ABSTRACT
Japanese quince (Chaenomeles japonica (Thunb.)) is a very important ornamental plant that is rich in many biochemical compounds such as antioxidants and secondary metabolites that have positive effects on human health. The aim of the study was to evaluate the biochemical compounds in the leaves of three Japanese quince (Chaenomeles japonica (Thunb.)) Lindl. ex Spach cultivars (ʻRasaʼ, ʻDariusʼ and ʻRondoʼ). The results showed that the leaves of Japanese quince have strong antiradical activity (1091 ± 22–1135 ± 15 µmol TE/g) in the CUPRAC reaction system. The analysis by the HPLC method revealed that the major polyphenol group in Japanese quince cultivars leaves was phenolic acids and the most common polyphenol compound is chlorogenic acid (11.2– 52.4 mg/g DW). The most common triterpene is ursolic acid (3.7– 6.8 mg/g DW). Japanese quince leaf powder can be used as a food or beverage additive and enriches our diet with compounds with strong antioxidant activity.
Introduction
Japanese quince Chaenomeles japonica (Thunb.) is grown in most part of the world as an ornamental plant and it belongs to the family Rosaceae. However, this species is widely grown in Nordic European countries. [1] Other plants from the same family (Cydonia oblonga Miller) are known for their medicinal properties and have shown to constitute a promising array of bioactive phytochemicals, suitable for application in foods and pharmaceutical fields.[Citation2–Citation5] Many studies have reported the nutritional and medicinal values of other Chaenomeles species and interestingly, most of the plant parts are good sources of biologically active compounds.[Citation6–Citation8]
One of the most important and most promising Japanese quince secondary metabolites are phenolic compounds with very strong antioxidant properties, manifested by different mechanisms in the human body. Phenolic compounds are capable of binding free radicals to form less reactive compounds.[Citation9] Also suppresses free radical formation processes by joining transition metal ions and inactivating free radical-catalyzing enzymes.[Citation10] Phenols protect other antioxidants from oxidative effects and promote the release of antioxidant enzymes.[Citation11]
Phenolic compounds – natural antioxidants acting with various mechanisms are necessary for human health because they have anticancer[Citation12], anti-inflammatory[Citation13] neuroprotective[Citation14], blood cholesterol and triglyceride lowering[Citation15], antiviral[Citation16], for improving cardiovascular activity[Citation17], an antidiabetic[Citation18] and many other body-enhancing effects. Another significant group of biologically active compounds found in quince organs is triterpenic compounds. Triterpenes are secondary plant metabolites generally found in the fruit peel, leaves and stem bark. Studies have found that the biological activity of triterpenoids exhibiting different pharmacological effects, including antitumor[Citation19,Citation20], anti-inflammatory[Citation21], inhibits human immunodeficiency virus (HIV)[Citation22,Citation23] antiviral and antimicrobial activity.[Citation24] Proanthocyanidins are a group of phenolic compounds with antioxidant and radical scavenging activities, which could enrich food products with antioxidants.[Citation25]
Studies have also shown that different plant organs of Chaenomeles genus accumulate valuable biochemical in different proportions, for example,[Citation26] reported that the leaves accumulate significantly higher polyphenol compounds than the fruits and are a valuable source of these bioactive substances with strong antioxidant activity. In order to maximize the potential of plants, it is necessary to investigate its biochemical composition. However, there are limited numbers of publications about the biochemical composition and potential use of Japanese quince leaves. This study aimed to determine the biochemical composition in three cultivars of quince (Chaenomeles japonica (Thunb.)) leaves.
Methods and materials
Leaves material
Quince Chaenomeles japonica leaves were collected in August 2017, at the same time, ie. then quince fruit was ripe, in the Latvia State Institute of Horticulture, Latvia University of Agriculture, GPS location: N56°36’39“ E:23°17’50”. Quince leaves were lyophilized with a ZIRBUS sublimator 3 × 4 × 5/20 (ZIRBUS technology, Bad Grund, Germany) at the pressure of 0.02 mbar (condenser temperature −85°C). The quince leaves using the ZM200 (Retsch GmbH & Co. KG Haan, Germany) ultracentrifugal mill was ground up to 0.2–0.5 mm fraction.
