ABSTRACT
In this study, milk was hydrolyzed using protease (Asperigillus oryzae), trypsin, pepsin, or papain at concentrations of 0.001, 0.005, or 0.01 g/100 g milk for 30 or 60 min to produce angiotensin-converting enzyme inhibitory and antioxidant peptides. Results showed that the proteolysis, antioxidant, and ACE-I activity gradually increased with the increase in the enzyme concentration and hydrolysis time. The protease-treated milk had the highest proteolytic and ACE-I activity, while the papain-treated milk had the lowest. The papain-treated milk exhibited the greatest Fe2+ chelating activity. The use of trypsin at concentration of 0.001 g/100 g milk for 60 min produced ACE-I and antioxidant activity without changes in the technological properties of milk.
Introduction
Protein hydrolysis leads to change the functional, sensory, and nutritional properties of the foods including solubility, gelation, emulsifying, foaming, texture, flavor, reduction of protein allergy, and bioactive peptides liberation [Citation1–Citation4]. Enzymatic hydrolysis is an efficient and reliable method to produce peptides with antihypertensive, antithrombotic, antioxidant, anticancer, immunomodulatory, opioid, and anti-inflammatory activities. Digestive enzymes (pepsin, trypsin, and chymotrypsin) and enzymes of animal, microbial, or plant origin (papain, alcalase, flavourzyme, pronase, ficin, thermolysin, and neutrase) are used to break large polypeptides into specific small peptides that contain 2–20 amino acid units with molecular weights ranging from 500 to 1800 Da [Citation3,Citation5,Citation6,Citation7], and its activity depends upon its amino acid composition and sequence. Enzymatic hydrolysis is conducted under mild conditions which can be easily controlled and allows one to obtain products with well-defined features [Citation8] depending on the protein substrate [Citation9], protease type [Citation10], enzyme concentration [Citation11], hydrolysis time [Citation3], temperature, and pH [Citation12].
Bioactive peptides with antihypertensive and antioxidative activities can be used as a functional ingredient in foods to deliver to consumers. ACE plays an important role in blood pressure regulation via inactivating the vasodilator bradykinin and producing the potent vasoconstrictor octapeptide angiotensin II [Citation13,Citation14]. ACE-I inhibitory peptides derived from food proteins as a natural alternative to ACE inhibitor drugs have attracted particular attention for their ability to prevent hypertension and may have reduced toxic effects of chemosynthetic drugs in humans [Citation13]. Elimination of free radicals resulting from oxidation in food and biological systems is very important to prevent food deterioration and to provide protection against serious diseases such as cancer, coronary heart disease, and Alzheimer’s [Citation15]. Cytotoxicity and carcinogenicity of synthetic antioxidants have shown an increased tendency to choose natural antioxidant peptides that have potential health benefits with no or little side effects [Citation16].
Milk proteins are considered the most important source of bioactive peptides. Most studies obtained the bioactive peptides by enzymatic hydrolysis of casein [Citation17], whey proteins [Citation12], and their fractions, i.e., κ-casein [Citation18], β-casein [Citation19], αs1–casein [Citation20], αs2-casein [Citation21], β-lactoglobulin [Citation22], and α-lactalbumin [Citation23]. There are no published data on the production of bioactive peptides by enzymatic hydrolysis of whole milk. Although the enzymatic hydrolysis of proteins provides targeted desirable characteristics, it has some limitations such as bitterness, coagulation of casein by heat, and the higher production cost of pure bioactive peptides. Modification of the food functional properties would be economical interest as well as processing significance [Citation24]. The choice of enzymatic hydrolysis conditions must be realized, taking into account taste, heat stability, and specific application properties of the product [Citation2]. Consequently, the main objective of the present study was to choose the proper conditions for milk proteolysis to produce bioactive peptides without changes in the technological milk properties.
