flavonoids were found as 80.34 μg GAEs/mg and 25.09 μg QEs/mg in the extract, respectively. Twenty-three fatty acids were found in the aerial parts, being oleic acid (24.30 g/100 g of total fatty acids) the most abundant, followed by linoleic (21.19 g/100 g of total fatty acids) and palmitic acids (17.50 g/100 g of total fatty acids).
INTRODUCTION
Free radicals, which are generated by normal metabolic process and from exogenous factors, and they affect DNA, proteins, and polyunsaturated fatty acids.[Citation1] Overproduction of these radicals, especially reactive oxygen species (ROS), have been associated with carcinogenesis, coronary heart disease, and many other health issues related to advancing age.[Citation2] This situation is called as “oxidative stress/oxidative damage.” Nevertheless, all aerobic organisms have natural antioxidant defense systems that protect against oxidative damage. If the natural protective system is insufficient, dietary intake of antioxidants become important.[Citation3] Synthetic antioxidants, such as BHA, BHT, and PG, are used to slow down lipid oxidation in the food industry, but it has been reported that these compounds may have side effects to human health.[Citation4] As a result, the importance of exploiting natural antioxidants, especially of plant origin, has deeply increased in recent years.
Interest in the search for new natural enzyme inhibitors from medicinal plants has grown dramatically over the past years because synthetic inhibitors have side effects. For example, acarbose and miglitol are used as α-amylase and α-glucosidase inhibitors in the management of diabetes. However, these compounds have some gastrointestinal side effects such as abdominal pain and diarrhea.[Citation5] Likewise, kojic acid and corticosteroids are known as tyrosinase inhibitors for skin disease but can cause adverse reaction such as dermatitis and skin irritation.[Citation6] From this direction, it becomes necessary to search for new enzyme inhibitors from natural sources having lesser side-effects.
The genus Anchusa belongs to the Boraginaceae family and consists of about 170 taxa native to temperate and subtropical areas of the world.[Citation7] This genus is represented by 15 species in Turkey. Some of them are endemic to Turkey.[Citation8] Anchusa species are used in Turkish folk medicine for wound healing and as a diuretic agent. In addition, several Anchusa species are used as demulcent, analgesic, sedative, and hypotensive purposes in the folk medicine practices of many countries.[Citation9] Some Anchusa species have been chemically studied and found to contain triterpene glycosides and flavonoid.[Citation10] On the other hand, as far as our literature survey could ascertain, there is only one report on the fatty acid contents of the seed of A. undulata subsp. hybrida.[Citation9] However, no report is available for neuroprotective, antioxidative, skin care, and antidiabetic properties of A. undulata subsp. hybrida. Additionally, fatty acid composition of aerial parts of this plant has not previously been reported. Therefore, data presented here will be the first record on A. undulata subsp. hybrida. Therefore, the aims of the present work were: (1) to evaluate the in vitro neuroprotective, antioxidative, skin care, and antidiabetic effects (2) to determine the total bioactive components of the methanolic extract, and (3) to assess the fatty acid composition of A. undulata subsp. hybrida.
MATERIALS AND METHODS
Plant Material
Anchusa undulata L. subsp. hybrida (Ten.) Coutinho plant was collected in 2012 from Mugla University campus, Mugla-Turkey at its flowering season. Taxonomic identification of the plant material was confirmed by the senior taxonomist Dr. Olcay Ceylan, in Department of Biology, Mugla University. The voucher specimen has been deposited at the Herbarium of the Department of Biology, Mugla University, Mugla-Turkey (Voucher No: B6055).
Preparation of the Methanol Extract
The air-dried samples (20 g) were extracted by using a Soxhlet extractor for 5 h with 250 mL of methanol under reflux conditions. Methanol was removed with a rotary evaporator to obtain an extract in the yield of 12.81% (w/w).
Enzyme Inhibitory Activity
Anticholinesterase (ChE) assay
ChE activity was measured using Ellman’s method, as previously reported[Citation11] with slight modification. Sample solution (50 μL) was mixed with DTNB (125 μL) and AChE (or BChE) solution (25 μL) in Tris-HCl buffer (pH 8.0) in a 96-well microplate and incubated for 15 min at 25°C. The reaction was then initiated with the addition of acetylthiocholine iodide (ATCI) or butyrylthiocholine chloride (BTCl) (25 μL). Similarly, a blank was prepared by adding sample solution to all reaction reagent without enzyme (AChE or BChE) solution. The sample and blank absorbances were read at 405 nm after 10 min incubation at 25°C. The absorbance of the blank was subtracted from that of the sample and the ChE activity was expressed as equivalents of galantamine.
