ABSTRACT
Malnutrition is increasing across the globe owing to urbanization, poverty, and climatic changes. In the current circumstances, alternative and unexplored sources of food and nutrients are getting attention. The current sources of food cannot meet the ever-increasing population demand. The demand for animal-source protein is estimated be double due to a projected 50% rise in the world population by the year 2050. Moreover, global animal protein supply chains are not only vulnerable to natural disasters but also a significant source of greenhouse gas emissions. Mycoprotein is considered an excellent alternative to animal protein due to its amino acid profile and cost-effectiveness. Mycoprotein is produced by Fusarium venenatum, a naturally occurring fungus that can be used as a substitute for conventional animal protein sources. Mycoprotein is high in protein and fiber while low in cholesterol, fat, sugar, and salt. Mycoprotein offers excellent functional and therapeutic potential in mitigating various health disorders. Furthermore, it helps maintain muscle synthesis and optimal plasma and cholesterol levels, regulating insulin, glucose, and satiety. This review is focused on the mycoprotein’s origin and production, with a particular emphasis on its nutritional, health, and economic opportunities and challenges.
Introduction
By 2050, the global population is projected to rise by 50%, following a 50% rise in food demand, with which worldwide demand for animal-sourced protein will increase nearly twice as much.[Citation1] Despite efforts to combat hunger, one in every nine individuals (821 million) on the globe are still malnourished, and recent trends indicate that malnutrition is expanding worldwide.[Citation2] While several causes contribute to the rise in global hunger, the main drivers are climate fluctuations and extremes, which result in severe food crises. According to research findings, climate change will worsen nutrient deficiencies among people already at risk of food insecurity. Agriculture is responsible for 29% of worldwide greenhouse gas emissions, with livestock accounting for a substantial portion of both GHG emissions and land-water shortages.[Citation3] Because of the ecological impact of livestock and concerns about animal welfare, the meat industry will be unable to respond to this rise in demand by using extra resources. There is an escalating demand for eco-friendly (eco-advanced) and cost-efficient sustainable food proteins.[Citation4] Plant proteins have acquired a lot of attention in modern times because of their potential to improve health indices, including glycemic management and blood lipid profiles in diabetics, when animal proteins replace them.[Citation5] Plant-based dietary proteins are gradually being incorporated into the emerging (FBDG) Food-Based Dietary Guidelines. According to a worldwide analysis of FBDG, one-half of the nations with protein diet key details (33 of 67) covered animal and plant-obtained protein sources.[Citation6] However, some well-known dietary proteins, like fungal-based proteins, seem to have received less attention. Fungal-originated mycoproteins are becoming more popular due to their beneficial nutritive value, low cost of production, ecological benefits, and resistance to terrain constraints such as drought and flood.[Citation7] The productive strain utilized to produce and reap mycoprotein, Fusarium Venenatum ATCC 2684, was identified in 1960.[Citation8] After extensive research, the Ministry of Agriculture, Fisheries & Food (MAFF) in the United Kingdom approved the marketing of mycoprotein as a food protein source in 1984,[Citation9] and it is now available for purchase in all EU member states. Further regulatory licenses followed in the United States, Norway, Australia, and Switzerland, as well as, more recently, Thailand, Japan, Canada, and Malaysia. Mycoprotein is mostly utilized as a part of the QuornTM brand of vegan and vegetarian cuisine. Mycoprotein is now synthesized at scale via fermentation, resulting in a good-quality protein with a low ecological impact.[Citation10] Despite their increasing popularity among customers, many health practitioners are still unaware of the potential of fungal-derived proteins to deliver a nutritious novel protein with minimal ecological consequences. In a research committee of health professionals, most nutritionists reported that most had no idea what fungal-based nutritive proteins were or that they were distinct from the plant kingdom.[Citation11] Because there is a growing desire for nutritious and sustained novel protein resources, misunderstandings about fungal-based proteins must be centered. This review describes the origin, production, and critical nutritional aspects of mycoprotein, its ecological impact, and future challenges.
Mycoprotein’s origin
During the 1960s, practitioners, and legislators worldwide were apprehensive that an anticipated population explosion might lead to future worldwide protein shortages. Food scientists struggled to develop a low-cost, tasty microbial protein source. Finally, a filamentous fungus often found in soil has been the focus of this investigation.[Citation12] In 1967, a fungus (Fusarium venenatum) was discovered in a garden in Marlow, Buckinghamshire, and was ultimately utilized to synthesize mycoprotein. The (ribonucleic acid) RNA-lowered biomass constituting the mycelium (cells) of F. venenatum A3/5 (ATCC PTA-2684) in a continual fermentation system is known as mycoprotein. Greater than 3000 fungus isolates from all over the globe were investigated at the start of the investigation and production process, and F. venenatum A3/5 (ATCC PTA-2684) was ultimately chosen as the ideal entity for mycoprotein biosynthesis. To make mycoprotein from F. venenatum A3/5 for retail, scientists have spent many years researching the safety of the organisms and ultimate products. When the RNA matter of the cells was decreased to secure levels, it became evident that mycoprotein could be ingested without causing harm to human participants or laboratory animals. Because the growing circumstances employed for manufacturing were inadequate for mycotoxin generation, the ATCC PTA-2684 strain did not create mycotoxins. In 1984, the Ministry of Agriculture, Fisheries & Food in the United Kingdom certified Fusarium venenatum A3/5 for trade as food.[Citation13]
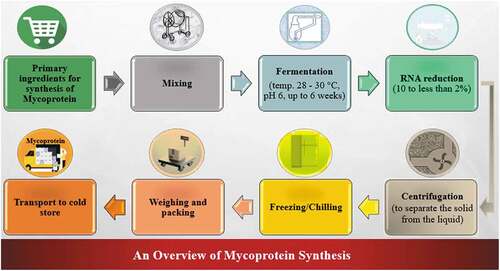
Mycoprotein production
Mycoprotein is produced industrially by continuously fermenting F. venenatum on a carbohydrate substrate.[Citation14] The flow rate is affected by the CO2 evolution rate, which indicates the biomass content. The temperature of the cultures is kept between 28–30°C, with a pH of 6. Typically, the continuous fermentation procedure lasts about six weeks. Mycotoxins are checked at 6-hour intervals during the production process to guarantee that the mycoprotein is devoid of them. To achieve the required safety criteria, the fungal biomass’s RNA content must be minimized.[Citation13] The culture-broth is exposed to a brief heat therapy after being harvested from the fermenter to lower its RNA concentration from 10 to less than 2% (dry matter), which is accomplished by activating the endogenic RNA enzymes with heat. This reduces the number of purines, which can cause an excess of uric acid and raise the risk of gout if consumed in large doses.[Citation12] The fungal biomass is heated in a separate tank for 30–45 min at over 68°C (ideal 72–74°C). After centrifuging the heat-treated culture broth, the mycoprotein is extracted as a paste.[Citation13] Lastly, the mycoprotein is blended with albumen from free-range chicken eggs as a binder, then flavored and textured to mimic meat.[Citation14] () illustrates the mycoprotein synthesis process.