Materials and methods
Materials
Cow milk, free from antibiotics, was obtained from open nucleus herd belonging to the Cattle Information System of Egypt (CISE) and Technology Center of Agricultural Production, Faculty of Agriculture, Cairo University, defatted and sterilized at 121°C for 15 min. The chemicals included O-Phthaldialdehyde (OPA), sodium tetraborate, sodium dodecyl sulfate, 2-mercaptoethanol, hippuryl-L- histidyl-L-leucine (Hip-His-Leu), 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-p, p′-disulfonic acid monosodium salt hydrate (ferrozine), 1,1-diphenyl-2-picrylhydrazyl (DPPH), sodium tetraborate buffer HPCE (pH 8.0), ferrous chloride (FeCl2), ACE (0.25U) and ferric chloride (FeCl3) were obtained from Sigma-Aldrich (Egyptian International Centre for Import, Cairo, Egypt). Four proteolytic enzymes were used to hydrolyze the various milk proteins ().
Table 1. Characteristics of proteolytic enzymes used to hydrolyze skim milk.
Milk proteolysis
Sterilized milk was used to avoid the effect of proteolytic agents in raw milk, although the heat treatment of milk reduces the degree of proteolysis as a result of κ-casein–β-Lg complex formation at the surface of casein micelles [Citation25]. Sterilized skim milk was hydrolyzed using commercial enzymes that included protease (EC No. 232.642.4, Asperigillus oryzae, 3.5 units/mg), trypsin, pepsin, and papain at ratio 0.001, 0.005, or 0.01 g/100 g milk. Treated milk with protease, pepsin, or trypsin was kept at 37°C and at 50°C with papain at normal pH of milk. Treated milk was heated in a boiling water bath for 10 min to inactivate the enzymes after 30 and 60 min from enzyme addition. The proteolytic, antioxidant, ACE-I activity, titratable acidity, pH as well as sensory evaluation were determined.
Methods
Preparation of soluble nitrogen extract (supernatant) from milk
The extract was prepared according to Li [Citation26]. Milk (2 ml) was added to distilled water (1 ml) and mixed thoroughly, and then 12% (w/v) trichloroacetic acid (TCA) (5 ml) was added and mixed together. After 10 min standing, the mixture was centrifuged at 10000 xg for 30 min, and the supernatant was collected.
Proteolytic activity
The proteolytic activity of the milk hydrolysates was determined by reacting free amino acids with o-phthaldialdehyde (OPA), according to Luo [Citation3]. The OPA working reagent was prepared from 25 ml of 100 mM sodium tetraborate, 2.5 ml of 20% (w/w) sodium dodecyl sulfate, 40 mg of OPA, and 100 μl of β-mercaptoethanol. The volume was made up to 50 ml by adding distilled water. Briefly, 50 μl of the supernatant was mixed with 3 ml of an OPA working reagent, then vortexed for 5 s, and incubated at room temperature for 2 min. The absorbance of the mixture was measured at 340 nm using spectrophotometer (Jenway® Genova Life Science Spectrophotometer UV/Visible).
Angiotensin I-converting enzyme (ACE) inhibitory activity
The ACE inhibitory activity was determined by a spectrophotometric method described by Luo [Citation3]. 50 μl sample of the supernatant was mixed with an equal volume of an ACE solution (0.25U). Following incubation at 37°C for 10 min, 150 μl of a hippuryl-L-histidyl-L-leucine (HHL) substrate solution was added that was previously prepared at 8.3 mM HHL in 50 mM sodium borate buffer containing 0.5 M NaCl at pH 8.3. The mixture was then incubated at 37°C for 60 min, followed by termination of the reaction by adding 250 μl of 1 N HCl. To extract hippuric acid, 1.4 ml of ethyl acetate was added to the mixture that was vortexed for 5 s and centrifuged at 14,100 g for 5 min. Then, 1 ml of the upper organic phase was transferred into glass vials. The ethyl acetate in the glass vials was evaporated at room temperature for 1.5 h in a vacuum oven. After the addition of 2 ml distilled water to dissolve the extracted hippuric acid, the absorbance was measured at 228 nm using the spectrophotometer. The control sample was prepared by replacing the test sample with distilled water. The inhibition (%) was calculated as follows:
where Acontrol and Asample are the absorbance values of the control and test sample, respectively.
