Abstract
Concentration of polyphenols in apples depends on factors such as harvest, cultivar, processing, and storage conditions. We aimed to identify essential polyphenols present in various apple varieties, and measure their free phenol and antioxidant capacities using 1,1-diphenyl-2-picylhydrazyl radical scavenging capacity, oxygen radical absorbance capacity, hydroxyl radical scavenging capacity, and ferric reducing antioxidant power methods, and correlating with the total polyphenol content and antioxidant capacity with high-performance liquid chromatography results. Thirteen apple varieties cultivated in northwest China showed significant polyphenol content. Hongxun 1 had the highest polyphenol content and antioxidant capacities in comparison with other apple varieties, while phlorizin showed the strongest 1,1-diphenyl-2-picylhydrazyl free radical scavenging rate (73.5 ± 2.7%), oxygen radical absorbance capacity value (44.6 ± 3.1 μmol TE/g), hydroxyl radical scavenging capacity value (61.9 ± 3.5 μmol TE/g), and ferric reducing antioxidant power value (0.53 ± 0.37 μmol TE/g). The antioxidant capacities of the polyphenol extract was related not only to its total polyphenol content but also to the phenolic composition.
Keywords:
INTRODUCTION
The consumption of fruit and vegetables has been associated with lower morbidity and mortality rates caused by degenerative diseases.[Citation1] Phytochemicals, which are naturally occurring bioactive components of plants, contain functional components such as polyphenols, thiols, carotenoids, tocopherols, and glucosinolates,[Citation2] which are thought to promote human health. Apples are one of the most cultivated and consumed tree fruits in the world. According to the 2012 national apple industry system statistical data, the total area of apple cultivation reached 2.57 million hectares with a total output of about 39.50 million tons in China alone. Apples also contain functional compounds such as polysaccharoses, vitamins, pectins, and polyphenols.[Citation3,Citation4] The concentration of polyphenols in apples depends on several factors such as the cultivar, harvest, storage, and processing conditions.[Citation4] Although polyphenols are distributed throughout the apple body, a high concentration is located in the peel. Polyphenols have been reported to exert a variety of positive biological activities such as antioxidant capacity,[Citation5,Citation6] metal chelation,[Citation7] anti-allergic activity,[Citation8,Citation9] and the prevention and treatment of cardiovascular and cerebrovascular diseases.[Citation10] Recent studies have demonstrated that the biological activities of polyphenols are also linked to other pathways involved in cellular metabolism and cellular survival. These activities are the result of manifold and complex actions of polyphenols that extend beyond their mere antioxidant actions. The extraction, separation, purification, and functional properties of polyphenols from apples have been previously reported. The characterization of polyphenols in apples, such as hydrocinnnamic acid, dihydrochalcones, flavonols, flavan-3-ols, and anthocyanins, has attracted much attention in recent years in different ways. Quercetin, epicatechin, hyperoside, catechin, phloridzin, anthocyanin, chlorogenic acid, phloretin, and caffeic acid are among the most important compounds reported. In addition, apple polyphenols demonstrate good stability after heat and pH treatment,[Citation11] possess antiproliferative,[Citation12,Citation13] and antimicrobial[Citation14] activity, and can be used in many types of food products.
Many methods for evaluating antioxidant capacity have been established. The scavenging of free radicals is a frequently used technique. Other assays used include the 1,1-diphenyl-2-picylhydrazyl (DPPH) radical scavenging capacity (DRSC), oxygen radical absorbance capacity (ORAC), hydroxyl radical scavenging capacity (HOSC), and ferric reducing antioxidant power (FRAP). In this study, polyphenols were extracted from apple peels to evaluate their in vitro antioxidant activity using these four antioxidant evaluation methods. Their chemical compositions were analyzed using high-performance liquid chromatography (HPLC).[Citation15,Citation16] This study aimed to ascertain the relationship between the types of polyphenols from apple peel and their antioxidant capacities, and it provides a theoretical basis for further research on polyphenols.