Safety
Mycoprotein was declared safe for use in food by the US Food & Drug Administration in 2001, except for poultry and meat foodstuffs. According to the FDA, 160 widely ingested foods cause an unusual allergic risk (USFDA, 2001). The “Top 8” allergens are peanuts, eggs, milk, fish, soy, shellfish, wheat, and tree nuts, which account for ninety percent of food allergy consequences. As a result, mycoprotein, like all other protein sources, has some allergic risks. A single company (Quorn®, Marlow Foods, UK) currently supplies mycoprotein foods in 19 nations.[Citation15] Mycoprotein foods are not recommended for babies under the age of three because of the high energy demands of quickly growing newborns, the comparatively low energy density of mycoprotein, and its high fiber content.[Citation12]
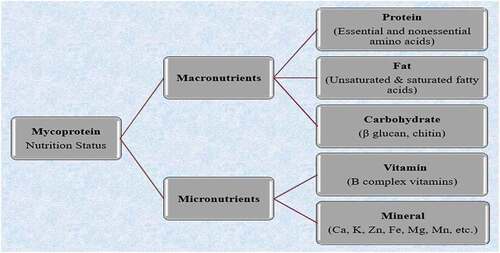
Nutrition status
Mycoprotein is crucial for inclusion in good nutrition because of its comparatively high protein quality, low saturated fat, and high fiber content (). Experimental research suggests that mycoprotein may have various nutritional benefits, such as increasing satiety and regulating blood glucose and cholesterol. shows the nutritional value of mycoprotein in its food ingredient composition.
Table 1. Mycoprotein amino acid and micronutrient content.
Table 2. Nutrition profile of quorn mince and mycoprotein (per 100 g).
Protein
Mycoprotein’s intended use in foodstuffs that serve as the centerpiece of a meal necessitates that it be of high protein quality. The amino acid content of mycoprotein, as shown in , indicates that it comprises all of the essential amino acids. The optimal protein bioavailability-assessed amino level is 0.996, suggesting that it is a good quality protein obtained by employing gold-standard ileostomy practices.[Citation18]
Fiber
According to European Commission guidelines, mycoprotein is “rich in fibre,” suggesting it comprises 6 g of fiber/100 g (EU, 2008). Mycoprotein contains natural dietary fiber, with one-third chitin and two-thirds glucan (resulting in a “fibrous chitin–glucan matrix” in the small intestine), 12% is soluble, and 88% is insoluble fiber. Branched A-glucans (from yeast) and linear A-glucans (from grains) have been found to stimulate the immune system and actively engage in physiological activities associated with fat metabolization.[Citation10,Citation19]
Fat
Because mycoprotein has a low accessible carbohydrate content, fat makes up around one-third of its energy composition. Mycoprotein fat is abundant in poly–and monounsaturated fatty acids (Table-2) while low in saturated fat (less than 1.5 g of saturated fatty acid/ 100 g of solid).[Citation14]
Micronutrients
A variety of water-soluble B vitamins can be found in mycoprotein (pyridoxine, folate, cobalamin) (). Mycoprotein also contains a diverse range of mineral compounds, and according to European Commission nutrient claims, it is “rich” in zinc, phosphorus, calcium, iron, potassium, etc.
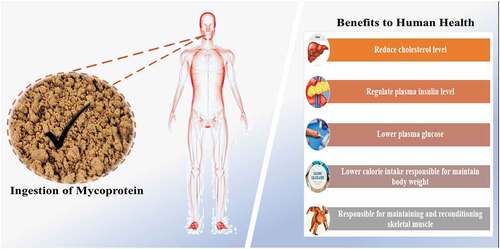
Health evidence
A modest amount of human research on the effects of mycoprotein on hypercholesterolemia, satiety, and insulinemia/glycemia has resulted from an interest in the potential role of mycoprotein in lowering blood cholesterol concentrations, reducing caloric intakes, and managing blood sugar levels ().