Antioxidant activity properties
1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH radical scavenging activity of hydrolysates was determined according to the method described by Li [Citation26]. The supernatant (1 ml) was mixed with 0.1 mmol /l DPPH (1 ml) dissolved in 95% ethanol. The mixture was shaken and left for 30 min at room temperature. Absorbance of the resulting solution was measured at 517 nm. Distilled water was used as blank instead of the sample. The scavenging activity was calculated using the following equation:
where, A0 = The absorbance at 517 nm of blank; As = The absorbance at 517 nm of supernatant.
Reducing power
The reducing power of samples was tested according to Luo [Citation3]. One millilitre of the supernatant was mixed with 1 ml of 0.2 M phosphate buffer saline (pH 6.6) and 1 ml of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min, followed by cooling to room temperature. After adding 2 ml of 10% trichloroacetic acid, the mixture was mixed with 0.3 ml of a 0.1% ferric chloride solution. Following incubation for 10 min at room temperature, the absorbance of the resulting solution was measured at 700 nm. The extent of absorbance increase was used to indicate the reducing power of a sample.
Metal ion chelating activity
The chelating activity of the samples was estimated by the ferrozine method [Citation3]. One ml of the supernatant was mixed with 1 ml distilled water and 50 μl of 2.0 mM FeCl2, followed by resting at room temperature for 30 s. After adding 0.1 ml of 5 mM ferrozine, vortexing and incubation at room temperature for 10 min, the absorbance was measured at 562 nm. The control was determined similarly by replacing the supernatant with distilled water. The chelating activity was calculated as follows:
where Asample and Acontrol are the absorbance of test sample and control, respectively.
Physico-chemical properties
The pH values were measured using a digital pH meter with a glass electrode (Jenway 3305, England). Titratable acidity was determined by titration with 0.1N NaOH using phenolphthalein as an indicator [Citation27]. Clot-on-boiling was examined according to Ghatak [Citation28]. The panel of sensory evaluation was monitored according to ISO standards (8586-1: 1993) by 10 panellists. Each sample of milk hydrolysate was evaluated for color and flavor [Citation29].
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). A randomize complete block design and analysis of variance of factorial methods were carried out using Mstat-C [Citation30]. All data were analyzed in three replications for each parameter. The least significant differences (L.S.D.) test was calculated to compare the significant differences between the mean of different treatments [Citation31]. Results were considered statistically significant at P ≤ 0.05.
Results and discussion
Proteolytic activity of proteases in milk
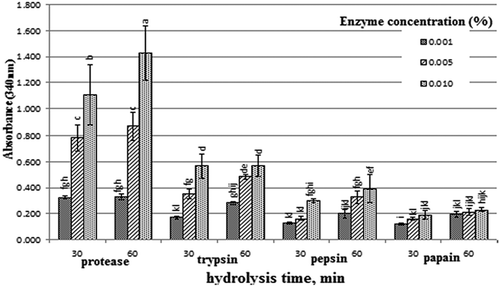
Proteolysis has been described by analyzing the rate of hydrolysis expressed as free amino groups and peptides concentration using OPA method. The results of proteolytic activity for protease, trypsin, pepsin, and papain in milk are shown in . From the obtained results, at the same concentration and the same time of hydrolysis, the proteolytic activity reached the maximum with protease treatment followed by trypsin, pepsin, and finally papain at the studied conditions. These results may be due to the type of used enzyme which attributed to the difference in enzyme cleavage specifity and the number of such sites present in the milk protein [Citation14]. The results in the same showed that the proteolytic activity increased gradually with the increase of hydrolysis time and the enzyme concentration. In this respect, Nurfatin et al. [Citation14] found that the degree of proteolysis of edible bird nest depended on the type of enzyme, the cutting site of enzymes, and the reaction time. Also, they reported that at a given hydrolysis time (30–240 min), the degree of hydrolysis values of the alcalase digestion were significantly higher than those of the papain treatment. Shanmugam [Citation32] found that proteolysis degree of casein varied according to the type of enzyme where it was 7.1, 7.9, 11.2, 14.8, 13.2, 16.7, and 17.6% for casein hydrolyzed by pepsin, trypsin, chymotrypsin, pepsin-trypsin, pepsin-chymotrypsin, trypsin-chymotrypsin, and pepsin-trypsin-chymotrypsin, respectively.