Dry matter content
Reduction of mass through dehydration before the examination was controlled by European Pharmacopoeia (2010).
Total phenolic content
Spectrophotometric measurements were done using the Genesys-10 UV/VIS spectrophotometer (Thermo Spectronic, Rochester, USA). The total phenolic content (mg GAE/100 g DW) in the methanol (99.0%, v/v) of quince leaves was determined by the Folin-Ciocalteu method and expressed as gallic acid equivalents (GAE).
Quantitative evaluation of phenolic compounds
Extraction. An amount of 0.25 g of lyophilized quince leaves powder (exact weight) was weighed, added to 10 mL of alcohol (70%, v/v), and extracted in a Sonorex Digital 10 P ultrasonic bath (Bandelin Electronic GmbH & Co. KG, Berlin, Germany) at 60°C for 40 min. Quantitative evaluation of phenolic compounds was determined by applying the technique described by.[Citation27]
Antioxidant activity of quince leaves
The DPPH free radical scavenging activity was determined using the method proposed by.[Citation28] 2 mL DPPH (2,2-diphenyl-1-picrylhydrazyl) solution in 99.0% v/v ethanol was mixed with 20 μL of the methanol extract of quince leaves. A decrease in absorbance was determined at a wavelength of 515 nm after storage the samples in the dark for 30 min. An ABTS radical cation decolorization assay was applied according to the methodology described by.[Citation29] A volume of 2 mL of ABTS (2,2‘-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)) solution (absorbance 0.800 ± 0.02) was mixed with 20 μL of the methanol extract of quince leaves. A decrease in absorbance was measured at a wavelength of 734 nm after storage the samples in the dark for 30 min.
A TFPH•+ radical cation decolorization assay was evaluated following the method of.[Citation30] A volume of 3 mL of TFPH•+ solution (absorbance 0.700 ± 0.02) was mixed with 10 μL of quince leaf extracts. A decrease in absorbance was measured at the wavelength of 502 nm with a double beam UV/VIS spectrophotometer M550 (Spectronic Camspec, Garforth, England, United Kingdom).
The ferric reducing antioxidant power (FRAP) assay was carried out as described by.[Citation31] The FRAP solution included TPTZ (0.01 M dissolved in 0.04 M HCl), FeCl3× 6H2O (0.02 M in water), and acetate buffer (0.3 M, pH 3.6) at the ratio of 1:1:10. A volume of 3 mL of a freshly prepared FRAP reagent was mixed with 10 μL of quince leaf extracts. An increase in absorbance was recorded after 30 min at the wavelength of 593 nm using the UV/VIS spectrophotometer M550 (Spectronic Camspec, Garforth, England, United Kingdom).
The Cupric Reducing Antioxidant Capacity (CUPRAC) assay was performed using the method of.[Citation32] The working CUPRAC solution included copper (II) chloride (0.01 M in water), ammonium acetate buffer solution (0.001 M, pH = 7), and neocuproine (0.0075 M in ethanol) at a ratio 1:1:1. A volume of 3 mL of a freshly prepared CUPRAC reagent was mixed with 10 μL of quince leaf extracts. An increase in absorbance was recorded after 30 min at a wavelength of 450 nm.
Determination of total proanthocyanidins content
Spectrophotometric measurements were done using a Genesys-10 UV/Vis spectrophotometer (Thermo Spectronic, Rochester, USA). Total proanthocyanidins were determined by applying the technique described by.[Citation33] 3 mL DMCA solution (0.1% 4-Dimethylamino cinnamaldehyde in methanol – HCl 9:1 v/v) was mixed with 20 μL of the methanol extract of quince leaves. A decrease in absorbance was determined at a wave length of 640 nm after 5 min. The concentration of condensed tannins in the extract was calculated based on a calibration curve established with catechin as a standard (calibration curve: catechin (mg/100 g) = (y – 0.0066)/3.1312), rCitation2 = 0.995.
