ABSTRACT
A fast, simple and sensitive salting-out assisted liquid-liquid extraction method for the simultaneous determination of thujones and pulegone in beverage samples was developed. The extraction was achieved by adding ammonium sulphate to a miscible mixture of sample (10 mL) and acetonitrile (1.5 mL). The upper layer was directly injected into a capillary gas chromatography unit and detected using flame ionisation detector. The method was validated and calibration curve was linear with r2 > 0.99 over the range of 0.1–15 mg.kg–1 and 1–300 mg.kg−1 for thujone and pulegone, respectively. The limits of detection were 4.3 and 5.1 µg.kg–1 and the limit of quantification were 12.9 and 15.3 µg.kg–1 for thujone and pulegone, respectively. The relative standard deviation for inter- and intra -day precision was less than 10.1%. The proposed method was conveniently applied for the determination of these compounds in various herbal and fruit drinks, alcoholic beer and soft drinks. The average recoveries of the analyzed compounds ranged from 97 to 110% for thujone and from 87 to 119% for pulegone in three types of beverages. Thujones were not detected in all the 43 samples analysed while pulegone was detected in two samples (< quantitation limit and 1.78 mg.kg−1). Positive identification of the thujone and pulegone peaks were provided by GC-MS.
Introduction
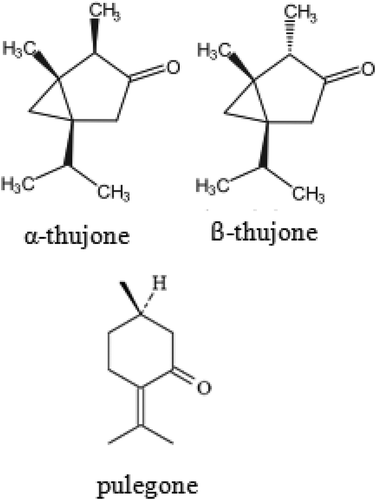
Thujones and pulegone () are interesting compounds that are constituents of many essential oils. Thujone occurs naturally as a mixture of α- and β-diastereoisomers. These isomers are neurotoxins that inhibit the γ-aminobutyric acid-gated chloride channel. [Citation1] Earlier studies demonstrated that the α-isomer had greater ability to elicit seizures than the β-isomer. [Citation2] On the other hand, it had been reported that isomers of thujone helps to decrease levels of cholesterol and improve lipid profile in blood of diabetic rats.[Citation3] Thujones are also the active ingredient found in absinthe, a popular alcoholic beverage in 19th-century Europe. Long term drinking of absinthe caused seizures, blindness, hallucination and depression. [Citation4]
Pulegone is an optically active monoterpene ketone present in the essential oils from many mint species (up to 4%) and is used industrially as flavouring agent, cosmetics, medical, etc. Pulegone caused cancer of the urinary bladder in female rats, and cancer of the liver in male and female mice. [Citation5,Citation6] The toxicological effects of pulegone on liver and lung are mainly ascribed to the R-isomer [Citation7], while the S-isomer of pulegone had been reported to have much lower toxicity in man. [Citation8]
Due to the potential risks associated with the intake of α- and β-thujone as well as pulegone, many countries have implemented strict rules limiting their content in food products. According to the European Union regulation EC 1334/2008 [Citation9], thujone and pulegone are not allowed to be added to food products. Annex III of the same regulation sets a maximum level if they are naturally present in approved flavouring and food ingredient with flavouring properties. The levels of α- plus β-thujones should not exceed 10 mg.kg−1 in alcoholic beverages, with exception of 35 mg.kg−1 for those produced from Artemisia species, and 0.5 mg.kg−1 for non-alcoholic beverages produced from Artemisia species. The maximum limit of pulegone in that regulation is 20 mg.kg−1 in mint/peppermint containing non-alcoholic beverage and 100 mg.kg−1 for mint/peppermint containing alcoholic beverages.
