Abstract
The total antioxidant capacity of Brassica nigra seeds (black mustard seeds) and the presence of phenolic compounds which are known to exist in seeds of this family were quantified. Oxygen radical absorbance capacity assay, ferric reducing antioxidant power, and the 2, 2-diphenyl-1-picrylhydrazyl and 2, 2-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging assays were conducted on dichloromethane, ethanol, ethyl acetate, hexane, methanol, and water extracts of the seeds. Water and ethanol extracts displayed the highest antioxidant activity. Phenolic compounds which have been known to demonstrate antioxidant properties such as catechin, epicatechin, gallic acid, myricetin, quercetin, and rutin were present in all extracts.
INTRODUCTION
Traditional knowledge of medicinal plants has been a common guide for the search for new medicinal remedies. In spite of the advent of modern high throughput drug discovery and screening techniques, traditional knowledge systems have given clues to the discovery of valuable drugs with better efficacies.[Citation1,Citation2] Traditional medicinal plants are often cheaper, locally available, and easily consumable, either in raw form or as simple medicinal preparations. Currently, traditional medicinal practices form an integral part of complementary or alternative medicine. Although their efficacy and mechanisms of action have not been tested scientifically in most cases, these simple medicinal preparations often mediate beneficial responses due to their active chemical constituents. From a more modern perspective, free radicals, produced as a result of normal biochemical reactions in the body, are implicated in contributing to disease conditions such as cancer, atherosclerosis, cardiovascular disease, diabetes, and neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease.[Citation3] Given the mechanisms of action of the free radicals, the human body itself possesses innate defense mechanisms to counter them in the form of enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. Apart from these, plant secondary metabolites such as flavonoids and terpenoids play an important role in the defense against free radicals.[Citation4]
Brassica nigra (Family: Brassicaceae) popularly known as black mustard has been consumed in different parts of the world for its medicinal value as well as a flavoring agent. In the former aspect, the seeds of this plant has been incorporated into traditional medicinal recipes for treatment of ailments such as cardiovascular disease, diabetes, and its complications.[Citation5] Additionally, it has also been used in the treatment of rheumatism as well as a means of reducing congestion in internal organs. Only a few studies have been carried out to date on the antioxidant potential of Brassica nigra and the measurement of its antioxidant compounds, although antioxidant compounds have been discovered to be present in seeds of other members of the Brassicaceae family.[Citation6] In this study, the total antioxidant capacity of different solvent extracts of Brassica nigra were examined using the oxygen radical absorbance capacity (ORAC) assay, ferric reducing antioxidant power (FRAP) and the 2, 2-diphenyl-1-picrylhydrazyl (DPPH•) and 2, 2-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid; ABTS•+) radical scavenging assays. The total polyphenol content of the seeds were also measured in order to draw comparisons and to provide more detailed information about this sub-group of antioxidants, since they are known to be particularly important in the pathologies of heart disease, hypertension, and age-related degeneration. Characterization and quantification of well-known antioxidant compounds such as catechin, epicatechin, gallic acid, myricetin, quercetin, and rutin which are typically known to be present in members of the Brassicaceae family was carried out using high-performance liquid chromatography (HPLC) to provide a better identification as to the contributors of the observed antioxidant capacity.
Materials and Methods
Brassica nigra seeds were obtained from Johor Bahru, Malaysia during the month of October 2010 and was identified by the Miss Lee Yian Hoon of the Temasek School of Applied Sciences, Temasek Polytechnic, Singapore. Reference samples were placed in the herbarium of the Temasek School of Applied Sciences (reference no. 31984). Anhydrous sodium carbonate, Folin-Ciocalteu’s phenol reagent, KH2PO4, and K2HPO4, were obtained from Merck (Darmstadt, Germany). 4,6-Tripryridyl-s-triazine (TPTZ), gallic acid and trolox were purchased from Acros Organics (Morris plains, NJ, USA). 2, 2-Azobis (2-amidinopropane) dihydrochloride (AAPH), Fluorescein disodium salt, vitamin C, neoxanthin, violaxanthin, lutein, zeaxanthin, lycopene, α- and β-carotene, and meta-phosphoric acid were purchased from Sigma Chemicals (St. Louis, MO, USA). Sulphuric acid (95%) was obtained from BDH (London, UK). Tetrahydrofuran, n-hexane (HEX), methanol (MeOH), n-butanol, ethyl acetate (EtOAc), acetone, and acetonitrile (HPLC/Spectro grade) were purchased from Tedia (Fairfield, OH, USA). Absolute ethanol (EtOH), butylated hydroxytoluene (BHT) and disodium sulphate (analytical grade) were obtained from Merck (Darmstadt, Germany). All other reagents, chemicals and HPLC standards used for the study were purchased from Sigma Chemicals (St. Louis, MO, USA).
