 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study investigated the effects of different heating times (30–150 min) at 100°C on nutraceuticals and antioxidant properties of Lonicera japonica Thunb. (LJ). Total phenolic, phenolic acid (chlorogenic acid, caffeic acid, 4,5-dicaffeoylquinic acid, and 3,5-dicaffeoylquinic acid) and flavonoids (rutin, quercetin, and luteolin) in LJ were significantly increased after heat treatments. Antioxidant activities, such as DPPH radical scavenging activity, ABTS radical scavenging activity, FRAP and reducing power, of LJ, were improved after heating. Antioxidant activities were positively correlated with total phenolic, total flavonoid, chlorogenic acid, caffeic acid, and quercetin contents.
Introduction
Lonicera japonica Thunb. (LJ), belonging to the Caprifoliaceae family, is native in East Asia. Nowadays, LJ has been widely used in the food industry because of its health benefits, such as antioxidant activity,[Citation1–Citation3] anti-inflammatory activity,[Citation2,Citation4] and antibacterial activity.[Citation5] It has been reported that LJ has numerous phytochemicals, and the main active constituents are phenolic compounds such as phenolic acids and flavonoids.[Citation6] Among them, chlorogenic acid, a phenolic acid is, a standard compound used to assess the chemical quality of LJ.[Citation7]
Most phenolic compounds form covalent bonds with cellular structures, including cellulose, hemicellulose, and lignin, and are present in bound forms in plants. Especially, bound phenolic acids can form ester linkages with the structural carbohydrates and ether linkages with lignin.[Citation8] Since bound phenolic compounds can not function as antioxidants, one must break down those linkages to convert bound phenolics into free forms. Acosta-Estrada et al.[Citation9] demonstrated that several food-processing methods, such as heating, malting, and fermentation, can increase the liberation of bound phenolic compounds in a food matrix and can improve the biological activities of food.
Heating is a common method employed in food processing. In general, heating causes the loss of heat-sensitive compounds, thus reducing the nutritional quality.[Citation10] Therefore, heating can be an unfavorable method in food processing, because consumers demand high-quality, cheap, and convenient products with great nutritional value.[Citation11]
However, improvement of phenolic compounds contents and antioxidant properties of various heated foods has been reported. According to Kim et al.,[Citation12] heating at 100°C for 30–90 min significantly improved total phenolic contents in grape-seed extracts. Furthermore, significant increases in the FRAP value of grape-seed flour were observed after heating at 100°C for 50–60 min.[Citation13] Heating can improve nutrition values in various foods by destroying cell structures and can lead to the release of bound phenolics, which implies great bioaccessibility.[Citation14]
To date, there is limited information on how nutraceuticals and antioxidant properties of LJ are affected by heating for different heating times. Therefore, the objectives of this study were to elucidate the effect of heating time (30–150 min) on (1) individual phenolic acid contents (chlorogenic acid, caffeic acid, 4,5-dicaffeoylquinic acid, and 3,5-dicaffeoylquinic acid) and individual flavonoids (rutin, quercetin, and luteolin) in LJ and (2) the antioxidant properties (DPPH radical scavenging activity, ABTS radical scavenging activity, FRAP value, and reducing power) of LJ.
Materials and methods
Materials
The whole plants of LJ were purchased from an online market (Goseong, Korea) and were deposited in the herbarium of the Department of Food and Nutrition, Kyunghee University under the voucher number KCL213. Chlorogenic acid, caffeic acid, 4,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, rutin, quercetin, luteolin, quinic acid, malic acid, shikimic acid, citric acid, and succinic acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals and reagents were HPLC or analytical grade.
Heat treatment
LJ samples (12 g, dw), in Erlenmeyer flasks, were sealed and equilibrated at 4°C for 24 h. Samples were then heated at 100°C for different periods (30, 60, 90, 120, and 150 min) in a dry oven (Thermo Stable EOF-155, DAIHAN Scientific Co., Korea). All samples were stored in a freezer (-18°C) until analysis.
pH and soluble solids
The pH values of heat-treated LJ samples were measured by a pH-meter (ORION 3 STAR pH Benchtop, Thermo Scientific, Waltham, MA, USA). Soluble solids of heat-treated LJ were estimated with a portable refractometer (PAL-alpha, ATAGO CO. LTD., Tokyo, Japan) previously adjusted to zero with distilled water.
Chlorophyll contents
Chlorophyll in heat-treated LJ samples was measured according to Medina-Meza et al.[Citation15], with slight modifications. One gram of sample was homogenized at 15,000 rpm in 25 mL of 80% (v/v) acetone solution for 1 min and centrifuged at 3,500 rpm for 10 min. The supernatant was obtained and absorbance was measured at 647 and 663 nm using a spectrophotometer (Thermo Scientific, Vantaa, Finland). Chlorophyll a, b, and total chlorophyll contents were calculated as follows:
Where A663 is the absorbance at 663 nm and A647 is the absorbance at 647 nm.
Color values
The color values of heat-treated LJ samples were measured using a Minolta Chroma Meter CR-400 (Konica Minolta, Tokyo, Japan) calibrated using a white tile. The results were expressed as CIE L* (lightness), a* (redness), and b* (yellowness). The net color difference (ΔE), hue angle (H°), and chroma or saturation index (C*) were calculated using L*, a*, and b* values as follows:
Total phenolic and total flavonoid contents
The total phenolic contents in heat-treated LJ samples were measured using the Folin-Ciocalteau reagent method described by Kähkönen et al.[Citation16], with slight modifications. Briefly, 10 mL of a 70% (v/v) methanol solution was added to 0.1 g of heat-treated LJ samples. Each suspension was vortexed for 1 min, sonicated for 30 min at room temperature and then centrifuged at 3,500 rpm for 20 min. Then, the supernatant was diluted 5 times with distilled water. The sample solution (0.5 mL) was mixed with 0.5 mL of 1 N Folin-Ciocalteau reagent. After 3 min, 1.5 mL of 10% (w/v) sodium carbonate solution was added to the mixture. Next, the mixture was incubated in darkness at room temperature for 60 min. The absorbance of the mixture was measured at 725 nm using a spectrophotometer (Thermo Scientific, Vantaa, Finland). Gallic acid (0–100 ug/mL) was used as a standard, and the results were expressed as micrograms of gallic acid equivalent per 100 mg of sample (ug GAE/100 mg).
The total flavonoids in the heat-treated LJ samples were measured using the aluminum-chloride colorimetric method described by Zhu et al.[Citation17], with slight modifications. Briefly, 10 mL of 70% (v/v) methanol solution was added to 0.1 g of heat-treated LJ samples. Each suspension was vortexed for 1 min, sonicated for 30 min at room temperature and then centrifuged at 3,500 rpm for 20 min. Then, the supernatant was diluted 5 times with 70% (v/v) methanol. The sample solution (0.3 mL) was mixed with an equal volume of distilled water followed by an addition of 30 uL of 5% (w/v) NaNO2. The mixture was incubated at room temperature for 5 min and the 60 uL of 10% (w/v) AlCl3 was added to the mixture. Next, the mixture was incubated at room temperature for 5 min, and then 200 uL of 1 M NaOH was added to the mixture. The absorbance of the mixture was measured at 500 nm using a spectrophotometer (Thermo Scientific, Vantaa, Finland). Rutin (0–200 ug/mL) was used as a standard, and the results were expressed as micrograms of rutin equivalent per 100 mg of sample (ug RE/100 mg).