Due to the concerns of the presence of these compounds, several analytical methods for their determination have been reported. Techniques such as liquid-liquid extraction (LLE), solid phase extraction (SPE)[Citation2] and solid-phase microextraction (SPME)[Citation10] have been used for the isolation of thujone from absinthe or wormwood extracts[10,.[Citation11] As thujone and pulegone are not highly chromophoric, gas chromatography (GC) is commonly used for their analysis[12,.[Citation13,Citation14] However, thujones have been derivatised using dansylhydrazine, rendering it to be determined using HPLC with fluorescence detection.[Citation15] LLE, SPE and SPME were mainly used for the extraction of thujones in absinthes and other drinks. The LLE technique is not favoured as it consumes large amounts of organic solvents which can not only pose health risks to the operator but also increase costs of disposal. The SPE method, although requires less organic solvent but it involves multiple steps (e.g., conditioning, washing, elution). Often long evaporation time is further required to remove the eluting solvent. The SPME technique is an interesting option as it is a solvent less technique. However, the SPME fibres are fragile and carryover is common. A straightforward method to determine flavouring agents (including thujones and pulegone) in food using GC-MS was described by Lopez et al..[Citation16] The analytes were extracted using ethyl acetate prior to the GC-MS analysis.
Salting-out assisted liquid-liquid extraction (SALLE) is a relatively new technique that uses water miscible organic solvent (e.g., acetonitrile) as the extraction solvent. The addition of salts causes a phase separation and the analytes are transferred to the organic layer. Compared to LLE, it is simple and do not require long mechanical shaking since the two phases are miscible. The solvents and reagents used are more environmentally-friendly and lesser amounts are required. As the organic solvents used are suitable for direct injection into analytical instruments (e.g., HPLC and capillary electrophoresis (CE)), the evaporation step can therefore be avoided. The SALLE technique is not only cheaper but is also considerably simpler compared to SPE. Over the past decade, SALLE have been used for the determination of compounds in different types of matrices. [Citation17–Citation19]
In the present studies, a simple SALLE method targeted for the extraction of thujones and pulegone was for the first time described and the extract was analysed using GC with flame ionisation detector (FID). GC- mass spectrometry (MS) was used for confirmation of compounds. The advantages of this method over those found in the literature are discussed. As will be seen later, the proposed method is attractive for widespread adoption by laboratories as it is simple and uses common glasswares.
Materials and methods
Chemicals and reagents
Pulegone (98.7%) and thujone analytical standards (97.95% α-thujone and 1.88% β-thujone) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals and reagents used were obtained from the following sources: HPLC-grade acetonitrile (>99.9%) was from Merck (Darmstadt, Germany); ammonium sulphate was from QReC (Selangor, Malaysia). Ultrapure water (resistivity, 18.2 MΩ.cm−1) was produced by a Millipore water purification system (Molsheim, France) and was used throughout for the preparation of solutions.
Instrumentation
A Perkin Elmer Clarus 500 gas chromatograph equipped with FID and split/splitless injector (Waltham, MA, USA) was used for quantitative work. A Perkin Elmer Clarus 600 gas chromatograph equipped with split/splitless injector (Waltham, MA, USA) coupled with a Perkin Elmer Clarus 600T mass spectrometer detector (Waltham, MA, USA) was used. Separation for both GC-FID and GC-MS were achieved using a DB5 capillary column (30 m long, 0.25 mm inner diameter, 0.25 μm film thickness; J & W Scientific, Palo Alto, CA, USA). The split ratio was 5:1 for the GC-FID and 10:1 for GC-MS. The injection volume was 2 μL for GC-FID and 1 µL for GC-MS. Nitrogen was used as the carrier gas at a flow rate of 1.5 mL.min−1 for GC-FID while helium was used as the carrier gas (1.5 mL.min−1) for GC-MS. For both instruments, the injector temperature was 300°C. The initial oven temperature was 70°C, held for 3 min, increased at 20°C.min−1 to 190°C, held for 2 min. The FID temperature was set at 310°C. Electron ioniSation (EI) mode at 70 eV was used for the MS; the ion source temperature was 250°C. The mass spectra were measured in the range 35– 360 m/z. Qualitative analysis was carried out using NIST MS database version 2.2 (2014) library.