Energy Intake
The impacts of mycoprotein (fungal-derived) on energy consumption were examined in five different studies. Burley et al.[Citation20] studied 18 slim individuals (male and female). They discovered that consuming a mycoprotein-rich lunch lowered evening ad libitum caloric intake by 18%, contrasted to a poultry control, with males experiencing the most significant decline. Turnbull et al.[Citation21] reported that after consuming 130 g of chicken or mycoprotein as an isoenergetic supper, healthy women’s energy consumption decreased by 24%, and these effects persisted the next day, with energy consumption dropping by 16.5%. Bottin et al.[Citation22] expanded this work further by focusing on the impacts on overweight adults. Caloric intake was lowered by 132 g of mycoprotein in both randomized trials, resulting in a 10% reduction in later ad libitum energy consumption. The studies are at the point were unable to present clear mechanistic insights, although metabolomic assays suggested that only a few putative molecules would be worth investigating. Williamson et al.[Citation23] studied healthy, premenopausal women and revealed that a pasta preload with 44.3 g mycoprotein substantially decreased energy intake at the subsequent meal when matched to the chicken control. In obese, overweight, and lean adults, acute mycoprotein ingestion appeared to reduce consumption at later ad libitum meals and 24 hours after consumption. Further studies might be beneficial in determining whether these effects are long-lasting and the processes that underlie such activities.
Glucose levels
Glucose levels were examined in different investigations as a glycemia marker. The findings demonstrate that acute mycoprotein consumption lowers blood glucose levels, although the effects are not statistically significant. Turnbull & Ward[Citation24] noticed that the control and mycoprotein groups had lower glucose levels, though glycemia was substantially lower at 60 minutes (13% lower) following the mycoprotein meal. According to Ruxton & McMillan,[Citation25] blood sugar levels in the mycoprotein state decreased over time while they rose in the control group. However, the results of the post-hoc test were not statistically significant. Bottin,[Citation26] measured glucose levels and reported them as (IAUC) incremental Area Under the Curve estimates, which were reduced in over-weight people who consumed 30g of mycoprotein (dry-weight) relative to the control (whey protein) but not statistically prominent. Later research by Bottin et al.[Citation22] found no significant differences in glucose levels in overweight and obese subjects when they ate minor, moderate, or high mycoprotein or calorie-equivalent poultry meals. Dunlop et al.[Citation16] assessed blood sugar profiles following mycoprotein investigation. They found some proof of a decline in the late post-prandial stages, though the data evaluation was not involved or debated in the core body of the article. Coelho et al.[Citation17] conducted two investigations that measured glucose levels and found no considerable variations related to controls. Colosimoa et al.[Citation27] found that the availability of fungal cell walls in the mycoprotein decreases the kinetics of glucose released during the digestive process compared to the absence of cell walls. The penetrability of the mycoprotein fungal cell wall enables α-amylase to diffuse into the cells, resulting in trapped enzymes inside the hyphal matrix and decreased enzymatic reactions and starch digestion. These actions are the underlying pathways for mycoproteins’ ability to control post-prandial glycemia.
Insulin levels
The response of acute mycoprotein intake on insulin levels was studied in multiple studies. Turnbull & Ward[Citation24] noticed that post-meal insulinemia was substantially decreased in the mycoprotein group compared to the control at 30 and 60 minutes after intake. Bottin[Citation26] examined IAUC and PPIR in 10 active, overweight individuals, finding considerable reductions in insulin readings at 15, 30, and 45 minutes after intake. Compared to whey protein consumption, PPIR (post-prandial insulin resistance) improved dramatically following mycoprotein ingestion. Bottin et al.[Citation22] found that using IAUC calculations, mycoprotein meals with (44 g) small, (88 g) moderate, or (132 g) high mycoprotein considerably decreased insulin concentrations when compared to poultry, with these estimated to be −8% (IAUC small), −12% (IAUC moderate), and −21% (IAUC high), correspondingly. These findings are intriguing since they happened under an energy-balanced state, implying that they are not the result of a reduction in total energy consumption or weight loss. According to Dunlop et al.,[Citation16] mycoprotein intake resulted in delayed but prolonged hyperinsulinemia more than protein-match milk. Likewise, Monteyne et al.[Citation28] observed that, compared to milk protein, mycoprotein consumption (70 g) resulted in a slower but more prolonged rise in insulin serum levels, with a climax at 30 minutes after intake. Coelho et al.[Citation17,Citation29] found no variations in plasma insulin responses following mycoprotein intake when comparing fish/meat and nucleotide-deficient mycoprotein controls. In people who are overweight or obese at the beginning, mycoprotein ingestion may be extra beneficial in controlling insulin levels.[Citation22] More prominent and more extended research is required to study such impacts further. Current studies are varied in model and laboratory-based, limiting anticipation and diligence of outcomes to community-based settings.
Cholesterol levels
Adding a moderate amount of fungal mycoprotein to the diet may help lower overall cholesterol levels. There are currently limited human studies looking at the impacts of mycoprotein (a fungal protein) on overall cholesterol levels. Turnbull conducted two trials (1990; 1992). In the first trial, individuals who had 191 g of mycoprotein at dinner and lunch for three weeks saw a 13% drop in total cholesterol in their blood.[Citation30] In the second[Citation31] trial, related declines in overall blood cholesterol were observed in an extensive 8-week study. However, the intervention (130 g of mycoprotein; wet-weight) was supplied as cookies. Ruxton & McMillan[Citation25] employed 88 g of wet-weight mycoprotein per day in a community context and found that individuals with higher cholesterol levels at standard had significantly reduced cholesterol value. Although no randomization or blinding was utilized in this trial, which could have altered the control group’s regular meal compliance, these intriguing findings indicated that mycoprotein could be a critical food ingredient for treating high plasma cholesterol. Lately, Coelho et al.[Citation29] supplied a diet comprising 1.2 g of protein per kg of mass weight, which was provided from QuornTM, fish, or meat, examining positive impacts on the blood lipidome-specific lipid subfractions declined, which contained free-cholesterol and overall plasma cholesterol in the mycoprotein contrasted with controlling. It was suggested that a decline in cholesterol levels might be due to the type or amount of fiber present. Some of these benefits may be attributed to short-chain fatty acids (SCFAs) like propionate, butyrate, and acetate, which are significant fiber commodities fermentation.[Citation32] Such potential mechanisms require further investigation.