MATERIALS AND METHODS
Apple Samples
Approximately 2.5 kg of apples, including 13 different varieties, were sampled by hand from different parts of five apple trees located in Baishui County, Shaanxi Province (China) at three different stages of ripeness. After harvest, all apples were stored in controlled atmosphere conditions (0°C, relative humidity 95, 2 O2, 3% CO2) for no longer than 1 week before being analyzed. All apple varieties tested are shown in .
TABLE 1 Varieties and producing areas of apples
Reagents
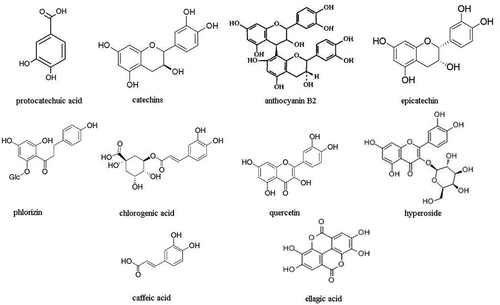
Folin–Ciocalteu reagent was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). DPPH, phosphate buffer (0.2 mol/l, pH 6.6), ethanol, sodium carbonate, sodium nitrite, sulfanilic acid, N-(1-naphthyl) ethylenediamine dihydrochloride, pyrogallic acid, HCl, H2O2, FeSO4, salicylic acid, potassium ferricyanide, trichloroacetic acid, FeCl3 were obtained from Kermel Chemical Co., Ltd. (Tianjin, China). Water was purified with a Milli-Q system Millipore (Bedford, MA, USA). Solvents were of analytical grade. Ten phenolic standards (protocatechuic acid, catechins, anthocyaninB2, epicatechin, phloridzin, chlorogenic acid, quercetin, hyperoside, caffeic acid, and ellagic acid; ) were purchased from Sigma-Aldrich Chemical Co., Ltd (St. Louis, MO, USA).
Preparation of Apple Polyphenol Extracts
Three apples for each variety were separately chosen at random. The apple stalks were removed after cleaning with water while the peels were carefully separated from the pulps using a vegetable peeler and then chopped into very small pieces. The peel samples were ground in a porcelain mortar with 1% ascorbic acid prior to analysis. One gram of apple peel was weighed and mixed with 30 mL of 70% ethanol in a 100 mL triangular flask, employing an ultrasonic extractor (KQ-2300E, Hechuang Inc., Kunshan, China) and extracted (80 Hz, 30°C) for 20 min. Polyphenol extracts were collected twice, and the samples were then concentrated in an R-120 rotary evaporator (BÜCHI Labortechnik, AG, Flawil, Switzerland) for 25 min at 1000 rpm. Ten milliliters of sample solvent was filtered through 0.22 μm polyvinyl difluoride (PVDF) syringe filters (Merck Millipore, Billerica, Switzerland). The extracts were stored at −20°C. The total polyphenol content of apple peel extracts were assessed using a modified version of the Folin–Ciocalteu assay.[Citation17]
HPLC Analysis
A Waters 2695 system, equipped with a quaternary pump, a degasser, an auto-sampler and a diode array detector (DAD), was used. The column used for chromatographic separation was a Diamonsil C18 column (250 mm × 4.6 mm, particle size: 5 μm). The chromatographic data was acquired and processed by Empower software. During the process, the mobile phases were A: 1 mL/100 mL acetic acid in water (v/v) and B: methanol. The polyphenols were eluted in a gradient system: 0–10 min, linear gradient of 5–30% B; 10–25 min, linear gradient of 30–50% B; 25–35min, linear gradient of 50–70% B and 35–40 min, linear gradient of 70–5% B. Finally, the column was washed and reconditioned. The flow rate was 1.0 mL/min and the injection volume was 20 μL, each sample was replicated three times. Protocatechuic acid, catechins, anthocyaninB2, epicatechin, phloretin, chlorogenic acid, quercetin, hyperoside, caffeic acid, and ellagic acid were monitored at wavelengths of 280 and 320 nm, all polyphenols were monitored in the above wavelengths. For quantification and identification, standard curves for the analytes of interest were prepared for each standard polyphenol. The system was operated at 30°C.[Citation18]
Analysis of Antioxidant Capacities
DRSC
The free radical scavenging activity quantitatively tested using DPPH based on the method of Shimada.[Citation19] The initial absorbance of DPPH in methanol was measured at 517 nm until the absorbance remained constant. A total of 2 mL of extract was added to 2 mL of 0.2 mmol/L methanolic DPPH solution. The mixture was reacted at room temperature for 30 min without the light. After that the absorbance at 517 nm was measured. The percentage of scavenging was calculated according to the equation:
where /DPPH is the DPPH radical scavenging rate (%), A0 is the absorbance of the control, Ax is the absorbance in the presence of the extract, Ax0 is the absorbance without DPPH radicals.
