Abstract
The antioxidation activities of Eriobotrya japonica (Lindl.) fruit peel and pulp extracts were determined using DPPH, β-carotene, and Rancimat methods. Results showed that ethanol-water extract of peel and ethanol extract of pulp had the highest antioxidant activity. Protective effects of extracts in stabilization of soybean oil were tested and compared to tert-butyl hydroquinone by measuring peroxide values, free fatty acids, thiobarbituric acid, oxidative stability, and conjugated dienes and trienes values during storage (65 days at 25°C). Results showed that the ethanol-water extract of peel at 400 ppm exhibited stronger antioxidant activity, but the highest effect was observed in tert-butyl hydroquinone.
INTRODUCTION
Oxidation of oils and fats is a chain of chemical reactions that causes changes in organoleptic properties, reduces the nutritional value and shelf life of food.[Citation1] Primary and secondary oxidation products of oils are harmful for humans and cause damage of the body’s biological cells.[Citation2] Oils that contain high levels of unsaturated fatty acid are more susceptible to oxidation.[Citation3] Therefore, evaluation of oxidation stability of oils during processing and storage is very important.
Synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butyl hydroquinone (TBHQ) are used to increase oxidative stability of oils and fats.[Citation4] However, studies conducted subsequently have demonstrated that synthetic antioxidants have toxic effects and consequently restrictions have been imposed on their use.[Citation5] For example, despite the high antioxidant activity of TBHQ, its use has been prohibited in some countries, such as Japan and Canada.[Citation6] Therefore, researchers have focused on plant-derived natural antioxidants.
Plants are a rich source of antioxidant compounds such as phenols, tocopherols, and ascorbate.[Citation7] Phenolic compounds are an important group of antioxidants which have appropriate chemical structure to absorb free radicals. Phenols are shown to have greater antioxidant activity in vitro than other bioactive compounds.[Citation8] Loquat (Eriobotrya japonica) is an Asian fruit and a member of the Rosaceae family.[Citation9] The species is native to southeastern China and mainly grow in subtropical and mild temperatures. Currently it is also cultivated in other areas, namely in South Africa, South America, Australia, and California.[Citation10] Loquat is a large evergreen shrub or small fruit tree with a rounded crown and short trunk. Loquats are unusual among other fruits, they flower in autumn or early winter, and the fruits ripen in late winter or early spring.[Citation11] Loquat fruit is eaten as a fresh fruit, and also recently it has been used in the preparation of jam, jelly, and chutney.[Citation12] Phenolic compounds of loquat fruits have been characterized in a few studies which state that the antioxidant activity of loquat fruits is due to the presence of hydroxycinnamic and benzoic acids derivatives and cyanidine glycoside.[Citation13] Therefore, the aim of this study was to evaluate the antioxidation effects of loquat fruit (Eriobotrya japonica) skin and pulp extracts during oxidation of soybean oils by measuring both primary and secondary oxidation products and to compare its antioxidant activity with a synthetic antioxidant (TBHQ).
MATERIALS AND METHODS
Plant Material and Chemicals
Loquat fruits were collected from fields in Sari in the Mazandaran province, Iran. Refined, bleached, and deodorized soybean oil with no added antioxidants (SBO) was supplied by Rana (Gorgan, Iran) and was stored at –20ºC until analysis. All reagents used in this study were of analytical grade and obtained mainly from Sigma-Aldrich (St. Louis, USA). Solvents used for extraction of plant samples were purchased from Merck (Darmstadt, Germany).
Preparation of Plant Extracts
The peel and pulp of loquat fruits were separated and dried in shadow in natural conditions. The bioactive compounds of dried powders of peel and pulp (20 g) were extracted with 100 mL of water, ethanol, and ethanol-water (1:1) by shaker at room temperature for 48 h. The extracts were filtered and solvents evaporated using a rotary evaporator (Heidolph, Germany) at 50°C. The extracts were stored at –20°C until testing.[Citation14]
Total Phenolic Content
The content of phenolic compounds in the extracts was determined with Folin-Ciocalteu reagent following the colorimetric method adapted by Taghvaei et al.[Citation15] Measurements were carried out in triplicate and calculations were based on a calibration curve obtained with gallic acid. The contents of total phenols were expressed as micrograms of gallic acid equivalents per gram of dry weight (µg GAE.g–1 DW).
