ABSTRACT
This study aimed to investigate the effect of adding binary mixtures of disodium phosphate and tetrasodium pyrophosphate during milk powder production. Overall, the addition of disodium phosphate–tetrasodium pyrophosphate caused an increase in pH and a decrease in the acidity and turbidity of reconstituted milk samples. The decrease in turbidity was attributed to either dispersion or swelling of the casein micelles. The addition of mixtures containing the lowest amounts of tetrasodium pyrophosphate considerably reduced the insolubility index, whereas mixtures containing higher levels of tetrasodium pyrophosphate exerted a detrimental effect on solubility. Interestingly, microscopic observations showed large agglomerated particles in mixtures with the highest level of tetrasodium pyrophosphate. We hypothesized that the formation of casein calcium–pyrophosphate complexes led to the higher insolubility index in this mixture.
Introduction
Dairy powders are commonly used ingredients and are incorporated into a wide range of food products. Their functional attributes play important roles in their contribution to food formulations.[Citation1] The technological properties of most dairy products are influenced by casein micelles,[Citation2,Citation3] which represent approximately 80% of the total milk protein.[Citation4] Various molecular forces, including hydrophobic and electrostatic interactions, and colloidal calcium phosphate (ccp) maintain micelle cohesion.[Citation5] CCP is considered to be a cementing and neutralizing agent which contributes to micellar integrity and stability.[Citation6]
One of the common methods to modify the structure and functionality of casein micelles is modulating the CCP content of the micelles[Citation3] by several methods: heating and cooling,[Citation7] acidification,[Citation3,Citation8] alkalization,[Citation9] and sequestration of calcium by calcium chelating salts (CCSs), also known as melting or emulsifying salts (ESs).[Citation10–Citation12] The caseins and minerals are in dynamic equilibrium. The alteration of mineral balance, especially the distribution of calcium and phosphate between the soluble and colloidal phase, results in significant changes in the conformation and stability of the casein micelles.[Citation13,Citation14] It is well known that the addition of ESs to milk and dairy products changes the calcium balance of the system, leading to a decline in calcium ion concentration, dissolution of CCP, and release of caseins from the micelles.[Citation15,Citation16] The decrease in free calcium ions and removal of CCP result in swelling and hydration of casein micelles. These phenomena have a great influence on turbidity and solubility of a dairy product.[Citation16] Recent studies on the addition of NaCl to concentrated casein micelle suspensions demonstrated that dissolution of CCP, dissociation of casein micelles, and the subsequent changes in the composition of serum phase have an important effect on the viscosity and turbidity of the continuous phase.[Citation17,Citation18] The ESs are composed of phosphates, citrates, tartarates, and lactates as polyvalent anions, and sodium, ammonium, or potassium as monovalent cations. Monosodium phosphate (MSP), disodium phosphate (DSP), sodium tripolyphosphate (STPP), tetrasodium pyrophosphate (TSPP), and sodium hexametaphosphate (SHMP) are frequently used in the dairy industry.[Citation2]
Addition of CCSs to milk systems is a well-known practice to improve the functional characteristics of the derived products. There are many investigations considering the role of ESs in manufacturing processed and imitation cheese, in which ESs are used to alter textural and viscoelastic properties.[Citation13,Citation19–Citation21] In yogurt processing, ESs are added to improve the gel network.[Citation22] There are few published reports available on the use of ESs in milk powders. Schuck et al.[Citation23] reported that water transfer in milk protein concentrate during drying and reconstitution is influenced by the addition of mineral salts including phosphates and citrates. Schuck et al.[Citation24] studied the rehydration properties of casein powder following salt and mineral salt addition. It was concluded that protein micellar structure was modified as a result of sodium phosphate addition. Anema[Citation25] has shown that addition of SHMP during skim milk powder production decreased pH, serum and ionic calcium, micellar zeta potential, and particle size, and increased serum phase orthophosphate levels and dissociation of caseins. In a recent study, Sikand et al.[Citation26] investigated the effect of mineral chelators on selected properties of skim milk powders and found that solubility and turbidity decreased with increasing levels of chelating salts.
As discussed above, a combination of CCSs is extensively used in the dairy industry. It has been reported that the application of a mixture composed of TSPP and DSP in dairy systems (milk puddings and milk protein concentrate (MPC) solutions) results in the formation of new types of complexes with casein and calcium, which lead to the gelation of milk.[Citation2,Citation27] However, the current knowledge does not include the determination of the effects of TSPP and DSP mixtures on the functional properties of spray-dried milk powders. The aim of this study was to determine the physicochemical attributes of milk powders prepared with varying proportions of TSPP and DSP (at a constant total concentration of 0.2% w/v). Rehydration properties of powders were assessed through solubility measurement. The turbidity, pH, and titratable acidity (TA) of reconstituted powders were also investigated. Scanning electron microscopy was used to observe the morphology of powders.
Materials and methods
Materials
Ultra high temperature (UHT) milk was provided by Mihan Dairy Manufacturing (Tehran, Iran). Tetrasodium pyrophosphate anhydrous (CAS No. 7722-88-5; Na4P2O7; TSPP) was obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA), and disodium phosphate anhydrous (CAS No. 7558-79-4; Na2HPO4; DSP) was purchased from Merck KGaA (Darmstadt, Germany).