Adaptation of skeletal muscle
Maintaining and reconditioning skeletal muscle mass necessitates appropriate nutritive protein consumption. Skeletal muscle volume and protein quality are sustained through functional alterations in muscle protein formation and degradation rates. Muscle protein degradation outnumbers in the overnight starved state, resulting in net muscle protein loss. Protein intake enhances muscle protein synthesis rates transiently (for 2–5 hours), owing to higher plasma (EAAs) essential amino acids, among which leucine is essential.[Citation17] Protein consumption also stimulates pancreatic insulin secretion, which retards muscle protein disintegration, resulting in net muscle protein accretion (the so-called anabolic reaction) in the post-prandial state and countering protein losses during fasting.[Citation33] Individuals can retain muscle mass due to these diurnal alternations in muscle-protein balance.[Citation34] Proteins based on animals generally have a high bioavailability, which results in rapid and prolonged post-prandial leucinemia and aminoacidemia. Therefore, animal-based proteins have been proven to be better than plant-derived protein sources in terms of stimulating human muscle protein synthesis processes.[Citation35–37] However, only soy and wheat (deficient in essential amino acids and leucine) have been studied for their anabolic potential.[Citation38] Mycoprotein has a great PDCAAS (protein digestibility corrected amino acid score, an approximate indicator of a protein’s digestibility) of 0.99. It is high in essential amino acids (41% of the overall protein) and moderately high in leucine (6% of the overall protein). Mycoprotein’s in vivo amino acid digestibility was recently compared to extracted milk protein explained by Dunlop et al.[Citation16] Milk protein was chosen as the control reference point because it comprises high levels of EAAs (49% protein) and leucine (11% of the total protein), has a PDCAAS of 1.0, and is thus widely regarded as a near-gold standard source of protein for stimulating muscle protein synthesis,[Citation39] and optimizing muscle strength.[Citation40] The analyses showed that inactive men, the digestibility of EAA and leucine in the hrs following protein-matched boluses of milk-protein were comparable to that of mycoprotein (albeit mycoprotein absorption was slower and more sustained).[Citation16] It’s worth noting that because mycoprotein is a complete food source, about double the volume (and energy) of mycoprotein was ingested to match these protein content conditions. It was also noticed that until between 60 and 80 g of mycoprotein (i.e., 2.1–2.9 g of leucine; 27–36 g of protein) is ingested, the amino-acid bioavailability of fungal-derived protein (mycoprotein) rises a dose-response manner. As a result, it appears that mycoprotein would promote a strong muscle protein synthesis adaptation and, when consumed in higher amounts, would be a substitute supplier of protein to maintain muscle reconditioning during continuous training. When compared to adaptations triggered by other sources of protein, the degree of this response would generally be dependent on whether the entire systemic supply of amino acids (essential) or the rate at which amino acids become accessible is the most critical regulatory aspect.[Citation36,Citation41] Mycoprotein’s insulinotropic effects and bioavailability were recently investigated in a study. The study found that 40 g of mycoprotein (i.e., 18 g of whole protein) was adequate to activate a muscular protein production response, but 60 g of mycoprotein (i.e., 27 g of overall protein) was sufficient to optimally boost muscle protein production responses in young men described by Bottin et al.[Citation22] These key findings point to mycoprotein as an insulinotropic and bioavailable protein source that could help muscles produce more protein.
Environmental impact
Consumers are influenced by both nutritional content and perceptions of the food’s ecological consequences, according to Siegrist and Hartmann.[Citation42] As a result, the primary step in popularizing meat alternatives is raising community awareness about the ecological effect of their eating behaviors. There is a dearth of literature on mycoprotein life cycle analyses (LCA). However, they conclude that mycoprotein has a lower ecological effect than meat. Finnigan et al.[Citation43] utilized an LCA to examine Quorn® (mince) versus beef (mince) and concluded that the meat substitute creates just 48% of the greenhouse effect that animal protein does when measure by weight. Uncertainties concerning the requisite volumes of egg albumin and glucose in the mycoprotein product’s formulation can push this number up to 60%. The system limits in this study were set from the raw material production to the delivery point. The water, land, and energy utilized in the manufacturing of meat substitutes were reported by Smetana et al.[Citation44] When it comes to water use up (~ 500 L /kg) and land usage (< 2 m2a /kg, contrasted to 7–8 m2a for ham and poultry 5–7 m2a), mycoprotein is one of the most competent substitutes. Mycoprotein was effective as dairy substitutes (15–20 kWh /kg) in terms of energy expenditure but less economical than insects and vegetables (< 5–15 kWh /kg and 10 kWh /kg, correspondingly). It is critical to utilize LCA to assess protein sources to inform customers about the food’s ecological consequences. Therefore, the structural unity notion for product comparison must still be verified. Metrics like kg of protein, kg of product, and kg of protein adjusted by their digestibility value have been explored.[Citation45] Furthermore, while comparing protein levels, other nutrients are overlooked. Jungbluth et al.[Citation46] conducted a preliminary study using the Swiss ecological scarcity technique in 2013 to investigate the effects of a whole home-cooked meal provided using animal substitutes. The meals were designed to deliver a healthy blend of nutrients. Among the vegetarian options explored, the mycoprotein option performed negatively, although it outperformed the fish and meat options used as a benchmark. Another approach for minimizing the ecological consequences of this meat replacer is to exploit agro-industrial by-products for mycoprotein biosynthesis. Filamentous fungi can utilize lignocellulosic materials without pretreatment to produce mycoprotein in solid-state and submerged fermentation.[Citation47] The concern with this approach is to seek biodegradable streams with a beneficial nutritional quality to ensure efficient yield. Additionally, if these sources are already utilized for animal food, the ecological effect of substituting these substances in animal production could escalate environmental consequences. Smetana et al.[Citation44] found that successfully using agro-based leftovers in the manufacturing of mycoprotein might decrease its ecological footprint to 2–4 kg CO2eq, energy expenditure (10 kWh), water (250 L), and land usage (0.5 m2a). However, governing and safety authorization, large-scale experiments, and production scale-up must all be overcome before this innovation can be implemented on an industrial level.