DPPH• Scavenging Activity
The scavenging activity of the stable free radical; 2, 2-diphenyl-1-picrylhydrazyl (DPPH•) was evaluated by the method described by Burits and Bucar[Citation16] Briefly, 5 mL of DPPH• solution (0.004%) was added to 50 µL of varying concentrations of the plant extracts and absorbance was read at 517 nm (Cintra 20; GBC, Dandenong, Australia) following a 30 min incubation period. The capability to scavenge the DPPH• radical was calculated by using the following equation:
A0 and A1 are the absorbance of blank and sample, respectively.
β-Carotene Bleaching Assay
A solution of β-carotene was prepared by dissolving 5 mg of β-carotene in 10 mL of chloroform. Then, 600 µL of this solution was added to 40 mg linoleic acid and 400 mg of Tween 40 in a round-bottom flask. Chloroform was gently removed at 50°C under vacuum, and distilled water (100 mL) was added to the flask with vigorous shaking. About 200 µL of extracts and TBHQ were separately mixed with 5 mL of emulsion. Reading of all samples were taken immediately at t = 0 min by spectrophotometer at 470 nm. The remaining samples were placed in the dark for 24 h. Then, absorbance of samples at 470 nm was determined by spectrophotometer.[Citation17] Antioxidant activity was calculated as inhibition percentage according to the following equation:
where, the Abss24 is the absorbance of the antioxidant after 24 h, Absc24 is the absorbance of the control after 24 h, Abss0 is the absorbance of the antioxidant at t = 0 and Absc0 is the absorbance of the control at t = 0.
Rancimat/Oxidative Stability Index (OSI)
A Metrohm 743 Rancimat instrument (Herisan, Switzerland) was used in the experiment. Air supply was maintained at 15 l/h and the temperature was kept at 110°C.[Citation18]
Oil Storage Tests
The ethanol-water extract of peel (PEEW) at 400 and 1000 ppm, ethanolic extract of pulp (PUE) at 100 and 400 ppm, and TBHQ at 100 ppm were added to refined soybean oil. SBO was used as a control oil sample. Oil samples were stored at 25°C in dark for 60 days. At the end of each 15 days (0, 15, 30, 45, and 60), about 20 g of the oil samples were filtered into a screw-cap vial and promptly stored in freezer at –20°C until use.[Citation19]
Oil Stability Analysis
The stability of oil samples during 60 days oven storage at 25°C was evaluated by Rancimat test, peroxide value (PV), thiobarbituric acid (TBA) value, free fatty acids (FFA), and conjugated dienes (CD) and conjugated trienes (CT) values. The PV was performed according to Shantha and Decker.[Citation20] The TBA value was carried out by Senevirathne et al.[Citation21] and the FFA content was performed according to Farhoosh et al.[Citation22] The CD and CT values were calculated according to the method described by Chirinos et al.[Citation23]
Statistical Analysis
The data were subjected to analysis of variance (ANOVA) and the significant differences between means were determined by Duncan’s multiple range test (p < 0.05) using SPSS statistical software (version 19; SPSS Inc., Chicago, IL).
RESULTS AND DISCUSSION
Total Phenol Content
Antioxidant activities in plant extracts are related to their phenolic contents[Citation24] and the antioxidant activities of phenolic compounds are probably due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and single oxygen quenchers.[Citation2] There were varying phenolic contents in the loquat fruit peel and pulp extracts ranging from 147.4 to 879.5 µg GAE/g of dried weight. Phenolic contents in ethanol-water, ethanol and water extracts of peel were 879.5, 664.5, and 272.5 µg GAE/g, and in ethanol-water, ethanol and water extracts of pulp were 387.7, 249.9, and 147.4 µg GAE/g, respectively. Therefore, the ethanol-water extracts had higher phenolic levels than that the water and ethanol extracts of the same plant part. Very low phenol contents obtained for pulp sample using water is presumably due to the unexpected incomplete solubility of this extract in water. These results also indicated the peel extract had the highest phenolic content compared to pulp. Our results concurred with Koba et al.[Citation12] and Ferreres et al.[Citation10] results, as they mentioned that the content of total phenolics of loquat fruit pulp was significantly lower than that of peel. Vieira et al.[Citation25] reported that the total phenolic content in pulp of loquat fruits cultivars ranged from 128.33 to 212.01 µg GAE/g and in the peel ranged from 304.66 to 712.65 µg GAE/g. Also, Ferreres et al.[Citation10] calculated that the phenolic contents of loquat pulp and peel were in range of 72.5 to 664.4 and 125.7 to 882.4 µg GAE/g, respectively. The phenolic content and composition of fruits and vegetables depend on the genetic and environmental factors as well as post-harvest processing conditions.[Citation26]
DPPH• Radical Scavenging Activity
The DPPH• test has been widely used for antioxidant activity screening, probably due to the simplicity of the equipment required.[Citation27] Rajaei et al.[Citation28] suggested the DPPH• test as an easy and accurate method for measuring the antioxidant potential of fruit and vegetable extracts. shows DPPH• radical scavenging activity of loquat fruit peel and pulp extracts along with the reference standard TBHQ. The peel and pulp extracts showed that the concentration depends on scavenging activity by quenching DPPH• radicals. The DPPH• radical scavenging activities of extracts increased as the content increased, similar to previous studies results such as Suja et al.[Citation6] and Kamkar et al.[Citation29] It has been proven that antioxidant activity of plant extracts is mainly ascribed to the concentration of the phenolic compounds present in the plants.[Citation30] The highest scavenging activity was shown by ethanol extract of pulp (at 100, 200 and 300 ppm) and PEEW (at 300 and 400 ppm) with very close values to each other. In general, the peel extracts show better antioxidant activity against DPPH• radical than pulp extracts, but the highest antioxidant effect was observed in TBHQ. Our results for antioxidant activity were in agreement with previously published results such as the works by Koba et al.[Citation12] and Ferreres et al.,[Citation10] which concluded that antioxidant capacity of loquat peel extracts was higher than pulp extracts.