Quantitative evaluation of triterpenes
Quantitative evaluation of triterpenes was determined by applying the technique described by.[Citation34] During the analysis, 1 g of lyophilized powder of quince leaves (exact weight) was weighed, added to 10 mL of acetone (100%, v/v), and extracted in a Sonorex Digital ultrasonic bath 10 P (Bandelin Electronic GmbH & Co. KG, Berlin, Germany) for 10 min at 60°C. The ultrasound strength was 1100 W, and frequency – 80 kHz. The extract obtained was filtered through a paper filter, and the residue on the filter was washed with acetone (100%, v/v) in a 10 mL flask until the exact volume was reached. The quince leaves extract was filtered through a membrane filter with a pore size of 0.22 µm (Carl Roth GmbH, Karlsruhe, Germany).
Instrumentation and chromatographic conditions
For HPLC analysis a Waters 2695 chromatograph equipped with a Waters 2998 PDA detector (Waters, Milford, USA) was used. The chromatographic separation was controlled, chromatograms were recorded, and data were processed with the Empower® v. 3.0 software (Waters, Milford, USA). Chromatographic separations were carried out by using an ACE (5 μm, C18, 250 × 4.6 mm i.d.) column. The column was operated at a fixed temperature of 25°C. The volume of the investigated extract was 10 µL. The flow rate was 1 mL/min. The mobile phase consisted of 100% (v/v) acetonitrile (solvent A) and water (solvent). We applied isocratic elution, the eluents ratio being 88% (solvent A) and 12% (solvent). For quantitative analysis, the calibration curve was obtained by injection of known concentrations of different standard compounds. The concentrations of triterpene compounds identified in the quince leave extracts were within the limits of the calibration curves. All the identified triterpene compounds were quantified at 205 nm.
Statistical analysis
For statistical analysis, a single factor analysis of variance (ANOVA) along with the post hoc Tukey test was employed. Differences at P < .05 were considered significant.
Results and discussion
Antioxidant activity of quince chaenomeles japonica cultivars leaves
The highest antioxidant activity was determined by the CUPRAC and ABTS methods in cultivar ʻDariusʼ 1134.9 ± 14.7 and 1062.38 ± 90.1 µmol TE/g, respectively (). This may be due to the fact that CUPRAC and ABTS methods different from others because ABTS radical cation is soluble in water and organic solvents, which allows determining the antiradical activity of the hydrophilic and lipophilic compounds.[Citation35] The CUPRAC method evaluates not only the hydrophilic and lipophilic phenolic compounds but also vitamins C and E.[Citation32] DPPH radicals are only soluble in organic solvents. The FRAP and TFPH methods evaluated antioxidative activity in the low pH medium.
Table 1. Antioxidant activity in the methanol extracts of quince Chaenomeles japonica cultivars leaves
Determination of antioxidant activity (ABTS system) showed that quince leaves of cultivars ʻRasaʼ and ʻDariusʼ grew in Latvia, had significantly higher antioxidant activity values than in cultivar ʻRondoʼ and more than Chaenomeles japonica other cultivars, reported before.[Citation26] This difference may be due to the influence of genotype or different extracts preparation methods.
Total phenolic content and total proanthocyanidins content
The total amount of phenolic compounds in the methanol extracts of quince leaves varied from 5934 ± 70 (cv. Rondo) to 7866 ± 178 (cv. Darius) mg GAE/100 g (). Quince Cydonia oblonga Miller total phenolic contents of dry leaves was 5252.0 mg GAE/100 g and these values show the material is well suited for medicinal use.[Citation36] Our research results show that quince Chaenomeles japonica leaves accumulates more phenolic compounds and are the potentially good source of bioactive constituents.