Experimental design
Response surface methodology (RSM) was employed to investigate the influence of two independent variables, DSP (X1: 0.060%–0.127%, w/v) and TSPP (X2: 0.073%–0.140%, w/v) concentrations, on the response yields of pH, TA, turbidity, and insolubility index (ISI). Initially, ranges and levels of variables were decided on the basis of a literature survey.[Citation2,Citation27,Citation28] Thus, the preliminary experiments on turbidity and microscopic observations of the milk containing added phosphate enabled the range of salt concentrations to be fixed. During the preliminary tests, we intended to induce a gel-like texture. Each variable was coded at two levels (). An optimal mixture design, which included 13 runs with 5 replicates, was used. The experimental data were fitted to a quadratic (second order) polynomial model, and the regression coefficients were obtained for five responses. The polynomial equation used for the two variables is given below:
Table 1. Experimental range and levels of independent variables.
where Y represents the response variable; is the model constant; and
,
and
are linear, quadratic, and interaction effects, respectively.
and
are the levels of the independent variables. Validity of the model was evaluated by lack of fit, multiple correlation coefficient (R2), adjusted (adj-R2) and predicted (pre-R2) determination coefficients, adequate precision, the predicted residual error sum of squares (PRESS), and coefficient of variation (CV).[Citation29]
Preparation of emulsifying salts solutions (feed liquid of spray drier)
The salt solutions were prepared by dissolving various proportions of DSP–TSPP binary mixtures in UHT milk according to , at a constant total CCS concentration of 0.2% w/v. Afterward, solutions were stirred by a RW 20 digital overhead stirrer (IKA-Werke GmbH & Co., Staufen, Germany) at 25°C for 15 min to ensure that the ESs were completely dissolved.
Table 2. Equations of responses in terms of coded values.
Manufacture of milk powders
Milk powders were produced using a mini-spray dryer B290 (Buchi Labortechnik AG, Flawil, Switzerland) with the following operating conditions: 130°C inlet air temperature, outlet air temperature was kept between 70°C and 72°C, 90% aspirator, 25% pump rate, and with the nozzle cleaner set at 7. The atomizer was equipped with a pressure nozzle (1 mm orifice diameter). Control milk powders were prepared in the same conditions but without addition of phosphate salts. The spray drying conditions were primarily determined according to the manufacturer’s guidelines and Rulliere et al.,[Citation30] who investigated the impact of heat treatments on the composition of polyphosphates in aqueous solutions. Next, preliminary experiments were performed to obtain the optimum yield with the minimum drying temperature. Immediately after drying, powders were kept in glass jars sealed with parafilm and stored in a desiccator containing silica gel at room temperature until the analysis was performed. All experiments for each sample were performed within a week of production.
Reconstitution of milk powders
In order to determine pH, TA, and turbidity, powders were reconstituted at 10% (w/v) in deionized water and then stirred for 2 h,[Citation25] utilizing the overhead stirrer to ensure a complete rehydration.
Chemical analysis of UHT milk and milk powder
The chemical analysis was performed on UHT milk and control milk powder. Fat content in the milk and milk powder was determined by Gerber[Citation31] and gravimetric methods,[Citation32] respectively. The protein content of sterilized milk and the milk powder was determined by the Kjeldahl method, and a conversion factor of 6.38 was applied.[Citation33] The amount of total solid (TS) in the milk was measured according to ISO.[Citation34] The TS content of the powder was calculated by the oven method as described by Schuck et al.[Citation24] Briefly, 1 g of powder was dried at 105°C for 5 h. The weight difference before and after drying was considered to be the amount of TS. The concentration factor (Q) was calculated as described by Walstra et al.,[Citation35] who described that the degree of concentration could be determined from the ratio of dry matter content of the concentrated product to that in the initial solution (Eq. (2)):
The amount of protein, fat, and TS was 3.3%, 0.3%, and 9.6% w/w in UHT milk and 32.2%, 3%, and 97.33% w/w in milk powder, respectively. The calculated concentration factor was ~10.
Determination of TA and pH of reconstituted milk powders
The pH values of reconstituted milk samples (prepared as described before) were evaluated using a Metrohm 827 pH meter (Metrohm Ltd., Herisau, Switzerland) at 20°C. The pH probe was calibrated with standard buffer solutions of pH 4.0 and pH 7.0. TA was determined by titration with a 0.1 N NaOH solution.[Citation36]
Determination of turbidity of reconstituted milk powders
casein micelle dispersion caused by CCSs can be assessed by observing the decrease in turbidity.[Citation2–Citation35] The 10% (w/v) reconstituted milk solutions were prepared as described earlier and diluted twofold in deionized water to be within the detection limits of the spectrophotometer. Afterward, spectra were recorded on a Varian Cary 300 UV-visible spectrophotometer (Varian, Inc., Melbourne, Australia) at 20°C using a 1 cm path length plastic cuvette.[Citation16]
Determination of insolubility index of milk powders
The method of Westergaard,[Citation37] with slight modifications, was used to determine the ISI. Briefly, 10 g of milk powder was poured into 100 mL of distilled water at 20°C and stirred at 1000 rpm with a magnetic stirrer. The solution was allowed to rest for 15 min. Afterward, 50 mL of the solution was placed into a centrifuge tube and centrifuged at 1500 rpm for 5 min using a Kokusan H-200NR centrifuge (Kokusan Co., Taito, Tokyo, Japan). The sediment-free liquid was carefully removed and replaced by water. The content was vigorously shaken by hand. Next, the tubes were recentrifuged under the same conditions. The amount of sediment was read and expressed in milliliters.