β-Carotene Bleaching Assay
The ability of extracts to inhibit the bleaching of β-carotene was measured and compared to TBHQ as a positive control and the negative control which contained no antioxidant component.[Citation28] The effects of loquat peel and pulp extracts on oxidation of β-carotene-linoleic acid emulsion are shown in . It was clear that the presence of extracts reduced the oxidation of β-carotene. There were significant differences (p < 0.05) between antioxidant effects of the peel and pulp extracts, negative control, and reference standard (TBHQ). Effects of peel extracts on reducing oxidation of β-carotene were better than control and pulp extracts, but the best antioxidant activity was observed in TBHQ. In this test, similar to DPPH assay, it was indicated that the antioxidant activities of PEEW and ethanol extract of pulp were highest compared to the other solvents. Phenolic extracts of plants are generally mixtures of different classes of phenolic compounds which are soluble in the used solvent.[Citation29] It was found that the richest extracts in phenolic compounds are the most active extracts. Antioxidative effects of various plant extracts in β-carotene-linoleic acid emulsion have been already reported, for instance Sun and Ho[Citation30] and Mohdaly et al.[Citation31] compared the antioxidative effects of buckwheat extracts and sesame cake extract with BHT, BHA, and TBHQ by β-carotene-linoleic acid assay. They showed that extracts made higher inhibition to oxidation of β-carotene-linoleic acid emulsion than the BHT and BHA, but inhibitory effects of TBHQ was the highest.
TABLE 1 Antioxidant activities of loquat peel and pulp extracts at different conditions analyzed by DPPH• and β-carotene assays
Rancimat Analysis
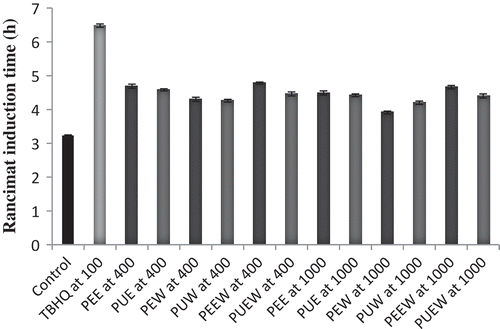
The Rancimat analysis was performed at 110°C and the induction period (h) was evaluated for soybean oils with or without loquat fruit peel and pulp extracts at 400 and 1000 ppm. As it is shown in , the presence of extracts retarded the oxidation of soybean oil. Our results demonstrated both peel and pulp extracts had strong antioxidant activity in soybean oil. Therefore, the lowest thermal stability in oils treated with extracts was for PEW sample (3.9 h), which was higher compared to control oil (3.32 h). On other hand, the highest thermal stability in oils containing loquat peel and pulp extracts was for PEEW at 400 and 1000 ppm (4.69 and 4.49 h), but the best protection effect was observed in oil containing 100 ppm of TBHQ. Results also showed that the peel extracts was more effective in prevention of soybean oil oxidation compared to pulp extracts. These results concurred with Sun and Ho[Citation30] and Aladedunye and Matthäus[Citation32] which reported the induction period of oils containing buckwheat, rowanberry, and crabapple extracts was higher than the control oil, but the most antioxidant effect was seen in TBHQ. From the evaluation of the antioxidant activity of loquat extracts by DPPH, β-carotene, and Rancimat assays, it was realized that PEEW at 400 and 1000 ppm and ethanol extract of pulp at 100 and 400 ppm had the highest antioxidant activity. Therefore, these extracts were used to assess their effects on stability of soybean oil during storage conditions.