Measurement of individual phenolic acids and flavonoids using RP-HPLC
Sample preparation for measuring individual phenolic acids and flavonoids: Accurately quantified 0.1 g of heat-treated LJ samples were mixed with 5 mL of 70% (v/v) methanol solution. Each suspension was vortexed for 1 min, sonicated for 30 min at room temperature, and then centrifuged at 3,500 rpm for 20 min. The supernatant was filtered through a 0.45 um membrane filter (Millipore, Ireland) prior to RP-HPLC analysis.
Quantification of individual phenolic acids using RP-HPLC: An Agilent 1,200 HPLC system (Agilent Technologies, Palo Alto, USA) equipped with a G1322A vacuum degasser, a G1311A quaternary pump, a G1315D diode array detector (DAD), a G1316A column compartment, and a G1313A autosampler was used to obtain HPLC chromatograms for heat-treated samples. The system was controlled with Agilent Chemstation software (Agilent Technologies, Palo Alto, USA). Chromatographic separations were done on a Zorbax Eclipse XDB-C18 reversed-phase column (4.6×250 mm i.d., 5 um) maintained at 25°C. The mobile phase was 0.2% (v/v) phosphoric acid aqueous water (A) and methanol (B). Gradient elution was done as follows: 0–30 min, 30–60% B. The flow rate was 1 mL/min and the injection volume was 10 uL.
Individual phenolic acids (chlorogenic acid, caffeic acid, 4,5-dicaffeoylquinic acid, and 3,5-dicaffeoylquinic acid) were verified at a wavelength of 340 nm and used for RP-HPLC quantification. The individual phenolic acids in heat-treated LJ samples were confirmed by comparing their retention times and UV spectra with those of standards using a concentration range of 10 to 500, 0.1 to 10, 1 to 100, and 10 to 250 ug/mL, respectively. The R2 values were 0.99996, 0.99991, 0.99996, and 0.99996, respectively.
Quantification of individual flavonoids using RP-HPLC: An Agilent 1,200 HPLC system (Agilent Technologies, Palo Alto, USA) equipped with a G1322A vacuum degasser, a G1311A quaternary pump, a G1315D diode array detector (DAD), a G1316A column compartment, and a G1313A autosampler was used to obtain HPLC chromatograms for heat-treated samples. The system was controlled with Agilent Chemstation software (Agilent Technologies, Palo Alto, USA). Chromatographic separations were done on a Zorbax Eclipse XDB-C18 reversed-phase column (4.6×250 mm i.d., 5 um) maintained at 25°C. The mobile phase was 0.2% (v/v) phosphoric acid aqueous water (A) and methanol (B). Gradient elution was done as follows: 0–30 min, 30–60% B. The flow rate was 1 mL/min and the injection volume was 10 uL.
Individual flavonoids (rutin, quercetin, and luteolin) were verified at a wavelength of 340 nm and used for RP-HPLC quantification. Rutin, quercetin, and luteolin in heat-treated LJ samples were confirmed by comparing their retention times and UV spectra with those of standards using a concentration range of 1 to 50, 0.1 to 5, and 0.1 to 10 ug/mL, respectively. The R2 values were 0.99953, 0.99388, and 0.99990, respectively.
Measurement of individual organic acids using HPLC
Sample preparation for measuring individual organic acids: Accurately quantified 0.1 g of heat-treated LJ samples were mixed with 5 mL of distilled water. Each suspension was vortexed for 1 min, sonicated for 30 min at room temperature and then centrifuged at 3,500 rpm for 20 min. The supernatant was filtered through a 0.45 um membrane filter (Millipore, Ireland) prior to HPLC analysis.
Quantification of organic acids using HPLC: The same Agilent 1,200 HPLC system as described was used to obtain HPLC chromatograms for heat-treated samples. Chromatographic separations were done on a Zorbax SB-Aq column (4.6×150 mm i.d., 5 um) maintained at 25°C. The mobile phase was 200 mM aqueous phosphate buffer (pH 2.0)/acetonitrile (99:1, v/v). The flow rate was 0.6 mL/min and the injection volume was 10 uL.
Quinic acid, malic acid, shikimic acid, citric acid, and succinic acid were verified at a wavelength of 214 nm, with a reference wavelength of 360 nm, and used for HPLC quantification. Organic acid compounds in heat-treated LJ samples were confirmed by comparing their retention times and UV spectra with those of standards using a concentration range of 20 to 1,000, 10 to 500, 1 to 10, 10 to 500, and 10 to 200 ug/mL, respectively. The R2 values were 0.99998, 1.00000, 0.99998, 0.99999 and 0.99977, respectively.
Measurement of antioxidant properties
Sample preparation for measuring antioxidant properties: Accurately quantified 0.1 g of heat-treated LJ samples were mixed with 10 mL of 70% (v/v) methanol solution. Each suspension was vortexed for 1 min, sonicated for 30 min at room temperature, and then centrifuged at 3,500 rpm for 20 min. The supernatant was diluted 10 times with 70% (v/v) methanol and was used for subsequent analysis.
DPPH radical scavenging activity: The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities of heat-treated LJ samples were measured by the method of Blois[Citation18], with slight modifications. The sample solution (0.2 mL) was mixed with 0.6 mL of 200 mM DPPH solution. The mixture was incubated in darkness at room temperature for 15 min. The absorbance of the mixture was measured at 517 nm using a spectrophotometer (Thermo Scientific, Vantaa, Finland). Ascorbic acid (0–60 ug/mL) was used as an antioxidant standard. The DPPH radical scavenging activity was calculated as follows:
Where A0 is the absorbance of the blank and A1 is the absorbance of the test compound.
ABTS radical scavenging activity: The 2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activities of heat-treated LJ samples were measured by the method of Shalaby et al.[Citation19], with slight modifications. The radical cation was prepared by mixing 7 mM ABTS stock solution with 2.45 mM potassium persulfate (1:1, v/v), leaving the mixture for 12–16 h until the reaction was completed and the absorbance was stable. The ABTS+ solution was diluted with methanol to the absorbance of 0.700 ± 0.005 at 734 nm. Then, the sample solution (0.1 mL) was mixed with 0.9 mL of ABTS+ solution. The mixture was incubated in darkness at room temperature for 6 min. The absorbance of the mixture was measured at 734 nm using a spectrophotometer (Thermo Scientific, Vantaa, Finland). Trolox (0–90 ug/mL) was used as an antioxidant standard. The ABTS radical scavenging activity was calculated as follows:
Where A0 is the absorbance of the blank and A1 is the absorbance of the test compound.
Ferric reducing antioxidant power (FRAP) assay:Ferric reducing antioxidant powers (FRAP) of heat-treated LJ samples were measured by the method of Benzie and Strain[Citation20], with slight modifications. FRAP reagent was prepared by mixing 300 mM sodium acetate buffer (pH 3.6) with 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl and 20 mM FeCl3 solution at a ratio of 10:1:1 (v/v/v) and was warmed at 37°C. Then, the sample solution (0.15 mL) was mixed with 2.85 mL of FRAP reagent and incubated in darkness at 37°C for 15 min. The absorbance of the mixture was measure at 593 nm using a spectrophotometer (Thermo Scientific, Vantaa, Finland). FeSO4 · 7H2O (0–1 mM) was used as a standard, and the results were expressed as millimole of Fe2+·7H2O equivalent per 100 mg of sample (mM Fe2+·7H2O/100 mg).