Antityrosinase assay
Antityrosinase activity was measured using the modified dopachrome method with L-DOPA as substrate, as previously reported[Citation12] with slight modification. Sample solution (25 μL) was mixed with tyrosinase solution (40 μL) and phosphate buffer (100 μL, pH 6.8) in a 96-well microplate and incubated for 15 min at 25°C. The reaction was then initiated with the addition of L-DOPA (40 μL). Similarly, a blank was prepared by adding sample solution to all reaction reagent without enzyme (tyrosinase) solution. The sample and blank absorbances were read at 492 nm after 10 min incubation at 25°C. The absorbance of the blank was subtracted from that of the sample and the antityrosinase activity was expressed as equivalents of kojic acid.
Anti-α-amylase assay
Anti-α-amylase activity was performed using Caraway-Somogyi iodine/potassium iodide (IKI) method[Citation13] with some modifications. Sample solution (25 μL) was mixed with α-amylase solution (50 μL) in phosphate buffer (pH 6.9 with 6 mM sodium chloride) in a 96-well microplate and incubated for 10 min at 37°C. After preincubation, the reaction was initiated with the addition of starch solution (50 μL, 0.06%). Similarly, a blank was prepared by adding sample solution to phosphate buffer (50 μL, pH 6.9 with 6 mM sodium chloride) and water (50 μL). The reaction mixture was incubated 10 min at 37°C. The reaction was stopped with the addition of HCl (25 μL, 1 M). This was followed by addition of the iodine-potassium iodide solution (100 μL). The sample and blank absorbances were read at 630 nm. The absorbance of the blank was subtracted from that of the sample and the anti-α-amylase activity was expressed as equivalents of acarbose.
Anti-α-glucosidase assay
Anti-α-glucosidase activity was performed by the previous method[Citation14] with some modifications. Sample solution (50 μL) was mixed with glutathione (50 μL), α-glucosidase solution (50 μL) in phosphate buffer (pH 6.8) and PNPG (50 μL) in a 96-well microplate and incubated for 15 min at 37°C. Similarly, a blank was prepared by adding sample solution to all reaction reagent without enzyme (α-glucosidase) solution. The reaction was then stopped with the addition of sodium carbonate (0.2 M, 50 μL). The sample and blank absorbances were read at 400 nm. The absorbance of the blank was subtracted from that of the sample and the anti-α-glucosidase activity was expressed as equivalents of acarbose.
Total Antioxidant Activity
β-carotene-linoleic acid method
In this assay antioxidant activity is determined by measuring the inhibition of the volatile organic compounds and the conjugated diene hydroperoxides arising from linoleic acid oxidation[Citation15] with slight modification. A stock solution of β-carotene-linoleic acid mixture was prepared as following: 0.5 mg β-carotene was dissolved in chloroform (1 mL, HPLC grade). Twenty-five μL linoleic acid and 200 mg Tween 40 was added. Chloroform was completely evaporated using a vacuum evaporator. Then 100 mL of oxygenated distilled water was added with vigorous shaking; 2.5 mL of this reaction mixture was dispersed to test tubes and sample solution (0.35 mL) were added and the emulsion system was incubated for up to 2 h at 50°C. The same procedure was repeated with the standards and a blank. After this incubation period, the sample absorbance was read at 490 nm. Measurement of absorbance was continued until the color of β-carotene disappeared. The bleaching rate (R) of β-carotene was calculated according to Eq. (1).
Phosphomolybdenum method
The total antioxidant activities of samples were evaluated by phosphomolybdenum method according to Berk et al.[Citation16] with slight modification. Sample solution (0.3 mL) was combined with 3 mL reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The sample absorbance was read at 695 nm after 90 min incubation at 95°C. The total antioxidant capacity was expressed as equivalents of ascorbic acid according to the equation that was obtained from the standard ascorbic acid graph.
Antiradical Activity
Free radical scavenging activity (DPPH)
The effect of samples on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was estimated according to Sarikurkcu.[Citation17] Sample solution (1 mL) was added to a 4 mL of a 0.004% methanol solution of DPPH. The sample absorbance was read at 517 nm after 30 min incubation at room temperature in dark.
