ABSTRACT
Physicochemical and antioxidant properties of raw honeys from Malaysia were used as markers for determining its entomological source of bee species of Apis dorsata, Apis mellifera, Apis cerana, or Heterotrigona itama. Physicochemical properties of moisture content, water activity, specific gravity, viscosity, pH, free acidity, electrical conductivity, colour (L*, a* and b*), colour intensity, and antioxidant properties including the DPPH free radical scavenging activity power (1/IC50), ascorbic acid equivalent antioxidant content (AEAC), ferric ion reducing antioxidant power (FRAP), and total phenolic content (TPC) were measured and analysed. Honeys were classified into two major groups of those from honey bees (Apis spp.) and Trigona stingless bees (Heterotrigona itama) from its physicochemical and antioxidant properties using hierarchical cluster and principal component analyses. The Kelulut honey produced by stingless bees, Heterotrigona itama was differentiable from honeys from the regular honey bee species, the Apis spp. with characteristics of high moisture content of 33.24 g/100 g, free acidity of 136.8 meq/kg, colour intensity of 990.3 mAU, AEAC of 26.64 mg/100 g, and FRAP of 41.95 mg AAE/100 g. Honey classification by its entomological origin helps in honey identification and it reduces honey fraudulence.
Introduction
Honey is obtained from two types of beekeeping in Malaysia, which are the commercial kind of Apiculture using honey bees (Apini) and Meliponiculture using stingless bees (Meliponini). Both bee types are highly eusocial corbiculate bees from the same bee family of Apidae. Honey bees consist of 11 species in the single genus Apis with Apis mellifera as the most widespread species in the world. There are more than 500 species (about 60 genera) of stingless bees and they are mostly found in the tropical and subtropical regions like Central and South America, Australia and Southeast Asia.[Citation1,Citation2] Honey bees build hexagonal-shaped combs with wax in the nests and the honey produced is known as comb honey. The stingless bees construct horizontal pots made of cerumen, a mixture of propolis and wax for their nests to store honey and honey produced is known as pot honey.[Citation3] The honey bees produce higher yields than the stingless bees, thus the Apis mellifera are more commonly reared to produce honey.[Citation3] Although the stingless bees produce less amount of honey, their honey fetches higher price than those from Apis honey bees. The price of stingless bee honey reaches up to ten times of the price of honey from Apis mellifera in tropical Africa, Colombia and Bolivia due to beliefs of stingless bee honey has higher medicinal features and healing qualities.[Citation4]
Honey is not only used as sweetener and flavour enhancer. It is also consumed as food and drinks, and used as ingredients for bakery, beauty, and health products. Honey provides a good source of natural antioxidants which are effective in reducing risk of heart disease, cancer, immune-system decline, cataracts, and different inflammatory process.[Citation5–Citation7] The antioxidant properties of stingless bee honey have raised the interest for many studies which evidenced that stingless bee honey has good antioxidant capacities. From the measurement of total phenolic compounds, flavonoids, 1,1-diphenyl-2-picryhydrazyl (DPPH) radical scavenging activity, ferric reducing antioxidant power (FRAP), 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical activity, β-carotene bleaching activity, and oxygen radical absorbance capacity (ORAC),[Citation2,Citation8–Citation11] a wide variation of antioxidant properties has been reported for honeys from the different stingless bee species. The high market value of honey for its therapeutic effect has invited various honey fraudulence issues such as its substitution with low-valued honey and other sugars as food ingredients or mislabeling of its source and origin to gain a higher selling price.[Citation12]
Physicochemical properties of honey are important parameters to be known. For example, colour of honey affects its price, its moisture helps in stability analysis, and its viscosity is needed for design of processing equipment. These properties are often used to determine the quality of honey.[Citation13] Vit et al.[Citation4] stated that physicochemical characteristics are varied following bee species and floral resources while the physicochemical properties of honey from stingless bees from Brazil[Citation2,Citation8,Citation9,Citation14], Thailand[Citation1,Citation15] and Australia[Citation16] have been reported. The physicochemical properties are used as parameters in honey authentication studies including determination of its botanical and geographical origins and detection of unauthorised substances.[Citation13] In recent years, improvement in the determination of botanical and geographical origins of honey was approached using multivariate analysis which is also known as the pattern recognition methods via principal component analysis (PCA), discriminant analysis (DA) and cluster analysis (CA).[Citation17–Citation22] However, work on determination of honey entomological origin is limited except for Duarte et al.[Citation8] who classified honeys into the bee species of Apis mellifera, Melipona spp., and Plebeia spp. using physiochemical and antioxidant properties, Silva et al.[Citation23] who classified honey into Apis mellifera and Melipona spp. using its physicochemical properties and minerals, Kek et al.[Citation24] who classified honey into groups of Apis and Trigona bees by its chemical profiles and minerals, and Vit et al.[Citation25] and Zuccato et al.[Citation26] who discriminated the entomological origin of honey by using the 1H NMR-based metabolomics approach. With stingless bee honey growing into high popularity, more studies on it is needed to find out suitable properties that can serve as parameters for determining honey origin in the effort of protecting consumers against honey frauds. In addition, data of stingless bee honey properties can contribute to build up its international quality standardisation as the properties of stingless bee honey such as moisture content, electrical conductivity, free acidity, and enzyme activity are different as compared to honey produced by Apis mellifera that are currently regulated in the Codex Standard for Honey.[Citation1,Citation3,Citation10]
The study has evaluated the physicochemical and antioxidant properties of honey from two major groups, i.e. those from honey bees (Apis spp.) which included the Apis dorsata, Apis mellifera, and Apis cerana and the Trigona stingless bees, Heterotrigona itama. Honeys produced by the different bee species were identified and classified into their entomological origin. Differentiation of the stingless bee honey from the more established Apis bee honey could help in identifying honey origin and safeguard consumers in terms of quality assurance and its authenticity.