ACE-I activity of milk protein treated by proteolytic enzymes
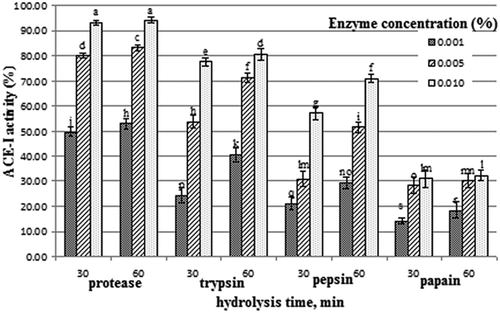
ACE-I activity depends on the peptide amino acid composition and sequence which are related to the specificity of the used enzyme and the hydrolysis conditions [Citation5,Citation33]. ACE-I activity assay was carried out relying on hydrolysis of hippuryl-L-histidyl-L-leucine by ACE [Citation7]. The ACE-I activity of milk treated by the used proteolytic enzymes is presented in . Results indicated that the ACE-I activity of milk treated by the proteolytic enzymes increased significantly with the increase of hydrolysis time and enzyme concentration. However, protease-treated milk had the significantly highest ACE-I activity followed by trypsin-, pepsin-, and papain-treated milk at all studied conditions. This is probably due to the higher proteolytic activity of protease. Our results are supported by Luo [Citation3] who found that the ACE-inhibitory activities of sodium casienate treated by papain and pancreatin increased gradually with the increase of hydrolysis time, while the treatment by trypsin reached a plateau after 1 h of hydrolysis. Also, Ming [Citation34] obtained ACE-I peptides from casein by digesting with pepsin and trypsin for 3 h each. Tavano [Citation2] reviewed that the most effective ACE-inhibitory peptides contain hydrophobic (aromatic or branched side chains) amino acids at C-terminal positions or are positively charged by Lys (ε-amino group) and Arg (guanidine group) as the C-terminal residue. In this respect, Ferreira [Citation35] found that the peptide consisted of Ala-Leu-Pro-Met-His-Ile-Arg exhibited ACE-I activity when the whey protein was hydrolyzed by trypsin. Also, Welderufael [Citation36] isolated and identified a tripeptide (Ile–Pro–Pro) and an octapeptide (Gln–Asp–Lys–Thr–Glu–Ile–Pro–Thr) from casein fractions hydrolyzed by the protease from Bacillus subtilitis. Statistically, there was a great positive correlation (r = 0.89) between ACE-I activity and proteolytic activity for all treatments at studied conditions.
Antioxidant activity of milk protein treated by proteolytic enzymes
In this study, three of methods were used to evaluate the antioxidant activity of the milk protein hydrolyzed by the proteolytic enzymes including DPPH radical scavenging, metal ion chelating, and reducing power assay.
DPPH radical scavenging activity
This method based on DPPH radical in ethanol encountering a proton-donating antioxidant and the radical would then be scavenged, reducing the absorbance at 517 nm [Citation37]. DPPH radical scavenging activity of milk treated by different proteolytic enzymes is tabulated in . From the obtained results, the DPPH radical scavenging activity improved gradually with the increase in the hydrolysis time and the concentration of used enzymes. Among the used proteolytic enzymes, papain-treated milk had the significant greatest DPPH radical scavenging activity except at concentration of 0.01 g/100 g milk after 60 min of hydrolysis where the protease-treated milk was the highest significance. On the other hand, the pepsin-treated milk exhibited the significant lowest activity except at the concentration of 0.005 and 0.01 g/100 g milk after 30 min of hydrolysis where the trypsin-treated milk had the significant lowest activity. Kumar [Citation38] found that DPPH radical scavenging activity of cow caseinate hydrolyzed by pepsin, trypsin, and chymotrypsin varied widely along with hydrolysis time (15–150 min), and a relationship between hydrolysis time and DPPH activity could not be established; however, the higher DPPH-scavenging activity was evidenced after 1 h of hydrolysis. Zhao [Citation10] interpreted the differences in radical scavenging activity to the differences in the amino acid compositions and their sequences as well as the peptide level resulting from the specificity of the used enzymes. Therefore, the presence of several amino acid residues in the peptide chain can increase antioxidative property as a result of the additive effects in terms of electron transfer to the free radicals [Citation3].