ORAC
The ORAC assay was described by Moore[Citation20] with modifications. Fluorescein was used as the fluorescent probe and the ORAC assay was performed on a VictorCitation3 multi-label plate reader (Perkin–Elmer, Turku, Finland) with fluorescence filters. Trolox was used as a standard. The initial reaction mixture contained 225 μL of 8.16 × 10−Citation8 M Fluorescein, 30 μL sample, blank, or standard, and put into preheated plate reader at 37°C for 20 min. Then, 25 μL of 0.36 MAAPH was added to each well and the fluorescence of the mixture was recorded every 2 min, over 2 h at 37°C. The results were expressed as μmol Tris-EDTA (TE)/g spice sample.
HOSC
The HOSC assay was measured using a method adapted by Moore[Citation21] using a VictorCitation3 multi-label plate reader (Perkin–Elmer, Turku, Finland). In brief, the reaction mixture consisted of 170 μL of 9.28 × 10−Citation8 M Fluorescein, 30 μL sample, blank, or Trolox standards, 40 μL of freshly prepared 0.199 M H2O2 and 60 μL FeCl3. The fluorescence was recorded every 4 min, for 4 h. The HOSC was quantified using the area under the curve and expressed relative to Trolox as micromoles of TE per gram of spice samples.
FRAP
The antioxidant activity was investigated using the FRAP assay for plant extracts, investigated according to the method of Pulido.[Citation22] The working FRAP reagent was freshly prepared every day by mixing 2.5 mL of 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) (10 mmol/L in 40 mmol/L hydrochloric acid), 2.5 mL of ferric chloride (20 mmol/L) and 25 mL of sodium acetate buffer (300 mmol/L, pH 3.6). The FRAP assay was carried out at 37°C, in 1 cm disposable plastic cells. Nine hundred microliters of the FRAP reagent were mixed with 90 μL of water and 30 μL of samples. After 120 min, the absorbance was measured at 595 nm.[Citation23,Citation24] The ferric reducing power was calculated by the formula:
where /FRAP is the ferric reducing power, A0 is the absorbance in the presence of the polyphenol enriched extract or phenol standard, A1 is the absorbance of the control (without extract), and A2 is the absorbance without FRAP. A standard curve was obtained using Trolox standard solution at various concentrations (0.5, 1.0, 1.5, 2.0 mmol/L). The absorbences of the samples were compared with that of the Trolox standard and the results were expressed in terms of micromoles Trolox equivalents per kilogram (μmol TE/kg). All measurements were performed in triplicate.
Statistical Analysis
The data is shown as the mean ± standard deviation of five repeated samples. The results obtained were analyzed using the SPSS 18.0 program for Windows (Munich, Germany). The statistical analysis was performed by analysis of variance (ANOVA) with a significance level of α = 0.05.