Materials and methods
Honey
This research has used five types of Malaysian raw honeys which were produced by four different bee species originating from the honey bee and stingless bee species. All the honey samples were obtained directly from the beekeepers or honey collectors in two batches for the Tualang, Gelam, Pineapple, and Borneo honeys except for the Kelulut honey in three batches over the span of two seasons at least from January 2013 to March 2014. The Tualang honey, harvested in Tasik Pedu, Kedah is produced by Apis dorsata bees which collect nectar from plants and blossoms and build their hives in the branches of tall Tualang (Koompassia excelsa) trees. The Gelam honey was collected from Merchang, Terengganu, is also produced by Apis dorsata bees which collected the nectar from the Gelam (Melaleuca cajupati) trees. The Pineapple honey was from Rengit, Johor and produced by Apis mellifera bees that collect nectar from the pineapple (Ananas comosus) flowers. The Borneo honey was collected from Kudat, Sabah and sourced from Acacia mangium trees and other flowers by the Apis cerana bees. The Kelulut honey was harvested from the forest in Teluk Intan, Perak is produced by the Heterotrigona itama stingless bees which the nectar source of this Kelulut honey is mainly from Acacia mangium trees and other flowers. Conmmercialised honey used for comparisons were the Manuka honey (MGO 120+, Berringa, Australia) and two randomly selected honeys from a local supermarket (Commercial Y and Commercial Z). lists the total number of honey samples from the five types of raw honeys and three different commercial honey samples (Manuka, Commercial Y, and Commercial Z) used for physicochemical and antioxidant properties studies.
Table 1. Physicochemical properties of various honey samples.
Physicochemical properties analysis
Moisture content
Moisture content of honey samples was measured using the oven method following AOAC Official Method 925.45.[Citation27] Five grams of honey in stainless steel flat dish with tight-fit-cover was heated at 105°C for 24 hours in a convection oven (UM500, Memmert GmbH, Schwabach, Germany). Moisture content was determined in unit of g/100 g honey.
Water activity
Water activity (aw) of honey samples was measured at room temperature by using a water activity meter (Fast-lab, GBX, Bourg de Peage, France).
Specific gravity
Specific gravity of honey samples was obtained as the ratio of the weight of a pycnometer (50 mL) filled with honey to the weight of the same pycnometer filled with water.
Viscosity
Viscosity of honey samples was measured using a rheometer (AR-G2, TA Instruments, New Castle, USA) with a 2° and 40 mm diameter cone-plate geometry fixed at a truncation gap of 56 µm. A shear stress versus shear rate curve was obtained in the range of shear rates from 0 to 100 s−1 for a steady-state flow experiment at 25°C which followed the method described by Trávníček et al.[Citation28] The viscosity was determined by the slope of the shear stress versus shear rate curve in unit of Pascal second (Pa·s).
pH and free acidity
The pH and free acidity of honey samples were determined based on AOAC Official Method 962.19.[Citation27] Ten grams of honey was dissolved in 75 mL of distilled water and pH was measured at room temperature by using a pH meter (SevenMulti™ S47, Mettler Toledo, Switzerland). The honey solution was subsequently titrated with 0.1 M NaOH to pH 8.30 and the amount of NaOH used was recorded. Free acidity of honey was determined as 10 times the volume (mL) of 0.1 M NaOH used in titration and expressed as miliequivalent acid per kg of honey (meq/kg).
Electrical conductivity
Electrical conductivity of 20% (w/v) honey solution on a dry matter basis was measured using a conductivity meter (SevenMulti™ S47, Mettler Toledo, Switzerland), following Harmonised Methods of the International Honey Commission.[Citation29] Results were expressed as milliSiemens per centimetre (mS/cm).
Colour (L*, a* and b*) and colour intensity
Honey samples were filled in an optically clear glass cell and their colour were measured using a Hunter Lab spectrophotometer (UltraScan PRO, Hunter Associates Laboratory, Inc., VA, USA) with D65 illumination, diffuse/8° geometry, and 10° observer. Colour of honey was reported in the International Commission on Illumination (CIE) L*a*b* coordinates where L* is lightness, a* is red/green (+a*/-a*), and b* is yellow/blue (+b*/-b*).
Colour intensity of honey samples was determined based on the net absorbance using the method of Beretta et al.[Citation30] Honey was diluted to 50% (w/v) with warm water (45–50°C) and the solution was filtered to remove any coarse particles. The absorbance was read at 450 nm and 720 nm using a UV-VIS spectrophotometer (Ultrospec 3100 pro, Amersham Biosciences, NJ, USA), and the difference in the absorbance readings was expressed as milli absorbance unit (mAU).
Antioxidant properties analysis
Free radical scavenging activity power and antioxidant content
Free radical scavenging activity of honey samples was determined using 1,1-diphenyl-2-picryhydrazyl (DPPH) assay with minor modification.[Citation31–Citation33] The DPPH stock solution was prepared by dissolving 24 mg of DPPH with 100 mL of methanol. A 10 mL DPPH stock solution was further diluted with 45 mL of methanol and this DPPH working solution’s absorbance was adjusted to 1.1 at 515 nm. The DPPH working solution (100 µL) was added into the 96-well plate containing 100 µL of honey solution (1.563–1000 mg/mL). After incubation in the dark for 30 minutes, the absorbance was measured at 515 nm using a microtiter-plate reader (µQuant, Bio-tek Instruments, Inc., Vermont, USA). The DPPH free radical scavenging activity was calculated in percentage using the formula DPPH = [(Acontrol – Asample)/Acontrol] × 100, where Acontrol and Asample are the absorbance readings of control and sample, respectively. The DPPH free radical scavenging activity was expressed as IC50, which corresponds to the concentration of sample required to inhibit 50% of DPPH free radicals. IC50 was determined graphically from the curve plot between the percentages of DPPH scavenging activity and sample’s concentrations. For better clarity, IC50 was transformed into the reciprocal values (1/IC50) to indicate the power of DPPH free radical scavenging activity.
The results were also expressed as AEAC in mg AEAC/100 g of honey which represents the antioxidant content of honey as described by Meda et al.[Citation33] The antioxidant content was determined by interpolating the absorbance reading obtained from the mixture of 100 µL of honey solution (0.1 g/mL) and 100 µL of DPPH working solution against the ascorbic acid standard curve (1.563–25 µg/mL). The AEAC was calculated using Eq. (1).
where CAA is concentration of ascorbic acid from the standard curve (mg/mL), V is volume of honey solution (mL), and DF is dilution factor.