ABTS (2,2 azino-bis [3-ethylbenzothiazloine-6-sulfonic acid]) radical cation scavenging activity
The scavenging activity against ABTS assay was measured according to the method of Aktumsek et al.[Citation11] with slight modification. Briefly, ABTS+ radical cation was produced directly by reacting 7 mM ABTS solution with 2.45 mM potassium persulfate and allowing the mixture to stand for 12–16 h in dark at the room temperature. Prior to assay, the solution was diluted with methanol to an absorbance of 0.700 ± 0.02 at 734 nm. Sample solution (1 mL) was added to ABTS solution (2 mL) and mixed. The sample absorbance was read at 734 nm after 30 min incubation at room temperature. The ABTS radical cation scavenging activity was expressed as equivalents of trolox according to the equation that was obtained from the standard trolox graph.
Hydroxyl (.OH) radical scavenging activity
The hydroxyl radical scavenging activity was measured by the method described by Halliwell et al.[Citation18] with slight modification. Ascorbic acid (1 mM, 0.1 mL) was added to premixed reaction mixture containing 10 mM deoxyribose (0.28 mL), 50 mM phosphate buffer (0.41 mL, pH 7.4), 10 mM ferric chloride (0.01 mL), 10 mM hydrogen peroxide (0.1 mL), 1 mM EDTA (0.1 mL), and sample solution (0.25 mL), then incubated for 12 h at 37°C. Similarly, a blank was prepared by adding sample solution (0.25 mL) to reaction mixture (1 mL) without ferric chloride. Afterwards, the incubated sample was mixed with 0.75 mL trichloroacetic acid (2.8% w/v) and 0.75 mL thiobarbituric acid reagent (1% w/v, in 50 mM NaOH), followed by heating at 100°C for 1 h and subsequent cooling to room temperature. The sample and blank absorbances were read at 532 nm. The absorbance of the blank was subtracted from that of the sample and the hydroxyl radical scavenging activity was expressed as equivalents of mannitol according to the equation that was obtained from the standard mannitol graph.
Superoxide anion (O2.-) radical scavenging activity
The superoxide anion radical scavenging activity was followed in the riboflavin-light-nitroblue tetrazolium (NBT) system[Citation19] with slight modification. Sample solution (0.25 mL) was added to reaction mixture containing riboflavin (0.1 mL, 0.1 mg/mL), EDTA (0.1 mL, 12 mM), and NBT (0.05 mL, 1 mg/mL), phosphate buffer (1 mL, 50 mM, pH 7.8), and 1-butanol (0.5 mL). The reaction mixture was illuminated for 10 min at room temperature and the sample absorbance was read at 560 nm. The unilluminated reaction mixture was used as a blank. The absorbance of the blank was subtracted from that of the sample and the superoxide radical scavenging activity was expressed as equivalents of trolox according to the equation that was obtained from the standard trolox graph.
Nitric oxide (.NO) radical scavenging activity
Sodium nitroprusside in aqueous solution at physiological pH spontaneously generated NO, which was measured by the Griess reaction.[Citation20] Sample solution (0.5 mL) was mixed with sodium nitroprusside (0.5 mL, 5 mM) in phosphate buffer (0.2 M, pH 7.4) and incubated for 150 min at room temperature. Similarly, a blank was prepared by adding sample solution (0.5 mL) to phosphate buffer (0.5 mL). The incubated sample was added to diluted Griess reagent (1 mL, 1:1) and allowed standing for 30 min. The sample and blank absorbances were read at 548 nm. The absorbance of the blank was subtracted from that of the sample and the NO radical scavenging activity was expressed as equivalents of trolox according to the equation that was obtained from the standard trolox graph.
Reducing Power Activity
Iron (III) to iron (II) reduction method
The reducing power was determined according to the method of Oyaizu[Citation21] with slight modification. The sample solution (0.5 mL) was mixed with phosphate buffer (0.5 mL, 0.2 M, pH 6.6) and potassium ferricynide (0.5 mL, 1%), and the mixture was incubated at 50°C for 20 min. Then, trichloroacetic acid (0.5 mL, 10%), deionized water (2.5 mL) and ferric chloride (0.5 mL, 0.1%) were added to this mixture. Finally, the sample absorbance was read at 700 nm. Iron (III) to iron (II) reduction activity was expressed as equivalents of trolox according to the equation that was obtained from the standard trolox graph.
Cupric ion reducing (CUPRAC) method
The CUPRAC was determined according to the method of Apak et al.[Citation22] Sample solution (0.5 mL) was added to premixed reaction mixture containing CuCl2 (1 mL, 10 mM), neocuproine (1 mL, 7.5 mM), and NH4Ac buffer (1 mL, 1 M, pH 7.0). Similarly, a blank was prepared by adding sample solution (0.5 mL) to premixed reaction mixture (3 mL) without CuCl2. Then, the sample and blank absorbances were read at 450 nm after 30 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. CUPRAC activity was expressed as equivalents of trolox according to the equation that was obtained from the standard trolox graph.