Future directions
Growing populations are imposing tremendous burdens on the planet’s food sources- enhanced animal-source protein synthesis boosts greenhouse gas emissions and soil and water demand- a “perform storm” that emphasizes the urgency for alternatives.[Citation48] Mycoprotein requires less resources, such as water than animal proteins and releases fewer greenhouse gases than meat production.[Citation49] Plant-based foods are less harmful to the climate. As a result, they are gaining popularity and gradually being integrated into the FBDG, even though specific ideas are not universally reflected throughout nations.[Citation50,Citation51] Compared to fish, meat eaters, and vegetarians, vegan lifestyles have been linked to low plasma total cholesterol levels, possibly due to dietary changes.[Citation52] However, there are still some misunderstandings, with some health experts interpreting fungal protein as a “plant-based” protein, which is not the reality.[Citation53] Fungal mycoprotein has been accumulating health and mechanistic evidence. The public’s attitude toward the intake of fungal protein seems to be altering.[Citation54,Citation55] Older consumers, for example, are now ready to receive alternate, better viable protein sources due to changing demographics. Asian countries are also expected to become critical markets for meat substitutes.[Citation40,Citation56]
Moreover, fungal biotechnology has the potential to convert organic substances into nutrient-dense dietary proteins, which can help to address some of the world’s most pressing issues.[Citation57] Even though humans have been utilizing biodiversity for long periods, there has never been a more critical time in history to further the research on how we may use it as a source of sustainable and healthy protein.[Citation58,Citation59] In the growing alternative food arena, other food manufacturing platforms for fungus are also emerging, and they are predicted to add to this body of support in the future.[Citation60] For example, the exploitation of low-cost agricultural wastes as a (filamentous fungi) mycoprotein manufacturing substrate.[Citation15] While wheat and soy proteins have long-held dominant positions in the protein industry, novel protein components are rapidly emerging, particularly from plants and fungi, underscoring the importance of protein diversity in modern diets.[Citation61] Considering these aspects, it is time to reconsider FBDG and manufacturing dietary protein from fungus, which comprises mycelium mycoprotein, which has demonstrated health and environmental benefits.[Citation6]
Conclusion
Dietary patterns have changed and substantially influenced human nutrition, society, and the climate. Climate-smart food systems can assist in alleviating the sector’s negative consequences. As a result, substituting meat with meat alternatives can positively impact both social and personal aspects. Mycoprotein is a novel protein alternative source with a high level of consumer acceptance. Its consumption could be ideal for non-meat eaters and those who desire to keep their meat dose within dietary limits. It’s a great source of dietary protein because it’s low in fat, calories, fiber, and protein. Numerous studies have demonstrated that eating mycoprotein provides many health benefits, including muscle maintenance, reducing LDL cholesterol, and regulating insulin and blood glucose levels. The high cost of this protein source restricts its application to emerging markets, and the raw materials required in its production have a high ecological effect compared to other plant-based alternatives. Innovative manufacturing strategies like solid-state fermentation and using agricultural leftovers as a substrate must be prioritized to meet these challenges.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Credit authorship contribution statement
Farhan Saeed and Muhammad Afzaal proposed this idea and drafted the initial manuscript. Armaghan Khalid, Yasir Abbas Shah, Arslan Shoukat, Huda Ateeq, Fakhar Islam, Gulzar, and Afaf helped in preparing figures and tables and the overall quality of the manuscript.
Consent to participate
Corresponding and all the co-authors are willing to participate in this manuscript.
Acknowledgments
The authors are thankful to Government College University Faisalabad for providing literature collection facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Even though adequate data has been given in the form of tables and figures, however, all authors declare that if more data is required then the data will be provided on a request basis.
Additional information
Funding
References
- Upcraft, T.; Tu, W.-C.; Johnson, R.; Finnigan, T.; Van Hung, N.; Hallett, J.; Guo, M. Protein from Renewable Resources: Mycoprotein Production from Agricultural Residues. Green Chem. 2021, 23(14), 5150–5165. DOI: 10.1039/D1GC01021B.
- FAO, I. UNICEF. WFP, WHO, the State of Food Security and Nutrition in the World; FAO: Rome, 2019.
- Li, J.; Xia, E.; Wang, L.; Yan, K.; Zhu, L.; Huang, J. Knowledge Domain and Emerging Trends of climate-smart Agriculture: A Bibliometric Study. Environ. Sci. Pollut. Res. 2022, 29(46), 70360–70379.
- Webb, L.; Fleming, A.; Ma, L.; Lu, X. Uses of Cellular Agriculture in plant-based Meat Analogues for Improved Palatability. ACS Food. Sci. & Tech. 2021, 1(10), 1740–1747. DOI: 10.1021/acsfoodscitech.1c00248.