Preparation of standard solutions
Thujone and pulegone stock solutions (50 and 10000 mg.L−1, respectively) were prepared by dissolving the desired amounts in acetonitrile and stored at 4°C. Working standard solutions were prepared in test tube (50 mL) by mixing appropriate volumes of standards stock solution with water (10 mL) and the required volume of acetonitrile to ensure that the final volume of acetonitrile was 1.5 mL. The mixture was vortexed vigorously (2500 rpm) for 10 s, and then subjected to the SALLE procedure (section 2.5).
Preparation of beverage samples
Beverage samples (43) were purchased from local stores in Kedah and Pulau Pinang, Malaysia. The beverage samples include. Alcoholic (24), soft drinks (9) and herbal and fruit drinks (10). The alcoholic drinks include beer from different sources (malt, cereals, hope, and cider) and lemon shandy. Soft drinks were cola and sports isotonic drinks. Herbal and fruit include ice tea fruit flavoured, herbal tea and mint flavoured lemonade. Each sample was filtered through 0.45 µm nylon filter. The filtrate (10 mL) was transferred to a test tube (50 mL) and acetonitrile (1.5 mL) was added. The mixture was vortexed vigorously (2500 rpm) for 10 s and then subjected to the SALLE procedure (section 2.5).
SALLE
For each working standard or sample solution, ammonium sulphate (12 g) was added and vortexed vigorously (2500 rpm) for 20 s, then centrifuged (4000 rpm) for 3 min. An aliquot of the upper layer (2 µL) was directly introduced into the GC unit. The extraction recovery (ER) was calculated by comparing peak areas of standards solution after subjecting to SALLE against standards prepared in acetonitrile. The enrichment factor (EF) was calculated using the following equation:
Results and discussion
SALLE
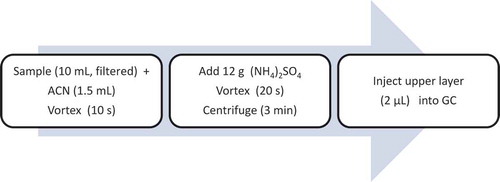
The scheme of the proposed SALLE procedure is shown in . In the current study, the extraction of thujones and pulegone from the different beverage samples was achieved by SALLE into acetonitrile. The SALLE procedure was suitable due to efficiency of the extraction, simplicity and suitability of the extract for the direct injection into the GC unit. The main factors affecting the extraction such as type of salt and its amount, type of organic solvent, vortex and centrifuge times were investigated.
Figure 2. Scheme of sample preparation using the SALLE procedure for the determination of thujone and pulegone in beverages.
Acetonitrile is the most widely used solvent for SALLE [Citation20] and has many attractive properties such as the good miscibility with water, its favourable polarity that facilitates the extraction of a wide range of compounds and furthermore, can be directly injected to GC instruments. Acetonitrile is also less harmful to the environment compared to the other common liquid extraction solvents.
Ammonium sulphate has been used in SALLE [Citation21,Citation22] due to its good solubility (75.4 g in 100 mL) and the sulphate anion is the most efficient anion, which contributes more than cations during the phase separation in accordance to the Hofmeister series. [Citation20,Citation23] The amount of ammonium sulphate was varied to obtain the highest peak area (). The optimum amount was found to be 11 and 12 g for pulegone and α-thujone, respectively. Therefore 12 g was used. The decrease in peak area when more than 12 g was used is due to analyte dilution as the reformed layer volume is increased. The extraction recovery (ER%) was found to be 95.2% and 94.7% for α-thujone and pulegone, respectively. The reformed organic layer was 0.9 mL (n = 10).