Ferric ion reducing antioxidant power
FRAP was determined based on the method described by Aljadi and Kamaruddin[Citation34] and Lachman et al.[Citation6] with minor modification. The FRAP reagent was prepared by mixing 2.5 mL of 10 mM TPTZ (2,4,6-tripyridyl-s-trizine) solution in 40 mM HCl, 2.5 mL of 20 mM ferric chloride (FeCl3), and 25 mL of 300 mM acetate buffer, pH 3.6. Each 1 g of honey sample was diluted to 10 mL with distilled water and aliquots of 10 µL honey solution was mixed with 190 µL of FRAP reagent in a 96-well microtitre plate. The sample was kept in dark for 30 minutes, and its absorbance read at 593 nm using a microtiter-plate reader (µQuant, Bio-tek Instruments, Inc., Vermont, USA). Ascorbic acid was used for the standard curve (3.125–200 µg/mL), and FRAP was calculated using Eq. (2). Results were expressed as mg ascorbic acid equivalent per 100 g of honey (mg AAE/100 g).
where CAA is concentration of ascorbic acid from the standard curve (mg/mL), V is volume of honey solution (mL), and DF is dilution factor.
Total phenolic content
The Folin-Ciocalteu method was used to determine TPC of honey samples as described by Singleton et al.[Citation35] with minor modification. Each 1 mL of honey sample (0.05 g/mL) was mixed with 5 mL of 0.2 N Folin-Ciocalteu reagent, followed by the addition of 4 mL sodium carbonate solution (7.5% w/v) after 5 minutes. The mixture was mixed vigorously and left to incubate for 2 hours in the dark. The absorbance was measured at 765 nm against water blank using a UV-VIS spectrophotometer (Ultraspec 3100 Pro, Amersham Biosciences, NJ, USA). The TPC was determined based on the gallic acid standard curve (5–100 µg/mL) and calculated using Eq. (3). Results were expressed as mg of gallic acid equivalent per 100 g of honey (mg GAE/100 g).
where CGA is concentration of gallic acid from the standard curve (mg/mL), V is volume of honey solution (mL), and DF is dilution factor.
Statistical analysis
All measurements of each honey sample were determined in triplicates. A univariate one-way analysis of variance (ANOVA) was performed to compare the mean of physicochemical and antioxidant properties of different honey samples. Significant differences between means were evaluated using Tukey’s test at a confidence level of 95%. Correlation analysis via the bivariate technique was used to calculate the relationship between various parameters. Results were expressed in Pearson correlation coefficients (r). The one-way ANOVA and Pearson correlation coefficients were performed using Minitab statistical software (Version 16, Minitab Inc., USA).
Unsupervised multivariate analyses using hierarchical cluster analysis (HCA) and PCA were performed to classify honey samples based on physicochemical and antioxidant properties using Statistica for Windows (Version 10, Statsoft, Inc., Tulsa, OK, USA). Prior to HCA and PCA analyses, the data matrix of physicochemical and antioxidant properties was auto-scaled by subtraction with mean value of each variable and further dividing with its standard deviation. This was to ensure all variables had an equal influence over the results. When two variables were found highly correlated from the Pearson correlation coefficient and bringing almost similar information, the variable with low Fisher F-ratio was considered redundant and removed. The Fisher F-ratio is defined as the ratio of between-groups to within-groups variances, which evaluates the discriminating ability of a variable where variables with low F-ratio value indicates low discriminating ability.[Citation19] The redundant and/or less discriminating variables was removed as they could affect predictive ability of chemometrics.[Citation19] The selected variables were proceeded for HCA and PCA analyses.
HCA was applied to classify honeys based on the similarities of physicochemical and antioxidant properties. Euclidean distance with complete linkage rule was used to calculate sample similarities, and a hierarchical agglomerative procedure was employed to establish clusters. The physicochemical and antioxidant properties were also subjected to PCA to classify the honey following its entomological origin.
Results and discussion
Physicochemical properties of honey
summarises physicochemical properties of honey samples. Moisture content is the most important parameter to evaluate honey as high moisture can easily lead to honey fermentation caused by osmotolerant yeasts, thus affects the quality of honey.[Citation36] The moisture content of selected raw honeys from Malaysia ranged between 21.96–33.24 g/100 g and this was higher than the limit of ≤ 20% as required by Codex Standard for Honey.[Citation37] The Manuka, Commercial Y and Z honeys were within the Codex limit of less than 20 g/100 g of moisture content. The high moisture content of Malaysian honey is most likely affected by the high yearly rain volume in the tropical region.[Citation38] Among the Apis spp. honeys, Tualang and Gelam honeys produced by Apis dorsata (wild giant bees) showed significant higher moisture content than the Pineapple and Borneo honeys produced by Apis mellifera and Apis cerana, respectively (cavity-nesting bees). The highest value of moisture content was found in Kelulut honey (33.24 g/100 g) significantly (P < 0.05). It was similar to the moisture content of stingless bee honey (34.1 ± 4.34 g/100g) from west Amazonian Ecuador reported by Guerrini et al.[Citation10] Stingless bee honey generally has higher moisture content than Apis mellifera honey.[Citation3,Citation10,Citation15,Citation39] This high moisture content of honey from stingless bee may also be affected by the different honey storage by the stingless bee honey using cerumen resin pots made of wax combined with propolis as compared to brood combs made from wax only by the Apis spp. bees. These moisture content of honey itself may tell the difference in honey source by its bee origin.