Table 2. DPPH radical scavenging activity of skim milk during hydrolysis with different proteases.
Reducing power
Reducing power method depends on the peptide ability to reduce the ferric (Fe3+)/ferricyanide complex to the ferrous (Fe2+) ion through the donation of an electron resulting ferrous ion (Fe2+) monitored spectrophotometrically at 700 nm [Citation10]. The reducing power of proteolytic enzymes-milk treatments is illustrated in . From the obtained results, the reducing power differed under all studied conditions of enzymatic hydrolysis where it increased with the increase of hydrolysis time and enzyme concentration. This is attributed to the increment of availability of protons and electrons resulting from enzymatic hydrolysis [Citation3]. The differences in the reducing power may be imputed to the composition and sequence of amino acids in the resultant peptides where the hydrolysates with a high level of peptide possessed a strong antioxidant activity. These peptides react with free radical to form more stable products. These results seem to agree with the study of Luo [Citation3] who found that the reducing power of sodium caseinate (NaCas) improved under all enzymatic hydrolysis conditions and it was more significant at longer hydrolysis time.
Table 3. The reducing power of skim milk during hydrolysis with different proteases.
The results in the same showed that there was different reducing power among the used enzymes. At enzyme concentration of 0.001 g/100 g milk after 30 or 60 min of hydrolysis, the pepsin- or protease-treated milk was significantly higher than trypsin- or papain-treated milk in the reducing power. At enzyme concentration of 0.005 g/100 g milk after 30 or 60 min of hydrolysis, there was no significant difference in the reducing power between all used enzymes. At enzyme concentration of 0.01 g/100 g milk after 30 min of hydrolysis, trypsin-treated milk had the significant highest reducing power followed by papain, and there was no significant difference between pepsin and protease. At enzyme concentration of 0.01 g/100 g milk after 60 min of hydrolysis, there was no significant difference in the reducing power between all used enzymes. Luo [Citation3] observed different reducing power among the used enzymes (papain, pancreatin, and trypsin) and reported that NaCas treated by pancreatin had the strong reducing power.
Metal ions chelating activity
In this method, the transition metals play a critical role in the production of free radicals which initiate autoxidation. Among metal ions, ferrous ions cause oxidative damage in cells which lead to lipid peroxidation [Citation39]. The disintegration of ferrozine/Fe2+ complex and thus the reduction of a violet color were used as an indicator to evaluate the Fe2+ chelating activity of hydrolysates [Citation40]. As shown in , the metal ions chelating activities of milk hydrolysates by all used enzymes increased significantly with the increase in the duration of hydrolysis and enzyme concentration. The Fe2+ chelating activity of papain-milk treatment was significantly stronger than other treatments at the studied conditions. However, the trypsin-milk treatment showed less activity at 30 min of hydrolysis. After 60 min of hydrolysis, the protease-milk treatment at concentration of 0.001 g/100 g milk had the lowest activity, while the pepsin-milk treatment at concentration of 0.005 g/100 g milk had the highest activity. The Fe2+ chelating ability of milk hydrolyzed by the used enzymes could be attributed to that peptide cleavage caused an enhanced metal ion binding as a result of an increased concentration of carboxylic groups and amino groups in branches of the acidic and basic amino acids, thus removing prooxidative free metal ions from the hydroxyl radical system. The direct relationship between proteolytic activity and the increase in the chelating capability supported this premise [Citation37]. Luo [Citation3] found that chelating activity of NaCas hydrolyzed by pancreatin, trypsin, and papain decreased at longer hydrolysis time (10 min to 24 h).
Table 4. The Fe2+ chelating activity of skim milk during hydrolysis with different proteases.
The correlation between antioxidant activity by three ways and the proteolytic activity of all treatments under studied conditions was calculated. Positive correlations (r = 0.24–0.56) were observed between the antioxidant and proteolytic activity at different conditions. In this respect, Liu [Citation37] found that the antioxidant activity increased with increasing degree of hydrolysis.