Figure 3. Peak area of (a) α-thujone, and (b) pulegone as a function of amount of ammonium sulfate; α-thujone, 0.5 mg.kg−1; pulegone, 100 mg.kg−1; volume of acetonitrile, 1.5 mL.
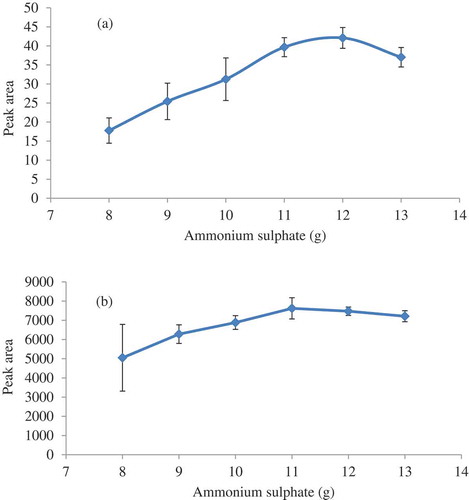
The limit of quantitation (LOQ) value obtained () is due to the analyte enrichments in the reformed organic layer after SALLE to about 10.5-fold and 10.6-fold for α-thujone and pulegone, respectively which is expressed as the EF.
Table 1. Validation parameters for the SALLE-GC method.
The sample preparation is simple and fast as each sample required only 3.5 min to be ready for the GC injection. This is because no long mechanical shaking is required since acetonitrile and the sample is fully miscible before the salt addition. Alcoholic beverages that were used in this study contain less than 10% of ethanol (v/v) and this did not affect the extraction, which is reflected by the good recoveries of the spiked alcoholic beverages samples (). This was also noticed by Lopez et al. [Citation16] where ethanol content up to 15% has no significant influence on the extraction efficiency for both thujones and pulegone. It is interesting to note that under the adopted GC conditions, baseline separation of the thujone isomers was possible. In the absence of β-thujone standard, the α- peak was used for its quantitation by assuming that the response factor is similar for both isomers. This assumption is valid as the calculated β- standard solution are in good agreement to that of the claimed manufacturer’s value. Similar approach was used by other researchers.[Citation2]
Table 2. %Recoveries of thujone and pulegone in drinks (n = 5).
Method validation
In order to demonstrate the suitability of the method, the following validation merits were investigated: linearity, limit of detection (LOD), LOQ, precision, accuracy and selectivity after subjecting the working standard and spiked beverage samples solutions to the SALLE procedure. and summarise the results of chromatographic method validation. A standard mixture (98% α-thujone) was used in the validation calculations. β-thujone, where no standard is available, was calculated from the response factor of α-thujone. The β-isomer was identified by injecting high concentration (1000 mg L−1) of the thujones standard mixture
Linearity was established using standards at concentration levels from 0.1 to 15 mg.kg–1 for thujone and from 1 to 300 mg.kg−1 for pulegone. Calibration curve was linear (r2 = 0.9953 and 0.9968 for α-thujone and pulegone, respectively) over the studied range. The LOQ were 12.9 and 15.3 µg.kg–1 and LOD were 4.3 and 5.1 µg.kg–1 for α-thujone and pulegone, respectively. LOQ and LOD were calculated using blank response at signal to noise ratio (S/N) of 10 and 3, respectively. The low value achieved is largely contributed to the extractions into smaller volumes in addition to the good clean-up of the matrix by the SALLE method. Precision (repeatability and intermediate precision) was measured by the relative standard deviation (%RSD) of three different standard solutions on five consecutive days (n = 6 for each day). The %RSD for three different concentration levels for both analytes was less than 10.1%.