Antioxidant Activity in Oil under Storage Conditions
Effect on PV
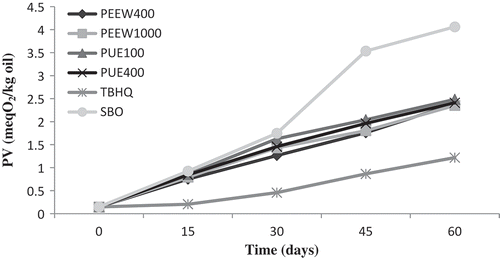
shows change in PV of the oil samples during storage at 25°C for 60 days. PV is one of the most common tests used to assess primary products of oxidation (hydroperoxide) in oils and fats. There was no significant difference (p < 0.05) between the initial PV (0.146 meq O2/kg) of oil samples. The PV of all oil samples, similar to those reported by Goli et al.,[Citation14] Chotimarkorn et al.,[Citation33] and Mohdaly et al.[Citation31] increased gradually during storage conditions. It was revealed that an increasing trend in PV during storage was slow at the first 15 days and then, a sharp increase was observed until the end of storage. The PV of soybean oil containing 100 and 400 ppm of PUE and 400 and 1000 ppm of PEEW and 100 ppm of TBHQ and control oil reached from 0.14 to 12.88, 11.21, 10.98, 9.05, 8.21, and 31.06 meq O2/kg, respectively. It was generally observed that the extracts significantly reduced (p < 0.05) PV compared to control oil. The PV of soybean oil containing 100 ppm of pulp extract reached to a maximum of 12.88 meq O2/kg after 60 days of storage, which was higher than other oils treated with extracts. The results showed peel extract is more effective than pulp extract in oxidation prevention of soybean oil. According to the PV results, PEEW at 400 ppm indicated a suitable antioxidant activity but the best protection was observed in soybean oil containing 100 ppm of TBHQ. These results were in agreement with other previous studies results such as works by Zia-ur-Rehman[Citation34] and Chotimarkorn et al.,[Citation33] which examined the antioxidant activity of citrus peel and rice bran extracts during 6 months and 30 days of storage in vegetable oils.
Effect on TBA Value
TBA value measurement is a good parameter for determination of secondary oxidation products.[Citation15] This method is based on measuring pink complex formed at an absorbance of 532 nm after reaction of one molecule of malondialdehyde (MDA) with two molecules of thiobarbituric acid.[Citation35] As seen in , TBA values of the oil samples increased gradually during storage period. Similar studies were conducted by Goli et al.[Citation14] and Zhang et al.[Citation4] There were significant differences (p < 0.05) between the TBA values of the oil samples during storage conditions. The initial TBA value for soybean oil was 0.047 which reached to a maximum of 0.31 (µmol/g MDA) in the control oil sample. Therefore, extracts prevented the increase of TBA value in soybean oil. These values for oil samples containing 100 and 400 ppm of PUE and 400 and 1000 ppm of PEEW and TBHQ at 100 ppm after 60 days of storage were 0.16, 0.154, 0.15, 0.152, and 0.134 (µmol/g MDA), respectively. The oxidation stability of oil samples during 60-day storage test were as follows:
FIGURE 3 Change in thiobarbituric acid value (TBA) of the oil samples during storage. PEEW: ethanol-water extract of loquat peel; PUE: ethanol extract of loquat pulp; SBO: soybean oil without any antioxidant.
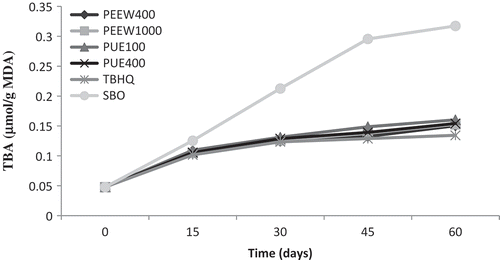
The TBA value results demonstrated that peel extract at 400 ppm (PEEW) had higher antioxidant activity than pulp extract, but lower than TBHQ. These results confirmed with previously published results such as the work of Kamkar et al.,[Citation29] which evaluated the antioxidative effect of various concentrations of Iranian Pulicaria gnaphalodes L. extracts in soybean oil during storage over a 35-day period.