Effect of partial enzymatic proteolysis on selected physico-chemical and sensory properties of milk
Proteolysis of milk reduces the heat stability of milk protein via the acid production during the hydrolysis and the casein micelles destabilization. Furthermore, the proteolysis of milk leads to release of tyrosine, which can be an indicator of milk organoleptic quality. The correlation between the tyrosine levels and bitterness flavor is strong [Citation41]. Therefore, this study is interested in the selection of the proper conditions of proteolysis by enzymes to produce bioactive peptides without changes in milk technological and sensory properties. The results listed in showed that titratable acidity and pH were changed among the used enzymes at all studied conditions where pH decreased gradually with the increase of the enzyme concentration and hydrolysis time, while titratable acidity was vice versa. These results may be attributed to protein digestion leading to break the peptide bonds and releasing H+ that causes decreases in pH value [Citation42]. Our findings also indicated that protease-milk treatment had positive clot-on-boiling (COB) at all concentrations after 30 min of proteolysis, while trypsin- and pepsin-milk treatment showed positive COB at concentration of 0.01 and 0.005 g/100 g milk after 30 min of proteolysis. However, the papain-milk treatment exhibited negative COB at all concentrations after 30 or 60 min of proteolysis. These observations may be due to the fact that the proteolysis decreased the pH leading to protein destabilization, and it can expose hydrophobic peptides which increase the peptides aggregation causing decrease of solubility and heat stability [Citation9,Citation37].
Table 5. Technological properties of skim milka hydrolyzed by proteolytic enzymes.
Regarding the flavor, bitterness appeared in the protease-milk treatment at concentration of 0.005 and 0.01 g/100 g milk after 30 and 60 min of hydrolysis, while the trypsin- and pepsin-milk treatment showed bitter flavor at concentration of 0.01 g/100 g milk after 60 min of hydrolysis. On the contrary, the papain-milk treatment did not exhibit any change in flavor at all studied conditions because of the decrease in the proteolysis. As for the color, there was no change in the color of milk treated by all proteolytic enzymes at all concentrations during the hydrolysis time.
Conclusion
The present work reveals that the use of trypsin for the limited milk proteolysis at concentration of 0.001 g/100 g milk for 60 min of hydrolysis is a suitable route and more effective to obtain peptides which possess different ACE-I and antioxidant activity with maintaining the milk properties and economical costs. Based on our findings, it is possible to provide opportunities for dairy industry application aiming at increasing the health benefits of functional dairy products, particularly those that are independent on the fermentation.
References
- Fernández, A.; Riera, F. β –lactoglobulin Tryptic Digestion: A Model Approach for peptide release. Biochemical Engineering Journal 2013, 70, 88–96.
- Tavano, O.L. Protein Hydrolysis using Proteases: An Important Tool For Food Biotechnology. Journal of Molecular Catalysis B: Enzymatic 2013, 90, 1–11.
- Luo, Y.; Pan, K.; Zhong, Q. Physical, Chemical and Biochemical Properties of Casein Hydrolyzed by Three Proteases: Partial Characterizations. Food Chemistry 2014, 155, 146–155.
- McSweeney, P.L.H.; O’Mahony, J.A. Advanced Dairy Chemistry; Springer Science+Business Media: New York, USA, 2016.
- Korhonen, H. Milk-derived Bioactive Peptides: From Science to Applications. Journal of Functional Foods 2009, 1, 177–187.
- Najafian, L.; Babji, A.S. Production of Bioactive Peptides using Enzymatic Hydrolysis and Identification Antioxidative Peptides from patin (Pangasius sutchi) Sarcoplasmic Protein Hydolysate. Journal Functional Foods 2014, 9, 280–289.
- Daud, N.A.; Babji, A.S.; Yusop, S.M. Effects of Enzymatic Hydrolysis on the Antioxidative and Antihypertensive Activities from Red Tilapia fish protein. Journal of Nutrition and Food Sciences 2015, 5, 1–5.
- Zambrowicz, A.; Polanowski, A.; Timmer, M.; Lubec, G.; Trziszka, T. Manufacturing of Peptides Exhibiting Biological Activity. Amino Acids 2013, 44, 315–320.