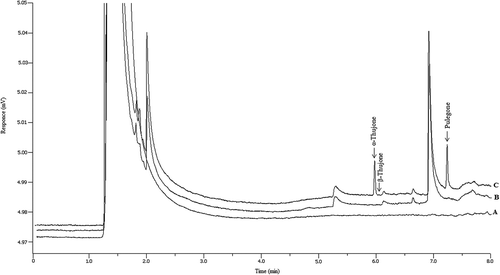
The accuracy of the method was determined from recovery of spiked beverage samples at three concentrations using stock standard solutions. Three types of beverages were used: alcoholic beer, soft drinks and herbal ice tea. The recovery results were in the range of (97–112) % for α-thujone and (87–120) % for pulegone with RSD% less than 19% for both. The selectivity of the method was verified as no interferences either from blank or beverage sample components were observed (). Positive identification of the analyte peaks was confirmed by the GC-MS spectra where the MS profiles are similar to those of the library database.
Analysis of beverage samples
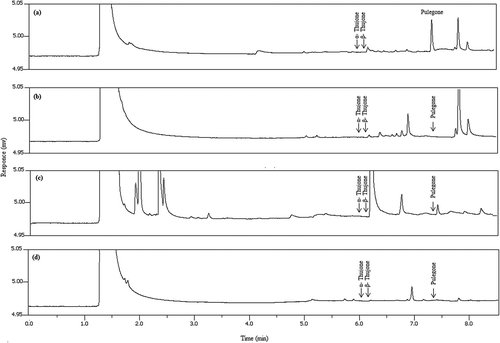
The proposed SALLE procedure was applied to a total of 43 beverage samples. Thujones (α and β isomers) were not detected in all the tested samples while pulegone was found in two samples, that is herbal and fruit drinks (below the LOQ of the method) and mint flavoured lemonade (1.78 ± 0.21 mg.kg−1). That pulegone was found in the second sample is not surprising as pulegone is naturally found in mint. [Citation24] Positive identification of the pulegone peak was confirmed by the GC-MS spectra where the MS profiles are similar to those of the library database. shows typical chromatograms for the different types of beverages.
Comparison to previously reported analytical methods
summarises the important analytical characteristics of the proposed SALLE method for the determination of thujones and pulegone in beverage compared to some previously reported methods. It is readily apparent that the proposed method offers numerous advantageous features in terms of sensitivity, simplicity and using the common and cheap detector (i.e., FID). The good enrichment of the SALLE method used (about 10-fold) is translated into low LOQ (12.9 and 15.3 µg.kg–1 for thujones and pulegone, respectively). This is superior even to MS detection for thujone (50 µg.kg–1) [Citation10,Citation16] and for pulegone (60 µg.kg–1).[Citation16] The obtained LOQ for thujone is superior to the SPME work reported by Schafer et al.[Citation25] using MS detector (21 µg.kg−1) and in the same work the FID was used but the LOQ was higher (300 µg.kg−1) . The proposed SALLE is simple and unlike the SPE method, does not require multi-steps washing, loading and elution. [Citation15,Citation26] An earlier GC-MS method used liquid-liquid extraction as sample preparation[Citation16] required 30 min for the mixing. On the other hand, our proposed method required only ~ 3 min for the entire sample preparation step. Also, the SALLE procedure can be easily performed by analytical chemists with minimum training and is simpler than the SPME procedure that requires delicate handling of the fibres.
Table 3. Comparison between the proposed SALLE-GC method and some reported methods for the determination of thujone and pulegone in beverages.
Conclusion
The use of the SALLE technique for the extraction of thujones and pulegone in beverages has been demonstrated for the first time. The enrichment of the analytes into the small volume of the organic layer enables direct injection into GC, achieving low quantitation levels. The hallmarks of the proposed method are the simplicity and use of readily available glasswares, thus making it attractive even for laboratories with basic infrastructure. The proposed procedure was successfully applied for the determination of thujones and pulegone in alcoholic and non-alcoholic beverages that were obtained from Malaysian markets.
Acknowledgement
USM fellowship scheme to Anas Alshishani are acknowledged. This article does not contain any studies with human participants or animals performed by any of the authors. All authors declare that there is no conflict of interest.