Ferric reducing antioxidant power (FRAP) method
The FRAP assay was measured by the method described by Aktumsek et al.[Citation11] with slight modification. Sample solution (0.1 mL) was added to premixed FRAP reagent (2 mL) containing acetate buffer (0.3 M, pH 3.6), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ; 10 mM) in 40 mM HCl and ferric chloride (20 mM) in a ratio of 10:1:1 (v/v/v). Then, the sample absorbance was read at 593 nm after 30 min incubation at room temperature. FRAP activity was expressed as equivalents of trolox according to the equation that was obtained from the standard trolox graph.
Metal chelating activity on ferrous ions
The metal chelating activity on ferrous ions was determined by the method described by Aktumsek et al.[Citation11] Briefly, sample solution (2 mL) was added to FeCl2 solution (0.05 mL, 2 mM). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL). Similarly, a blank was prepared by adding sample solution (2 mL) to FeCl2 solution (0.05 mL, 2 mM) and water (0.2 mL) without ferrozine. Then, the sample and blank absorbances were read at 562 nm after 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample.
Determination of Total Bioactive Components
Total phenolic content
The total phenolic content was determined by employing the methods given in the literature[Citation11] with slight modification. Sample solution (0.25 mL) was mixed with diluted Folin-Ciocalteu reagent (1 mL, 1:9) and shaken vigorously. After 3 min, Na2CO3 solution (0.75 mL, 1%) was added and the sample absorbance was read at 760 nm after 2 h incubation at room temperature. The total phenolic content was expressed as equivalents of gallic acid according to the equation that was obtained from the standard gallic acid graph.
Total flavonoid content
The total flavonoid content was determined using the Dowd method as adapted by Berk et al.[Citation16] Briefly, sample solution (1 mL) was mixed with the same volume of aluminium trichloride (2%) in methanol. Similarly, a blank was prepared by adding sample solution (1 mL) to methanol (1 mL) without AlCl3. The sample and blank absorbances were read at 415 nm after 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The total flavonoid content was expressed as equivalents of quercetin according to the equation that was obtained from the standard quercetin graph.
Total saponins content
The total saponins content was determined by the vanillin-sulfuric acid method.[Citation11] Sample solution (0.25 mL) was mixed with vanillin (0.25 mL, 8%) and sulfuric acid (2 mL, 72%). The mixture was incubated for 10 min at 60°C. Then the mixture was cooled for another 15 min, followed by the sample absorbance measurement at 538 nm. The total saponin content was expressed as equivalents of Quillaja according to the equation that was obtained from the standard Quillaja graph.
Total condensed tannin content
The total condensed tannin content was determined by the vanillin method[Citation23] with slight modification. Sample solution (0.5 mL) was mixed with vanillin reagent (1.5 mL, 1% in 7 M H2SO4) in an ice bath and then mixed well. Similarly, a blank was prepared by adding sample solution (0.5 mL) to 7 M H2SO4 (1.5 mL). The sample and blank absorbances were read at 500 nm after 15 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The total condensed tannin content was expressed as equivalents of (+)-catechin according to the equation that was obtained from the standard (+)-catechin graph.
Determination of fatty acid content
The oil extraction of dried and powdered aerial plants (10 g) was carried out at 60°C for 6 h by Soxhlet extractor using petroleum ether as a solvent. The solvent was evaporated by rotary evaporator. The obtained oil was esterified to determine fatty acid composition. The fatty acids in the total lipid were esterified into methyl esters by saponification with 0.5 N methanolic NaOH and transesterified with 14% BF3 (v/v) in methanol.
The fatty acid methyl esters were analyzed on a HP (Hewlett Packard) Agilent 6890N model gas chromatograph (GC), equipped with a flame ionization detector (FID) and fitted with a HP-88 capillary column (100 m, 0.25 mm i.d. and 0.2 μm). Injector and detector temperatures were 250 and 280°C, respectively. The oven was programmed at 60°C initial temperature and 1 min initial time. Thereafter, the temperature increased 20°C/min to 190°C held for 60 min then increased at 1°C/min to 220°C and held for 10 min at 220°C. Total run time was 107.5 min. Carrier gas was helium (1 mL/min).