Water activity (aw) is a parameter that governs food stability with respect to microbial growth and types of microorganisms encounter in food. The water activity of Apis spp. honeys varied from 0.60 to 0.67 while Kelulut honey had the highest water activity of 0.76 significantly (P < 0.05) (). All three commercial honeys had water activity values lower than 0.60. Beuchat[Citation40] reported that water activity of honey is generally in the range of 0.60–0.65 with note that the osmotolerant yeasts, xerophilic molds, and halophilic bacteria are able to grow in aw > 0.60, aw > 0.65, and aw > 0.70, respectively. The high water activity of Kelulut honey from stingless bee has tendency of fermentation from yeasts and bacteria[Citation4] and it was comparable to Melipona subnitida stingless bee honeys from Brazil with average values of 0.70 ± 0.02[Citation9] and Tetragonula (Trigona) carbonaria honey from Australia with average values of 0.74 ± 0.01.[Citation16]
The specific gravity of all honey samples ranged from 1.36 to 1.44 with no significant difference (). It was similar to the specific gravity of Palestinian multifloral honeys with average values of 1.424 ± 0.003.[Citation36] Viscosity is an important rheological property of honey which affects its quality and design of equipment for honey processing for its extraction, straining, mixing, pumping, and packaging processes.[Citation41] All the honey samples behaved as Newtonian fluid from the regressed shear stress versus shear rate function with R2 > 0.999. The viscosity of raw honeys was significantly lower than the commercial honeys (P < 0.05). The cavity-nesting bee honeys of Pineapple and Borneo showed highest viscosity, followed by wild giant bee honeys of Tualang and Gelam while the Kelulut honey had the lowest viscosity. The lower viscosity values of Malaysian raw honeys from 0.29 to 2.06 Pa·s in comparison to unprocessed Greek honeys ranging from 2.54 to 23.41 Pa·s at 25°C with 15–21% moisture content[Citation41] was due to their high moisture contents.
Honey is acidic in nature and the pH values of Malaysian raw honeys varied from 3.22 to 3.74 (). These values were similar to those reported for Malaysian Apis spp. honeys from 3.53 to 4.03[Citation42], Brazilian stingless bee honeys from 2.90 to 3.83[Citation9], and Thai stingless bee honeys from 3.10 to 3.90.[Citation1] The pH values for Pineapple (3.22) and Kelulut (3.26) were significantly lower (P < 0.05) than other raw honeys and this contributed to a sourly taste. The low pH of honey inhibits the presence and growth of microorganisms.[Citation9] The parameter of pH is important during honey extraction and storage because pH influences its texture, stability and shelf life.[Citation17]
Free acidity is a quality parameter related to honey fermentation[Citation22] which corresponds to the presence of organic acids in honey.[Citation13] The Codex Alimentarius[Citation37] stated that free acidity of honey shall not exceed 50 meq/kg where all three commercial honeys including Manuka were below this limit. The free acidity of Kelulut honey (136.8 meq/kg) was 2.7 times significantly higher than other Apis spp. honeys (<55.0 meq/kg) (). This high free acidity values of Kelulut honey did not comply with the Codex Standard of Honey where it reflected the Apis mellifera product. It is in agreement with Vit et al.[Citation3], who reported that free acidity of stingless bee honey is higher as compared to Apis mellifera honey. The free acidity of Kelulut stingless bee honey from Malaysia was similar to stingless bee honey produced by Tetragonula (Trigona) carbonaria from Australia with average 124.2 ± 22.9 meq/kg[Citation16], Melipona quadrifasciata anthidiodes and Tetragonisca angustula from Brazil with average 103.3 meq/kg and 109.0 meq/kg, respectively.[Citation39] Low and wide variation in the amount of free acidity in honey from different stingless bee species in Brazil was also reported by Duarte et al.[Citation8] and Biluca et al.[Citation2] which varied from 16.2 to 139.0 meq/kg. The free acidity that varied between honeys produced by different bee species may due to fermentation of sugars into organic acids.[Citation2]
Electrical conductivity is a parameter that relates to the concentration of mineral salts, organic acids, and protein in honey samples and measures all ionisable organic and inorganic substances.[Citation17,Citation38] shows that electrical conductivity values of Tualang, Pineapple, Commercial Y and Z honeys which are in the range of 0.10–0.73 mS/cm are within the Codex standard limit of less than 0.8 mS/cm.[Citation37] The Manuka honey had the highest electrical conductivity followed by Kelulut, Gelam and Borneo honeys which ranged from 0.96 to 1.22 mS/cm. Electrical conductivity is related to the ash (mineral) content and the higher the content, the higher the electrical conductivity. Biluca et al.[Citation2] reported that stingless bee honeys from the same predominant floral source of Sylvan but produced by nine different stingless bees species in São Miguel do Oeste of Brazil differed significantly in the electrical conductivity that ranged from 0.15 to 1.34 mS/cm. This suggested that the electrical conductivity in honey can vary by its different geographical, botanical and even entomological origins.
Colour of honey is the first quality appearance judgement that affects consumer preference. Colour of honey is related to the floral origin or plant source, minerals, phenolic contents, storage time and temperature.[Citation5] Dark honeys are preferred by consumers because honeys with dark colour have a higher mineral and antioxidant capacity.[Citation38] The L* parameter represents lightness of honey and Commercial Y honey was the brightest (29.03) while Manuka honey was the darkest (23.70) as shown in . The a* and b* values for honey samples were all in the positive range suggesting reddish and yellowish (). Statistical results shows that colour parameter of a* had no significant different (P > 0.05) between Malaysian raw honey samples which therefore a* was not selected as variables for HCA and PCA analyses. Manuka honey with the lowest L*, a* and b* values and the highest colour intensity had significant different colour compared to all raw honeys from Malaysia (P < 0.05) probably due to different geographical origin and the native plant sources (Leptospermum Polygalifolium) of the Manuka honey from Australia.
Antioxidant properties of honey
The scavenging activity of honey samples measured by a stable nitrogen-based radical, the 1,1-diphenyl-2-picryhydrazyl (DPPH), was monitored by reduction of deep purple coloured DPPH radicals after receiving a hydrogen donated by free radical scavenging antioxidant from honey. Ascorbic acid was used as positive control in determining free radical scavenging activity. The IC50 value for 50% of scavenging activity of ascorbic acid was 16.07 µg/mL. A higher 1/IC50 value indicates a higher scavenging activity power and a lower concentration of sample is required to scavenge 50% of DPPH radicals. shows that the Manuka honey had the highest 1/IC50 value of 99.06 mL/g, followed by Kelulut honey from Heterotrigona itama of 26.63 mL/g, and Apis spp. honeys (2.90–7.25 mL/g). The stingless bee honey from West Amazonian Ecuador was also reported to have higher DPPH inhibition than honeys produced by Apis mellifera.[Citation10] No significant difference of 1/IC50 values was observed between honeys from the Apis spp., i.e. Tualang, Gelam, Pineapple, and Borneo honeys.