Preparation of Brassica Nigra Seed Extracts
Brassica nigra seeds were ground into a powder in a grinder (Philips HL 1606, Bangkok, Thailand) for 5 min at an ambient temperature of 22 ± 3°C. The solvents used for subsequent extraction were dichloromethane (DCM), EtOH, EtOAc, HEX, MeOH, and water. The powder (0.5–1.0 g, approximately 4–6 µm particle size) was extracted three times with each solvent. After the addition of solvent, the tube was vortexed for 30 s followed by sonication for 15 min at 4.5 W min−1 with temperature maintained between 37 and 39°C. The tube was shaken once in the middle of sonication to suspend the sample. After the sonication, the tube was allowed to cool to room temperature. The tube was then centrifuged and the supernatant was collected in a 25 mL volumetric flask and topped up to the mark with each solvent prior to conducting the various assays and analyses.
Total Phenolics Content
The total phenolics contents of the various solvent extracts were determined according to a modified version of the procedure described by Singleton and Rossi[Citation7] using the Folin and Ciocalteu’s phenol reagent. The value was determined using a standard curve prepared from gallic acid and expressed as mg gallic acid equivalents per gram fresh weight (mg GAE/g) of sample.
ORAC Assay
The assay was carried out according to the method by Prior et al.[Citation5] with a few modifications in 96-well microplate format using a Thermo Scientific Multiskan FC Microplate Reader. Fluorescein disodium was used for the kinetic monitoring of free radical quenching and AAPH was used as the free radical source. The excitation and emission wavelengths were 485 nm and 528–538 nm, respectively. The following components were added to a single well: (1) Blank (phosphate buffered saline)/trolox standard/sample – 20 μL; (2) fluorescein working solution – 160 μL; and (3) AAPH – 20 μL. The reaction kinetics were monitored for two hours at 37°C, following which the area under the curve was used to calculate the ORAC value compared with those of the trolox standards. BHT was used as the positive control. Results were expressed as µmol trolox equivalents per gram fresh weight (µmol TE/g) of sample.
Determination of the DPPH Radical Scavenging Activity
Extract concentrations of 62.5, 125, 250, 500, and 1000 mg of extract per kg raw material (mg/kg) were prepared by dilution with 75 mM phosphate buffer (pH = 7.4). A 96-well microplate was used for the analysis where 140 µL of the respective extracts were pipetted along with 60 µL of 400 µM of DPPH (prepared in the 75 mM phosphate buffer solution). The blank wells consisted of 200 µL of the phosphate buffer solution, while the control wells consisted of 140 µL of the phosphate buffer solution and 60 µL of the DPPH solution. The microplate was incubated at 37°C for 30 min and the absorbance was measured at 517 nm, using a Thermo Scientific Multiskan FC Microplate Reader. Each sample concentration was added in triplicate into the microplate. BHT was used as the positive control. The antioxidant activity was calculated as % DPPH radical scavenging activity, by substituting the absorbance values into the following equation:
The % DPPH scavenging activity of ten replicates of each sample was used to calculate the EC50 values (the concentration required to obtain 50% antioxidant effect) in milligrams per kilogram fresh weight (mg/kg) of the samples.