Identification of fatty acids were carried out by comparing sample FAME peak relative retention times with those obtained for Alltech standards. Results were expressed as FID response area relative percentages. Each reported result is the average value of three GC analyses. The results are offered as mean ± SD.
RESULTS AND DISCUSSION
Determination of Total Bioactive Components
shows the extraction yield, total phenolic, flavonoid, tannin, and saponin content of A. undulata subsp. hybrida. The total phenolic and flavonoid content were determined as 80.34 mg GAEs/g extract and 25.09 mg QEs/g extract, respectively. Folin-Ciocalteu assay were used to determine total phenolics of the methanolic extract. The assay is considered to be very valuable because it is low cost, quick, and reproducible. In many previous studies, the strong antioxidant properties of phenolics have been reported. The antioxidant activity of phenolics is mainly due to their redox properties which allow them to act as reducing agents, hydrogen donators, singlet oxygen quenchers, and metal chelator.[Citation24] Total condensed tannin and saponin contents were also calculated as spectrophotometric. These values were detected as 80.29 mg CEs/g extract and 88.39 mg QAE/g extract, respectively. In the previously published articles, there is no information about total bioactive components of A. undulata subsp. hybrida.
TABLE 1 Total antioxidant (by phosphomolybdate assay), reducing power, antiradical and enzyme inhibitory activities of the methanolic extract from A. undulata subsp. hybrida
Total Antioxidant Activity
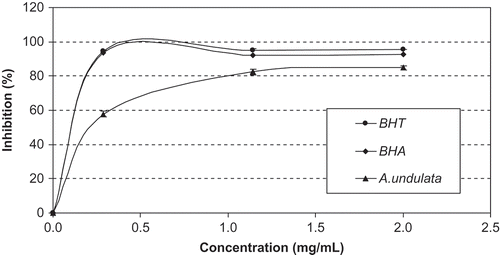
Total antioxidant capacity of the methanolic extract was evaluated by phosphomolybdenum and β-carotene/linoleic acid bleaching assays. The result of phosphomolybdenum assay was expressed ascorbic acid equivalents and the activity was detected as 1.531 mmol AAEs/g extract (). shows the antioxidant activity of the extract and synthetic antioxidants using the β-carotene/linoleic acid bleaching assay. This assay is based on the loss of the yellow color of β-carotene due to its reaction with radicals formed by linoleic acid oxidation in an emulsion. In this system, the inhibition values of linoleic acid oxidation were estimated as 84.90, 92.42, and 95.50% in the presence of the methanolic extract, BHA, and BHT at 2.0 mg/mL concentration, respectively. Apparently, synthetic antioxidants possessed a stronger inhibition activity than that of the methanolic extract from A. undulata subsp. hybrida.
Reducing Power Activity
Antioxidative activity has been proposed to be related to reducing power. Therefore, in order to assess the electron-donating power of the methanolic extract, its ability to reduce iron (III) and Cu(II) were investigated. These activities were evaluated as trolox equivalents (mmol TEs/g extract). In potassium ferricyanide method, Fe3+/ferricyanide complex is reduced to the ferrous form by antioxidants. The Fe2+ formed is monitored by measuring the formation of Perl’s Prussian blue at 700 nm.[Citation21] FRAP method is based on the principle of the reduction of the Fe(III)/TPTZ complex to the Fe(II), upon which an intense blue color develops, and the change of absorbance is measured at 593 nm. The results of the reducing power assays are shown in . The trolox equivalents value was observed as close to ferric reducing power assays (0.329 mmol TEs/g extract for potassium ferricyanide, 0.425 mmol TEs/g extract for FRAP). Reduction of Cu(II) ability of the extract was tested by CUPRAC assay and the activity was detected 0.081 mmol TEs/g extract. As far as our literature survey could ascertain, there is no study on the reducing potential of A. undulata subsp. hybrida. Hence, our study is the first report about reducing potential of A. undulata subsp. hybrida.