Scanning electron microscopy (SEM)
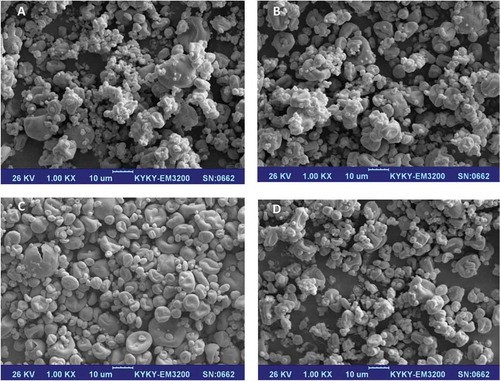
The morphology was studied by a KYKY-EM3200 digital scanning electron microscope (KYKY Technology Development Ltd., Beijing, China) operated at 26 kV. Particles were coated with gold to ~10 nm thickness using a KYKY SBC 12 sputter coater in an argon environment at low pressure (~2 × 10–3 bar). Only control milk powder and powders manufactured with DSP–TSPP mixtures of 0.127:0.073, 0.105:0.095, and 0.060:0.140 were observed.
Statistical analysis
Design Expert, trial version 8.0.5 (State-Ease Inc., Minneapolis, MN, USA), was used for regression analysis and analysis of variance (ANOVA). p-Values of less than 0.05 were considered to be statistically significant. The fitted polynomial equation was then expressed in the form of response surface curves () in order to illustrate the relationship between responses and the independent variables. In addition to RSM to compare the phosphate-added milk powders with the control sample, all experiments were conducted in triplicate. The data were subjected to one-way ANOVA. Duncan’s multiple-range test was used to compare the mean values. Statistical analysis was carried out using the Statistical Packages for the Social Sciences (SPSS 22.0, SPSS, Inc., Chicago, IL, USA).
Results and discussion
Response surface model fitting
The data obtained from the optimal mixture design were fitted to quadratic polynomial equations. The obtained equations that show the relationship between the significant independent variables (DSP and TSPP levels) and responses in terms of coded values are given in . The positive and negative values of the coefficients represent the synergistic and antagonistic effects of independent variables on the response, respectively.[Citation38] ANOVA for the response surface model showed a large number of model F-values with a low probability value (p < 0.01) for all of the responses indicate that the model is statistically significant. Lack of fit was insignificant for all of the equations. The nonsignificant lack of fit is good and indicates that the model fitted the experimental data. The relatively high values of the multiple correlation coefficients (R2 > 0.89) for all responses further supported the model proficiency. Additionally, the predicted R2 was in reasonable agreement with the adjusted R2 ((adj-R2 – pre-R2) < 0.2).[Citation29] Adequate precision for all responses was above 4, indicating adequate model discrimination. The value of CV (%) was less than 10 in all responses, which shows the precision and reliability of the experiments.[Citation39]
Titratable acidity
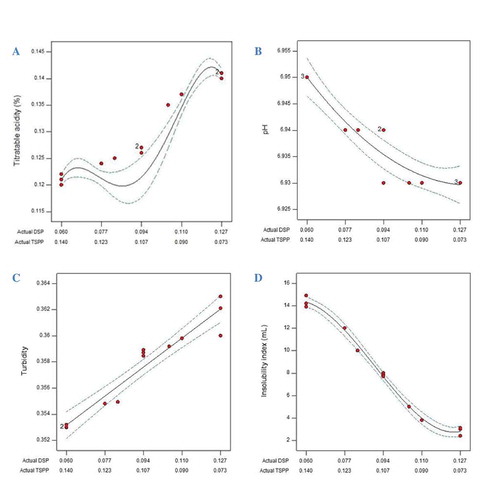
A one-way ANOVA test was conducted to compare the characteristics of ES-containing powders with control milk powder (). There was a significant (p < 0.01) decrease in TA of the reconstituted samples with addition of DSP–TSPP mixtures. TA and pH are commonly used to measure the acidity of milk and milk products. The activity of the hydronium ions (H3O+) is expressed as a pH value, while TA is a measure of the total buffer capacity between the milk pH and the phenolphthalein end point (~8.3). The caseins and phosphate are the primary components of milk that determine the buffering capacity. Casein reacts to NaOH similar to an acid substance, through its ionizable groups (e.g., amino acid residues and CCP).[Citation40] It is generally recognized that the addition of ESs results in the destruction of CCP clusters associated with serine residues. This phenomenon is based on the calcium ion exchange ability of the CCP clusters; thus, the replacement of monovalent sodium ions of the ES with divalent calcium ions of the CCP will occur.[Citation41] This exchange of calcium by sodium within the casein micelles lead to the solubilization of CCP and the formation of the sodium–phosphate clusters accompanied by the release of specific caseins into the continuous phase and noticeable changes in the serum composition.[Citation17] The newly formed sodium–phosphate casein complexes decrease the protein–protein interactions and weaken the internal structure of casein micelles.[Citation17,Citation18] Further, we hypothesize that the newly formed sodium–phosphate casein complexes are no longer capable of reacting with NaOH. As a result, the amount of NaOH in the titration decreased, which was indicated by the decreased TA. The response plot of represents the TA of reconstituted powders prepared with DSP–TSPP mixtures. Powders containing higher TSPP proportions showed lower acidity. The lower TA of the samples with higher levels of TSPP is presumably due to the number of Na atoms in the TSPP structure. TSPP with higher number of sodium ions and calcium affinity[Citation10] participate to the formation of sodium–phosphate complexes to a larger extent compared with DSP.