RESULTS AND DISCUSSION
Total Polyphenol Content
The polyphenol content in the 13 apple varieties ranged from 2.57 to 5.46 mg gallic acid equivalent (GAE)/g dry weight (DW), presenting significant differences (p < 0.05) among the apple varieties (). As shown in , all apple varieties had a difference in total polyphenol content. The order of total polyphenol content of the different apple varieties determined by the Folin–Ciocalteu assay was: hongxun 1 > honeycrisp > maowen > sinkiang 2 > nagafu 2 > cameo > yuhuazaofu > golden delicious > liangxiang > huaniu > pinova > pacific rose > honggailu. Hongxun 1 had the highest polyphenol content of 5.45 ± 0.04 mg GAE/g DW, whereas the apple variety with the lowest content was honggailu with 2.07 ± 0.03 mg GAE/g DW. Other studies have reported different values of total polyphenol content in different apple varieties with the content ranging from 0.71 mg GAE/g DW to 3.36 mg GAE/g DW.[Citation25,Citation26]
TABLE 2 Total polyphenol content and antioxidant capacities of 13 apple varieties
The polyphenol content determined in this study demonstrated higher levels than other reported studies because the initial apple sample used was different. Other studies used a fresh apple sample including the peel and pulp while this research only used apple peel. The content of polyphenols varies depending on the different fruit tissues. The polyphenol content in apple peel is approximately three times higher than that found in the pulp.[Citation26] The different results obtained can also be explained by the complexity of these compounds and the extraction and analysis methods. The polyphenols present in fruits occur freely or are combined with glycosides in nature. When combined with glycosides; these phenolic compounds cannot be completely extracted. For that reason, the total phenolic content may be underestimated.[Citation27] During the sample preparation stage, the way in which apples are cut and the homogenization process may induce a reaction of the polyphenol oxidase enzyme, thus decreasing the concentration of phenolic compounds. Furthermore, the concentration of phenolic compounds may be affected by apple variety, cultivar and genus and also by extrinsic factors, such as soil, seasonality, agronomic factors, and light exposure.[Citation28]
Comparison of Antioxidant Capacities of Various Apples
The results of antioxidant and radical scavenging capacities determined by DRSC, ORAC, HOSC, and FRAP assay are shown in . The DPPH free radical scavenging ability is widely used to evaluate the antioxidant activity of polyphenols extracted from fruits and vegetables. The results showed that polyphenol extracts had good DPPH free radical scavenging capacities. Hongxun 1 polyphenol extracts had the highest DPPH free radical scavenging rates of 97.13 ± 3.84%, and the lowest rate of 81.11 ± 4.59% was observed in honggailu polyphenol extracts. There was a significant difference among the values of these apple polyphenol extracts. Yinrong Lu[Citation29] reported that glycosides, procyanidins, chlorogenic acid, hydroxyphloridzin, and phloridzin had significant DPPH free radical scavenging capacity. Rababah[Citation30] evaluated the effect of jam processing using strawberry, cherry, apricot, fig, and orange and their antioxidant activity using the DPPH radical scavenging method during months of storage at 25°C.
The ORAC of the polyphenol extracts from the different apple varieties was limited within a certain range of fluctuation. These results are displayed in . The highest value of 34.33 ± 3.42 μmol TE/g was found for the Honeycrisp apple, while the lowest value of 9.90 ± 1.01 μmol TE/g was found in the Pacific rose apple.
Hydroxyl radicals may be generated under physiological conditions, which can damage lipids, DNA, and proteins.[Citation31] The HOSC assay measures HOSC under physiological pH. All tested apple polyphenols demonstrated HOSC as shown in . Hydroxyl free radical scavenging capacities were observed in all polyphenol extracts of about 30 μmol TE/g. Lesser scavenging capacities were found for the apple varieties of pacific rose, pinova, honggailu, and huaniu. HOSC was found to be significantly different among the apple varieties.