Antiradical Activities
Free radical scavenging activities of the methanolic extract were evaluated using different in vitro models like scavenging of DPPH radical, ABTS cation radical, NO radical, superoxide anion radical, and hydroxyl radical. The results obtained from these assays are expressed trolox (mmol TEs/g extract) and mannitol (mmol MEs/g extract) equivalents and are given in . DPPH stable free radical method is an easy, rapid and sensitive way to survey the antioxidant activity of a specific compound or plant extracts.[Citation25] This method based on the reduction DPPH solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H by the reaction. The DPPH scavenging activity of the methanolic extract was found to be 2.086 mmol TEs/g extract. ABTS radical cation decolorization assay is another assay widely used to radical scavenging activity. In this method, in presence of antioxidants, the colored (blue) radical is converted back to colorless ABTS. Trolox equivalent value obtained for ABTS assay was 0.112 mmol TEs/g extract. Measurement of superoxide radical scavenging activity of the extract was done by using riboflavin-NBT method. The superoxide radical scavenging potential was determined as 0.364 mmolTEs/g extract. NO radical scavenging activity is based on generation of nitric oxided from sodium nitroprusside. NO level can be measured by using Griess reagent. Antioxidant compounds are leading to reduced production of NO. The NO scavenging activity was observed as 3.866 mmol TEs/g extract. The hydroxyl radical scavenging activity of the methanolic extract was assessed using deoxyribose assasy. Deoxyribose degradation occurs by hydroxyl radicals generated by a Fenton reaction. The hydroxyl radical scavenging activity was expressed mannitol equivalents and the activity was detected as 0.208 mmol MEs/g extract. No literature is available regarding the radical scavenging activities of A. undulata subsp. hybrida. In this work, it was reported for the first time antiradical potentials of this plant.
Metal Chelating Activity
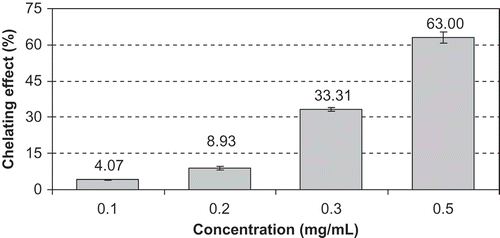
Transition metal ions, especially iron, are generated radicals through the Fenton and Haber-Weiss reaction. Hence, metal chelating activity is claimed as one of antioxidant mechanisms.[Citation26] In the present study, the chelating activity can compete with ferrozine for ferrous ion. The iron-ferrozine complex has maximal absorbance at 562 nm and a large decrease in absorbance indicates strong chelating power. The chelating activity of the methanolic extract increased in a concentration-dependent manner (). At the concentration of 0.1, 0.2, 0.3, and 0.5 mg/mL, the chelating activity was 4.07, 8.93, 33.31, and 63.00% for the methanolic extract, respectively. However, EDTA showed an excellent chelating ability of 98.08 ± 0.18% at 0.1 mg/mL.
Enzyme Inhibitory Activity
In this work, the methanolic extract from A. undulata subsp. hybrida was examined for cholinesterase, tyrosinase, α-amylase, and α-glucosidase inhibitory activities. The results obtained from enzyme inhibitory assays are given . Alzheimer’s disease (AD) is the most common form of neurodegenerative disorders. AD characterized by a consistent deficit in cholinergic neurotransmission. AD is a progressive and neurologically disorder illustrated by memory disfunction and dementia.[Citation27] Symptoms of AD can be treated by the use of agents which restore the level of acetylcholine through inhibition of both the two major forms of cholinesterase (AChE and BChE). Therefore, cholinesterase inhibitors play important role in the treatment of AD. The cholinesterase inhibitory activities of the methanolic extract were determined using spectrophotometric Elmann’s methods and the results were expressed as equivalents of galantamine. The results for AChE and BChE were observed as 2.238 and 1.239 μmol GALAEs/g extract, respectively.
Tyrosinase is known to be a key enzyme in melanin biosynthesis. Several dermatological disorders, such as age spots, melanoma, and freckles arise from excessive accumulation of melanin.[Citation28] For this reason, tyrosinase inhibitors have become increasingly important above mentioned skin disorders. Kojic acid is well-known a tyrosinase inhibitior and the result of tyrosinase inhibitory activity assay was expressed as equivalents of kojic acid. This activity for the methanolic extract was detected as 0.339 mmol KAEs/g extract.
Diabetes mellitus is a metabolic disorder resulting from the defect in insulin secretion and is characterized by increased concentrations of glucose in the blood. Many studies indicated that inhibiton of carbohydrate metabolism enzymes such as α-glucosidase and α-amylase is one way for treating diabetes.[Citation14,Citation29] Acarbose is conventionally used in the management of diabetes and the results of enzyme inhibitory activities were evaluated as equivalents of acarbose. The methanolic extract showed a higher α-glucosidase inhibitor activity (0.219 mmol ACEs/g extract) compared to α-amylase inhibitory activity (0.193 mmol ACEs/g extract). To the best of the author’s knowledge, this study is the first report on cholinesterase, tyrosinase, α-amylase, and α-glucosidase inhibitory activities in A.undulata subsp. hybrida.