FRAP Assay
A modification of the FRAP assay of Benzie and Strain[Citation8] was carried out. Briefly, FRAP reagent was prepared from 300 mM acetate and glacial acetic acid buffer (pH 3.6), 20 mM ferric chloride and 10 mM TPTZ made up in 40 mM HCl. All three solutions were mixed together in the ratio 10:1:1. The FRAP assay was performed by warming 1 mL of deionized H2O to 37°C before adding 25 μL of sample and 1 mL of reagent and incubating at 37°C for 4 min. Absorbance at 593 nm was determined relative to a reagent blank also incubated at 37°C. The total antioxidant capacity of samples was determined against a standard of known FRAP value, ferrous sulphate (1000 μM). BHT was used as the positive control.
ABTS•+ Radical Cation Scavenging Activity
Antioxidant activities of the samples were analyzed by investigating their ability to scavenge the ABTS•+ free radical using a methodology previously reported by Ozgen et al.[Citation9] BHT was used as the positive control.
HPLC Determination of the Antioxidant Compounds
A Shimadzu (Kyoto, Japan) HPLC system equipped with a diode array detector (SPDM10Avp) and a phenomenex Luna C-18(2) column (4.6 mm i.d. × 25 cm, 5 μm) was used for the quantification of the phenolic compounds of catechin, epicatechin, gallic acid, myricetin, quercetin, and rutin. A gradient profile using two solvents was applied at room temperature following the method by Wijeratne et al.[Citation10] with a few modifications. The solvents used were as follows: Solvent A: 8% aqueous formic acid and solvent B: acetonitrile/MeOH (10:90, v/v). A flow rate of 0.9 mL/min was maintained. The gradient was as follows: 0 min – 20% B; 7 min – 35% B; 14 min – 45% B; 21 min – 65% B; 25 min – 85% B; 32 min – 95% B. The wavelengths of the diode array detector were set at 260, 280, and 320 nm for monitoring of the phenolic compounds. The concentrations of all the compounds in the extracts were quantified using standard curves and expressed as micrograms per gram fresh weight of powder (µg/g).
Statistical Analysis
All data are presented as mean ± standard error mean (SEM) of at least three independent experiments (n ≥ 3), where each experiment had a minimum of three replicates of each sample. For comparisons between samples, data was analyzed by ANOVA and Tukey’s multiple comparison test (SPSS, version 17). A probability of 5% or less was accepted as statistically significant (p < 0.05).
Results and Discussion
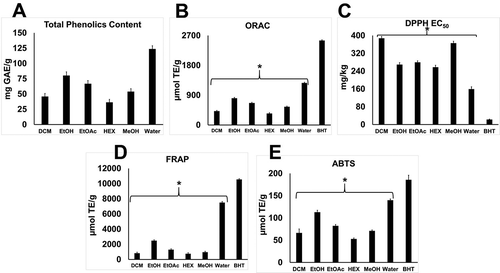
The yield of the dried extract as a percentage weight of the starting fresh seed powder are shown in . The total phenolics contents of the extracts and the results from the antioxidant capacity assays are shown in . The total phenolics contents of the extracts decreased in the order of water > EtOH > EtOAc > MeOH > DCM > HEX. The ABTS•+, FRAP, and ORAC values of the extracts decreased in the same order as the total phenolics contents, while the DPPH EC50 values increased in the reversed order. The increases and differences of the various antioxidant values correlated with the total phenolics content, indicating the water extract to contain the highest amount of total phenolics and antioxidant potential of HEX being the lowest. Therefore, water was observed to be the best solvent for the extraction of the phenolic compounds from the Brassica nigra powder. All the antioxidant values were statistically significantly lower (p < 0.05) than those of BHT. The correlation between the total phenolics content and the ORAC assay values (R2 = 0.981) were higher than ABTS•+ (R2 = 0.798), DPPH EC50 (R2 = 0.811), and FRAP (R2 = 0.851). Given the high R2 value in relation to the ORAC values, the total phenolics content was shown to be a good indicator of the presence of antioxidant compounds for the solvent extracts.
TABLE 1 Yield of the dried extract (g) as a percentage weight of the starting fresh Brassica nigra seed powder
FIGURE 1 (A) Total phenolics contents; (B) ORAC; (C) DPPH EC50; (D) FRAP; and (E) ABTS radical scavenging activities of DCM, EtOH, EtOAc, HEX, MeOH, and water extracts of Brassica nigra. Results are expressed as mean ± SEM per gram fresh weight. *p < 0.05 versus the antioxidant values of BHT.