Figure 1. Antioxidant properties of (a) DPPH free radical scavenging activity power (1/IC50); (b) ascorbic acid equivalent antioxidant capacity (AEAC); (c) ferric ion reducing antioxidant power (FRAP); and (d) total phenolic content (TPC) of various honey samples (T: Tualang; G: Gelam; P: Pineapple; B: Borneo; K: Kelulut; M: Manuka; CY: Commercial Y; CZ: Commercial Z). Values presented are mean ± standard error (n = 3 × N) with different letters indicate significant differences (P < 0.05).
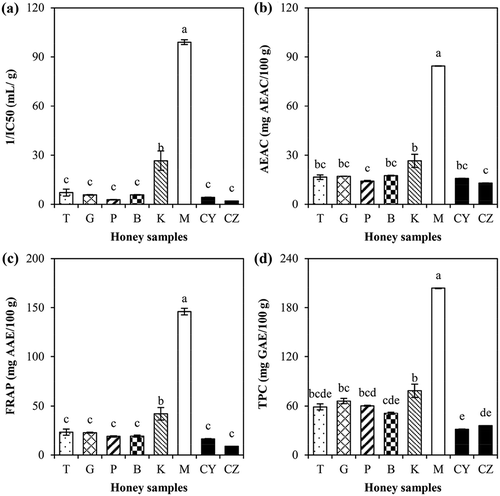
shows that antioxidant content in terms of AEAC of Malaysian raw honey samples ranged from 14.23 to 26.64 mg AEAC/100 g. These values were comparable to those reported in Pakistani natural honeys having 8.30–22.10 mg AEAC/100 g[Citation7], multifloral Burkina Fasan honeys from 10.20 to 37.87 mg AEAC/100 g[Citation33], and Czech honeys from 14.15 to 40.71 mg AEAC/100 g.[Citation6] The AEAC value in Manuka honey of 84.47 mg/100 g was the highest. For Malaysian raw honeys, Kelulut had the highest AEAC value of 26.64 mg/100 g followed by Tualang, Gelam and Borneo while Pineapple had the lowest AEAC content of 14.23 mg/100 g. Meda et al.[Citation33] reported that the Vitellaria honey with the highest AEAC value had the highest antioxidant content among the Burkina Fasan honeys.
The antioxidant activity of honey was also determined by FRAP assay. shows that the FRAP values for Malaysian raw honeys produced by Apis spp. ranged from 19.05 to 23.34 mg AAE/100 g with no significant differences between them. The Kelulut honey produced by Heterotrigona itama bees was 2 times higher significantly (41.95 mg AAE/100 g) than the Apis spp. honeys. This indicates Kelulut honey had higher reducing power and also stronger antioxidant activity than Apis spp. honeys. The FRAP values of Malaysian raw honeys were comparable with honeys found in Czech with FRAP values of 29.54–77.61 mg AAE/100 g honey.[Citation6]
For TPC, shows that Manuka honey contained the highest value with 203.52 mg GAE/100 g. The Malaysian raw honeys ranged from 51.04 to 78.43 mg GAE/100 g where the TPC of Kelulut honey was higher than other Apis spp. honeys. This is in accordance with TPC of honey from Plebeia stingless bees (106.01 ± 9.85 mg GAE/100 g) which was slightly higher than those from Apis mellifera (92.34 ± 13.55 mg GAE/100 g) as reported by Duarte et al.[Citation8]
The Manuka honey from the Leptospermum Polygalifolium trees is well known for various medicinal properties from its antioxidant capacity and it was used as benchmarking for this honey study. All four analysis of 1/IC50, AEAC, FRAP, and TPC revealed that the Kelulut honey from Heterotrigona itama stingless bees had the highest antioxidant properties in ranking after the Manuka honey. It had better antioxidant properties compared to the Apis spp. honeys while Commercial Y and Z honeys were the lowest ranked. The high antioxidant properties of Kelulut honey makes it favourable for promotion of the local stingless bee honey beekeeping industry in the country.
Pearson correlation (r)
Pearson correlation is a number between −1 and +1 and measures the degree of linear relationship between two parameters. A correlation of positive value indicates a positive (increasing) linear relationship, while a negative correlation indicates a negative (decreasing) linear relationship. shows that Pearson correlations between physicochemical and antioxidant properties of honey samples. Strong and significant positive correlations between physicochemical properties of honey were found for moisture content and water activity (r = 0.982, P < 0.0005), and moisture content and free acidity (r = 0.896, P < 0.0005). Moisture content was also negatively correlated with specific gravity (r = −0.990, P < 0.0005) and viscosity (r = −0.816, P < 0.0005). This explains that honey which contains more moisture content is higher in water activity, and is less viscous. High moisture content increases the tendency for fermentation which may increase the free acidity of honey.
Table 2. Pearson correlation coefficients between various properties of honey.
Colour intensity is the only parameter among the physicochemical properties that is highly correlated with the antioxidant properties of honey.[Citation43] The correlation values between colour intensity and 1/IC50, AEAC, FRAP, and TPC were 0.944, 0.968, 0.966, and 0.953, respectively. The positive correlation indicates that colour pigments increased with the observed antioxidant properties and TPC of honey. It implies that honey with dark colour showed a higher antioxidant capacity and a higher total phenolic content.[Citation5,Citation17,Citation18,Citation30]
Hierarchical cluster analysis
For the successive analyses of HCA and PCA, high correlated and redundant variables could be removed by considering the F-ratio values. Moisture content, viscosity and free acidity with high F-ratio values of 89.37, 1239.07, and 392.16, respectively, were selected for HCA and PCA whereas water activity (131.66) and specific gravity (1.9) with low F-ratio values were reduced. Moisture content was selected instead of water activity due to moisture is a parameter required in Codex Standard for Honey.[Citation37] Among the four antioxidant properties of honey, FRAP and AEAC with high F-ratio of 62.19 and 52.94, respectively, were retained for HCA and PCA analyses.