Table 3. Mean values of the physicochemical characteristics of milk powders prepared with and without emulsifying salts.ª
pH
The one-way ANOVA test was used to compare the pH of reconstituted control powder with powders prepared with added DSP–TSPP mixtures (). Addition of ES caused a significant increase (p < 0.01) in pH of the reconstituted powders. The mechanism by which the DSP–TSPP mixtures increased pH probably involves calcium chelation by the CCS and destruction of the CCPs, releasing phosphate ions. The subsequent protonation of the phosphate anions cause an increase in pH.[Citation11] Various forms of phosphate (PO34–, HPO42−, H2PO4−, or H3PO4) with corresponding pKa values of 2.12, 7.21, and 12.30 can exist depending on the pH of the milk system.[Citation42] The destruction of CCP clusters releases the bound inorganic phosphate which readily buffers potential pH changes caused by the addition of DSP–TSPP mixtures.[Citation6] This pH-stabilizing effect of ES is probably the reason of the small variations in pH of powders containing DSP–TSPP mixtures.
According to response surface plots for pH () of the ES-containing powders, an increase in pH values was observed by the growing amount of TSPP in the binary mixtures. Higher pH values in mixtures with higher TSPP proportions can be explained by the affinity of the chelating salt for calcium;[Citation15] the better calcium-masking ability of TSPP[Citation14,Citation28] leads to more widespread CCP destruction, thus more phosphate ions will be present for accepting hydronium ions and a concomitant pH increase will occur. Similar results were obtained by Dimitreli and Thomareis,[Citation13] Gupta et al.,[Citation43] and Sádlíková et al.,[Citation21] who reported an increase in the final pH of processed cheese made with various ESs, with TSPP being more efficient in increasing the pH than DSP. In a study on addition of ternary mixtures of phosphate and citrate ESs to processed cheese, Salek et al.[Citation44] showed that the pH values of model processed cheese samples with added TSPP are higher than those prepared with DSP.
Turbidity
In general, addition of DSP–TSPP mixtures resulted in a decrease in turbidity of reconstituted samples (); a significant (p < 0/01) difference was observed between control sample and sample with highest proportion of TSPP (0.060:0.140 DSP–TSPP mixture) (). The mechanism by which ESs decreased the turbidity of reconstituted milk samples is primarily related to calcium sequestration leading to destruction of CCP crosslinks and a decrease in serum phase calcium. The loss of CCP salt bridges, one of the casein micelle structural elements, and a concomitant exposure of charged phosphoserine residues, increasing electrostatic repulsion, results in casein dissociation, which was measured as a decrease in turbidity ().[Citation2,Citation16,Citation28] The subsequent formation of casein calcium–CCS complexes upon addition of DSP–TSPP mixtures may also result in an increase in charge repulsion and further dispersion.[Citation10] The small differences in turbidity observed in ES-containing reconstituted samples () can be explained by the narrow range of DSP–TSPP mixtures we chose to use in this study. The response surface plot for turbidity shown in Fig. 1c indicates that the turbidity decreased linearly with increasing proportion of added TSPP in binary mixtures, suggesting the dispersion of casein micelles.[Citation2] The extent of the effect of ESs on casein dissociation depends on the calcium complexing ability of the salt.[Citation16,Citation19] Compared with orthophosphates, pyrophosphates, which have a higher number of phosphate groups, have a stronger affinity for calcium[Citation10,Citation20] and provide more interactions with protein molecules, thus affecting the micellar integrity.[Citation16,Citation21] Consistent with our results, Kaliappan and Lucey[Citation2] reported that the turbidity of MPC solutions containing CCS binary mixtures considerably decreased when TSPP was dominant in a blend with DSP. Mizuno and Lucey[Citation10] also stated that at a constant concentration, TSPP was more efficient at dispersing casein than DSP.
Turbidity is related to particle size, concentration, and refractive index of particles. The casein micelles and CCP are the main light scatterers in milk solutions, and CCP makes significant contribution to refractive index of the micelles.[Citation17,Citation45,Citation46] Interestingly, in a study on CCS usage in concentrated micellar casein solutions, de Kort et al.[Citation16] suggested a decline in turbidity is related to either swelling of intact casein micelles or dispersion of the casein micelles, and the latter has a more pronounced effect in decreasing turbidity. It was suggested that the swelling is due to the chelation of loosely bound calcium associated with negatively charged amino acid side chains and phosphate groups, and the dissociation of micelles is a result of CCP destruction. We hypothesize that in this study, the decrease in the turbidity of samples in which the DSP proportion is predominant is most likely due to the swelling of casein micelles because DSP may chelate the loosely bound calcium due to its lower calcium masking ability compared with TSPP. As a result of swelling of the casein micelles, more continuous phase will be present in the micellar structure which thus reduces the difference between the refractive index of serum and casein micelles, leading to a decrease in turbidity.[Citation16,Citation17] In samples with a higher proportion of TSPP, the turbidity decrease is probably due to CCP destruction and this may be the reason for lower turbidity in these samples compared with those with a higher proportion of DSP (Fig. 1c). It is shown that removal of CCP from casein micelles leads to a decline in the refractive index of casein micelles which is measured as a decrease in turbidity.[Citation16,Citation45] However, further research on the voluminosity of casein micelles is needed to prove this theory.
Similarly, Zhao and Corredig[Citation17] demonstrated that addition of NaCl to concentrated milk protein causes a decrease in turbidity. They attributed the changes in turbidity to the difference in the refractive index between the serum and protein particles. It was concluded that the dissolution of CCP and release of caseins, as a result of NaCl addition, led to the alteration of the refractive index of the serum phase and decrease in turbidity.