Fatty Acid Composition
Fatty acid composition of the aerial parts of A. undulata subsp. hybrida was analyzed by GC. The results of the fatty acid analysis were summarized in and 23 fatty acids were identified in the oil. Major fatty acids were oleic (C 18:1 ω9), linoleic (C 18:2 ω6), and palmitic (C 16:0) acid. Similarly, oleic and linoleic acids were reported as the major fatty acids in the A. undulata subsp. hybrida seed oil.[Citation9] In the present study, α-linolenic (C 18:3 ω3) and γ-linolenic acid (C 18:3 ω6) were observed as the other predominant fatty acids. These results were agreement with many previous studies which was investigated fatty acid profile of some Boraginaceae species.[Citation9] Members of Boraginaceaae species are found to be the most common source of γ-linolenic acid in these studies. Linoleic and α-linolenic acid are known to be “essential fatty acids” (EFAs) which cannot be synthesized by human body. Because the studied oil had high levels of EFAs (32.90 g/100 g of total fatty acids), are considered as a source of EFAs. The high levels of stearic (C 18:0) and myristic (C 14:0) acid were observed in the saturated fatty acids.
TABLE 2 Fatty acid composition of aerial part of Anchusa undulata subsp. hybrida
The total unsaturated fatty acids levels were considerably higher than total saturated fatty acids in the studied oil. The results for unsaturated fatty acids agree with values mentioned by other researchers.[Citation30] Polyunsaturated fatty acids, especially ω3 fatty acids, have been associated with various health benefits relating to treatment of some diseases including rheumatoid arthritis and coronary artery disease.[Citation31] ΣSFA, ΣMUFA, and ΣPUFA accounted for 28.26, 29.29, and 42.45 g/100 g of total fatty acids, respectively. Some parameters and indexes, such as ω3/ω6 ratio, atherogenic (AI) and thrombogenicity index (TI), are used as an indicator to evaluate nutritional quality of lipid. The ratio and indexes were determined as 0.38, 0.36, and 0.40 for A. undulata subsp. hybrida oil, respectively.
CONCLUSION
A. undulata subsp. hybrida exhibited strong neuroprotective, antioxidative, skin care, and antidiabetic properties and, had high levels of phenolic, flavonoids, and unsaturated fatty acids. We identified for the first time the fatty acid composition, antioxidant ability, and enzyme (AChE, BChE, tyrosinase, α-amylase, and α-glucosidase) inhibitors effect of this plant. Therefore, the aboveground part of the species could be helpful in developing drug preparations and functional foods in the pharmaceutical and food industries. However, further studies should be carried out for the evaluation of the in vivo potential of the extract in animal model.
NOMENCLATURE
| AAEs | = | Ascorbic acid equivalents |
| ACEs | = | Acarbose equivalents |
| BHA | = | Butylated hydroxyanisole |
| BHT | = | Butylated hydroxyltoluene |
| CEs | = | Catechin equivalents |
| DPPH | = | 1,1-diphenyl-2-picrylhydrazyl |
| GAEs | = | Gallic acid equivalents |
| GALAEs | = | Galantamine equivalents |
| GC | = | Gas chromatography |
| KAEs | = | Kojic acid equivalents |
| MEs | = | Mannitol equivalents |
| PNPG | = | 4-N-trophenyl-α-D-glucopyranoside |
| TEs | = | Trolox equivalents |
| QAEs | = | Quillaja equivalents |
| QEs | = | Quercetin equivalents |
REFERENCES
- Liochev, S.I.; Fridovich, I. The role of O−2 in the production of HO in vitro and in vivo. Free Radical Biology and Medicine 1994, 16, 29–36.
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radical Biology and Medicine 2000, 28, 1685–1696.
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Science and Technology 2007, 40 (9), 1664–1669.
- Kehrer, J.P.; DiGiovanni, J. Comparison of lung injury induced in 4 strains of mice by butylated hydroxytoluene. Toxicology Letters 1990, 52, 55–61
- Singh, S.K.; Rai, P.K.; Jaiswal, D.; Watal, G. Evidence based critical evaluation of glycemic potential of Cynodon dactylon. Evidence Based Complementary and Alternative Medicine 2007, 6 (4), 415–420.