Table 2. The total amount of dry matter, phenolic compounds and proanthocyanidins of Chaenomeles japonica leaves
The total amount of proanthocyanidins in the methanol extracts of quince leaves varied from 662 ± 16 (cv. Rondo) to 1325 ± 31 (cv. Rasa) mg/100 g (). Determination of proanthocyanidins mg/100 g showed that quince leaves of cultivars Rasa 1325.1 ± 31.4 and Darius 910.4 ± 20.1 grown in Latvia contained significantly more proanthocyanidins than cultivar Rondo 662 ± 16. A correlation was found between the radical-scavenging capacity (ABTS) of methanol extract of quince leaves and the total contents of phenolic compounds and total proanthocyanidins (r = 0.93 and r = 0.47, respectively). Similar results were reported before.[Citation26] Proanthocyanidins have a strong antioxidant activity and can protect against various diseases. The proanthocyanidins, extracted from the grape seed, inhibited osteoclast formation and might be useful for the treatment of osteoporosis.[Citation37] The proanthocyanidins, extracted from cranberry, significantly reduced E. coli adhesion to fresh buccal epithelial cells and can be used to reduce oropharyngeal colonization and prevent lung infection.[Citation38] The oligomeric proanthocyanidins extracted from grape seed with chemotherapeutic drugs significantly inhibited the growth of the chemoresistant cells and may serve as additional treatments in patients with refractory colorectal cancer.[Citation39] Our results show that the Japanese quince leaf extracts can also be used as a great source of proanthocyanidins.
Quantitative evaluation of phenolic compounds
Determination of quantitative evaluation of phenolic compounds mg/g () showed that quince leaves of cv. Rasa (38.84 mg/g) and cv. Darius (64.79 mg/g) grown in Latvia, contained significantly more phenolic compounds than cv. Rondo (12.94 mg/g). Compared to the results of previous Japanese quince studies, it showed similar results, but other species of quince Cydonia oblonga Miller leaves content more phenolic compounds (117.97 mg/g).[Citation26]
Table 3. Quantitative evaluation of phenolic compounds mg/g (expressed for absolute dry weight) in alcohol extracts obtained from the quince leaves of cultivars grown in Latvia
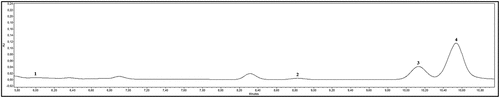
The major phenolic group in quince Chaenomeles japonica leaves was phenolic acids, identified and quantified by the HPLC method (.), same results was reported before.[Citation26] (+) – Catechin was identified only in cv. Darius, in the other two cultivars it was not detected. The most common phenolic compound in all three cultivars is chlorogenic acid, which accounts for about 80% of total phenolic compound quantified by the HPLC method. Accumulating evidence has demonstrated that chlorogenic acid has many biological properties, including antibacterial, antioxidant, and anticarcinogenic activities, especially hypoglycemic and hypolipidemic effects.[Citation40–Citation43] These results show that supplementing the diet with quiche leaf powders may have application as a preventive or therapeutic agent in many diseases.
Figure 1. Chromatogram of alcohol extract of quince Chaenomeles japonica leaf sample investigate (λ = 280 nm)
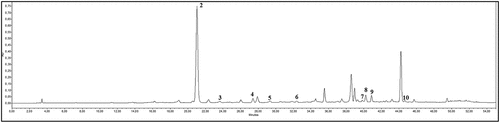
Triterpenes of quince chaenomeles japonica cultivars leaves
The most common triterpene in all three cultivars is ursolic acid (UA): 3689 ± 178 (cv.Rasa), 5231 ± 209 (cv. Darius) and 6750 ± 154 (cv. Rondo) µg/g DW (). Studies have found that ursolic acid has potential use as a cardioprotective compound[Citation44] and strong immunomodulatory compounds to modulate the innate immune.[Citation45] The ursolic acid has been shown to suppress nuclear factor-κB (NF-κB), which regulates the expression of a number of genes whose products are involved in tumorigenesis[Citation46] and may be used as a valuable antimetastatic agent for the treatment of cancer metastasis.[Citation47] The ursolic acid may effectively inhibit the development of benign prostatic hyperplasia (BPH) and it may be a useful agent in BPH treatment.[Citation48]
Table 4. Triterpenes of quince Chaenomeles japonica cultivars leaves
Second most common triterpene in all three cultivars is oleanolic acid (OA): 1062.4 ± 43.9 (cv. Rasa), 1265 ± 74 (cv.Darius) and 2142 ± 194 (cv.Rondo) µg/g DW (). The supplement of OA or UA might be helpful for the prevention or alleviation of glycation associated renal diseases.[Citation49] In fresh quince, Chaenomeles japonica fruit was found 88 ± 17 µg/g oleanolic acid and 224 ± 52.0 µg/g ursolic acid.[Citation6] In quince, Chaenomeles japonica leaves also has been identified betulinic and corosolic acids (). The abundance of triterpenes proves that Chaenomeles japonica leaves are great material to be used for functional food and medical purposes.