Solubility
The ability of a powder to dissolve in water, termed solubility, indicates the complete rehydration of powders.[Citation47] The main factors affecting the solubility of milk powders are drying conditions (which were similar in the production of all of the samples) and the physical characteristics of the feed liquid (e.g., viscosity).[Citation48] Casein is considered to be the main water-attracting component in milk powders at low and intermediate water activity. The interaction between water and casein is largely influenced by casein’s structural organization.[Citation49] Addition of CCSs modifies the structure of casein.[Citation2] Modification of the structural organization of casein micelles induces changes in the interactions between water and casein molecules. It is known that the state of protein aggregation have an important role in the quantity of water bound to the supramolecular structure.[Citation50] Conformational modifications are probably responsible for differences in the binding of water molecules because it is known that the reaction of proteins with phosphate causes variations in their sensitivity to hydration.[Citation51]
The results in indicate that powders with lower proportions of TSPP (≤47%) had a lower ISI than the control sample, whereas for mixtures with higher proportions of TSPP (≥53%) the ISI was greater than the control. Indeed, the effect observed on insolubility index of powders depended on the proportions of DSP and TSPP in the added mixtures. In samples containing lower proportions of TSPP (DSP–TSPP mixtures of 0.127:0.073, 0.110:0.90, and 0.105:0.095), the decrease in the ISI compared with control samples could be related to the role of ESs in the destruction of casein micelles[Citation24] and unraveling of the protein chains, which makes more hydrophilic phosphate sites available and promotes casein hydration.[Citation51,Citation52] The latter supposition supports the suggestion that a disruption in protein–protein interactions is required for the protein–water interactions to take place.[Citation20,Citation50,Citation53] Further, according to de Kort et al.[Citation16] the decrease in calcium ion activity upon addition of DSP and TSPP results in an increase in the electrostatic repulsion between the casein micelles and an increase in hydration of the micelles. It is interesting to compare these results with those reported by Zhao and Corredig.[Citation18] They stated that the addition of NaCl to concentrated casein micelle suspensions causes the solubilization of CCP, increases disruption of caseins, and improves the solubility of milk concentrates. Schuck et al.[Citation24] also reported that the reduction of calcium ions improves the solubility of spray-dried MPC85.
In samples containing higher proportions of TSPP (DSP–TSPP mixtures of 0.094:0.107, 0.082:0.118, 0.077:0.123, and 0.060:0.140), the increase in the ISI compared with the control can be explained by the reassociation of dispersed caseins with calcium–pyrophosphate complexes. We can assume that at these ratios the formation of casein calcium–pyrophosphate complexes and large casein aggregates occurred, which consequently caused an increase in the amount of sediment during ultracentrifugation as large particles are known to precipitate more easily.[Citation12,Citation54]
An alternative explanation for increased ISI in high ratio of TSPP can be attributed to the viscosity of suspensions. It has been demonstrated that the viscosity of liquid feed and solubility of the powder are inversely correlated.[Citation1] Addition of CCS is known to increase the viscosity of milk systems.[Citation16,Citation46,Citation55] The increase in viscosity can be due to (1) the extensive release of proteins from casein micelles and the subsequent increase in the concentration of soluble caseins in the serum phase,[Citation18] (2) cross-links between casein micelles via calcium pyrophosphate complexes.[Citation16] de Kort et al.[Citation16] reported that addition of SHMP increased the viscosity of concentrated micellar casein solutions due to the aggregation of casein micelles through formation of cross-links between micelles.
The response plot of ISI versus added DSP–TSPP mixtures is shown in Fig. 1d. The solubility of powders decreased with increasing TSPP addition. It is likely that the higher dissociation of casein micelles at higher proportions of TSPP (as indicated by lower turbidity, Fig. 1c) enabled more interactions between proteins due to the unfolding of casein chains, leading to aggregation of casein micelles[Citation44,Citation52] and a subsequent increase in the insoluble fraction. It is also possible that reassociation of dispersed casein through calcium–pyrophosphate complexes caused the similarity in the turbidity of samples ().
Morphology of milk powders
Samples with the highest (0.060:0.140, ), intermediate (0.105:0.095, ), and lowest (0.127:0.073, Fig. 2c) level of TSPP in the added DSP–TSPP mixtures, as well as the control sample, were observed with SEM. Microscopic images showed that powders generally appeared as collapsed spheres with smooth dimples on the surface. In powders prepared with an added DSP–TSPP mixture of 0.127:0.073 (Fig. 2c), the powder consisted of single particles and less compact structures (Fig. 2c) than the control (Fig. 2d), likely due to the dispersion of casein micelles. It is assumed that dispersed proteins did not reassociate through calcium–pyrophosphate bridges at the 0.127:0.073 DSP–TSPP ratio, leading to the formation of more open and distinct structures (Fig. 2c) in the powder. This is in line with the results observed for the ISI in , in which the 0.127:0.073 DSP–TSPP mixture exhibited a lower ISI than the control sample, possibly due to the dispersion of casein micelles.[Citation23] The aggregation of smaller particles into larger complex aggregates is clearly observed in the DSP–TSPP mixtures of 0.060:0.140 () and 0.105:0.095 () which may be related to extensive cross-links between casein micelles via calcium–pyrophosphate complexes. In this work, the investigation of the surface composition of milk powders was neglected but it has been reported that powder surfaces are primarily dominated by surface-active components (lipid and proteins).[Citation56,Citation57] It is possible that calcium–pyrophosphate complexes bound to casein particles are the predominant species on the surface of milk powders containing phosphate, which can render them capable of interacting readily with other particles. This may result in the agglomeration of particles, as was observed in the 0.060:0.140 DSP–TSPP mixture (). Presumably, this complex network inhibited further rehydration of the powder (indicated by the high ISI, Fig. 1d). Similarly, Anema et al.[Citation58] and Mimouni et al.[Citation59] reported that the poor solubility of MPC85 during rehydration was possibly due to the formation of a skin of cross-linked casein micelles at the surface of powder particles. These aggregated and cross-linked surfaces decreased the solubility of powders. However, the exact explanation is unknown and further research on the surface composition of powder particles is needed to support this theory.