Reducing power: The reducing powers of heat-treated LJ samples were measured by the method of Sanjukta et al.[Citation21], with slight modifications. Briefly, the sample solution (0.1 mL) was mixed with 0.9 mL of 200 mM phosphate buffer (pH 6.6) and 1% (w/v) potassium ferric cyanide. The mixture was incubated at 50°C for 20 min. Then, 0.9 mL of 10% (w/v) trichloroacetic acid was added to the mixture and centrifuged at 3,500 rpm for 20 min. The supernatant (0.9 mL) was mixed with 0.9 mL of distilled water and 0.9 mL of 0.1% (w/v) FeCl3. The absorbance of the mixture was measured immediately at 700 nm using a spectrophotometer (Thermo Scientific, Vantaa, Finland). Ascorbic acid (0–100 ug/mL) was used as a standard, and the results were expressed as micrograms of ascorbic acid equivalent per one milligram of sample (ug AAE/mg).
Statistical analysis
All statistical analyses were done using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). Analysis of variance (ANOVA) was done using the general linear models (GLM) procedure to find significant differences between the samples. Means were compared by using Fisher’s least significant difference (LSD) procedure. Significance was defined at the 5% level. In addition, Pearson correlation coefficients between the samples were calculated. The CORR procedure was used to obtain correlation coefficients.
Results and discussion
pH values and soluble solid contents
The pH values and soluble solid contents of LJ samples heated for 30–150 min are given in . The pH values of heat-treated LJ samples were almost constant during heating for 60 min but began to decrease significantly after 90 min of heating. The decrease in pH values after heating for more than 90 min can be attributed to the increasing organic acid contents in LJ. Soluble solid contents in LJ were not significantly affected by heating for different heating times (30–150 min).
Table 1. Effect of different heating times on pH and soluble solid contents of Lonicera japonica Thunb. at 100°C
Chlorophyll contents
Chlorophylls are the important group of biologically active compounds with health promoting properties. Structurally, chlorophylls are composed of a porphyrin ring replaced by a centrally bound Mg2+ atom and a highly hydrophobic esterified phytol tail (C20H39). This structure is sensitive to high temperatures; therefore, heat can degrade chlorophylls.[Citation22]
Changes of chlorophyll contents in LJ during heating at 100°C for 30–150 min are shown in . Chlorophyll a, b and total chlorophyll contents gradually decreased with increasing heating time from 30 to 150 min. After heating for 150 min, contents of chlorophyll a and b were decreased about 45% and 27% compared to those of the control, respectively. According to Benlloch-Tinoco et al.,[Citation22] structures of chlorophylls are sensitive to high temperatures, so heating degraded the chlorophyll a and b into pheophytins and pyropheophytins. Pheophytins were formed by the displacement of the central Mg2+ atom from the chlorophyll phorphyrin ring with hydrogen. Under more extreme heating, the carbomethoxy group at the C10 of pheophytins was removed, and pyropheophytins were newly formed. In this study, we suspected that more chlorophylls were converted to pheophytins or pyropheophytins with longer heating times.
Table 2. Effect of different heating times on chlorophyll content in Lonicera japonica Thunb. at 100°C
Color values
Changes of the color values (L*, a*, b*, ΔE, H°, and C*) of LJ after heating at 100°C with different heating time (30–150 min) are shown in . The L* values of LJ samples heated for 30–150 min were significantly decreased compared to those of the control, showing that LJ became dark after heating. Values of a* and b* of LJ were elevated with increasing heating time from 30 to 150 mi; that is, the green component of color became less intense (increase in a*) and the yellow component of color became more intense (increase in b*). These results could be related to the degradation of chlorophylls in LJ, because chlorophylls were degraded into pheophytins and pyropheophytins under the high temperature, resulting in discoloration of LJ from bright green to yellowish-brown.[Citation22]
Table 3. Effect of different heating times on color values of Lonicera japonica Thunb. at 100°C
In general, a net color difference (ΔE) higher than 1.8 is perceptible by the human eye and a value greater than 5.0 is regarded as a remarkable difference.[Citation23] As shown in , all values of ΔE of LJ samples heated for 30–150 min were higher than 1.8. These values were generally elevated with increasing heating time from 30 to 150 min. After heating for 120 and 150 min, the ΔE values were greater than 5.0 (5.0 ± 0.1 and 5.7 ± 0.2, respectively), presenting a remarkable color difference from the control. Therefore, heating for less than 90 min was desirable for retaining the original color of LJ.
Hue angle (H°) is a qualitative indicator of the chromatic nature of the color. It is expressed in degrees (0° or 360° for red, 90°, 180°, and 270° for yellow, green, and blue, respectively). With increasing heating time from 30 to 150 min, the H° value of LJ was less than 90°. Chroma (C*) value characterizes the quantitative attribute of colorfulness and is proportional to its intensity.[Citation24] The C* value of LJ significantly increased up to 60 min of heating. This marked effect on the color of LJ by heating for 30–150 min is related to the decrease in chlorophyll contents (), possibly because of the formation of pheophytins and pyropheophytins from the degradation of chlorophylls.[Citation23]
Total phenolic and total flavonoid contents
Changes of total phenolic and total flavonoid contents in LJ during heating with different heating times (30–150 min) are given in . Total phenolic and total flavonoid contents in the control were 2013 ± 23 ug GAE/100 mg and 4495 ± 99 ug RE/100 mg, respectively. Compared to the control, significant increases in total phenolic and total flavonoid contents were found in the LJ samples heated for 30–60 min. In particular, the maximum contents of total phenolic (2594 ± 73 ug GAE/100 mg) and total flavonoid (5644 ± 57 ug RE/100 mg) contents in LJ were observed at 60 min of heating time. According to Uslu & Özcan,[Citation25] total phenolic contents in the heat-treated sour cherry extract (160°C, 60 min) were increased compared to those in the non-heated one. In addition, Sharma et al.[Citation10] reported that total flavonoid contents in onion were increased after heating (80–150°C, 30 min). Uslu & Özcan[Citation25] proposed that the increases in total phenolic and total flavonoid contents after heating were caused by disruption of cell walls, resulting in conversion or releasing of bound phenolics into free forms. Furthermore, Papoutsis et al.[Citation26] suggested that thermal dehydration can release the bound phenolic groups by breaking down the cell structures of the food matrix, because phenolic compounds were primarily present in bound forms linked to the cell walls. Therefore, we suggest in this study that the increases in total phenolic and total flavonoid contents in LJ samples heated for 30–60 min resulted from the disruption of cell walls during heating, subsequently leading to the conversion or release of bound phenolics into free forms.
Table 4. Effect of different heating times on total phenolic and total flavonoid contents in Lonicera japonica Thunb. at 100°C
In contrast, there decreasing trends of the total phenolic contents and total flavonoid contents in LJ after longer heating times of 120 and 90 min, respectively, although both contents were still significantly higher than those of the control. As previously described by Henríquez et al.,[Citation27] total phenolic contents in apple peel by-products were decreased by drum-drying (110°C for 120 sec and 140°C for 30 sec). Moreover, Sharma et al.[Citation10] reported that total flavonoid contents in onions were decreased after heating at 150°C for 30 min. Those authors suggested that the decreases in total phenolic and total flavonoid contents after heating are induced by too high a temperature. Accordingly, in this study, the reductions of the total phenolic and total flavonoid contents in LJ after heating (120 and 90 min, respectively) were induced by the long exposure periods and the low thermal stability of phenolics and flavonoids. Therefore, we think that heating at 100°C up to 150 min could improve the total phenolic and total flavonoid contents in LJ, but heating for 60 min was the best treatment for increasing total phenolic and total flavonoid contents in LJ.