Conclusion
The results of this study showed, that two of three varieties ʻRasaʼ and ʻDariusʼ of quince Chaenomeles japonica leaves are more suitable as additives in food and beverages because of the strong antioxidant activities, high phenols, and proanthocyanidins. These cultivars leaf powder can be a great raw material for creating functional food products. The cultivar ʻRondoʼ had a higher significant amount of triterpenes and can be potentially used for pharmaceutical research. However, further studies are needed to validate the clinical significance of Rondo leaves extracts for human health. Finally, Quince (Chaenomeles japonica) shows a promising beneficial impact on human health and can serve an important component in the food industry.
Acknowledgement
The research was supported by the ERAF project Nr. 1.1.1.1/16/A/094, Environment-friendly cultivation of emerging commercial fruit crop Japanese quince – Chaenomeles japonica and waste-free methods of its processing.
Additional information
Funding
References
- Rumpunen, K.; (2002). Chaenomeles: Potential New Fruit Crop for Northern Europe. Trends in new crops and new uses. ASHA Press, Alexandria, VA, 385–392.
- Ashrafi, H.; Ghabili, K.; Alihemmati, A.; Jouyban, A.; Shoja, M. M.; Aslanabadi, S.; … Hajhosseini, L. The Effect of Quince Leaf (Cydonia Oblonga Miller) Decoction on Testes in Hypercholesterolemic Rabbits: A Pilot Study. Afr. J. Traditional, Complementary Altern. Med. 2013, 10, 2. DOI: 10.4314/ajtcam.v10i2.12.
- Khademi, F.; Danesh, B.; Mohammad Nejad, D.; Soleimani Rad, J. The Comparative Effects of Atorvastatin and Quince Leaf Extract on Atherosclerosis. Iran. Red Crescent Med. J. 2013, 15(8), 639–643. DOI: 10.5812/ircmj.4030.
- Oliveira, A. P.; Costa, R. M.; Magalhães, A. S.; Pereira, J. A.; Carvalho, M.; Valentão, P.; Silva, B. M. Targeted Metabolites and Biological Activities of Cydonia Oblonga Miller Leaves. Food Res. Int. 2012, 46(2), 496–504. DOI: 10.1016/j.foodres.2010.10.021.
- Osman, A. G. M.; Koutb, M.; Sayed, A. E.-D. H. Use of Hematological Parameters to Assess the Efficiency of Quince (Cydonia Oblonga Miller) Leaf Extract in Alleviation of the Effect of ultraviolet–A Radiation on African Catfish Clarias Gariepinus (Burchell, 1822). J. Photochem. Photobiol. B Biol. 2010, 99(1), 1–8. DOI: 10.1016/j.jphotobiol.2010.01.002.
- Du, H.; Wu, J.; Li, H.; Zhong, P.-X.; Xu, Y.-J.; Li, C.-H.; Wang, L.-S. Polyphenols and Triterpenes from Chaenomeles Fruits: Chemical Analysis and Antioxidant Activities Assessment. Food Chem. 2013, 141(4), 4260–4268. DOI: 10.1016/j.foodchem.2013.06.109.
- Thomas, M.; Crépeau, M. J.; Rumpunen, K.; Thibault, J. F. Dietary Fibre and Cell-Wall Polysaccharides in the Fruits of Japanese Quince (Chaenomeles Japonica). LWT - Food Sci. Technol. 2000, 33(2), 124–131. DOI: 10.1006/fstl.1999.0628.