Conclusion
The study described in this article has demonstrated that the addition of DSP–TSPP mixtures to milk powder during processing could have several effects. The study found that the addition of a DSP–TSPP mixture, especially when containing higher levels of TSPP, caused an increase in pH and a decrease in TA. The latter varied more significantly among the samples. The decrease in the TA was attributed to the reaction of sodium salts with casein micelles, which presumably decreased the role of casein molecules as acid-like substances, leading to a decrease in the amount of NaOH needed for neutralization. These observations provide insights into the use of TA as an interesting parameter for estimating the extent of the reaction between sodium phosphate salts and casein micelles. The decrease in turbidity of reconstituted milk powders can be attributed to the decrease in the difference between the refractive index of the serum phase and casein particles. The decrease in turbidity in samples with higher proportions of DSP is possibly due to the swelling of the casein micelles. In samples with higher proportions of TSPP, the decrease in turbidity can be explained by the removal of CCP and destruction of the micelles. The study concludes that the 0.060:0.140 DSP–TSPP mixture exhibited the lowest solubility among the samples possibly due to the formation of cross-links between caseins. Furthermore, SEM images of this mixture showed large grape-structured agglomerates. Considering the physicochemical characteristics of the milk powders with added phosphate described in this study, further studies are recommended to examine the functional properties of these powders in manufacturing gel-like products, including yogurt and dairy desserts.
Acknowledgments
The authors thank the Department of Food Science and Technology and the National Nutrition and Food Technology Research Institute (Shahid Beheshti University of Medical Sciences) for providing the spray dryer facility and scientific and technical assistance in milk powder production.
References
- Reddy, R.S.; Ramachandra, C.; Hiregoudar, S.; Nidoni, U.; Ram, J.; Kammar, M. Influence of Processing Conditions on Functional and Reconstitution Properties of Milk Powder Made from Osmanabadi Goat Milk by Spray Drying. Small Ruminant Research 2014, 119(1), 130–137.
- Kaliappan, S.; Lucey, J.A. Influence of Mixtures of Calcium-Chelating Salts on the Physicochemical Properties of Casein Micelles. Journal of Dairy Science 2011, 94(9), 4255–4263.
- Silva, N.N.; Piot, M.; de Carvalho, A.F.; Violleau, F.; Fameau, A.-L.; Gaucheron, F. pH-induced Demineralization of Casein Micelles Modifies their Physico-Chemical and Foaming Properties. Food Hydrocolloids 2013, 32(2), 322–330.
- Fox, P.F.; Brodkorb, A. The Casein Micelle: Historical Aspects, Current Concepts and Significance. International Dairy Journal 2008, 18(7), 677–684.
- Horne, D.S. Casein Interactions: Casting Light on the Black Boxes, the Structure in Dairy Products. International Dairy Journal 1998, 8(3), 171–177.
- Lucey, J.A.; Fox, P. Importance of Calcium and Phosphate in Cheese Manufacture: A Review. Journal of Dairy Science 1993, 76(6), 1714–1724.
- Holt, C.; Fox, P. Effect of Heating and Cooling on the Milk Salts and their Interaction with Casein. Heat-Induced Changes in Milk 1995, (Ed. 2), 105–133.
- Anema, S.G. Role of colloidal Calcium Phosphate in the Acid Gelation Properties of Heated Skim Milk. Food Chemistry 2009, 114(1), 161–167.
- Ozcan-Yilsay, T.; Horne, D.; Lucey, J. Effect of Increasing the Colloidal Calcium Phosphate of Milk on the Texture and Microstructure of Yogurt. Journal of Dairy Science 2011, 94(11), 5278–5288.
- Mizuno, R.; Lucey, J. A. Effects of Emulsifying Salts on the Turbidity and Calcium-Phosphate–Protein Interactions in Casein Micelles. Journal of Dairy Science 2005, 88(9), 3070–3078.
- Odagiri, S.; Nickerson, T. Complexing of Calcium by Hexametaphosphate, Oxalate, Citrate, and Ethylenediamine-Tetraacetate in Milk. II. Dialysis of Milk Containing Complexing Agents. Journal of Dairy Science 1965, 48(1), 19–22.
- Tsioulpas, A.; Koliandris, A.; Grandison, A.S.; Lewis, M.J. Effects of Stabiliser Addition and In-Container Sterilisation on Selected Properties of Milk Related to Casein Micelle Stability. Food Chemistry 2010, 122(4), 1027–1034.
- Dimitreli, G.; Thomareis, A.S. Instrumental Textural and Viscoelastic Properties of Processed Cheese as Affected by Emulsifying Salts and in Relation to its Apparent Viscosity. International Journal of Food Properties 2009, 12(1), 261–275.
- Caric, M.; Gantar, M.; Kalab, M. Effects of Emulsifying Agents on the Microstructure and other Characteristics of Process Cheese-A Review. Food Structure 1985, 4(2), 13.
- Gao, R.; van Halsema, F.; Temminghoff, E.; van Leeuwen, H.; van Valenberg, H.; Eisner, M.; van Boekel, M. Modelling Ion Composition in Simulated Milk Ultrafiltrate (SMUF) II. Influence of pH, Ionic Strength and Polyphosphates. Food Chemistry 2010, 122(3), 710–715.