As shown in , significant differences in total reducing properties were found among the varieties of apple polyphenols extracts. The lowest value of 1.12 ± 0.03 μmol TE/g was detected in pacific rose, while higher values were observed in liangxiang (1.46 ± 0.06 μmol TE/g) and honeycrisp (1.46 ± 0.07 μmol TE/g). Direct dose-response relationships of FRAP and polyphenol contents were not relevant. The types of polyphenol within the varieties of apple might lead to this phenomenon. The value of overall effects were lower than other indicators and can most likely be attributed to the less sensitive FRAP assay. The values obtained in the present study were higher than those determined by Khanizadeh.[Citation32] The great variability observed in different studies regarding the apple antioxidant activity may be explained by the influence of the fruit tree’s location, harvest season, type of soil, agronomic factors, as well as post-harvest conditions. The antioxidant capacity measured by FRAP in apple peel was 6.6 times higher than that found in the pulp in apples from eight different varieties.[Citation32]
HPLC Analysis of Various Apple Polyphenols
Seventy percent ethanol extracts of apple, as well as extract hydrolysates of nagafu2, honggailu, hongxun1, huaniu, golden delicious, cameo, liangxiang, maowen, honeycrisp, pinova, pacific rose, sinkiang 2, and yuhuazaofu varieties, were analyzed by HPLC. and show the chromatograms of hongxun 1 and honggailu, respectively (chromatograms of other varieties are not shown). The tolerably small differences between the retention times of individual compounds ( and ) may be attributed to the slight differences in the column properties that occur during the relatively long recording time of the chromatogram.
FIGURE 2 Chromatograms of Hongxun 1 at A: 280 nm and B: 320 nm. Peaks: (1) protocatechic acid, (2) catechin, (3) anthocyaninB2, (4) epicatechin, (5) phlorizin, (6) ellagic acid, (7) quercitin, (8) chlorogenic acid, (9) caffeic acid, (10) hyperoside.
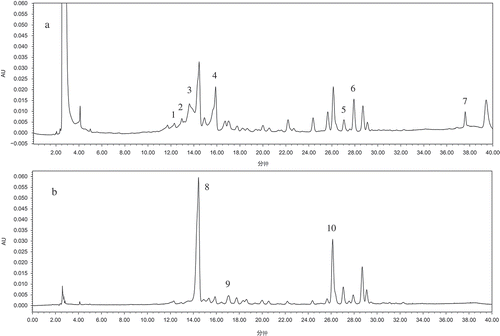
FIGURE 3 Chromatograms of Honggailu at A: 280 nm and B: 320 nm. Peaks: (1) protocatechic acid, (2) catechin, (3) anthocyaninB2, (4) epicatechin, (5) phlorizin, (6) ellagic acid, (7) quercitin, (8) chlorogenic acid, (9) caffeic acid, (10) hyperoside.
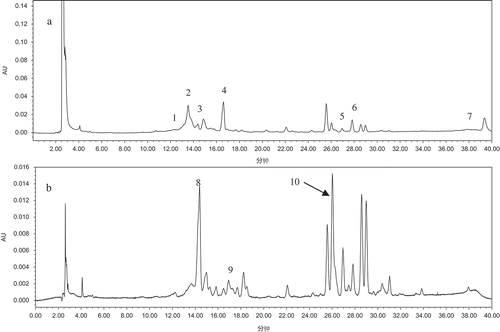
HPLC analysis showed that phenol standards were identified and quantified in most of the samples. The polyphenols were confirmed by comparing the sample peak retention times with those of the standards, and were quantified using the external standard method. The results are shown in . It was easily observed that there were significant differences between the polyphenols and their contents in the different apple varieties, with hyperoside, anthocyaninB2, chlorogenic acid, and ellagic acid being the major polyphenols in most apple varieties. The concentrations of these compounds varied greatly from one variety to another, exhibiting strong characteristics depending on the apple variety.