- Zhang, S. Y.; Han, L. Y.; Zhang, H.; Xin, H. L. Chaenomeles Speciosa: A Review of Chemistry and Pharmacology. Biomed. Rep. 2014, 2(1), 12–18. DOI: 10.3892/br.2013.193.
- Amić, D.; Davidović-Amić, D.; Beslo, D.; Rastija, V.; Lucić, B.; Trinajstić, N. SAR and QSAR of the Antioxidant Activity of Flavonoids. Curr. Med. Chem. 2007, 14(7), 827–845. DOI: 10.2174/092986707780090954.
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.; Bektaşoğlu, B.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules. 2007, 12(7), 1496–1547. DOI: 10.3390/12071496.
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and Prooxidant Properties of Flavonoids. Fitoterapia. 2011, 82(4), 513–523. DOI: 10.1016/j.fitote.2011.01.018.
- Chahar, M. K.; Sharma, N.; Dobhal, M. P.; Joshi, Y. C. Flavonoids: A Versatile Source of Anticancer Drugs. Pharmacogn. Rev. 2011, 5(9), 1–12. DOI: 10.4103/0973-7847.79093.
- Boots, A. W.; Wilms, L. C.; Swennen, E. L. R.; Kleinjans, J. C. S.; Bast, A.; Haenen, G. R. M. M. In Vitro and Ex Vivo Anti-Inflammatory Activity of Quercetin in Healthy Volunteers. Nutrition. 2008, 24(7–8), 703–710. DOI: 10.1016/j.nut.2008.03.023.
- Kovacsova, M.; Barta, A.; Parohova, J.; Vrankova, S.; Pechanova, O. Neuroprotective Mechanisms of Natural Polyphenolic Compounds. Act Nerv. Super Rediviva. 2010, 52(3), 181–186.
- Ngamukote, S.; Mäkynen, K.; Thilawech, T.; Adisakwattana, S. Cholesterol-Lowering Activity of the Major Polyphenols in Grape Seed. Molecules. 2011, 16(6), 5054–5061. DOI: 10.3390/molecules16065054.
- El-Toumy, S. A.; Salib, J. Y.; El-Kashak, W. A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral Effect of Polyphenol Rich Plant Extracts on Herpes Simplex Virus Type 1. Food Sci. Hum. Wellness. 2018, 7(1), 91–101. DOI: 10.1016/j.fshw.2018.01.001.
- Vita, J. A.;. Polyphenols and Cardiovascular Disease: Effects on Endothelial and Platelet Function. Am. J. Clin. Nutr. 2005, 81(1Suppl), 292S–297S. DOI: 10.1093/ajcn/81.1.292S.
- Ali Asgar, M.;. Anti-Diabetic Potential of Phenolic Compounds: A Review. Int. J. Food Prop. 2013, 16(1), 91–103. DOI: 10.1080/10942912.2011.595864.
- Li, J.; Guo, W.-J.; Yang, Q.-Y. Effects of Ursolic Acid and Oleanolic Acid on Human Colon Carcinoma Cell Line HCT15. World J. Gastroenterol. 2002, 8(3), 493–495. DOI: 10.3748/wjg.v8.i3.493.
- Zuco, V.; Supino, R.; Righetti, S. C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective Cytotoxicity of Betulinic Acid on Tumor Cell Lines, but Not on Normal Cells. Cancer Lett. 2002, 175(1), 17–25. DOI: 10.1016/S0304-3835(01)00718-2.
- de Brum, T. F.; Camponogara, C.; da Silva Jesus, R.; Belke, B. V.; Piana, M.; Boligon, A. A.; de Freitas Bauermann, L. Ethnopharmacological Study and Topical Anti-Inflammatory Activity of Crude Extract from Poikilacanthus Glandulosus (Nees) Ariza Leaves. J. Ethnopharmacol. 2016, 193, 60–67. DOI: 10.1016/j.jep.2016.07.075.