- Mittermeier-Kleßinger, V. K.; Hofmann, T.; Dawid, C. Mitigating off-flavors of plant-based Proteins. J. Agric. Food Chem. 2021, 69(32), 9202–9207. DOI: 10.1021/acs.jafc.1c03398.
- Derbyshire, E. J.; Delange, J. Fungal protein–what Is It and What Is the Health Evidence? A Systematic Review Focusing on Mycoprotein. Front. Sust. Food Syst. 2021, 5, 581682.
- Zhang, C.; Guan, X.; Yu, S.; Zhou, J.; Chen, J. Production of Meat Alternatives Using Live Cells, Cultures and Plant Proteins. Curr. Opin. Food Sci. 2022, 43, 43–52. DOI: 10.1016/j.cofs.2021.11.002.
- Fungal-Derived Mycoprotein, D. E. Health across the Lifespan: A Narrative Review. J. Fungi 2022, 8(7), 653.
- Whittaker, J. A.; Johnson, R. I.; Finnigan, T. J.; Avery, S. V.; Dyer, P. S. The Biotechnology of Quorn Mycoprotein: Past, Present and Future Challenges. Grand challenges fungal biotechnol. Springer. 2020, 59–79.
- Ahmad, M. I.; Farooq, S.; Alhamoud, Y.; Li, C.; Zhang, H. A Review on Mycoprotein: History, Nutritional Composition, Production Methods, and Health Benefits. Trends Food Sci. Technol. 2022, 121, 14–29. DOI: 10.1016/j.tifs.2022.01.027.
- Derbyshire, E. Protein guidance—Is It Time for an Update. Dietetics Today 2020, 22–23.
- Denny, A.; Aisbitt, B.; Lunn, J. Mycoprotein and Health. Nutr. Bull. 2008, 33(4), 298–310. DOI: 10.1111/j.1467-3010.2008.00730.x.
- Asgar, M.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9(5), 513–529. DOI: 10.1111/j.1541-4337.2010.00124.x.
- Finnigan, T.; Needham, L.; Abbott, C. Mycoprotein: A Healthy New Protein with a Low Environmental Impact. Sustain. protein sources, Elsevier. 2017, 305–325.
- Souza Filho, P. F.; Andersson, D.; Ferreira, J. A.; Taherzadeh, M. J. Mycoprotein: Environmental Impact and Health Aspects. World J. Microbiol. Biotechnol. 2019, 35(10), 1–8. DOI: 10.1007/s11274-019-2723-9.
- Dunlop, M. V.; Kilroe, S. P.; Bowtell, J. L.; Finnigan, T. J.; Salmon, D. L.; Wall, B. T. Mycoprotein Represents a Bioavailable and Insulinotropic non-animal-derived Dietary Protein Source: A dose–response Study. Br. J. Nutr. 2017, 118(9), 673–685. DOI: 10.1017/S0007114517002409.
- Coelho, M. O.; Monteyne, A. J.; Dunlop, M. V.; Harris, H. C.; Morrison, D. J.; Stephens, F. B.; Wall, B. T. Mycoprotein as a Possible Alternative Source of Dietary Protein to Support Muscle and Metabolic Health. Nutr. Rev. 2020, 78(6), 486–497. DOI: 10.1093/nutrit/nuz077.
- Edwards, D.; Cummings, J. The Protein Quality of Mycoprotein. Proc. Nutr. Soc. 2010, 69(OCE4). DOI: 10.1017/S0029665110001400.
- Derbyshire, E.; Ayoob, K.-T. Mycoprotein: Nutritional and Health Properties. Nutr. today. 2019, 54(1), 7–15. DOI: 10.1097/NT.0000000000000316.
- Burley, V.; Paul, A.; Blundell, J. Influence of a high-fibre Food (myco-protein^*) on Appetite: Effects on Satiation (Within Meals) and Satiety (Following Meals). Eur. J. Clin. Nutr. 1993, 47, 709.
- Turnbull, W. H.; Walton, J.; Leeds, A. R. Acute Effects of Mycoprotein on Subsequent Energy Intake and Appetite Variables. Am. J. Clin. Nutr. 1993, 58(4), 507–512. DOI: 10.1093/ajcn/58.4.507.
- Bottin, J. H.; Swann, J. R.; Cropp, E.; Chambers, E. S.; Ford, H. E.; Ghatei, M. A.; Frost, G. S. Mycoprotein Reduces Energy Intake and Postprandial Insulin Release without Altering glucagon-like Peptide-1 and Peptide tyrosine-tyrosine Concentrations in Healthy Overweight and Obese Adults: A randomised-controlled Trial. Br. J. Nutr. 2016, 116(2), 360–374. DOI: 10.1017/S0007114516001872.
- Williamson, D. A.; Geiselman, P. J.; Lovejoy, J.; Greenway, F.; Volaufova, J.; Martin, C. K.; Arnett, C.; Ortego, L. Effects of Consuming Mycoprotein, Tofu or Chicken upon Subsequent Eating Behaviour, Hunger and Safety. Appetite. 2006, 46(1), 41–48. DOI: 10.1016/j.appet.2005.10.007.
- Turnbull, W. H.; Ward, T. Mycoprotein Reduces Glycemia and Insulinemia When Taken with an oral-glucose-tolerance Test. Am. J. Clin. Nutr. 1995, 61(1), 135–140. DOI: 10.1093/ajcn/61.1.135.
- Ruxton, C. H.; McMillan, B. The Impact of Mycoprotein on Blood Cholesterol Levels: A Pilot Study. Br. Food J. 2010, 112(10), 1092–1101. DOI: 10.1108/00070701011080221.