TABLE 3 Content of 10 polyphenols in apple fruits of different varieties
Antioxidant Capacities of Ten Free Phenols
To identify the potential contributors of antioxidant activity in polyphenol enriched extracts from different apple varieties, 10 free phenol standards detected in the fractions were evaluated using DRSC, ORAC, HOSC, and FRAP. The individual antioxidant contents of the tested apples showed significant changes depending on the apple variety. As shown in , the 10 phenols had different antioxidant capacity at the same concentration of 1 mg/mL. By comparison of the antioxidant capacity with other phenolic compounds, anthocyaninB2, phlorizin, protocatechuic acid, hyproside, and chlorogenic acid were more effective. In particular, phlorizin had the strongest antioxidant capacities with a DPPH free radical scavenging rate of 73.54 ± 2.73%, an ORAC value of 44.55 ± 3.08 μmol TE/g, a HOSC value of 61.93 ± 3.52 μmol TE/g, and a FRAP value of 0.53 ± 0.37 μmol TE/g. The antioxidant activity of ellagic acid was weaker than any of the other polyphenols. The results also indicated that the order of the antioxidant capacity of these ten free phenols was phloridzin > protocatechuic acid > hyperoside > chlorogenic acid >catechins > anthocyaninB2 > epicatechin > caffeic acid > quercetin > ellagic acid. The findings presented in were generally in accordance with literature reports showing significant variations in the composition and contents of phenolic antioxidants. In our study, it was found that there were high correlations between the antioxidant capacity and the content of phenolic compounds.
TABLE 4 Antioxidant capacities of 10 free phenols detected in 13 apple varieties
CONCLUSIONS
Apple polyphenols have multiple health promoting biological effects, but the most interesting are the antioxidant capacities associated with preventing free radical formation and scavenging free radicals.[Citation33] The free radical-scavenging antioxidant plays important roles in the physiological defense network against oxidative stress. The assessment of antioxidant capacity has been the subject of extensive studies and arguments over the past two decades. The antioxidant capacity may be assessed for a pure antioxidant compound, mixtures of antioxidant compounds, extracts, or commercial products. This capacity may be evaluated in terms of an absolute number or a relative value. Our investigation enabled the qualitative and quantitative determination of some of the main polyphenols in various apple varieties contributing to the antioxidant capacity of apples measured with DRSC, ORAC, HOSC, and FRAP methods.[Citation34,Citation35] Among the total polyphenol content values of the apples tested, hongxun 1 had the highest polyphenol content and antioxidant capacities. There were 10 main free phenols existing in apple polyphenols, these included anthocyaninB2, phlorizin, protocatechuic acid, hyproside, and chlorogenic acid, which exhibited higher antioxidant capacities than the other free phenols. Antioxidant assays of the 10 free phenols detected in 13 apple varieties showed that these phenols were positively correlated with their antioxidant activity. Protocatechuic acid, hyperoside, phloridzin, and chlorogenic acid played more important roles in the antioxidant assays, which was consistent with that previously reported.[Citation36,Citation37] Based on our research, obtaining polyphenol extracts from different apple varieties using solid–liquid extraction under ultrasonic condition was not complicated and did not require expensive additional equipment or reagents. This process could be easily scaled up and a large proportion of the apple antioxidants could be identified and their contributions to the observed antioxidant capacity more precisely estimated by this proposed methodology.
ACKNOWLEDGMENTS
We would like to thank the apple experimental station, Baishui County, Shaanxi Province, China, for providing the apple samples. We would also like to acknowledge the Morden Agriculture Technology System (nycytx-08-01-03).
FUNDING
This work was supported by The Doctoral Scientific Research Foundation of Henan Institute of Science and Technology (205010616003) and “Climbing” program – innovation fund (205010915002).
Additional information
Funding
REFERENCES
- Mirmiran, P.; Noori, N.; Zavareh, M.B.; Azizi, F. Fruit and Vegetable Consumption and Risk Factors for Cardiovascular Disease. Metabolism 2009, 58(4), 460–468.