Quantification of individual phenolic acids using RP-HPLC
The effects of different heating time (30–150 min) on changes in the contents of the four phenolic acids in LJ were identified and quantified by RP-HPLC (). Chlorogenic acid, the ester of caffeic acid and quinic acid, is the major component in LJ.[Citation28] It has been reported that it has antioxidant activities in vitro by scavenging reactive oxygen species,[Citation29] antidiabetic activities by suppression of hepatic gluconeogenesis,[Citation30] and anti-obesity activities in vivo by reducing preadipocyte population growth.[Citation31]
Table 5. Quantification of individual phenolic acids and flavonoids in Lonicera japonica Thunb. after heating at 100°C with different heating time
In this study, chlorogenic acid in LJ was significantly increased by up to 60 min of heating. The chlorogenic acid content in the control was 1007 ± 37 ug/100 mg. After heating for 60 min, the chlorogenic acid content (1367 ± 2 ug/100 mg) was significantly increased and was retained up to 90 min, perhaps for the following reasons. First, the increases in chlorogenic acid contents in LJ samples heated for 30–90 min could result from the destruction of cell walls during heating. According to Acosta-Estrada et al.,[Citation9] chlorogenic acid in foods forms ester linkages with carbohydrates and proteins through their carboxyl groups and ether linkages with lignin through their hydroxyl groups. They reported that heat can break down the ester and ether linkages between bound phenolic groups and other food matrixes, consequently releaseing bound chlorogenic acid into free forms.
Second, the increases in chlorogenic acid contents in LJ after heating for 30–90 min could result from the hydrolysis of dicaffeoylquinic acid into chlorogenic acid. Clifford et al.[Citation32] reported that the amounts of chlorogenic acid doubled after 5 min of roasting time because of the partial hydrolysis of dicaffeoylquinic acid such as 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid to chlorogenic acid. Accordingly, we think the chlorogenic acid contents in LJ samples heated for 30–90 min were increased by the release of chlorogenic acids by the destruction of cell walls and the hydrolysis of dicaffeoylquinic acid to chlorogenic acid.
However, when the heating time was over 120 min, chlorogenic acid in LJ began to significantly decrease. We can suggest four mechanisms on for these. First, the acyl migration could induce the reductions of chlorogenic acid in LJ during heating for 120–150 min. Chlorogenic acid is referred to 5-caffeoylquinic acid according to IUPAC numbering, and it has isomers, such as neochlorogenic acid (3-caffeoylquinic acid) and cryptochlorogenic acid (4-caffeoylquinic acid).[Citation33] According to Liang et al.,[Citation34] the chlorogenic acid in coffee was significantly decreased after roasting at 210°C for 12 min, whereas the levels of neochlorogenic acid and cryptochlorogenic acid were significantly increased. These authors mentioned that the reason for the decreases in chlorogenic acid and increases in neochlorogenic acid and cryptochlorogenic acid after roasting was acyl migration via the formation of an ortho-ester intermediate. Acyl migration took place by the heating before dehydration at the quinic acid moiety (lactonization), which moved the 5-acyl group in chlorogenic acid to position C3 (neochlorogenic acid) and C4 (cryptochlorogenic acid).[Citation35]
Second, the decreases in chlorogenic acid content in LJ during heating for 120–150 min could be caused by lactonization of chlorogenic acid. Jeon et al.[Citation33] explained that the content of chlorogenic acid in medium roasted coffee was about 3–5 times higher than that in medium-dark roasted coffee, regardless of the coffee’s country of origin. They showed that chlorogenic acid was dehydrated during the roasting, thereby producing the corresponding lactones by the loss of a water molecule from the quinic acid moiety. Moreira et al.[Citation36] also mentioned that chlorogenic acid newly generated an intramolecular ester bond in quinic acid between C1 and C5, mainly by losing a water molecule from the quinic acid moiety, consequently converting into chlorogenic acid lactones (3-caffeoylquinic acid lactone and 4-caffeoylquinic acid lactone).
Third, hydrolysis could be why chlorogenic acid is reduced in LJ after heating for 120–150 min. Moreira et al.[Citation36] showed that chlorogenic acid was hydrolyzed to the phenolic moiety (caffeic acid) and non-phenolic moiety (quinic acid) by roasting. Moisture content (6.06%) in fresh LJ and released moisture by lactonization could act as a substrate for hydrolysis of chlorogenic acid to caffeic acid and quinic acid during heating.
Last, thermal degradation (pyrolysis) could be the reason for this decrease in chlorogenic acid in LJ. According to Moreira et al.,[Citation36] upon heating, chlorogenic acid resulted in decarboxylation forming simple phenols, such as pyrogallol, hydroxyhydroquinone, catechol, 4-ethylcatechol, and 4-methylcatechol. These authors said that the caffeic acid moiety of chlorogenic acid changed into catechol, 4-ethylcatechol, and 4-methylcatechol, and the quinic acid moiety of chlorogenic acid formed pyrogallol, hydroxyhydroquinone, and catechol during heating, subsequently causing the decreases in chlorogenic acid.
The amount of caffeic acid in LJ was significantly increased during 60 min of heating and was then retained up to 90 min, representing about a 54.2% increase over that in the control (). Del Pino-García et al.[Citation37] reported that the increases in caffeic acid in powdered red-wine pomace seasonings were observed after heating (90°C, 90 min) and suggested that this could be caused by release of caffeic acid bound to the food matrixes and by partial degradation of lignin, which liberated caffeic acid. Moreover, they also said that heating could break esterified forms of hydroxycinnamic acids, such as chlorogenic acid, leading to the increase in caffeic acid content. Therefore, in this study, the increases in caffeic acid in LJ samples heated for 30–90 min could have resulted from the the conversion of bound caffeic acids into free forms and hydrolysis of chlorogenic acid to caffeic acid.
In contrast, the amount of caffeic acid in LJ samples heated for more than 120 min began to decrease significantly. According to Dawidowicz & Typek,[Citation38] when coffee was roasted at 170–230°C for 10–30 min, caffeic acid was not found, because of its low thermal stability. Accordingly, we think that caffeic acid in LJ was degraded by 120 min of heating because the long exposure reduced the caffeic acid content in LJ.
Changes in the contents of the other phenolic acids (4,5-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid) in LJ after heating are shown in . The contents of 4,5-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid in LJ were significantly increased by 90 and 30 min of heating time, respectively. The 4,5-dicaffeoylquinic acid content in LJ heated for 90 min was about 2.26 times that of the control, and 3,5-dicaffeoylquinic acid content in LJ heated for 30 min was about 1.23 times that of the control. In general, isochlorogenic acids, such as 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid, are covalently bound to structural carbohydrates, protein, and lignin.[Citation8] Heating broke the linkages between isochlorogenic acids and cell walls, and consequently bound compounds became free forms.[Citation9] Therefore, in this study, the increases in 4,5-dicaffeoylquinic acid in LJ samples heated for 30–90 min and in 3,5-dicaffeoylquinic acid in LJ heated for 30 min could result from the transformation of bound isochlorogenic acids into free forms.
However, the contents of 4,5-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid in LJ were significantly decreased by 120 and 90 min of heating, respectively. Especially, the 3,5-dicaffeoylquinic acid content in LJ heated for 150 min was significantly lower than that of the control, with a retention rate of 71.2%, although 4,5-dicaffeoylquinic acid content in the control was doubled after 150 min of heating. According to Li et al.,[Citation39] the order of thermal stability was 4,5-dicaffeoylquinic acid >3,5-dicaffeoylquinic acid. Therefore, in this study, 3,5-dicaffeoylquinic acid in LJ was thermally degraded more than was the 4,5-dicaffeoylquinic acid in LJ during heating.