- Aiken, C.; Chen, C. H. Betulinic Acid Derivatives as HIV-1 Antivirals. Trends Mol. Med. 2005, 11(1), 31–36. DOI: 10.1016/j.molmed.2004.11.001.
- Zhou, J.; Chen, C. H.; Aiken, C. Human Immunodeficiency Virus Type 1 Resistance to the Small Molecule Maturation Inhibitor 3-O-(3ʹ,3‘-Dimethylsuccinyl)-Betulinic Acid Is Conferred by a Variety of Single Amino Acid Substitutions at the CA-SP1 Cleavage Site in Gag. J. Virol. 2006, 80(24), 12095–12101. DOI: 10.1128/JVI.01626-06.
- Pavlova, N. I.; Savinova, O. V.; Nikolaeva, S. N.; Boreko, E. I.; Flekhter, O. B. Antiviral Activity of Betulin, Betulinic and Betulonic Acids against Some Enveloped and Non-Enveloped Viruses. Fitoterapia. 2003, 74(5), 489–492. DOI: 10.1016/S0367-326X(03)00123-0.
- Bagchi, D.; Bagchi, M.; Stohs, S. J.; Das, D. K.; Ray, S. D.; Kuszynski, C. A.; Pruess, H. G. Free Radicals and Grape Seed Proanthocyanidin Extract: Importance in Human Health and Disease Prevention. Toxicology. 2000, 148(2–3), 187–197. DOI: 10.1016/S0300-483X(00)00210-9.
- Teleszko, M.; Wojdyło, A. Comparison of Phenolic Compounds and Antioxidant Potential between Selected Edible Fruits and Their Leaves. J. Funct. Foods. 2015, 14, 736–746. DOI: 10.1016/j.jff.2015.02.041.
- Liaudanskas, M.; Viškelis, P.; Raudonis, R.; Kviklys, D.; Uselis, N.; Janulis, V. Phenolic Composition and Antioxidant Activity of Malus Domestica Leaves. Thescientificworldjournal. 2014, 2014, 306217. DOI: 10.1155/2014/306217.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Sci. Technol. 1995, 28(1), 25–30. DOI: 10.1016/S0023-6438(95)80008-5.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26(9–10), 1231–1237. DOI: 10.1016/S0891-5849(98)00315-3.
- Asghar, M. N.; Khan, I. U. Measurement of Antioxidant Activity with Trifluoperazine Dihydrochloride Radical Cation. Braz. J. Med. Biol. Res. 2008, 41(6), 455–461. DOI: 10.1590/S0100-879X2008000600003.
- Benzie, I. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239(1), 70–76. DOI: 10.1006/abio.1996.0292.
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S. E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52(26), 7970–7981. DOI: 10.1021/jf048741x.
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, K. E.; McKey, D. Extraction and Quantification of “Condensed Tannins” as a Measure of Plant Anti-Herbivore Defence? Revisiting an Old Problem. Die Naturwissenschaften. 2002, 89(11), 519–524. DOI: 10.1007/s00114-002-0366-3.
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, K.; Raudonis, R.; Viškelis, J.; Janulis, V. Detection and Analysis of Triterpenic Compounds in Apple Extracts. Int. J. Food Prop. 2018, 21(1), 1716–1727. DOI: 10.1080/10942912.2018.1506478.
- Prior, R. L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53(10), 4290–4302. DOI: 10.1021/jf0502698.
- Ashraf, M. U.; Muhammad, G.; Hussain, M. A.; Bukhari, S. N. A. Cydonia Oblonga M., A Medicinal Plant Rich in Phytonutrients for Pharmaceuticals. Front. Pharmacol. 2016, 7, 163. DOI: 10.3389/fphar.2016.00163.
- Zhu, W.; Yin, Z.; Zhang, Q.; Guo, S.; Shen, Y.; Liu, T.; Peng, D. Proanthocyanidins Inhibit Osteoclast Formation and Function by Inhibiting the NF-κB and JNK Signaling Pathways during Osteoporosis Treatment. Biochem. Biophys. Res. Commun. 2018. DOI: 10.1016/j.bbrc.2018.12.125.