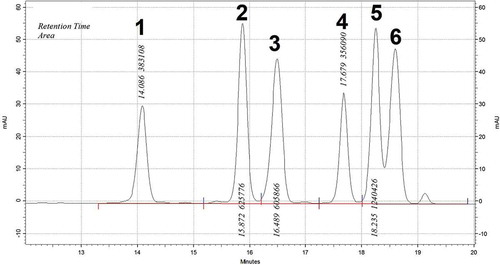
The list of phenolic compounds which were quantified to be present in the solvent extracts are shown in . These values, however, are tentatively determined and identified. Further complementing the total phenolics content values, the water extract was observed to contain the highest amount of phenolic compounds with EtOH coming a close second. Catechin and epicatechin had the highest presence out of all the phenolic compounds quantified. Their amounts present in the water and EtOH extracts were 62.9 and 22.8, and 24.7 and 25.9 micrograms per gram fresh weight of powder (µg/g), respectively. A representative HPLC diagram of the water extract is shown in . Further complementing the results from , the results from ascertained water to be the better solvent for the extraction of antioxidant compounds from the Brassica nigra seeds. This observation was somewhat contradictory given the fat content which has been reported in these seeds and the expectancy of a lipophilic solvent to be the better medium for the extraction of antioxidant compounds. However, it is also possible that despite the high content of fat, the overall antioxidant compounds in these seeds are more soluble in water, and, therefore, water proved to be the better solvent for their extraction.
TABLE 2 Phenolic compounds present in the Brassica nigra extractsa
FIGURE 2 Representative HPLC chromatogram of the water extract where the peaks were identified as follows: 1: Catechin; 2: Quercetin; 3: Myricetin; 4: Epicatechin; 5: Rutin; 6: Gallic acid.
This study mainly focused on quantifying the phenolic compounds which are commonly found in seeds belonging to the Brassicaceae family, and have been identified to be present in their extracts as per previous studies.[Citation6] The compounds studied here are also well known for their antioxidant potential and therapeutic benefits. Nevertheless, the possibility exists that novel antioxidant compounds may be present in the extracts which requires extensive characterization procedures. Following this, whether these novel compounds possess any therapeutic potential also requires further elucidation through in vitro and in vivo studies. However, the existence of known antioxidant compounds with established therapeutic properties in considerable amounts itself is a highlight of this study, given that black mustard seeds in general have not exhibited to contain these antioxidant compounds in these particular quantities (regardless of the type of solvent used for the extraction).[Citation11] Nevertheless, despite their association with therapeutic effects, the antioxidant content of plant material in general, could be affected by the cultivar, maturity level as well as growing conditions such as the location, soil state, climate, and agriculture practices.[Citation12] In addition, it is possible that the phytochemicals contained in the black mustard seeds could work synergistically with each other to demonstrate the observed antioxidant properties. Thus, although only the phenolic compounds were given special attention in terms of antioxidant properties, it is possible that their antioxidant characteristics work synergistically with other groups of phytochemical compounds.
Several methods have been used to assess the total antioxidant capacity of seeds and cereals in general. ORAC is based on the antioxidant’s ability to react with or neutralize free radicals generated in the assay systems, whereas FRAP measures the reduction of Fe3+ (ferric iron) to Fe2+ (ferrous iron) in the presence of antioxidants. The total antioxidant capacity measure was considered appropriate for assessing the cumulative antioxidant properties of plant foods.[Citation13,Citation14] However, the impossibility of comparing results obtained with different methodologies has seriously limited understanding of the role of total antioxidant capacity in disease prevention.[Citation15] As a result, in the recent years, ambiguous results have been published about the possible effect of seeds on oxidative stress status.[Citation16,Citation17] This was the rationale for further characterizing the antioxidant compounds present in the extracts. The antioxidants which were characterized to be present in Brassica nigra seeds have been identified to possess functional properties and health benefits. They are known to affect nutritional value, appearance, taste, and texture of these foods and promote better health by preventing cardiovascular diseases, cancers, urinary tract infections, and other aging-related metabolic complications.[Citation18–Citation20]
Conclusions
The study was able to provide insights as to the solubility of antioxidant compounds present in Brassica nigra in different types of solvents. Water was observed to be the better solvent for the efficient extraction of antioxidant compounds. Although the study only examined six pure solvents, their combinations may also be worthy of exploring for the efficacy of the extraction. The bioactivity and the radical scavenging efficacy of the extracts in in vitro or in vivo systems is a worthy exploration and could be considered as the next step following this screening process. Whether the identified antioxidant activities could be initiated in actual physiological conditions has always been the challenge since the chemical assays have not been essentially able to capture the true potential of antioxidants and plant-based food products containing antioxidant compounds. The possible synergistic effects between different antioxidant compounds also need to be explored. Such studies may help elucidate the mechanisms explaining the effect of consuming different types of seeds or their byproducts on oxidative stress status and their resulting or associated disease conditions in humans.