Quantification of individual flavonoids using RP-HPLC
The effect of different heating times (30–150 min) on changes in the contents of three flavonoids (rutin, quercetin, and luteolin) in LJ were identified and quantified by RP-HPLC (). In this study, the contents of rutin, quercetin, and luteolin in LJ began to increase by heating after 30 min. The contents of rutin, quercetin, and luteolin in the control were 36 ± 3, 6.5 ± 0.3, and 0.09 ± 0.02 ug/100 mg, respectively. The increases in rutin, quercetin, and luteolin in LJ were observed during 30, 60, and 90 min of heating time, respectively. According to Li et al.,[Citation40] when orange by-products were dried at 100°C for 48 h, the contents of rutin and luteolin were significantly increased. Furthermore, Juániz et al.[Citation41] reported that quercetin and luteolin in green pepper were significantly increased after heating twice at 150°C for 10 min and then 110°C for 5 min; they reported that heating softened the structures of vegetables by cell ruptures, leading to conversion of bound quercetin and luteolin into free forms. Therefore, we think the increases in rutin, quercetin, and luteolin in LJ during heating for 30, 60, and 90 min, respectively, were induced by conversion of those bound compounds into free compounds.
In contrast, both rutin and quercetin in LJ began to decrease significantly during 120 min of heating time. In particular, the contents of rutin and quercetin in LJ heated for 150 min were significantly lower than those of the control. The decreases in rutin and quercetin in LJ samples heated for 120–150 min were consistent with Chaaban et al.[Citation42] who reported that rutin was degraded by heating from 70 to 130°C for 120 min. In addition, Sharma et al.[Citation10] found that six onion varieties showed decreases in flavonoid contents, especially quercetin, after heating at 150°C for 30 min.
Quantification of individual organic acids using HPLC
The changes of contents of individual organic acids (quinic acid, malic acid, shikimic acid, citric acid, and succinic acid) in LJ during different heating times (30–150 min) were quantified by HPLC, as shown in . In this study, quinic acid was the major organic acid in LJ. As heating time passed from 30 to 150 min, quinic acid significantly increased from 2488 ± 4 to 2777 ± 15 ug/100 mg. Increases in quinic acid in LJ during heat treatments at 100°C for 30–150 min could result from the hydrolysis of chlorogenic acid and isochlorogenic acids into quinic acid. According to Moreira et al.,[Citation36] coffee roasting promoted hydrolysis of chlorogenic acid and isochlorogenic acid, thus forming quinic acid.
Table 6. Quantification of individual organic acids in Lonicera japonica Thunb. after heating at 100°C with different heating time
Shikimic acid content in LJ was significantly increased by 150 min of heating. Under high temperature, quinic acid could be transformed into quinic acid lactone or shikimic acid.[Citation35,Citation43] Jaiswal et al.[Citation43] showed that shikimic acid was formed by dehydration of quinic acid, losing 1-OH functionality. Deshpande et al.[Citation35] found that the conversion rate of quinic acid into shikimic acid was less than that of lactonization of quinic acid. Therefore, the significant but slight increments of the contents of shikimic acid in LJ during heating for 30–150 min could result from the transformation of quinic acid to shikimic acid.
Assessment of antioxidant properties
Antioxidant properties of LJ samples heated for 30–150 min were evaluated for DPPH radical scavenging activity, ABTS radical scavenging activity, FRAP value, and reducing power, and the results are given in . DPPH radical scavenging activity and ABTS radical scavenging activity reflect the ability of hydrogen-donating antioxidants and electron transfer to scavenge DPPH and ABTS+ radicals. In this study, findings on DPPH and ABTS radical scavenging activities of LJ during heating for 30–150 min showed similar tendencies. DPPH and ABTS radical scavenging activity of LJ were significantly increased during 60 min of heating and were retained for 90 min. In particular, after 60 min of heating, DPPH and ABTS radical scavenging activities of LJ were significantly improved by about 29 and 35%, respectively.
Figure 1. Effect of different heating times on antioxidant properties of Lonicera japonica Thunb. at 100°C. Different letters on the bars indicate significant differences (p < 0.05)
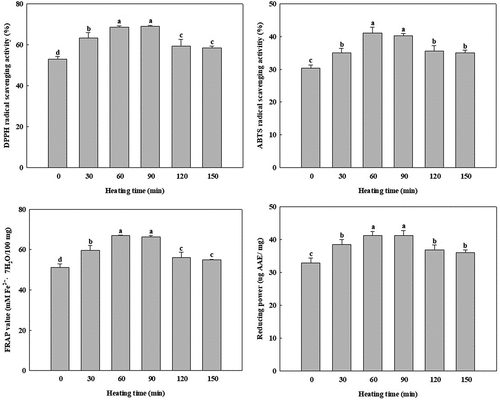
FRAP and reducing power assays represent their ability to reduce the of ferric (Fe3+) form to the ferrous (Fe2+) form. In the FRAP assay, the reaction of an antioxidant compound with a Fe3+-TPTZ complex formed a blue-colored Fe2+-TPTZ. Similarly, in the reducing power assay, a Prussian blue-colored complex was formed by adding FeCl3 to the Fe2+ form, and consequently reduction can be evaluated. We observed that the FRAP values and reducing powers of LJ were significantly increased by heating for 60 min.
The increasing trends of antioxidant properties of LJ after heating for 60 min corresponded to the results reported by Kao et al.[Citation44] and De Santiago et al.[Citation45] Kao et al.[Citation44] reported that Trolox equivalent antioxidant capacities of cilantro and sweet potato leaf were increased after boiling for 1 min. Also, De Santiago et al.[Citation45] reported that antioxidant properties of cactus cladodes were significantly improved after griddling (150°C, 5 min and then 110°C, 5 min). These authors proposed that the antioxidant properties were increased by the breakage of linkages between bound phenolics and cell walls by thermal hydrolysis of cell walls and subcellular compartments, leading to increases in free phenolics contents. Therefore, the improvement of antioxidant properties of LJ during heating for 60 min could result from the increase in free phenolic contents related to antioxidant properties of LJ.
When the heating time was over 120 min, we observed that antioxidant properties of LJ were significantly decreased. Nevertheless, DPPH radical scavenging activities, ABTS radical scavenging activities, FRAP values, and reducing powers of LJ samples heated for 120 and 150 min were significantly still higher than those of the control. According to Oancea et al.,[Citation46] 10% and 17% losses of DPPH radical scavenging activities were observed in sour-cherry extracts heated for 60 min at 100 and 150°C, respectively. They reported that DPPH radical scavenging activity declined during heating because phenolic or other compounds responsible for antioxidant capacity were degraded. Therefore, the declines of antioxidant properties of LJ after heating for 120 min resulted because the heating reduced the contents of phenolic compounds relevant to antioxidant capacities (). In conclusion, heating at 100°C for 60 min was the optimal treatment for improving antioxidant properties of LJ.