Additional information
Funding
References
- Sirisoma, N. S.; Höld, K. M.; Casida, J. E. Alpha- and beta-Thujones (Herbal Medicine and Food Additives): Synthesis and Analysis of Hydroxy and Dehydro Metabolites. J. Agric. Food Chem. 1915–1921, 49(2001). doi:10.1021/jf001445+.
- Dybowski, M. P.; Dawidowicz, A. L. The Determination of α- and β-thujone in Human Serum – Simple Analysis of Absinthe Congener Substance. Forensic Sci. Int. 2016, 259, 188–192. doi:10.1016/j.forsciint.2015.12.015.
- Baddar, N. W. A.-H.; Aburjai, T. A.; Taha, M. O.; Disi, A. M. Thujone Corrects Cholesterol and Triglyceride Profiles in Diabetic Rat Model. Nat. Prod. Res. 2011, 25, 1180–1184. doi:10.1080/14786419.2010.496116.
- Lachenmeier, D. W.; Emmert, J.; Kuballa, T.; Sartor, G. Thujone - Cause of Absinthism? Forensic Sci. Int.. 2006, 158, 1–8. doi:10.1016/j.forsciint.2005.04.010.
- Da Rocha, M. S.; Dodmane, P. R.; Arnold, L. L.; Pennington, K. L.; Anwar, M. M.; Adams, B. R.;, et al. Mode of Action of Pulegone on the Urinary Bladder of F344 Rats. Toxicological Sci. 2012, 128, 1–8. doi:10.1093/toxsci/kfs135.
- Box, P. O.;, Ntp Technical Report on the Toxicology and Carcinogenesis Studies of Α, Β -Thujone in F344/N Rats and B6C3F1 Mice National Toxicology Program, (2011).
- Moorthy, B.; Vijayasarathi, S. K.; Basu, A.; Madyastha, K. M. Biochemical, Histopathological and Ultrastructural Changes in Rat Liver Induced by R‐(+)‐Pulegone, a Monoterpene Ketone. Toxicological Environ. Chem. 1991, 33, 121–131.
- Gordon, W. P.; Huitric, A. C.; Seth, C. L.; McClanahan, R. H.; Nelson, S. D. The Metabolism of the Abortifacient terpene,(R)-(+)-pulegone, to a Proximate Toxin, Menthofuran. Drug Metabol. Disposit. 1987, 15, 589–594.
- European Commission. Regulation (EC) No 1334/2008. Off. J. Eur. Union. 2008, L 354(34), 34–50. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32008R1334.
- Bach, B.; Cleroux, M.; Saillen, M.; Schönenberger, P.; Burgos, S.; Ducruet, J.;, et al. A New Chemical Tool for Absinthe Producers, Quantification of α/β-thujone and the Bitter Components in Artemisia Absinthium. Food Chem. 2016, 213, 813–817. doi:10.1016/j.foodchem.2016.06.045.
- Lachenmeier, D. W.; Nathan-Maister, D.; Breaux, T. A.; Sohnius, E. M.; Schoeberl, K.; Kuballa, T. Chemical Composition of Vintage Preban Absinthe with Special Reference to Thujone, Fenchone, Pinocamphone, Methanol, Copper, and Antimony Concentrations. J. Agric. Food Chem. 2008, 56, 3073–3081. doi:10.1021/jf703568f.
- Kupska, M.; Wasilewski, T.; Jędrkiewicz, R.; Gromadzka, J.; Namieśnik, J. International Journal of Food Properties Determination of Terpene Profiles in Potential Superfruits. Int. J. Food Prop. 2016, 2912, 1532–2386. doi:10.1080/10942912.2016.1144066.
- Wang, L.; Hu, G.; Lei, L.; Lin, L.; Wang, D.; Wu, J. Identification and Aroma Impact of Volatile Terpenes in Moutai Liquor. Int. J. Food Prop. 2016, 19, 1335–1352. doi:10.1080/10942912.2015.1064442.
- Kıvançlı, J.; Elmacı, Y. Characterization of Turkish-Style Boiled Coffee Aroma by Gas Chromatography and Mass Spectrometry and Descriptive Analysis Techniques. Int. J. Food Prop. 1671–1686, 19(2016). doi:10.1080/10942912.2015.1080726.