- Alghazeer, R.; Saltani, H.; Saleh, N.; Najjar, A.; Hebail, F. Antioxidant and Antimicrobial Properties of Five Medicinal Libyan Plants Extracts. Natural Science 2012, 4(5), 324–335.
- Schieber, A.; Keller, P.; Carle, R. Determination of Phenolic Acids and Flavonoids of Apple and Pear by High-Performance Liquid Chromatography. Journal of Chromatography A 2001, 910(2), 265–273.
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of Polyphenolic Content and in Vitro Antiradical Characteristics of Apple Pomace. Food Chemistry 2008, 109(2), 340–347.
- Adil, I.H.; Cetin, H.; Yener, M.; Bayındırlı, A. Subcritical (carbon Dioxide+ Ethanol) Extraction of Polyphenols from Apple and Peach Pomaces, and Determination of the Antioxidant Activities of the Extracts. The Journal of Supercritical Fluids 2007, 43(1), 55–63.
- Sudha, M.; Baskaran, V.; Leelavathi, K. Apple Pomace as a Source of Dietary Fiber and Polyphenols and Its Effect on the Rheological Characteristics and Cake Making. Food Chemistry 2007, 104(2), 686–692.
- Chien, P.-J.; Sheu, F.; Huang, W.-T.; Su, M.-S. Effect of Molecular Weight of Chitosans on Their Antioxidative Activities in Apple Juice. Food Chemistry 2007, 102(4), 1192–1198.
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Totsuka, M.; Teshima, R.; Sawada, J.; Goda, Y.; Maitani, T. Dietary Unripe Apple Polyphenol Inhibits the Development of Food Allergies in Murine Models. FEBS Letters 2005, 579(20), 4485–4491.
- Kojima, T.; Akiyama, H.; Sasai, M.; Taniuchi, S.; Goda, Y.; Toyada, M.; Kobayashi, Y. Anti‐Allergic Effect of Apple Polyphenol on Patients with Atopic Dermatitis: A Pilot Study. Allergology International 2000, 49(1), 69–73.
- D’Angelo, S.; Cimmino, A.; Raimo, M.; Salvatore, A.; Zappia, V.; Galletti, P. Effect of Reddening–Ripening on the Antioxidant Activity of Polyphenol Extracts from cv. “Annurca” Apple Fruits. Journal of Agricultural and Food Chemistry 2007, 55(24), 9977–9985.
- Chen, J.; Sun, H.; Wang, Y.; Wang, S.; Tao, X.; Sun, A. Stability of Apple Polyphenols as a Function of Temperature and pH. International Journal of Food Properties 2014, 17(8), 1742–1749.
- Rice-Evans, C. Flavonoid Antioxidants. Current Medicinal Chemistry 2001, 8(7), 797–807.
- Lee, K.W.; Lee, H.J.; Lee, C.Y. Antioxidant and Antitumor Promoting Activities of Apple Phenolics. Paper presented at: Phenolic Compounds in Foods and Natural Health Products, American Chemical Society Symposium Series Book, Columbus, Ohio, 2005.
- Cushnie, T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. International Journal of Antimicrobial Agents 2005, 26(5), 343–356.
- Yue, T.; Shao, D.; Yuan, Y.; Wang, Z.; Qiang, C. Ultrasound‐Assisted Extraction, HPLC Analysis, and Antioxidant Activity of Polyphenols from Unripe Apple. Journal of Separation Science 2012, 35(16), 2138–2145.
- Bai, X.; Zhang, H.; Ren, S. Antioxidant Activity and HPLC Analysis of Polyphenol-Enriched Extracts from Industrial Apple Pomace. Journal of the Science of Food and Agriculture 2013, 93(10), 2502–2506.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods in Enzymology 1999, 299, 152–178.
- Junjian, R.; Mingtao, F.; Yahui, L.; Guowei, L.; Zhengyang, Z.; Jun, L. Optimisation of Ultrasonic-Assisted Extraction of Polyphenols from Apple Peel Employing Cellulase Enzymolysis. International Journal of Food Science & Technology 2013, 48(5), 910–917.