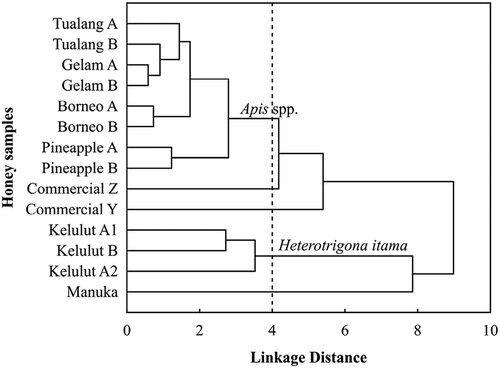
shows the dendrogram obtained from HCA based on variables of moisture content, viscosity, pH, free acidity, electrical conductivity, L*, b*, colour intensity, AEAC, and FRAP. The vertical lines in dendrogram represented honey samples while horizontal lines represented linkage distance between samples. Small linkage distance means high similar characteristics. Honey samples from different batches of A and B were grouped together into their varieties and five clusters were obtained at linkage distance of 4.0 (). From the top, the first cluster of honey samples comprised of the Tualang A and B, Gelam A and B, Borneo A and B, and Pineapple A and B. The second and third clusters were Commercial Y and Z honeys, respectively. The fourth cluster was the three Kelulut honey samples and the fifth cluster consisted of Manuka honey alone. Manuka honey was distinguishable from the groups of raw honeys and Commercial Y and Z honeys. This differentiation is contributed by the significant difference in high values of antioxidant properties found in Manuka honey. The different clusters of Tualang, Gelam, Borneo, and Pineapple honeys produced by Apis spp. (honey bees) from the Kelulut honey produced by Heterotrigona itama (Trigona stingless bees) suggest that honeys from different bee species of honey bees and stingless bees have different physicochemical and antioxidant properties. This helps identification of honey origin entomologically.
Principal component analysis
The PCA was applied to classify all 11 raw honey samples following its entomological origin using ten tested variables of physicochemical and antioxidant properties. The first three PCs that accounted for 88.27% of total variance with eigenvalues greater than 0.9713 were extracted to determine the best parameters to classify honey samples. is the loading plot of PC2 versus PC1 variables while is for PC3 versus PC2 variables. Moisture content (MC), viscosity, free acidity (FA), electrical conductivity (EC), colour b*, colour intensity (CI), AEAC, and FRAP were attributes corresponded to PC1 which explained about 61.15% of the total variance while PC2 mainly contributed by pH that accounted for 17.40% of total variance.
Figure 3. The (a) loading plot for PC2 versus PC1; (b) loading plot for PC3 versus PC2; (c) score plot for PC2 versus PC1; and (d) score plot for PC3 versus PC2 from PCA based on physicochemical and antioxidant properties for honey classification following its entomological origin (I: Heterotrigona itama; II: Apis dorsata and Apis cerana; III: Apis mellifera; IV: Apis cerana). The codes of A and B of raw honeys indicate the samples from different batches.
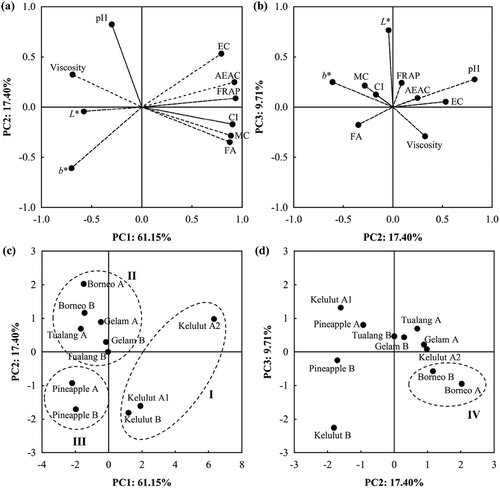
The score plot in shows that Kelulut honey produced by Heterotrigona itama was positioned at positive scores of PC1 while Tualang, Gelam, Pineapple, and Borneo honeys from Apis spp. (Apis dorsata, Apis mellifera, and Apis cerana) were positioned at negative scores of PC1. This indicates physicochemical and antioxidant properties were effective in classifying and differentiating honeys following bee types of Heterotrigona itama (Trigona stingless bees) and Apis spp. (honey bees). Although all three Kelulut honeys were sourced from same location, Kelulut A2 (collected in April 2013) showed variations in physicochemical and antioxidant properties compared to Kelulut A1 (collected in January 2013) and Kelulut B (collected in March 2014). In reality, it is acknowledged that honey properties are affected by the seasons and weather. Moisture content, free acidity, colour intensity, AEAC, and FRAP which had the highest absolute value (≥ 0.8769) on positive PC1 as shown in indicated those properties were high in Kelulut honey having the highest positive scores on PC1 (). This agreed with Vit et al.[Citation3] and Oddo et al.[Citation16] who reported that the moisture content and free acidity of stingless bee honey are generally higher than Apis mellifera honey. Moisture content, free acidity, colour intensity, AEAC, and FRAP are suggested to be the most dominant parameters used for the differentiation of honeys between Trigona stingless bees and Apis honey bees.
Pineapple A and B honeys produced by Apis mellifera could be distinguished from the group of Apis spp. honeys. Pineapple honey was observed to locate at negative scores of PC1 and PC2 compared to Tualang, Gelam and Borneo honeys that are located at negative scores of PC1 and positive scores of PC2 (). Pineapple honey was characterised acidic (low pH values) compared to other Apis spp. honeys. Tualang and Gelam honeys produced by Apis dorsata and Borneo honey produced by Apis cerana showed less discrimination in PC1 and PC2 scores space. However, PC3 which explained 9.71% of total variance could separate Borneo honeys to negative PC3 scores while Tualang and Gelam honeys to positive PC3 scores as shown in . Borneo honeys had lower colour L* values than Tualang and Gelam honeys. The loading plot in also showed that the colour L* had highest positive loading on PC3 which suggested colour L* had contributed in differentiating honeys from Apis cerana (Borneo honey) and Apis dorsata (Tualang and Gelam honeys). These PCA results suggest that raw honeys from Malaysia are classifiable to its entomological origin of bee species by its physiochemical and antioxidant properties.