- Tocco, G.; Fais, A.; Meli, G.; Begala, M.; Podda, G.; Fadda, M.B. … Berretta, S. PEG-immobilization of cardol and soluble polymer-supported synthesis of some cardol–coumarin derivatives: Preliminary evaluation of their inhibitory activity on mushroom tyrosinase. Bioorganic & Medicinal Chemistry Letters 2009, 19, 36–39.
- Selvi, F.; Bigazzi, M. Revision of genus Anchusa (Boraginaceae Boragineae) in Greece. Botanical Journal of the Linnean Society 2003, 142, 431–454.
- Davis, P.H. Boraginaceae. In: Flora of Turkey and the East Aegean Islands; Davis P.H.; Ed.; Vol. 6, Edinburgh Free Press: Edinburgh, 1988; 237–437.
- Ozcan, T. Analysis of the total oil and fatty acid composition of seeds of some Boraginaceae taxa from Turkey. Plant Systematics and Evolution 2008, 274, 143–153.
- Uz-Kuruuzum, A.; Guvenalp, Z.; Kazaz, C.; Salih, B.; Demirezer, O. Four new Triterpenes from Anchusa azurea var. azurea. Helvetica Chimica. Acta 2010, 93, 457–465.
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chemical and Toxicology 2013, 55, 290–296.
- Erdogan Orhan, I.; Senol, F.S.; Gulpinar, A.R.; Sekeroglu, N.; Kartal, M.; Sener, B. Neuroprotective potential of some terebinth coffee brands and the unprocessed fruits of Pistacia terebinthus L. and their fatty and essential oil analyses. Food Chemistry 2012, 130, 882–888.
- Yang, X.W.; Huang, M.Z.; Jin, Y.S.; Sun, L.N.; Song, Y.; Chen, H.S. Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia 2012, 83, 1169–1175.
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chemistry 2011, 127, 21–27.
- Sarikurkcu, C.; Eryigit, F.; Cengiz, M.; Tepe, B.; Cakir, A.; Mete, E. Screening of the antioxidant activity of the essential oil and methanol extract of Mentha pulegium L. from Turkey. Spectroscopy Letters 2012, 45, 352–358.
- Berk, S.; Tepe, B.; Arslan, S.; Sarikurkcu, C. Screening of the antioxidant, antimicrobial, and DNA damage protection potentials of the aqueous extract of Asplenium ceterach DC. African Journal of Biotechnology 2011, 10, 8902–8908.
- Sarikurkcu, C. Antioxidant activities of solvent extracts from endemic Cyclamen mirabile Hildebr. tubers and leaves. African Journal of Biotechnology 2011, 10, 831–839.
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method: A simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry 1987, 165, 215–219.
- Dasgupta, N.; De, B. Antioxidant activity of Piper betel L. leaf extract in vitro. Food Chemistry 2004, 88, 219–224.
- Srivastava, A.; Shivanandappa, T. Antioxidant and cytoprotective properties of 2-(hydroxymethyl)-3-methoxybenzaldehyde. Food Chemistry 2011, 128, 458–464.
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of browning reaction prepared from glucosamine. Japanese Journal of Nutrition 1986, 44, 307–315.
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E.; Ercag, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. International Journal of Food Science and Nutrition 2006, 57, 292–304.
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, antiinflammatory, anti-cholinesterase, and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chemical Toxicology 2013, 55, 470–475.
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant derived polyphenolic flavonoids. Free Radical Research 1995, 22, 375–383.
- Szabo, M.; Iditoiu, C.; Chambre, D.; Lupea, A.X. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chemical Papers 2007, 61, 214–216.
- Isbilir, S.S.; Sagiroglu, A. An assessment of in vitro antioxidant activities of different extracts from Papaver rhoeas L. Leaves. International Journal of Food Properties 2012, 15, 1300–1308.
- Iwata, N.; Higuchi, M.; Saido, T.C. Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacology & Therapeutics 2005, 108, 129–148.
- Chiari, M.E.; Vera, D.M.A.; Palacios, S.M.; Carpinella, M.C. Tyrosinase inhibitory activity of a 6-isoprenoid-substituted flavanone isolated from Dalea elegans. Bioorganic & Medicinal Chemistry 2011, 19, 3474–3482.
- Asgar, M.A.; Anti-diabetic potential of phenolic compounds: A review. International Journal of Food Properties 2013, 16, 91–103.
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Fatty acid distribution in the seed flour of wild Vicia species from Southern Spain. Journal American Chemical Society 2009, 86 (10), 977–983.
- Freeman, M.P. Omega-3 fatty acids in psychiatry. A review. Annual Review of Clinical Psychology 2000, 12, 159–165.