Pearson correlations
Pearson correlation coefficients between nutraceuticals (total phenolics, total flavonoids, chlorogenic acid, caffeic acid, 4,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, rutin, quercetin, and luteolin) and antioxidant properties (DPPH and ABTS radical scavenging activity, FRAP value, and reducing power) of LJ are shown in . Total phenolic content in LJ was positively correlated with the DPPH radical scavenging activity (r = 0.99, p < 0.01), ABTS radical scavenging activity (r = 0.96, p < 0.01), FRAP value (r = 0.96, p < 0.01), and reducing power (r = 0.99, p < 0.001). Also, total flavonoid content in LJ was positively correlated with the DPPH radical scavenging activity (r = 0.94, p < 0.01), ABTS radical scavenging activity (r = 0.86, p < 0.05), FRAP value (r = 0.91, p < 0.05), and reducing power (r = 0.95, p < 0.01). These findings were consistent with Foo et al.[Citation47] and Lin et al.[Citation48] who reported that total phenolic and total flavonoid contents had high correlation coefficient with antioxidant properties such as DPPH and ABTS radical scavenging activity, FRAP value, and reducing power (p < 0.05).
Table 7. Pearson correlations between nutraceuticals (total phenolic, total flavonoid, chlorogenic acid, caffeic acid, 4,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, rutin, quercetin, and luteolin) and antioxidant properties (DPPH radical scavenging activity, ABTS radical scavenging activity, FRAP, and reducing power) of heat-treated Lonicera japonica Thunb
Individual phenolic acids (chlorogenic acid and caffeic acid) and flavonoids (quercetin) in LJ were positively related to the antioxidant properties of LJ. Chlorogenic acid and caffeic acid in LJ were positively correlated with DPPH radical scavenging activity (r = 0.94–0.96, p < 0.01) and ABTS radical scavenging activity (r = 0.88–0.89, p < 0.05), and quercetin content in LJ was also positively correlated with DPPH radical scavenging activity (r = 0.83, p < 0.05). According to Miao et al.,[Citation49] the contents of chlorogenic acid, caffeic acid, and quercetin were negatively correlated with EC50 value of DPPH radical scavenging activity (p < 0.01). Also, Aires et al.[Citation50] showed that chlorogenic acid and caffeic acid were positively correlated with antioxidant activity (p < 0.001). In general, the antioxidant properties of phenolic acids depend on the position and number of hydroxyl groups linked to the aromatic ring. According to Kono et al.,[Citation51] chlorogenic acid and caffeic acid can donate an electron to stabilize nitrogen-centered free DPPH radicals. Wu[Citation52] also reported that chlorogenic acid can scavenge the DPPH radicals.
Furthermore, chlorogenic acid and caffeic acid in LJ had positive correlations with FRAP value (r = 0.94–0.97, p < 0.01) and reducing power (r = 0.95, p < 0.01), and quercetin content in LJ was also significantly related to FRAP value (r = 0.87, p < 0.05) and reducing power (r = 0.82, p < 0.05). Miao et al.[Citation49] showed that the contents of chlorogenic acid, caffeic acid, and quercetin were positively correlated with FRAP value (p < 0.01), and Lee et al.[Citation53] demonstrated that quercetin content was positively correlated with FRAP value (p < 0.01). According to Wu,[Citation52] chlorogenic acid reduced Fe3+ to Fe2+, and Firuzi et al.[Citation54] reported that quercetin could effectively reduce Fe3+ ions. Therefore, we found that the total phenolic and total flavonoid contents in LJ had strongly positive correlations with antioxidant properties. In particular, chlorogenic acid, caffeic acid, and quercetin contents significantly influenced the antioxidant properties of LJ, and those compounds were the main active compounds in LJ.
Conclusion
In this study, heating at 100°C with different heating times (30–150 min) were applied to LJ. According to the changes in chlorophyll contents in LJ after heating for 30–150 min, the decrease of chlorophyll of LJ. The total phenolic and total flavonoid contents in LJ, which were relevant to antioxidant properties, were significantly improved by 60 min of heating, so heating at 100°C for 60 min was the best treatment for improving total phenolic and total flavonoid contents in LJ. The analysis of individual phenolic acids (chlorogenic acid, caffeic acid, 4,5-dicaffeoylquinic acid, and 3,5-dicaffeoylquinic acid) and flavonoids (rutin, quercetin, and luteolin) in LJ by RP-HPLC revealed that the contents of individual phenolic acids and flavonoids were significantly increased during heating. In particular, 60 min of heating was adequate for improving the contents of chlorogenic acid, caffeic acid, and quercetin in LJ. The destruction of cell structures of LJ by heating made bound phenolic compounds into free forms, resulting in increases of phenolic compounds. Based on the results of antioxidant properties (DPPH and ABTS radical scavenging activity, FRAP value, and reducing power), we also observed that 60 min of heating was enough to elevate antioxidant properties of LJ. Since phenolic compounds can function as antioxidants, total phenolic, total flavonoid, chlorogenic acid, caffeic acid, and quercetin contents were positively correlated with the antioxidant properties of LJ. Consequently, these results suggest that heating at 100°C for a moderate duration (60 min in this study) can be used in LJ processing, improving several nutraceutical and antioxidant properties of LJ.
Acknowledgments
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01347103)” Rural Development Administration, Republic of Korea.
Additional information
Funding
References
- Gao, W.; Wang, R.; Li, D.; Liu, K.; Chen, J.; Li, H. J.; Xu, X.; Li, P.; Yang, H. Comparison of Five Lonicera Flowers by Simultaneous Determination of Multi-Components with Single Reference Standard Method and Principal Component Analysis. J. Pharm. Biomed. Anal. 2016, 117, 345–351. DOI: 10.1016/j.jpba.2015.09.008.
- Hsu, H. F.; Hsiao, P. C.; Kuo, T. C.; Chiang, S. T.; Chen, S. L.; Chiou, S. J.; Ling, X. H.; Liang, M. T.; Cheng, W. Y.; Houng, J. Y. Antioxidant and Anti-Inflammatory Activities of Lonicera Japonica Thunb. Var. Sempervillosa Hayata Flower Bud Extracts Prepared by Water, Ethanol and Supercritical Fluid Extraction Techniques. Ind. Crop. Prod. 2016, 89, 543–549. DOI: 10.1016/j.indcrop.2016.05.010.
- Choi, C. W.; Jung, H. A.; Kang, S. S.; Choi, J. S. Antioxidant Constituents and a New Triterpenoid Glycoside from Flos Lonicerae. Arch. Pharm. Res. 2007, 30(1), 1. DOI: 10.1007/bf02977770.
- Xu, Y.; Oliverson, B. G.; Simmons, D. L. Trifunctional Inhibition of COX-2 by Extracts of Lonicera Japonica: Direct Inhibition, Transcriptional and Post-Transcriptional down Regulation. J. Ethnopharmacol. 2007, 111(3), 667–670. DOI: 10.1016/j.jep.2007.01.017.
- Shi, Z.; Liu, Z.; Liu, C.; Wu, M.; Su, H.; Ma, X.; Zang, Y.; Wang, J.; Zhao, Y.; Xiao, X. Spectrum-Effect Relationships between Chemical Fingerprints and Antibacterial Effects of Lonicerae Japonicae Flos and Lonicerae Flos Base on UPLC and Microcalorimetry. Front. Pharmacol. 2016, 7, 12. DOI: 10.3389/fphar.2016.00012.
- Peng, X.; Duan, M. H.; Yao, X. H.; Zhang, Y. H.; Zhao, C. J.; Zu, Y. G.; Fu, Y. J. Green Extraction of Five Target Phenolic Acids from Lonicerae Japonicae Flos with Deep Eutectic Solvent. Sep. Purif. Technol. 2016, 157, 249–257. DOI: 10.1016/j.seppur.2015.10.065.