Effect on FFA Content
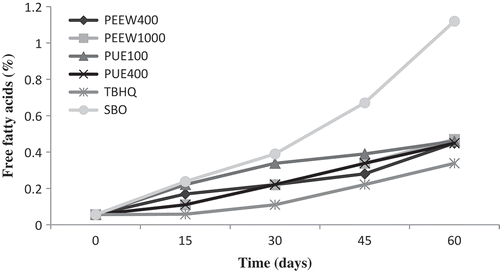
shows changes in the FFA of oil samples during storage conditions. FFA formation is an important measurement of rancidity in food, which is formed by hydrolysis of triglycerides. Thus, the rate of oil degradation can be examined by measuring the amount of production of FFA.[Citation36] Initial FFA was the same in oil samples. Our results indicated that peel and pulp extracts reduced FFA at different concentrations compared to control oil. However, in oil samples FFA showed an increasing trend from the beginning of the storage period to the end of experiment. Similar results were found by Rehman et al.[Citation19] and Chotimarkorn et al.[Citation33] The FFA of control oil sample without any additives reached to a maximum value of 1.12% after 60 days of storage. The FFA of soybean oil containing 400 and 1000 ppm of PEEW and 100 and 400 ppm of PUE and TBHQ were 0.44, 0.47, 0.46, 0.44, and 0.33%, respectively. The results showed that peel extract at 400 ppm (PEEW) exhibited very strong antioxidant activity which was almost equal to pulp extract at 400 ppm, but the best protection was observed in TBHQ.
Effect on CD and CT
Absorbance at 234 nm provides a measurement for the content of CD, which represent the degree of production of the primary oxidation products. Changes in CD of the oil samples during storage conditions are shown in . Absorbance of soybean oils treated with peel and pulp extracts were significantly different (p < 0.05) from the control oil, and from the synthetic antioxidant added oil which was also significantly protected compared to the control oil. At the end of the storage period, CD values for the oil samples containing 100 and 400 ppm of PUE, 400 and 1000 ppm of PEEW, TBHQ and control oil were 2.91, 2.83, 2.72, 2.81, 2.51, and 3.6 (mmol/l), respectively. The CD contents for soybean oil containing 400 ppm of PEEW at days of 15, 45, and 60, and for 1000 ppm PEEW at day 30 were lower than other oil samples, but more than of TBHQ significantly (p < 0.05). Therefore, CD results showed that the most oxidation stability for soybean oil containing extracts was obtained by adding 400 ppm of peel extract.
FIGURE 5 Change in conjugated dienes (CD) and trienes (CT) of the oil samples during storage. PEEW: ethanol-water extract of loquat peel; PUE: ethanol extract of loquat pulp; SBO: soybean oil without any antioxidant.
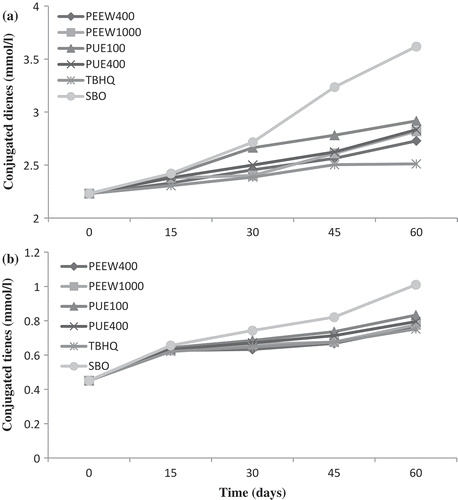
There were also significant effects on the absorbance at 270 nm with the use of extracts, and least absorbance was for the peel extract (). This absorbance for the control oil at the end of 60 days was greater than that for the oils treated with extracts. The CT values for the soybean oils containing 400 and 1000 ppm of PEEW and 100 and 400 ppm of PUE and TBHQ at 100 ppm and control oil at the end of 60 days of storage were 0.76, 0.77, 0.83, 0.79, 0.75, and 1.01 (mmol/l), respectively. According to CT value results, peel extract was more effective in oxidation prevention of soybean oil than pulp extract. The results showed that the best protection was observed in soybean oil consisting 400 ppm of peel (no significant difference with TBHQ) during storage conditions. The CD and CT value results concerned with previously published results such as the work by Mohdaly et al.,[Citation31] which examined the antioxidant potential of sesame cake extract compared to synthetic antioxidants (BHA, BHT, and TBHQ) in stabilization of sunflower and soybean oils. CD and CT were present initially in the fresh soybean oil in small amounts (2.31 and 0.45 mmol/l), which both increased as the storage time was increasing, similar results have been reported by Mohdaly et al.[Citation37] and Urbančič et al.[Citation5] Overall, increase in the CD was considerably higher compared to the CT, which will be specifically due to the high content of linoleic acid in the soybean oil.