Conclusion
Variations in physicochemical and antioxidant properties of Malaysian raw honeys from different species of Apis honey bees and Trigona stingless bees were measured. Use of HCA and PCA for pattern recognition on the physicochemical and antioxidant properties showed that these honeys could be classified following its bee species. The Kelulut honey produced by Trigona stingless bees, Heterotrigona itama exhibited significant higher values in moisture content, water activity, free acidity, colour intensity, and showed better antioxidant properties when compared to honey produced by Apis spp., that is Tualang, Gelam, Pineapple, and Borneo which possess similar characteristics. Moisture content, free acidity, colour intensity, AEAC, and FRAP are properties identified suitable for differentiating honeys produced by Apis spp. or Trigona stingless bees. Higher values of moisture content of 33 g/100 g and free acidity of 140 meq/kg are suggested as maximum standard limits for stingless bee honey from Heterotrigona itama, the Kelulut.
Additional information
Funding
References
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical Profiles of Stingless Bee (Apidae: Meliponini) Honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. DOI: 10.1016/j.foodchem.2015.06.089.
- Biluca, F. C.; Braghini, F.; Gonzaga, L. V.; Costa, A. C. O.; Fett, R. Physicochemical Profiles, Minerals and Bioactive Compounds of Stingless Bee Honey (Meliponinae). J. Food Composit. Anal. 2016, 50, 61–69. DOI: 10.1016/j.jfca.2016.05.007.
- Vit, P.; Medina, M.; Enriquez, M. E. Quality Standards for Medicinal Uses of Meliponinae Honey in Guatemala, Mexico and Venezuela. Bee World. 2004, 85, 2–5. DOI: 10.1080/0005772X.2004.11099603.
- Vit, P.; Pedro, S. R.; Roubik, D. Pot-Honey: A Legacy of Stingless Bees; Springer: New York, USA. 2013; pp 654.
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the Phenolic Content, Antioxidant Activity and Colour of Slovenian Honey. Food Chem. 2007, 105, 822–828. DOI: 10.1016/j.foodchem.2007.01.060.
- Lachman, J.; Orsák, M.; Hejtmánková, A.; Kovářová, E. Evaluation of Antioxidant Activity and Total Phenolics of Selected Czech Honeys. LWT-Food Sci. Technol. 2010, 43, 52–58. DOI: 10.1016/j.lwt.2009.06.008.
- Noor, N.; Sarfraz, R. A.; Ali, S.; Shahid, M. Antitumour and Antioxidant Potential of Some Selected Pakistani Honeys. Food Chem. 2014, 143, 362–366. DOI: 10.1016/j.foodchem.2013.07.084.
- Duarte, A. W. F.; Vasconcelos, M. R. S.; Menezes, A. P. D.; Silva, S. C.; Oda-Souza, M.; López, A. M. Q. Composition and Antioxidant Activity of Honey from Africanized and Stingless Bees in Alagoas (Brazil): A Multivariate Analysis. J. Apic. Res. 2012, 51, 23–35. DOI: 10.3896/IBRA.1.51.1.04.
- Silva, T. M. S.; Santos, F. P.; Evangelista-Rodrigues, A.; Silva, E. M. S.; Silva, G. S.; Novais, J. S.; Santos, F. A. R.; Camara, C. A. Phenolic Compounds, Melissopalynological, Physicochemical Analysis and Antioxidant Activity of Jandaíra (Melipona Subnitida) Honey. J. Food Composit. Anal. 2013, 29, 10–18. DOI: 10.1016/j.jfca.2012.08.010.
- Guerrini, A.; Bruni, R.; Maietti, S.; Poli, F.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Scalvenzi, L.; Sacchetti, G. Ecuadorian Stingless Bee (Meliponinae) Honey: A Chemical and Functional Profile of an Ancient Health Product. Food Chem. 2009, 114, 1413–1420. DOI: 10.1016/j.foodchem.2008.11.023.
- Sousa, J. M.; De Souza, E. L.; Marques, G.; Meireles, B.; De Magalhães Cordeiro, Â. T.; Gullón, B.; Pintado, M. M.; Magnani, M. Polyphenolic Profile and Antioxidant and Antibacterial Activities of Monofloral Honeys Produced by Meliponini in the Brazilian Semiarid Region. Food Res. Int. 2016, 84, 61–68. DOI: 10.1016/j.foodres.2016.03.012.
- Moore, J. C.; Spink, J.; Lipp, M. Development and Application of a Database of Food Ingredient Fraud and Economically Motivated Adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. DOI: 10.1111/j.1750-3841.2012.02657.x.
- Da Silva, P. M.; Gauche, C.; Gonzaga, L. V.; Costa, A. C. O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. DOI: 10.1016/j.foodchem.2015.09.051.
- De Sousa, J. M. B.; De Souza, E. L.; Marques, G.; De Toledo Benassi, M.; Gullón, B.; Pintado, M. M.; Magnani, M. Sugar Profile, Physicochemical and Sensory Aspects of Monofloral Honeys Produced by Different Stingless Bee Species in Brazilian Semi-Arid Region. LWT-Food Sci. Technol. 2016, 65, 645–651. DOI: 10.1016/j.lwt.2015.08.058.
- Suntiparapop, K.; Prapaipong, P.; Chantawannakul, P. Chemical and Biological Properties of Honey from Thai Stingless Bee (Tetragonula Leaviceps). J. Apic. Res. 2012, 51, 45–52. DOI: 10.3896/IBRA.1.51.1.06.
- Oddo, L. P.; Heard, T. A.; Rodríguez-Malaver, A.; Pérez, R. A.; Fernández-Muiño, M.; Sancho, M. T.; Sesta, G.; Lusco, L.; Vit, P. Composition and Antioxidant Activity of Trigona Carbonaria Honey from Australia. J. Med. Food. 2008, 11, 789–794. DOI: 10.1089/jmf.2007.0724.
- Alves, A.; Ramos, A.; Gonçalves, M. M.; Bernardo, M.; Mendes, B. Antioxidant Activity, Quality Parameters and Mineral Content of Portuguese Monofloral Honeys. J. Food Composit. Anal. 2013, 30, 130–138. DOI: 10.1016/j.jfca.2013.02.009.
- Khalafi, R.; Goli, S. A. H.; Behjatian, M. Characterization and Classification of Several Monofloral Iranian Honeys Based on Physicochemical Properties and Antioxidant Activity. Int. J. Food Prop. 2016, 19, 1065–1079. DOI: 10.1080/10942912.2015.1055360.