- de Castro, R.J.S.; Sato, H.H. Comparison and Synergistic Effects of Intact Proteins and their Hydrolysates on the Functional Properties and Antioxidant Activities in a Simultaneous Process of enzymatic Hydrolysis. Food and Bioproducts Processing 2014, 92, 80–88.
- Zhao, Q.; Selomulya, C.; Wang, S.; Xiong, H.; Chen, X.D.; Li, W.; Peng, H.; Xie, J.; Sun, W.; Zhou, Q. Enhancing the Oxidative Stability of Food Emulsions with Rice Dreg Protein Hydrolysate. Food Research International 2012, 48, 876–884.
- Yao, J.; Lin, C.; Tao, T.; Lin, F. The Effect of Various Concentrations of Papain on the Properties and Hydrolytic Rates of β-casein Layers. Colloids and Surfaces B: Biointerfaces 2013, 101, 272–279.
- Le Maux, S.; Nongonierma, A.B.; Barre, C.; FitzGerald, R.J. Enzymatic Generation of Whey Protein Hydrolysates under pH-controlled and non pH-controlled Conditions: Impact on Physicochemical and Bioactive Properties. Food Chemistry 2016, 199, 246–251.
- Campos, M.R.S.; González, F.P.; Guerrero, L.C.; Ancona, D.B. Angiotensin I-converting enzyme inhibitory peptides of chia (Salvia hispanica) produced by Enzymatic Hydrolysis. International Journal of Food Sciences 2013, 1–8.
- Nurfatin, M.H.; Syarmila, E.I.K.; Nur‘Aliah, D.; Zalifah, M.K.; Babji, A.S.; Ayob, M.K. Effect of Enzymatic Hydrolysis on Angiotensin Converting Enzyme (ACE) Inhibitory Activity in Swiftlet Saliva. International Food Research Journal 2016, 23, 141–146.
- Luo, H.; Wang, B.; Li, Z.; Chi, C.; Zhang, Q.; He, G. Preparation and Evaluation of Antioxidant Peptide From Papain Hydrolysate of Sphyrna Lewini Muscle Protein. LWT-Food Science and Technology 2013, 51, 281–288.
- Sarmadi, B.H.; Ismail, A. Antioxidative Peptides from Food Proteins: A Review. Peptides 2010, 31, 1949–1956.
- Espejo-Carpio, F.J.; García-Moreno, P.J.; Perez-Galvez, R.; Morales-Medina, R.; Guadix, A.; Guadixalvez, E.M. Effect of Digestive Enzymes on the Bioactive Properties of Goat Milk Protein Hydrolysates. International Dairy Journal 2016, 54, 21–28.
- Kawasaki, Y.; Isoda, H.; Tanimoto, M.; Dosako, S.; Idota, T.; Ahiko, K. Inhibition by Lactoferrin and κ-casein Glycomacropeptide of Binding of Cholera Toxin to its Receptor. Bioscience Biotechnology and Biochemistry 1992, 56, 195–198.
- Teschemacher, H.; Koch, G.; Brantl, V. Milk Protein-Derived Opioid Receptor Ligands. Biopolymers 1997, 43, 99–117.
- Lahov, E.; Regelson W. Antibacterial and Immunostimulating Casein-derived Substances from Milk: Casecidin, Isracidin Peptides. Food and Chemical Toxicology 1996, 34, 131–145.
- Zucht, H.; Raida, M.; Adermann, K.; Mägert, H.; Forssmann W. Casocidin-I: a casein-αs2 Derived Peptide Exhibits Antibacterial Activity. FEBS Letters 1995, 372, 185–188.
- Hernández-Ledesma, B.; Recio, I.; Ramos, M.; Amigo, L. Preparation of Ovine and Caprine β-lactoglobulin Hydrolysates with ACE-inhibitory Activity. Identification of Active Peptides from Caprine β-lactoglobulin Hydrolyzed with Thermolysin. International Dairy Journal 2002, 12, 805–812.
- Pellegrini, A. Antimicrobial Peptides from Food Proteins. Current Pharmaceutical Design 2003, 9, 1225–1238.
- Qian, L.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant Activity and Functional Properties of Porcine Plasma Protein Hydrolysate as influenced by the Degree of Hydrolysis. Food Chemistry 2010, 118, 403–410.