- Scott, P. M.; Lawrence, G. A.; Lau, B. P. Y. Determination of Thujone by Derivatization with Dansylhydrazine and Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 1071–1081. doi:10.1081/JLC-120030179.
- Lopez, P.; Van Sisseren, M.; De Marco, S.; Jekel, A.; De Nijs, M.; Mol, H. G. J. A Straightforward Method to Determine Flavouring Substances in Food by GC-MS. Food Chem. 2015, 174, 407–416. doi:10.1016/j.foodchem.2014.11.011.
- Wen, Y.; Li, J.; Yang, F.; Zhang, W.; Li, W.; Liao, C.;, et al. Salting-Out Assisted Liquid-Liquid Extraction with the Aid of Experimental Design for Determination of Benzimidazole Fungicides in High Salinity Samples by High-Performance Liquid Chromatography. Talanta. 2013, 106, 119–126. doi:10.1016/j.talanta.2012.12.011.
- Park, H. J.; Jung, M. Y. One Step Salting-Out Assisted Liquid-Liquid Extraction Followed by UHPLC-ESI-MS/MS for the Analysis of Isoflavones in Soy Milk. Food Chem. 2017, 229, 797–804. doi:10.1016/j.foodchem.2017.02.145.
- Moreno-González, D.; Rodríguez-Ramírez, R.; Olmo-Iruela, M.; García-, A. M. Talanta Validation of a New Method Based on Salting-Out Assisted Liquid-Liquid Extraction and UHPLC-MS/MS for the Determination of Betalactam Antibiotics in Infant Dairy Products. Talanta. 2017, 167, 493–498. doi:10.1016/j.talanta.2017.02.045.
- Valente, I. M.; Gonçalves, L. M.; Rodrigues, J. A. Another Glimpse over the Salting-Out Assisted Liquid-Liquid Extraction in Acetonitrile/Water Mixtures. J. Chromatogr. 2013, 1308, 58–62. doi:10.1016/j.chroma.2013.08.014.
- Fan, Y.; Hu, S.; Liu, S. Salting-Out Assisted Liquid-Liquid Extraction Coupled to Dispersive Liquid-Liquid Microextraction for the Determination of Chlorophenols in Wine by High-Performance Liquid Chromatography. J. Sep. Sci. 2014, 37, 3662–3668. doi:10.1002/jssc.201400869.
- Yu, C.; Han, J.; Wang, Y.; Yan, Y.; Hu, S.; Li, Y.;, et al. Ionic Liquid/Ammonium Sulfate Aqueous Two-Phase System Coupled with HPLC Extraction of Sulfadimidine in Real Environmental Water Samples. Chromatographia. 2011, 74, 407–413. doi:10.1007/s10337-011-2079-2.
- Zhang, Y.; Cremer, P. S. Interactions between Macromolecules and Ions: The Hofmeister Series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. doi:10.1016/j.cbpa.2006.09.020.
- Siano, F.; Catalfamo, M.; Cautela, D.; Servillo, L.; Castaldo, D. Analysis of Pulegone and Its Enanthiomeric Distribution in Mint-Flavoured Food Products. Food Addit. Contam. 2005, 22, 197–203. doi:10.1080/02652030500041581.
- Schäfer, N.; Lachenmeier, D. W. Bestimmung Von α - Und β -Thujon in Spirituosen Mittels Festphasenmikroextraktion (HS-SPME): Vergleich Mit Konventionellen Extraktionsmethoden. Deutsche Lebensmittle-Rundschau. 2005, 101, 534–539.
- Dawidowicz, A. L.; Dybowski, M. P. Fast Determination of α- and β-thujone in Alcoholic Beverages Using Solid-Phase Extraction and Gas Chromatography. Food Contr. 2012, 25, 197–201. doi:10.1016/j.foodcont.2011.10.045.