- Marini, F.; Magrı̀, A.; Balestrieri, F.; Fabretti, F.; Marini, D. Supervised Pattern Recognition Applied to the Discrimination of the Floral Origin of Six Types of Italian Honey Samples. Anal. Chim. Acta. 2004, 515, 117–125. DOI: 10.1016/j.aca.2004.01.013.
- Truzzi, C.; Illuminati, S.; Annibaldia, A.; Finale, C.; Rossetti, M.; Scarponi, G. Physicochemical Properties of Honey from Marche, Central Italy: Classification of Unifloral and Multifloral Honeys by Multivariate Analysis. Nat. Prod. Commun. 2014, 9, 1595–1602.
- Kropf, U.; Korošec, M.; Bertoncelj, J.; Ogrinc, N.; Nečemer, M.; Kump, P.; Golob, T. Determination of the Geographical Origin of Slovenian Black Locust, Lime and Chestnut Honey. Food Chem. 2010, 121, 839–846. DOI: 10.1016/j.foodchem.2009.12.094.
- Silvano, M. F.; Varela, M. S.; Palacio, M. A.; Ruffinengo, S.; Yamul, D. K. Physicochemical Parameters and Sensory Properties of Honeys from Buenos Aires Region. Food Chem. 2014, 152, 500–507. DOI: 10.1016/j.foodchem.2013.12.011.
- Silva, A. S.; Alves, C. N.; Fernandes, K. G.; Müller, R. C. S. Classification of Honeys from Pará State (Amazon Region, Brazil) Produced by Three Different Species of Bees Using Chemometric Methods. J. Braz. Chem. Soc. 2013, 24, 1135–1145.
- Kek, S. P.; Chin, N. L.; Tan, S. W.; Yusof, Y. A.; Chua, L. S. Classification of Honey from Its Bee Origin via Chemical Profiles and Mineral Content. Food Anal. Methods. 2017, 10, 19–30. DOI: 10.1007/s12161-016-0544-0.
- Vit, P.; Zuccato, V.; Uddin, J.; Schievano, E.; Maza, F. Entomological Origin of Honey Discriminated by NMR Chloroform Extracts in Ecuadorian Honey. International Journal of Biological. Biomol. Agric. Food Biotechnol. Eng. 2015, 9, 458–461.
- Zuccato, V.; Finotello, C.; Menegazzo, I.; Peccolo, G.; Schievano, E. Entomological Authentication of Stingless Bee Honey by 1H NMR-based Metabolomics Approach. Food Control. 2017, 82, 145–153. DOI: 10.1016/j.foodcont.2017.06.024.
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2005.
- Trávníček, P.; Vítěz, T.; Přidal, A. Rheological Properties of Honey. Sci. Agric. Bohemica. 2012, 43, 160–165. DOI: 10.7160/sab.
- Bogdanov, S.; Martin, P.; Lullmann, C. Harmonised Methods of the International Honey Commission; Swiss Bee Research Centre: FAM: Liebefeld, Switzerland. 2002; pp 62.
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R. M. Standardization of Antioxidant Properties of Honey by a Combination of Spectrophotometric/Fluorimetric Assays and Chemometrics. Anal. Chim. Acta. 2005, 533, 185–191. DOI: 10.1016/j.aca.2004.11.010.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. DOI: 10.1016/S0023-6438(95)80008-5.
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D. H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Composit. Anal. 2006, 19, 669–675. DOI: 10.1016/j.jfca.2006.01.003.
- Meda, A.; Lamien, C. E.; Romito, M.; Millogo, J.; Nacoulma, O. G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. DOI: 10.1016/j.foodchem.2004.10.006.
- Aljadi, A. M.; Kamaruddin, M. Y. Evaluation of the Phenolic Contents and Antioxidant Capacities of Two Malaysian Floral Honeys. Food Chem. 2004, 85, 513–518. DOI: 10.1016/S0308-8146(02)00596-4.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 265–275.
- Abdulkhaliq, A.; Swaileh, K. M. Physico-Chemical Properties of Multi-Floral Honey from the West Bank, Palestine. Int. J. Food Prop. 2017, 20, 447–454. DOI: 10.1080/10942912.2016.1166128.
- Codex Alimentarius. Revised Codex Standard for Honey. Codex STAN 12-1981, Rev. 1 (1987), Rev. 2 (2001); 2001.
- Chua, L. S.; Abdul-Rahaman, N.-L.; Sarmidi, M. R.; Aziz, R. Multi-Element Composition and Physical Properties of Honey Samples from Malaysia. Food Chem. 2012, 135, 880–887. DOI: 10.1016/j.foodchem.2012.05.106.
- Souza, B.; Roubik, D.; Barth, O.; Heard, T.; Enriquez, E.; Carvalho, C.; Villas-Bôas, J.; Marchini, L.; Locatelli, J.; Persano-Oddo, L.; Almeida-Muradian, L.; Bogdanov, S.; Vit, P. Composition of Stingless Bee Honey: Setting Quality Standards. Interciencia. 2006, 31, 867–875.
- Beuchat, L. R.;. Microbial Stability as Affected by Water Activity. Cereal Foods World. 1981, 26, 345–349.
- Yanniotis, S.; Skaltsi, S.; Karaburnioti, S. Effect of Moisture Content on the Viscosity of Honey at Different Temperatures. J. Food Eng. 2006, 72, 372–377. DOI: 10.1016/j.jfoodeng.2004.12.017.
- Moniruzzaman, M.; Khalil, M. I.; Sulaiman, S. A.; Gan, S. H. Physicochemical and Antioxidant Properties of Malaysian Honeys Produced by Apis Cerana, Apis Dorsata and Apis Mellifera. BMC Complement. Altern. Med. 2013, 13, 1–12. DOI: 10.1186/1472-6882-13-43.
- Kek, S. P.; Chin, N. L.; Yusof, Y. A.; Tan, S. W.; Chua, L. S. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis Spp. And Trigona Spp. Bees. Agric. Agric. Sci. Procedia. 2014, 2, 150–155. DOI: 10.1016/j.aaspro.2014.11.022.