- de Kort, E.; Minor, M.; Snoeren, T.; van Hooijdonk, T.; van der Linden, E. Effect of Calcium Chelators on Physical Changes in Casein Micelles in Concentrated Micellar Casein Solutions. International Dairy Journal 2011, 21(12), 907–913.
- Zhao, Z.; Corredig, M. Changes in the Physico-Chemical Properties of Casein Micelles in the Presence of Sodium Chloride in Untreated and Concentrated Milk Protein. Dairy Science & Technology, 2015, 95(1), 87–99.
- Zhao, Z.; Corredig, M. Influence of Sodium Chloride on the Colloidal and Rennet Coagulation Properties of Concentrated Casein Micelle Suspensions. Journal of Dairy Science, 2016, 99(8), 6036–6045.
- Cavalier‐Salou, C.; Cheftel, J. Emulsifying Salts Influence on Characteristics of Cheese Analogs from Calcium Caseinate. Journal of Food Science 1991, 56(6), 1542–1547.
- El-Bakry, M.; Duggan, E.; O’Riordan, E.; O’Sullivan, M. Effects of Emulsifying Salts Reduction on Imitation Cheese Manufacture and Functional Properties. Journal of Food Engineering 2010, 100(4), 596–603.
- Sádlíková, I.; Buňka, F.; Budínský, P.; Barbora, V.; Pavlínek, V.; Hoza, I. The Effect of Selected Phosphate Emulsifying Salts on Viscoelastic Properties of Processed Cheese. LWT-Food Science and Technology 2010, 43(8), 1220–1225.
- Ozcan-Yilsay, T.; Lee, W.-J.; Horne, D.; Lucey, J. Effect of Trisodium Citrate on Rheological and Physical Properties and Microstructure of Yogurt. Journal of Dairy Science 2007, 90(4), 1644–1652.
- Schuck, P.; Briard, V.; Méjean, S.; Piot, M.; Famelart, M.; Maubois, J. Dehydration by Desorption and by Spray Drying of Dairy Proteins: Influence of the Mineral Environment. Drying Technology 1999, 17(7–8), 1347–1357.
- Schuck, P.; Davenel, A.; Mariette, F.; Briard, V.; Méjean, S.; Piot, M. Rehydration of Casein Powders: Effects of Added Mineral Salts and Salt Addition Methods on Water Transfer. International Dairy Journal 2002, 12(1), 51–57.
- Anema, S. G. The Effect of Hexametaphosphate Addition During Milk Powder Manufacture on the Properties of Reconstituted Skim Milk. International Dairy Journal 2015, 50, 58–65.
- Sikand, V.; Tong, P.S.; Vink, S.; Roy, S. Physicochemical Properties of Skim Milk Powders Prepared with the Addition of Mineral Chelators. Journal of Dairy Science 2016, 99(6), 4146–4153.
- Clausi, A. S. Pudding Composition and Process of Producing the Same. General Foods Corporation assignee 1957. US Patent 2,801,924.
- Mizuno, R.; Lucey, J. A. Properties of Milk Protein Gels Formed by Phosphates. Journal of Dairy Science 2007, 90(10), 4524–4531.
- Erbay, Z.; Koca, N.; Kaymak-Ertekin, F.; Ucuncu, M. Optimization of Spray Drying Process in Cheese Powder Production. Food and Bioproducts Processing 2014, 93, 156–165. doi:10.1016/j.fbp.2013.12.008
- Rulliere, C.; Perenes, L.; Senocq, D.; Dodi, A.; Marchesseau, S. Heat Treatment Effect on Polyphosphate Chain Length in Aqueous and Calcium Solutions. Food Chemistry 2012, 134(2), 712–716.
- ISO. Milk - Determination of Fat Content. ISO Standard 2008, No. 2446. Geneva, Switzerland: International Standardisation Organisation.
- ISO. Dried Milk and Dried Milk Products - Determination of Fat Content - Gravimetric Method (Reference Method). ISO Standard 2008, No. 1736. Geneva, Switzerland: International Standardisation Organisation.
- ISO. Milk and Milk Products - Determination of Nitrogen Content - Part 1: Kjeldahl Principle and Crude Protein Calculation. ISO Standard 2014, No. 8968-1. Geneva, Switzerland: International Standardisation Organisation.
- ISO. Milk, Cream and Evaporated Milk - Determination of Total Solids Content (Reference Method). ISO Standard 2010, No. 6731. Geneva, Switzerland: International Standardisation Organisation.
- Walstra, P.; Wouters, J.T.; Geurts, T.J. Dairy Science and Technology (2nd edn., Chapt. 10). BocaRaton, FL,USA: CRC Press, 2005.
- ISO. (2010). Dried milk - Determination of Titratable Acidity (Reference Method). ISO Standard No. 6091. Geneva, Switzerland: International Standardisation Organisation.
- Westergaard, V. Analytical Methods Raw Milk, Concentrate and Powder Properties. In Milk Powder Technology: Evaporation and Spray Drying. Westergaard, V.; Ed.; Copenhagen, Denmark: Niro A/S, 2004; 203–213.
- Podstawczyk, D.; Witek-Krowiak, A.; Dawiec, A., Bhatnagar, A. Biosorption of Copper (II) Ions by Flax Meal: Empirical Modeling and Process Optimization by Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Simulation. Ecological Engineering 2015, 83, 364–379.