- Bottin, J.; Cropp, E.; Ford, H.; Bétrémieux, L.; Finnigan, T.; Frost, G. Mycoprotein Reduces Insulinemia and Improves Insulin Sensitivity. Proc. Nutr. Soc. 2011, 70(OCE6). DOI: 10.1017/S0029665111004575.
- Colosimo, R.; Warren, F. J.; Edwards, C. H.; Finnigan, T. J.; Wilde, P. J. The Interaction of α-amylase with Mycoprotein: Diffusion through the Fungal Cell Wall, Enzyme Entrapment, and Potential Physiological Implications. Food Hydrocolloids. 2020, 108, 106018. DOI: 10.1016/j.foodhyd.2020.106018.
- Monteyne, A. J.; Coelho, M. O.; Porter, C.; Abdelrahman, D. R.; Jameson, T. S.; Jackman, S. R.; Blackwell, J. R.; Finnigan, T. J.; Stephens, F. B.; Dirks, M. L. Mycoprotein Ingestion Stimulates Protein Synthesis Rates to a Greater Extent than Milk Protein in Rested and Exercised Skeletal Muscle of Healthy Young Men: A Randomised Controlled Trial. Am. J. Clin. Nutr. 2020, 112(2), 318–333. DOI: 10.1093/ajcn/nqaa092.
- Coelho, M. O.; Monteyne, A. J.; Kamalanathan, I. D.; Najdanovic-Visak, V.; Finnigan, T. J.; Stephens, F. B.; Wall, B. T. Short-communication: Ingestion of a nucleotide-rich Mixed Meal Increases Serum Uric Acid Concentrations but Does Not Affect Postprandial Blood Glucose or Serum Insulin Responses in Young Adults. Nutrients. 2020, 12(4), 1115. DOI: 10.3390/nu12041115.
- Turnbull, W. H.; Leeds, A. R.; Edwards, G. D. Effect of Mycoprotein on Blood Lipids. Am. J. Clin. Nutr. 1990, 52(4), 646–650. DOI: 10.1093/ajcn/52.4.646.
- Turnbull, W. H.; Leeds, A. R.; Edwards, D. G. Mycoprotein Reduces Blood Lipids in free-living Subjects. Am. J. Clin. Nutr. 1992, 55(2), 415–419. DOI: 10.1093/ajcn/55.2.415.
- Coelho, M. O.; Monteyne, A. J.; Dirks, M. L.; Finnigan, T. J.; Stephens, F. B.; Wall, B. T. Daily Mycoprotein Consumption for 1 Week Does Not Affect Insulin Sensitivity or Glycaemic Control but Modulates the Plasma Lipidome in Healthy Adults: A Randomised Controlled Trial. Br. J. Nutr. 2021, 125(2), 147–160. DOI: 10.1017/S0007114520002524.
- Wall, B. T.; Hamer, H. M.; de Lange, A.; Kiskini, A.; Groen, B. B.; Senden, J. M.; Gijsen, A. P.; Verdijk, L. B.; van Loon, L. J. Leucine co-ingestion Improves post-prandial Muscle Protein Accretion in Elderly Men. Clin. Nutr. 2013, 32(3), 412–419. DOI: 10.1016/j.clnu.2012.09.002.
- Murphy, C.; Oikawa, S.; Phillips, S. Dietary Protein to Maintain Muscle Mass in Aging: A Case for per-meal Protein Recommendations. J Frailty Aging. 2016, 5(1), 49–58. DOI: 10.14283/jfa.2016.80.
- Tang, J. E.; Moore, D. R.; Kujbida, G. W.; Tarnopolsky, M. A.; Phillips, S. M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and following Resistance Exercise in Young Men. J. Appl. Physiol. 2009, 107(3), 987–992. DOI: 10.1152/japplphysiol.00076.2009.
- Pennings, B.; Boirie, Y.; Senden, J. M.; Gijsen, A. P.; Kuipers, H.; van Loon, L. J. Whey Protein Stimulates Postprandial Muscle Protein Accretion More Effectively than Do Casein and Casein Hydrolysate in Older Men. Am. J. Clin. Nutr. 2011, 93(5), 997–1005. DOI: 10.3945/ajcn.110.008102.
- Gorissen, S. H.; Horstman, A. M.; Franssen, R.; Crombag, J. J.; Langer, H.; Bierau, J.; Respondek, F.; Van Loon, L. J. Ingestion of Wheat Protein Increases in Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomised Trial. J. Nutr. 2016, 146(9), 1651–1659. DOI: 10.3945/jn.116.231340.
- van Vliet, S.; Burd, N. A.; van Loon, L. J. The Skeletal Muscle Anabolic Response to plant-versus animal-based Protein Consumption. J. Nutr. 2015, 145(9), 1981–1991. DOI: 10.3945/jn.114.204305.
- Mitchell, C. J.; McGregor, R. A.; D’Souza, R. F.; Thorstensen, E. B.; Markworth, J. F.; Fanning, A. C.; Poppitt, S. D.; Cameron-Smith, D. Consumption of Milk Protein or Whey Protein Results in a Similar Increase in Muscle Protein Synthesis in Middle Aged Men. Nutrients. 2015, 7(10), 8685–8699. DOI: 10.3390/nu7105420.
- Hartmann, C.; Siegrist, M. Consumer Perception and Behaviour regarding Sustainable Protein Consumption: A Systematic Review. Trends Food Sci. Technol. 2017, 61, 11–25. DOI: 10.1016/j.tifs.2016.12.006.