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. Journal of Agricultural and Food Chemistry 1992, 40(6), 945–948.
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, Tocopherol, Phenolic Acid, and Antioxidant Properties of Maryland-Grown Soft Wheat. Journal of Agricultural and Food Chemistry 2005, 53(17), 6649–6657.
- Moore, J.; Yin, J.-J.; Yu, L. Novel Fluorometric Assay for Hydroxyl Radical Scavenging Capacity (HOSC) Estimation. Journal of Agricultural and Food Chemistry 2006, 54(3), 617–626.
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. Journal of Agricultural and Food Chemistry 2000, 48(8), 3396–3402.
- Arts, M.J.; Sebastiaan Dallinga, J.; Voss, H.-P.; Haenen, G.R.; Bast, A. A New Approach to Assess the Total Antioxidant Capacity Using the TEAC Assay. Food Chemistry 2004, 88(4), 567–570.
- Wong, C.-C.; Li, H.-B.; Cheng, K.-W.; Chen, F. A Systematic Survey of Antioxidant Activity of 30 Chinese Medicinal Plants Using the Ferric Reducing Antioxidant Power Assay. Food Chemistry 2006, 97(4), 705–711.
- Rop, O.; Juríková, T.; Sochor, J.; Mlcek, J.; Kramarova, D. Antioxidant Capacity, Scavenging Radical Activity and Selected Chemical Composition of Native Apple Cultivars from Central Europe. Journal of Food Quality 2011, 34(3), 187–194.
- Drogoudi, P.D.; Pantelidis, G. Effects of Position on Canopy and Harvest Time on Fruit Physico-Chemical and Antioxidant Properties in Different Apple Cultivars. Scientia Horticulturae 2011, 129(4), 752–760.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chemistry 2006, 99(1), 191–203.
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Di Pietro, P.F.; Nunes, Ed.C.; Fett, R. Phenolic Compounds and Antioxidant Activity of the Apple Flesh and Peel of Eleven Cultivars Grown in Brazil. Scientia Horticulturae 2011, 128(3), 261–266.
- Lu, Y.; Yeap Foo, L. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chemistry 2000, 68(1), 81–85.
- Rababah, T.M.; Al‐Mahasneh, M.A.; Kilani, I.; et al. Effect of Jam Processing and Storage on Total Phenolics, Antioxidant Activity, and Anthocyanins of Different Fruits. Journal of the Science of Food and Agriculture 2011, 91(6), 1096–1102.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 2005, 53(10), 4290–4302.
- Khanizadeh, S.; Tsao, R.; Rekika, D.; Yang, R.; Charles, M.T.; Rupasinghe, H.V. Polyphenol Composition and Total Antioxidant Capacity of Selected Apple Genotypes for Processing. Journal of Food Composition and Analysis 2008, 21(5), 396–401.
- Gülçin, İ. Antioxidant Activity of Food Constituents: An Overview. Archives of Toxicology 2012, 86(3), 345–391.
- Takashima, M.; Horie, M.; Shichiri, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Assessment of Antioxidant Capacity for Scavenging Free Radicals in Vitro: A Rational Basis and Practical Application. Free Radical Biology and Medicine 2012, 52(7), 1242–1252.
- Niki, E. Antioxidant Capacity: Which Capacity and How to Assess It? Journal of Berry Research 2011, 1(4), 169–176.
- Lee, K.W.; Kim, Y.J.; Kim, D.-O.; Lee, H.J.; Lee, C.Y. Major Phenolics in Apple and Their Contribution to the Total Antioxidant Capacity. Journal of Agricultural and Food Chemistry 2003, 51(22), 6516–6520.
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which Polyphenolic Compounds Contribute to the Total Antioxidant Activities of Apple? Journal of Agricultural and Food Chemistry 2005, 53(12), 4989–4995.