Effect on OSI
Rancimat method is frequently used for predicting oxidative stabilities under heating conditions. OSI measures secondary products of lipid peroxidation (Such as aldehydes and ketones) and represents resistance of oil against oxidation.[Citation38] The oxidative stability of soybean oil was greatly improved in the presence of extracts (). Oil samples stability decreased gradually during storage, similar results were reported by Gámez-Meza et al.,[Citation39] Gámez‐Meza et al.[Citation40] and Taghvaei et al.[Citation15] The change percentage for soybean oil containing 400 ppm of PEEW at days 0 to 15 (2.07%), 30 to 45 (0.48%), and 400 ppm of PUE at days 15 to 30 of storage (2.5%) was lower than other oil samples. A Rancimat test failed to show clearly the difference between oil samples OSI at days 45 to 60. At the end of 60 days of storage, the change percentage for soybean oils containing 400 and 1000 ppm of PEEW, 100 and 400 ppm of PUE, TBHQ and control oil were 10.9, 11.13, 19.7, 17.09, 17.89, and 21.66 %, respectively. Therefore, the rate of oxidation of oil samples was as follows:
FIGURE 6 Change in oxidative stability index (OSI) of the oil samples during storage. PEEW: ethanol-water extract of loquat peel; PUE: ethanol extract of loquat pulp; SBO: soybean oil without any antioxidant.
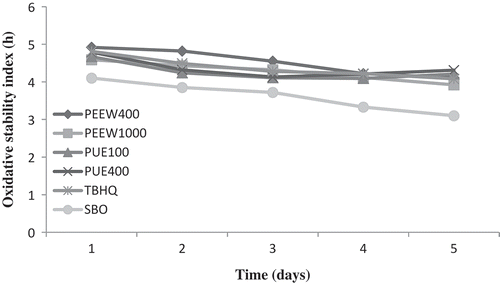
The results indicated that the maximum OSI in soybean oil was obtained by addition of 400 ppm of peel extract compared to other oil samples. The Rancimat results were in agreement with previously published results such as the works of Taghvaei et al.[Citation15] and Aladedunye and Matthäus.[Citation32]
Conclusions
Results from the present study showed that loquat fruit peel extract had more effective antioxidant activity compared to pulp extract in the DPPH• free radical assay, β-carotene bleaching assay, and the Rancimat method. In terms of PV, OSI, FFA, TBA, CD, and CT values the soybean oils treated with 400 ppm of peel extract indicated the highest antioxidant potential compared to the pulp extract, but the best protection effect was observed in soybean oil containing TBHQ. In general, loquat fruit peel and pulp extracts showed suitable antioxidant capacity in soybean oil and could be used as a substitute for synthetic antioxidants to increase the shelf life of vegetable oils.
REFERENCES
- Iqbal, S.; Bhanger, M. Stabilization of Sunflower Oil by Garlic Extract during Accelerated Storage. Food Chemistry 2007, 100(1), 246–254.
- Lagha-Benamrouche, S.; Madani, K. Phenolic Contents and Antioxidant Activity of Orange Varieties (Citrus sinensis L. and Citrus aurantium L.) Cultivated in Algeria: Peels and Leaves. Industrial Crops and Products 2013, 50, 723–730.
- Cha, D.S.; Eun, J.S.; Jeon, H. Anti-Inflammatory and Antinociceptive Properties of the Leaves of Eriobotrya japonica. Journal of Ethnopharmacology 2011, 134(2), 305–312.
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants during Accelerated Storage. Food Chemistry 2010, 118(3), 656–662.
- Urbančič, S.; Kolar, M.H.; Dimitrijević, D.; Demšar, L.; Vidrih, R. Stabilisation of Sunflower Oil and Reduction of Acrylamide Formation of Potato with Rosemary Extract During Deep-Fat Frying. LWT-Food Science and Technology 2014, 57(2), 671–678.
- Suja, K.; Jayalekshmy, A.; Arumughan, C. Antioxidant Activity of Sesame Cake Extract. Food Chemistry 2005, 91(2), 213–219.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis vinifera L. Varieties. International Journal of Food Properties 2013, 16(1), 45–60.
- Omena, C.M.B.; Valentim, I.B.; Guedes, G.d.S.; Rabelo, L.A.; Mano, C.M.; Bechara, E.J.H.; Sawaya, A.C.; Trevisan, M.T.S.; da Costa, J.G.; Ferreira, R.C.S. Antioxidant, Anti-Acetylcholinesterase and Cytotoxic Activities of Ethanol Extracts of Peel, Pulp, and Seeds of Exotic Brazilian Fruits: Antioxidant, Anti-Acetylcholinesterase, and Cytotoxic Activities in Fruits. Food Research International 2012, 49(1), 334–344.