- West, D. W.; Burd, N. A.; Coffey, V. G.; Baker, S. K.; Burke, L. M.; Hawley, J. A.; Moore, D. R.; Stellingwerff, T.; Phillips, S. M. Rapid Aminoacidemia Enhances Myofibrillar Protein Synthesis and Anabolic Intramuscular Signaling Responses after Resistance Exercise–. Am. J. Clin. Nutr. 2011, 94(3), 795–803. DOI: 10.3945/ajcn.111.013722.
- Siegrist, M.; Hartmann, C. Impact of Sustainability Perception on Consumption of Organic Meat and Meat Substitutes. Appetite. 2019, 132, 196–202. DOI: 10.1016/j.appet.2018.09.016.
- Finnigan, T.; Lemon, M.; Allan, B; Mycoprotein, P. I. Life Cycle Analysis and the Food 2030 Challenge. Asp. Appl. Biol. 2010, 102, 81–90
- Smetana, S.; Aganovic, K.; Irmscher, S.; Heinz, V. Agri-food Waste Streams Utilisation for Development of More Sustainable Food Substitutes. In Designing Sustainable Technologies, Products and Policies, Springer, Cham, 2018; pp 145–155.
- Sonesson, U.; Davis, J.; Flysjö, A.; Gustavsson, J.; Witthöft, C. Protein Quality as Functional unit–A Methodological Framework for Inclusion in Life Cycle Assessment of Food. J. Cleaner Prod. 2017, 140, 470–478. DOI: 10.1016/j.jclepro.2016.06.115.
- Jungbluth, N.; Eggenberger, S.; Nowack, K.; Keller, R. Life Cycle Assessment of Meals Based on Vegetarian Protein Sources. In proceedings from: the 10th international conference on life cycle assessment of food (LCA Food 2016), 2016.
- Satari, B.; Karimi, K. Mucoralean Fungi for Sustainable Production of Bioethanol and Biologically Active Molecules. Appl. Microbiol. Biotechnol. 2018, 102(3), 1097–1117. DOI: 10.1007/s00253-017-8691-9.
- Batini, N. Transforming agri-food Sectors to Mitigate Climate Change: The Role of Green Finance. Vierteljahrshefte zur Wirtschaftsforschung. 2019, 88(3), 7–42. DOI: 10.3790/vjh.88.3.7.
- Warner, R. Analysis of the Process and Drivers for Cellular Meat Production. Animal. 2019, 13(12), 3041–3058. DOI: 10.1017/S1751731119001897.
- Stipanuk, M. H.; Caudill, M. A. Biochemical, Physiological, and Molecular Aspects of Human nutrition-E-book; Elsevier health sciences, 2018.
- Osowski, C. P.; Sydner, Y. M. Traditional or Cultural Relativist School Meals?: The Construction of Religiously Sanctioned School Meals on Social Media. In What Is Food?; England, UK: Routledge, 2019; pp 72–87.
- Tong, T. Y.; Appleby, P. N.; Bradbury, K. E.; Perez-Cornago, A.; Travis, R. C.; Clarke, R.; Key, T. J. Risks of Ischaemic Heart Disease and Stroke in Meat Eaters, Fish Eaters, and Vegetarians over 18 Years of follow-up: Results from the Prospective EPIC-Oxford Study. bmj. 2019, 366.
- Van Loo, E. J.; Caputo, V.; Lusk, J. L. Consumer Preferences for farm-raised Meat, lab-grown Meat, and plant-based Meat Alternatives: Does Information or Brand Matter? Food Policy. 2020, 95, 101931.
- Grasso, A. C.; Hung, Y.; Olthof, M. R.; Verbeke, W.; Brouwer, I. A. Older Consumers’ Readiness to Accept Alternative, More Sustainable Protein Sources in the European Union. Nutrients. 2019, 11(8), 1904.
- Lonnie, M.; Johnstone, A. The Public Health Rationale for Promoting Plant Protein as an Important Part of a Sustainable and Healthy Diet. Nutr. Bull. 2020, 45(3), 281–293.
- Hobbs, J. E. The Covid-19 Pandemic and Meat Supply Chains. Meat Sci. 2021, 181, 108459.
- Meyer, V.; Basenko, E. Y.; Benz, J. P.; Braus, G. H.; Caddick, M. X.; Csukai, M.; De Vries, R. P.; Endy, D.; Frisvad, J. C.; Gunde-Cimerman, N. Growing a Circular Economy with Fungal Biotechnology: A White Paper. Fungal biol. biotechnol. 2020, 7(1), 1–23.
- Antonelli, A.; Smith, R.; Fry, C.; Simmonds, M. S.; Kersey, P. J.; Pritchard, H.; Abbo, M.; Acedo, C.; Adams, J.; Ainsworth, A. State of the World’s Plants and Fungi. Royal Botanic Gardens (Kew); Sfumato Foundation, 2020. White, C. Why Regenerative Agriculture? Am. J. Econ. Sociol. 2020, 79(3), 799–812.
- Rowan, N. J.; Galanakis, C. M. Unlocking Challenges and Opportunities Presented by COVID-19 Pandemic for cross-cutting Disruption in agri-food and Green Deal Innovations: Quo Vadis? Sci. Total Environ. 2020, 748, 141362.
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food Proteins from Plants and Fungi. Curr. Opin. Food Sci. 2020, 32, 156–162.
- Cherta-Murillo, A.; Frost, G. S. The Association of mycoprotein-based Food Consumption with Diet Quality, Energy Intake and non-communicable Diseases’ Risk in the UK Adult Population Using the National Diet and Nutrition Survey (NDNS) Years 2008/2009–2016/2017: A cross-sectional Study. Br. J. Nutr. 2022, 127(11), 1685–1694.