ACKNOWLEDGMENT
The authors would like to express their appreciation for the analytical support provided by the Temasek Applied Science Research Center, Temasek Polytechnic, Singapore.
FUNDING
The authors wish to extend their gratitude for the financial assistance provided by the TOTE Fund of Temasek Polytechnic, Singapore.
Additional information
Funding
References
- Buenz, E.J.; Schnepple, D.J.; Bauer, B.A.; Elkin, P.L.; Riddle, J.M.; Motley, T.J. Techniques: Bioprospecting historical herbal texts by hunting for new leads in old times. Trends in Pharmacological Sciences 2004, 25, 494–498.
- Cai, Y.Z.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. Life Sciences 2004, 74, 2157–2184.
- Serafini, M. Back to the origin of the “antioxidant hypothesis:” The lost role of the antioxidant network in disease prevention. Journal of Agricultural and Food Chemistry 2006, 86, 1989–1991.
- Chang, Y.L. Challenges in providing credible scientific evidence of health benefits of dietary polyphenols. Journal of Functional Foods 2013, 5, 524–526.
- Prior, R.L.; Hoang, H.; Gu, L.W.; Wu, X.L.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M; Huang, D.J.; Ou, B.X.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (Oxygen radical absorbance capacity [ORACFL]) of plasma and other biological food samples. Journal of Agricultural and Food Chemistry 2003, 51, 3273–3279.
- Chen, C.Y.; Blumberg, J.B. Phytochemical composition of nuts. Asia Pacific Journal of Clinical Nutrition 2008, 17 (Suppl 1), S329–S332.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power:” The FRAP assay. Analytical Biochemistry 1996, 239, 70−76.
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) methods. Journal of Agricultural and Food Chemistry 2006, 54, 1151−1157.
- Wijeratne, S.S.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant polyphenols in almond and its co-products. Journal of Agricultural and Food Chemistry 2006, 54, 312–318.
- Brock, A.; Herzfeld, T.; Paschke, R.; Koch, M.; Drager, B. Brassicaceae contain nortropane alkaloids. Phytochemistry 2006, 67, 2050–2057.
- Wu, X.; Gu, L.W.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Beecher, G.; Prior, R.L. Development of a database for total antioxidant capacity in foods: A preliminary study. Journal of Food Composition and Analysis 2004, 17, 407–422.
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals, and sweets consumed in Italy assessed by three different in vitro assays. Molecular Nutrition and Food Research 2006, 50, 1030–1038.
- Halliwell, B. Free radicals, antioxidants and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724.
- Mukuddem-Petersen, J.; Oosthuizen, W.; Jerling, J.C. A systematic review of the effects of nuts on blood lipid profiles in humans. Journal of Nutrition 2005, 135, 2082–2089.
- Koleva, I.I.; van Beek; T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochemical Analysis 2002, 13, 8–17
- Halliwell, B.; Aeschbach, R.; Loliger, J.; Aruoma, O.I. The characterization of antioxidants. Food and Chemical Toxicology 1995, 33, 601–617.
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. Journal of Nutrition 2005, 135, 2291–2294.
- Zhao, B. Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochemical Research 2009, 34, 630–638.
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chemistry 2004, 88, 411–417.