- Ercisli, S.; Gozlekci, S.; Sengul, M.; Hegedus, A.; Tepe, S. Some Physicochemical Characteristics, Bioactive Content, and Antioxidant Capacity of Loquat (Eriobotrya japonica (Thunb.) Lindl.) Fruits from Turkey. Scientia Horticulturae 2012, 148, 185–189.
- Ferreres, F.; Gomes, D.; Valentão, P.; Gonçalves, R.; Pio, R.; Chagas, E.A.; Seabra, R.M.; Andrade, P.B. Improved Loquat (Eriobotrya japonica Lindl.) Cultivars: Variation of Phenolics and Antioxidative Potential. Food Chemistry 2009, 114(3), 1019–1027.
- Wells, J.; Raju, B.; Huang, H.; Weisburg, W.; Mandelco-Paul, L. Evaluation of Loquats (Eriobotrya japonica [thunb.] Lindl.) at the Tropical Research and Education Center, Homestead. in Proc. Ha. State Hort. Soc. 1999, 112.
- Koba, K.; Matsuoka, A.; Osada, K.; Huang, Y.S. Effect of Loquat (Eriobotrya japonica) Extracts on LDL Oxidation. Food Chemistry 2007, 104(1), 308–316.
- Tosun, M.; Ercisli, S.; Karlidag, H.; Sengul, M. Characterization of Red Raspberry (Rubus idaeus L.) Genotypes for Their Physicochemical Properties. Journal of Food Science 2009, 74(7), 575–579.
- Goli, A.H.; Barzegar, M.; Sahari, M.A. Antioxidant Activity and Total Phenolic Compounds of Pistachio (Pistachia vera) Hull Extracts. Food Chemistry 2005, 92(3), 521–525.
- Taghvaei, M.; Jafari, S.M.; Mahoonak, A.S.; Nikoo, A.M.; Rahmanian, N.; Hajitabar, J.; Meshginfar, N. The Effect of Natural Antioxidants Extracted from Plant and Animal Resources on the Oxidative Stability of Soybean Oil. LWT-Food Science and Technology 2014, 56(1), 124–130.
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella Sativa Essential Oil. Phytotherapy Research 2000, 14(5), 323–328.
- Amarowicz, R.; Estrella, I.; Hernández, T.; Robredo, S.; Troszyńska, A.; Kosińska, A.; Pegg, R.B. Free Radical-Scavenging Capacity, Antioxidant Activity, and Phenolic Composition of Green Lentil (Lens culinaris). Food Chemistry 2010, 121(3), 705–711.
- Farhoosh, R.; Tavassoli-Kafrani, M.H. Simultaneous Monitoring of the Conventional Qualitative Indicators during Frying of Sunflower Oil. Food Chemistry 2011, 125(1), 209–213.
- Rehman, Z.-U.; Habib, F.; Shah, W. Utilization of Potato Peels Extract as a Natural Antioxidant in Soy Bean Oil. Food Chemistry 2004, 85(2), 215–220.
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. Journal of AOAC International 1993, 77(2), 421–424.
- Senevirathne, M.; Kim, S.H.; Siriwardhana, N.; Ha, J.H.; Lee, K.W.; Jeon, Y.J. Antioxidant Potential of Ecklonia Cavaon Reactive Oxygen Species Scavenging, Metal Chelating, Reducing Power, and Lipid Peroxidation Inhibition. Food Science and Technology International 2006, 12(1), 27–38.
- Farhoosh, R.; Khodaparast, M.H.H.; Sharif, A.; Rafiee, S.A. Olive Oil Oxidation: Rejection Points in Terms of Polar, Conjugated Diene, and Carbonyl Values. Food Chemistry 2012, 131(4), 1385–1390.
- Chirinos, R.; Huamán, M.; Betalleluz-Pallardel, I.; Pedreschi, R.; Campos, D. Characterisation of Phenolic Compounds of Inca Muña (Clinopodium bolivianum) Leaves and the Feasibility of Their Application to Improve the Oxidative Stability of Soybean Oil during Frying. Food Chemistry 2011, 128(3), 711–716.
- Barros, L.; Cabrita, L.; Boas, M.V.; Carvalho, A.M.; Ferreira, I.C. Chemical, Biochemical, and Electrochemical Assays to Evaluate Phytochemicals and Antioxidant Activity of Wild Plants. Food Chemistry 2011, 127(4), 1600–1608.