- Margetis, D.; Roux, D.; Gaudry, S.; Messika, J.; Bouvet, O.; Branger, C.; Ricard, J.-D. Effects of Proanthocyanidins on Adhesion, Growth, and Virulence of Highly Virulent Extraintestinal Pathogenic Escherichia Coli Argue for Its Use to Treat Oropharyngeal Colonization and Prevent Ventilator-Associated Pneumonia. Crit. Care Med. 2015, 43(6), e170–8. DOI: 10.1097/CCM.0000000000000972.
- Ravindranathan, P.; Pasham, D.; Goel, A. Oligomeric Proanthocyanidins (Opcs) from Grape Seed Extract Suppress the Activity of ABC Transporters in Overcoming Chemoresistance in Colorectal Cancer Cells. Carcinogenesis. 2018. DOI: 10.1093/carcin/bgy184.
- Rodriguez de Sotillo, D. V.; Hadley, M. Chlorogenic Acid Modifies Plasma and Liver Concentrations Of: Cholesterol, Triacylglycerol, and Minerals in (Fa/Fa) Zucker Rats. J. Nutr. Biochem. 2002, 13(12), 717–726. DOI: 10.1016/S0955-2863(02)00231-0.
- Bassoli, B. K.; Cassolla, P.; Borba-Murad, G. R.; Constantin, J.; Salgueiro-Pagadigorria, C. L.; Bazotte, R. B.; de Souza, H. M. Chlorogenic Acid Reduces the Plasma Glucose Peak in the Oral Glucose Tolerance Test: Effects on Hepatic Glucose Release and Glycaemia. Cell Biochem. Funct. 2008, 26(3), 320–328. DOI: 10.1002/cbf.1444.
- dos Santos, M. D.; Almeida, M. C.; Lopes, N. P.; de Souza, G. E. P. Evaluation of the Anti-Inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid. Biol. Pharm. Bull. 2006, 29(11), 2236–2240. DOI: 10.1248/bpb.29.2236.
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of Chlorogenic Acid in Vegetables and Fruits as an Inhibitor of 8-Hydroxydeoxyguanosine Formation in Vitro and in a Rat Carcinogenesis Model. Food Chem. Toxicol. 2000, 38(5), 467–471. DOI: 10.1016/S0278-6915(00)00014-4.
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskiene, D. M.; Bernatoniene, J. Uncoupling and Antioxidant Effects of Ursolic Acid in Isolated Rat Heart Mitochondria. J. Nat. Prod. 2011, 74(7), 1640–1644. DOI: 10.1021/np200060p.
- Mawa, S.; Jantan, I.; Husain, K. Isolation of Terpenoids from the Stem of Ficus Aurantiaca Griff and Their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils. Molecules. 2016, 21(1), 9. DOI: 10.3390/molecules21010009.
- Garg, A.; Aggarwal, B. B. Nuclear Transcription factor-kappaB as a Target for Cancer Drug Development. Leukemia. 2002, 16(6), 1053–1068. DOI: 10.1038/sj.leu.2402482.
- Cha, D. S.; Shin, T. Y.; Eun, J. S.; Kim, D. K.; Jeon, H. Anti-Metastatic Properties of the Leaves of Eriobotrya Japonica. Arch. Pharmacal Res. 2011, 34(3), 425–436. DOI: 10.1007/s12272-011-0310-1.
- Kaplan, S. A.;. Re: Ursolic Acid Reduces Prostate Size and Dihydrotestosterone Level in a Rat Model of Benign Prostatic Hyperplasia. J. Urol. 2013, 189(1), 388. DOI: 10.1016/j.juro.2012.09.143.
- Wang, Z.; Hsu, C.; Huang, C.; Yin, M. Anti-Glycative Effects of Oleanolic Acid and Ursolic Acid in Kidney of Diabetic Mice. Eur. J. Pharmacol. 2010, 628(1–3), 255–260. DOI: 10.1016/j.ejphar.2009.11.019.