- Yuan, Y.; Wang, Z.; Jiang, C.; Wang, X.; Huang, L. Exploiting Genes and Functional Diversity of Chlorogenic Acid and Luteolin Biosyntheses in Lonicera Japonica and Their Substitutes. Gene. 2014, 534(2), 408–416. DOI: 10.1016/j.gene.2012.09.051.
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z. Improving Free, Conjugated, and Bound Phenolic Fractions in Fermented Oats (Avena Sativa L.) With Monascus Anka and Their Antioxidant Activity. J. Funct. Food. 2017, 32, 185–194. DOI: 10.1016/j.jff.2017.02.028.
- Acosta-Estrada, B. A.; Gutiérrez-Uribe, J. A.; Serna-Saldívar, S. O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. DOI: 10.1016/j.foodchem.2013.11.093.
- Sharma, K.; Ko, E. Y.; Assefa, A. D.; Ha, S.; Nile, S. H.; Lee, E. T.; Park, S. W. Temperature-Dependent Studies on the Total Phenolics, Flavonoids, Antioxidant Activities, and Sugar Content in Six Onion Varieties. J. Food Drug Anal. 2015, 23(2), 243–252. DOI: 10.1016/j.jfda.2014.10.005.
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of Thermal and High Pressure Processing on Antioxidant Activity and Instrumental Colour of Tomato and Carrot Purées. Innov. Food Sci. Emerg. Technol. 2009, 10(1), 16–22. DOI: 10.1016/j.ifset.2008.09.008.
- Kim, S. Y.; Jeong, S. M.; Park, W. P.; Nam, K. C.; Ahn, D. U.; Lee, S. C. Effect of Heating Conditions of Grape Seeds on the Antioxidant Activity of Grape Seed Extracts. Food Chem.. 2006, 97.3, 472–479. DOI: 10.1016/j.foodchem.2005.05.027.
- Ross, C. F.; Hoye, J.; Fernandez-Plotka, C. V. C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76(6). DOI: 10.1111/j.1750-3841.2011.02280.x.
- Leong, S. Y.; Oey, I. Effects of Processing on Anthocyanins, Carotenoids and Vitamin C in Summer Fruits and Vegetables. Food Chem. 2012, 133(4), 1577–1587. DOI: 10.1016/j.foodchem.2012.02.052.
- Medina-Meza, I. G.; Barnaba, C.; Villani, F.; Barbosa-Cánovas, G. V. Effects of Thermal and High Pressure Treatments in Color and Chemical Attributes of an Oil-Based Spinach Sauce. LWT-Food Sci. Technol. 2015, 60(1), 86–94. DOI: 10.1016/j.lwt.2014.09.033.
- Kähkönen, M. P.; Hopia, A. I.; Vuorela, H. J.; Rauha, J. P.; Pihlaja, K.; Kujala, T. S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47(10), 3954–3962. DOI: 10.1016/j.lwt.2014.09.033.
- Zhu, H.; Wang, Y.; Liu, Y.; Xia, Y.; Tang, T. Analysis of Flavonoids in Portulaca Oleracea L. By UV–Vis Spectrophotometry with Comparative Study on Different Extraction Technologies. Food Anal. Meth. 2010, 3(2), 90–97. DOI: 10.1007/s12161-009-9091-2.
- Blois, M. S.;. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958, 181(4617), 1199. DOI: 10.1038/1811199a0.
- Shalaby, E. A.; Shanab, S. M. Comparison of DPPH and ABTS Assays for Determining Antioxidant Potential of Water and Methanol Extracts of Spirulina Platensis. Indian J. Geo-Mar. Sci. 2013, 42(5), 556–564. DOI: 10.4172/2324-8661.1000105.
- Benzie, I. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239(1), 70–76. DOI: 10.1006/abio.1996.0292.
- Sanjukta, S.; Rai, A. K.; Muhammed, A.; Jeyaram, K.; Talukdar, N. C. Enhancement of Antioxidant Properties of Two Soybean Varieties of Sikkim Himalayan Region by Proteolytic Bacillus Subtilis Fermentation. J. Funct. Food. 2015, 14, 650–658. DOI: 10.1016/j.jff.2015.02.033.
- Benlloch-Tinoco, M.; Kaulmann, A.; Corte-Real, J.; Rodrigo, D.; Martínez-Navarrete, N.; Bohn, T. Chlorophylls and Carotenoids of Kiwifruit Puree are Affected Similarly or Less by Microwave than by Conventional Heat Processing and Storage. Food Chem. 2015, 187, 254–262. DOI: 10.1016/j.foodchem.2015.04.052.
- Finten, G.; Agüero, M. V.; Jagus, R. J.; Niranjan, K. High Hydrostatic Pressure Blanching of Baby Spinach (Spinacia Oleracea L.). LWT-Food Sci. Technol. 2016, 73, 74–79. DOI: 10.1016/j.lwt.2016.05.043.
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, JS.; Lemmenus, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M; Van Loey, A. Colour and Carotenoid Changes of Pasteurised Orange Juice during Storage. Food Chem. 2015, 171, 330–340. DOI: 10.1016/j.foodchem.2014.09.007.
- Uslu, N.; Özcan, M. M. Effect of Microwave Heating on Phenolic Compounds and Fatty Acid Composition of Cashew (Anacardium Occidentale) Nut and Oil. J. Saudi Soc. Agric. Sci. Online 1. 2017. DOI: 10.1016/j.jssas.2017.10.001.
- Papoutsis, K.; Pristijono, P.; Golding, J. B.; Stathopoulos, C. E.; Bowyer, M. C.; Scarlett, C. J.; Vuong, Q. V. Enhancement of the Total Phenolic Compounds and Antioxidant Activity of Aqueous Citrus Limon L. Pomace Extract Using Microwave Pretreatment on the Dry Powder. J. Food Process. Preserv. 2017, 41(5), e13152. DOI: 10.1111/jfpp.13152.
- Henríquez, C.; Córdova, A.; Almonacid, S.; Saavedra, J. Kinetic Modeling of Phenolic Compound Degradation during Drum-Drying of Apple Peel By-Products. J. Food Eng. 2014, 143, 146–153. DOI: 10.1016/j.jfoodeng.2014.06.037.
- Kang, M.; Jung, I.; Hur, J.; Kim, S. H.; Lee, J. H.; Kang, J. Y.; Jung, K. C.; Kim, K. S.; Yoo, M. C.; Park, D. S.;, et al. The Analgesic and Anti-Inflammatory Effect of WIN-34B, a New Herbal Formula for Osteoarthritis Composed of Lonicera Japonica Thunb and Anemarrhena Asphodeloides BUNGE in Vivo. J. Ethnopharmacol. 2010, 131(2), 485–496. DOI: 10.1016/j.jep.2010.07.025.
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403(1–2), 136–138. DOI: 10.1016/j.ijpharm.2010.09.035.
- Ong, K. W.; Hsu, A.; Tan, B. K. H. Anti-Diabetic and Anti-Lipidemic Effects of Chlorogenic Acid are Mediated by Ampk Activation. Biochem. Pharmacol. 2013, 85(9), 1341–1351. DOI: 10.1016/j.bcp.2013.02.008.
- Cho, A. S.; Jeon, S. M.; Kim, M. J.; Yeo, J.; Seo, K. I.; Choi, M. S.; Lee, M. K. Chlorogenic Acid Exhibits Anti-Obesity Property and Improves Lipid Metabolism in High-Fat Diet-Induced-Obese Mice. Food Chem. Toxicol. 2010, 48(3), 937–943. DOI: 10.1016/j.fct.2010.01.003.