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Di Pietro, P.F.; Nunes, E.d.C.; Fett, R. Phenolic Compounds and Antioxidant Activity of the Apple Flesh and Peel of Eleven Cultivars Grown in Brazil. Scientia Horticulturae 2011, 128(3), 261–266.
- Rop, O.; Juríková, T.; Sochor, J.; Mlcek, J.; Kramarova, D. Antioxidant Capacity, Scavenging Radical Activity, and Selected Chemical Composition of Native Apple Cultivars from Central Europe. Journal of Food Quality 2011, 34(3), 187–194.
- Razali, N.; Mat-Junit, S.; Abdul-Muthalib, A.F.; Subramaniam, S.; Abdul-Aziz, A. Effects of Various Solvents on the Extraction of Antioxidant Phenolics from the Leaves, Seeds, Veins, and Skins of Tamarindus indica L. Food Chemistry 2012, 131(2), 441–448.
- Rajaei, A.; Barzegar, M.; Mobarez, A.M.; Sahari, M.A.; Esfahani, Z.H. Antioxidant, Anti-Microbial, and Antimutagenicity Activities of Pistachio (Pistachia vera) Green Hull Extract. Food and Chemical Toxicology 2010, 48(1), 107–112.
- Kamkar, A.; Ardekani, M.R.S.; Shariatifar, N.; Misagi, A.; Nejad, A.S.M.; Jamshidi, A.H. Antioxidative Effect of Iranian Pulicaria gnaphalodes L. Extracts in Soybean Oil. South African Journal of Botany 2013, 85, 39–43.
- Sun, T.; Ho, C.T. Antioxidant Activities of Buckwheat Extracts. Food Chemistry 2005, 90(4), 743–749.
- Mohdaly, A.A.; Smetanska, I.; Ramadan, M.F.; Sarhan, M.A.; Mahmoud, A. Antioxidant Potential of Sesame (Sesamum indicum) Cake Extract in Stabilization of Sunflower and Soybean Oils. Industrial Crops and Products 2011, 34(1), 952–959.
- Aladedunye, F.; Matthäus, B. Phenolic Extracts from Sorbus aucuparia (L.) and Malus baccata (L.) Berries: Antioxidant Activity and Performance in Rapeseed Oil during Frying and Storage. Food Chemistry 2014, 159, 273–281.
- Chotimarkorn, C.; Benjakul, S.; Silalai, N. Antioxidative Effects of Rice Bran Extracts on Refined Tuna Oil during Storage. Food Research International 2008, 41(6), 616–622.
- Zia-ur-Rehman. Citrus Peel Extract—A Natural Source of Antioxidant. Food Chemistry 2006, 99(3), 450–454.
- Zainol, M.; Abd-Hamid, A.; Yusof, S.; Muse, R. Antioxidative Activity and Total Phenolic Compounds of Leaf, Root, and Petiole of Four Accessions of Centella asiatica (L.) Urban. Food Chemistry 2003, 81(4), 575–581.
- Frega, N.; Mozzon, M.; Lercker, G. Effects of Free Fatty Acids on Oxidative Stability of Vegetable Oil. Journal of the American Oil Chemists’ Society 1999, 76(3), 325–329.
- Mohdaly, A.A.A.; Sarhan, M.A.; Mahmoud, A.; Ramadan, M.F.; Smetanska, I. Antioxidant Efficacy of Potato Peels and Sugar Beet Pulp Extracts in Vegetable Oils Protection. Food Chemistry 2010, 123(4), 1019–1026.
- Anwar, F.; Bhanger, M.; Kazi, T. Relationship Between Rancimat and Active Oxygen Method Values at Varying Temperatures for Several Oils and Fats. Journal of the American Oil Chemists’ Society 2003, 80(2), 151–155.
- Gámez-Meza, N.; Noriega-Rodríguez, J.; Medina-Juárez, L.; Ortega-García, J.; Cázarez-Casanova, R.; Angulo-Guerrero, O. Antioxidant Activity in Soybean Oil of Extracts from Thompson Grape Bagasse. Journal of the American Oil Chemists’ Society 1999, 76(12), 1445–1447.
- Gamez‐Meza, N.; Noriega‐Rodriguez, J.A.; Leyva‐Carrillo, L.; Ortega‐Garcia, J.; Bringas‐Alvarado, L.; García, H.S.; Medina‐Juarez, L.A. Antioxidant Activity Comparison of Thompson Grape Pomace Extract, Rosemary, and Tocopherols in Soybean Oil. Journal of Food Processing and Preservation 2009, 33(1), 110–120.