- García-Risco, M.R.; Ramos, M.; López-Fandiño, R. Modifications in Milk Proteins Induced by Heat Treatment and Homogenization and their Influence on Susceptibility to Proteolysis. International Dairy Journal 2002, 12, 679–688.
- Li, Y.; Liu, T.; He, G. Antioxidant Activity of Peptides from Fermented Milk with Mix Culture of Lactic Acid Bacteria and Yeast. Advanced Journal of Food Science and Technology 2015, 7, 422–427.
- A.O.A.C. Official Methods of Analysis; Association of Official Analytical Chemists USA: Washington, D.C., 2000.
- Ghatak, P.K.; Bandyopadhyay, A.K. Practical Dairy Chemistry; Kalyani Publishers: New Delhi, 2007; 291 p.
- Wróblewska, B.; Troszyñska, A. Enzymatic Hydrolysis of Cow’s Whey Milk Proteins in the Aspect of their Utilization for the Production of Hypoallergenic Formulas. Polish Journal of Food and Nutrition Sciences 2005, 14/55, 349–357.
- Mstat-c. Users guide: A Microcomputer Program for the Design, Management and Analysis of Agronomic Research Experiments; Michigan University: East Lansing, MC, USA, 1989.
- Snedecor, G.A.; Cochran, W.G. Statistical Method; Iowa State University Press: Ames, IA, USA, 1967.
- Shanmugam, V.P.; Kapila, S.; Sonfack, T.K.; Kapila, R. Antioxidative Peptide Derived from Enzymatic Digestion of Buffalo Casein. International Dairy Journal 2015, 42, 1–5.
- van der Ven, C.; Gruppen, H.; de Bont, D.B.A.; Voragen, A.G.J. Optimization of the Angiotensin Converting Enzyme Inhibition by Whey Protein Hydrolyzates using Response Surface Methodology. International Dairy Journal 2002, 12, 813–820.
- Ming, Y.; Zhi-he, H. Separation and Purification of ACE Inhibitory Peptides from Casein Hydrolysate by Two Enzymes. Food Science 2012, 33, 50–53.
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Mota, M.V.; Tavares, P.; Pereira, A.; Goncalves, M.P.; Torres, D.; Rocha, C.; Teixeira, J.A. Preparation of Ingredients Containing an ACE Inhibitory Peptide by Tryptic Hydrolysis of whey Protein Concentrates. International Dairy Journal 2007, 17, 481–487.
- Welderufael, F.T.; Gibson, T.; Jauregi, P. Production of Angiotensin-I Converting Enzyme Inhibitory Peptides from β-lactoglobulin- and Casein Derived Peptides: An integrative approach. Biotechnology Progress 2012, 28, 746–755.
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xi, X. Antioxidant Activity and Functional Properties of Porcine Plasma Protein Hydrolysateas influenced by the Degree of Hydrolysis. Food Chemistry 2010, 118, 403–410.
- Kumar, S.; Teotia, U.V.S.; Sanghi, A. Antioxidative Property of Cow Milk Caseinates Hydrolyzed with different Proteases. International Journal of Pharmacy and Pharmaceutical Sciences 2013, 5, 418–422.
- Huang, D.; Ou, D.; Hampsch-Woodi, M. Development and Validation of Oxygen Radical Absorbance Capacity Assay for Lipophilic Antioxidants using Randomly methylated β-cyclodextrin as the Solubility Enhancer. Journal of Agricultural and Food Chemistry 2002, 50, 1815–1821.
- Wu, H.C.; Chen, H.M.; Shiau, C.Y. Free Amino Acids and peptides as related to Antioxidant Properties in Protein Hydrolysates of mackerel (Scomber austriasicus). Food Research International 2003, 36, 949–957.
- Chen, L.; Daniela, R.M.; Coolbear, T. Detection and Impact of Protease and Lipase Activities in Milk and Milk Powders. International Dairy Journal 2003, 13, 255–275.
- Aluko, R.E. Bioactive Peptides Functional Foods and Nutraceuticals; Food Science Text Series, Springer Science+Business Media, LLC: NewYork, USA, 2012.