- Clifford, M. N.; Jaganath, I. B.; Ludwig, I. A.; Crozier, A. Chlorogenic Acids and the Acyl-Quinic Acids: Discovery, Biosynthesis, Bioavailability and Bioactivity. Nat. Prod. Rep. 2017, 34(12), 1391–1421. DOI: 10.1039/c7np00030h.
- Jeon, J. S.; Kim, H. T.; Jeong, I. H.; Hong, S. R.; Oh, M. S.; Park, K. H.; Shim, J. H.; El-Aty, A. A. Determination of Chlorogenic Acids and Caffeine in Homemade Brewed Coffee Prepared under Various Conditions. J. Chromatogr. B. 2017, 1064, 115–123. DOI: 10.1016/j.jchromb.2017.08.041.
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D. D. Interactions between Major Chlorogenic Acid Isomers and Chemical Changes in Coffee Brew that Affect Antioxidant Activities. Food Chem. 2016, 213, 251–259. DOI: 10.1016/j.foodchem.2016.06.041.
- Deshpande, S.; Jaiswal, R.; Matei, M. F.; Kuhnert, N. Investigation of Acyl Migration in Mono-And Dicaffeoylquinic Acids under Aqueous Basic, Aqueous Acidic, and Dry Roasting Conditions. J. Agric. Food Chem.. 2014, 62(37), 9160–9170. DOI: 10.1021/jf5017384.
- Moreira, A. S.; Nunes, F. M.; Domingues, M. R.; Coimbra, M. A. Coffee Melanoidins: Structures, Mechanisms of Formation and Potential Health Impacts. Food Funct.. 2012, 3(9), 903–915. DOI: 10.1039/c2fo30048f.
- Del Pino-García, R.; González-SanJosé, M. L.; Rivero-Pérez, M. D.; García-Lomillo, J.; Muñiz, P. The Effects of Heat Treatment on the Phenolic Composition and Antioxidant Capacity of Red Wine Pomace Seasonings. Food Chem. 2017, 221, 1723–1732. DOI: 10.1016/j.foodchem.2016.10.113.
- Dawidowicz, A. L.; Typek, R. Transformation of Chlorogenic Acids during the Coffee Beans Roasting Process. Eur. Food Res. Technol. 2017, 243(3), 379–390. DOI: 10.1007/s00217-016-2751-8.
- Li, Y. J.; Zhang, C. F.; Ding, G.; Huang, W. Z.; Wang, Z. Z.; Bi, Y. A.; Xiao, W. Investigating the Thermal Stability of Six Caffeoylquinic Acids Employing Rapid-Resolution Liquid Chromatography with Quadrupole Time-Of-Flight Tandem Mass Spectrometry. Eur. Food Res. Technol. 2015, 240(6), 1225–1234. DOI: 10.1007/s00217-015-2425-y.
- Li, C. C.; Hsu, H. J.; Wang, Y. S.; Cassidy, J.; Sheen, S.; Liu, S. C. Effects of Heat Treatment on the Antioxidative and Anti-Inflammatory Properties of Orange By-Products. Food Funct. 2017, 8(7), 2548–2557. DOI: 10.1039/c7fo00188f.
- Juániz, I.; Ludwig, I. A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J. M.; Cid, C.; De Peña, M. P. Influence of Heat Treatment on Antioxidant Capacity and (Poly) Phenolic Compounds of Selected Vegetables. Food Chem. 2016, 197, 466–473. DOI: 10.1016/j.foodchem.2015.10.139.
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gerardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process. Preserv. 2017, 41(5). DOI: 10.1111/jfpp.13203.
- Jaiswal, R.; Matei, M. F.; Subedi, P.; Kuhnert, N. Does Roasted Coffee Contain Chlorogenic Acid Lactones Or/And Cinnamoylshikimate Esters? Food Res. Int. 2014, 61, 214–227. DOI: 10.1016/j.foodres.2013.09.040.
- Kao, F. J.; Chiu, Y. S.; Chiang, W. D. Effect of Water Cooking on Antioxidant Capacity of Carotenoid-Rich Vegetables in Taiwan. J. Food Drug Anal. 2014, 22(2), 202–209. DOI: 10.1016/j.jfda.2013.09.010.
- De Santiago, E.; Domínguez-Fernández, M.; Cid, C.; De Peña, M. P. Impact of Cooking Process on Nutritional Composition and Antioxidants of Cactus Cladodes (Opuntia Ficus-Indica). Food Chem. 2018, 240, 1055–1062. DOI: 10.1016/j.foodchem.2017.08.039.
- Oancea, A. M.; Turturică, M.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. Phytochemicals and Antioxidant Activity Degradation Kinetics during Thermal Treatments of Sour Cherry Extract. LWT-Food Sci. Technol. 2017, 82, 139–146. DOI: 10.1016/j.lwt.2017.04.026.
- Foo, S. C.; Yusoff, F. M.; Ismail, M.; Basri, M.; Yau, S. K.; Khong, N. M. H.; Chan, K. W.; Ebrahimi, M. Antioxidant Capacities of Fucoxanthin-Producing Algae as Influenced by Their Carotenoid and Phenolic Contents. J. Biotechnol. 2017, 241, 175–183. DOI: 10.1016/j.jbiotec.2016.11.026.
- Lin, S.; Guo, H.; Gong, J. D. B.; Lu, M.; Lu, M. Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D. T. Phenolic Profiles, β-glucan Contents, and Antioxidant Capacities of Colored Qingke (Tibetan Hulless Barley) Cultivars. J. Cereal Sci. 2018, 81, 69–75. DOI: 10.1016/j.jcs.2018.04.001.
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active Compounds, Antioxidant Activity and α-glucosidase Inhibitory Activity of Different Varieties of Chaenomeles Fruits. Food Chem. 2018, 248, 330–339. DOI: 10.1016/j.foodchem.2017.12.018.
- Aires, A.; Carvalho, R.; Saavedra, M. J. Reuse Potential of Vegetable Wastes (Broccoli, Green Bean and Tomato) for the Recovery of Antioxidant Phenolic Acids and Flavonoids. Int. J. Food Sci. Technol. 2017, 52(1), 98–107. DOI: 10.1111/ijfs.13256.
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamato, Y.; Matsui, Y.; Shibata, H. Iron Chelation by Chlorogenic Acid as a Natural Antioxidant. Biosci. Biotechnol. Biochem. 1998, 62(1), 22–27. DOI: 10.1271/bbb.62.22.
- Wu, L.;. Effect of Chlorogenic Acid on Antioxidant Activity of Flos Lonicerae Extracts. J. Zhejiang Univ.-SCI. B. 2007, 8(9), 673–679. DOI: 10.1631/jzus.2007.b0673.
- Lee, L. S.; Choi, E. J.; Kim, C. H.; Sung, J. M.; Kim, Y. B.; Seo, D. H.; Choi, H. W.; Choi, Y. S.; Kum, J. S.; Park, J. D. Contribution of Flavonoids to the Antioxidant Properties of Common and Tartary Buckwheat. J. Cereal Sci. 2016, 68, 181–186. DOI: 10.1016/j.jcs.2015.07.005.
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the Antioxidant Activity of Flavonoids by “Ferric Reducing Antioxidant Power” Assay and Cyclic Voltammetry. Biochim. Biophys. Acta-Gen. Subj. 2005, 1721(1–3), 174–184. DOI: 10.1016/j.bbagen.2004.11.001.