- Ghorbani, F.; Younesi, H.; Ghasempouri, S.M.; Zinatizadeh, A.A.; Amini, M.; Daneshi, A. Application of Response Surface Methodology for Optimization of Cadmium Biosorption in an Aqueous Solution by Saccharomyces Cerevisiae. Chemical Engineering Journal 2008, 145(2), 267–275.
- Spreer, E. Milk and Dairy Product Technology (1st edn., Chapt. 2). NewYork, USA: Marcel Dekker, Inc, 1998.
- Tamime, A.Y. Processed Cheese and Cheese Analogues. In Structure of dairy products. Tamime, A.Y.; Ed.; Ayr, UK: Blackwell Publishing Ltd, 2008; 213–215.
- Upreti, P.; Metzger, L. Influence of Calcium and Phosphorus, Lactose, and Salt-to-Moisture Ratio on Cheddar Cheese Quality: pH Changes during Ripening. Journal of Dairy Science 2007, 90(1), 1–12.
- Gupta, S.; Karahadian, C.; Lindsay, R. Effect of Emulsifier Salts on Textural and Flavor Properties of Processed Cheeses. Journal of Dairy Science 1984, 67(4), 764–778.
- Salek, R.N.; Černíková, M.; Nagyová, G.; Kuchař, D.; Bačová, H.; Minarčíková, L.; Buňka, F. The Effect of Composition of Ternary Mixtures Containing Phosphate and Citrate Emulsifying Salts on Selected Textural Properties of Spreadable Processed Cheese. International Dairy Journal 2015, 44, 37–43.
- Martin, G.; Williams, R.; Dunstan, D. Comparison of Casein Micelles in Raw and Reconstituted Skim Milk. Journal of Dairy Science 2007, 90(10), 4543–4551.
- de Kort, E.; Minor, M.; Snoeren, T.; van Hooijdonk, T.; van der Linden, E. Effect of Calcium Chelators on Heat Coagulation and Heat-Induced Changes of Concentrated Micellar Casein Solutions: The Role of Calcium-Ion Activity and Micellar Integrity. International Dairy Journal 2012, 26(2), 112–119.
- Gaiani, C.; Banon, S.; Scher, J.; Schuck, P.; Hardy, J. Use of a Turbidity Sensor to Characterize Micellar Casein Powder Rehydration: Influence of Some Technological Effects. Journal of Dairy Science 2005, 88(8), 2700–2706.
- Chandan, R.C.; Kilara, A. Dry Milk Ingredients. In Dairy Ingredients for Food Processing; Chandan, R.C.; Kilara, A. Ed.; Iowa, USA: John Wiley and Sons Inc, 2011; 142–151.
- Fox, P.F.; McSweeney, P.L.H. Water in Milk and Dairy Products. In Dairy Chemistry and Biochemistry; Fox, P.F.; McSweeney, P.L.H.; Eds.;. London, UK: Blackie Academic & Professional, 1998; 308–309.
- Gaucher, I.; Piot, M.; Beaucher, E.; Gaucheron, F. Physico-Chemical Characterization of Phosphate-Added Skim Milk. International Dairy Journal 2007, 17(12), 1375–1383.
- Lamure, A.; Pommert, J.-F.; Klaebe, A.; Lacabanne, C.; Perie, J.-J. Effect of Polyphosphate Binding on the Chain Dynamic of Caseins: Investigation by Differential Scanning Calorimetry and Thermally Stimulated Currents. Journal of Dairy Research 1988, 55(03), 401–412.
- Lee, S.K.; Buwalda, R.; Euston, S.; Foegeding, E.; McKenna, A. Changes in the Rheology and Microstructure of Processed Cheese During Cooking. LWT-Food Science and Technology 2003, 36(3), 339–345.
- El-Bakry, M.; Duggan, E.; O’Riordan, E.; O’Sullivan, M. Effect of Chelating Salt Type on Casein Hydration and Fat Emulsification during Manufacture and Post-manufacture Functionality of Imitation Cheese. Journal of Food Engineering 2011, 102(2), 145–153.
- Straatsma, J.; Van Houwelingen, G.; Steenbergen, A.; De Jong, P. Spray Drying of Food Products: 2. Prediction of Insolubility Index. Journal of Food Engineering 1999, 42(2), 73–77.
- Fox, K.; Harper, M.K.; Holsinger, V.H.; Pallansch, M.J. Gelation of Milk Solids by Orthophosphate. Journal of Dairy Science 1965, 48(2), 179–185.
- Gaiani, C.; Mullet, M.; Arab-Tehrany, E.; Jacquot, M.; Perroud, C.; Renard, A.; Scher, J. Milk Proteins Differentiation and Competitive Adsorption during Spray-Drying. Food Hydrocolloids 2011, 25(5), 983–990.
- Kelly, G.M.; O’Mahony, J.A.; Kelly, A.L.; Huppertz, T.; Kennedy, D.; O’Callaghan, D.J. Influence of Protein Concentration on Surface Composition and Physico-Chemical Properties of Spray-Dried Milk Protein Concentrate Powders. International Dairy Journal 2015, 51, 34–40.
- Anema, S.G.; Pinder, D.; Hunter, R.; Hemar, Y. Effects of Storage Temperature on the Solubility of Milk Protein Concentrate (MPC85). Food Hydrocolloids 2006, 20(2), 386–393.
- Mimouni, A.; Deeth, H. C.; Whittaker, A. K.; Gidley, M. J.; Bhandari, B. R. Rehydration Process of Milk Protein Concentrate Powder Monitored by Static Light Scattering. Food Hydrocolloids 2009, 23(7), 1958–1965.