?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Date palm (Phoenix dactylifera L.) has a significant role in our daily life due to its diverse nutritional composition and potential health benefits. This study was designed to evaluate the phytochemicals, antioxidant, and antidiarrheal potential of various extracts of Ajwa whole fruit, date palm pit, and flesh. The extracts with the highest phytochemical quantities were further subjected to bio-assay assessment against castor oil-induced diarrhea using different animal models. The results indicated that Ajwa whole fruit extract (AWFE) exhibited higher amount of total phenolic contents (TPC: 36 ± 16 mg/g GAE), total flavonoid contents (TFC: 360 ± 135 mg/g RE), and 2, 2-diphenyl-1-picrylhydrazyl (DPPH: 60 ± 7%) in hydro-ethanolic extracts. In vivo results revealed that Ajwa whole fruit 60% hydro-ethanolic extract at high dose (2000 mg/kg) showed significantly (p < .05) highest protective effect against diarrheal changes in mice. In case of AWFE, the intestinal contents % inhibition was slightly higher (72%) as compared to that of loperamide control (70%). High dose of Ajwa whole (2000 mg/kg) demonstrated less effectiveness (66 ± 11%) in comparison with loperamide (78 ± 8%) regarding % inhibition of fluid accumulation in enteropooling studies. High dose (2000 mg/kg) of Ajwa also reduced intestinal transit time by decreasing the % distance (53 ± 1%) in comparison with loperamide (57 ± 1%). The % inhibition of charcoal meal traveling of AWFE (38 ± 2%) was also significantly higher as compared to loperamide (26 ± 1%). The findings of the current study reveal that AWFE might be consumed as an ingredient in daily food products to counter effect the symptoms of diarrhea.
Introduction
Diarrhea is well defined as frequent motility of bowel linked with abnormally loose or watery stools. The major symptoms such as number of diarrhea frequency, continuity, and mass of stool are usually measured to find diarrhea.[Citation1] Diarrhea is secreted by osmotic and active discharge, with reduced peristalsis and exudation.[Citation2] Three main types of diarrhea syndrome are watery diarrhea (loss of 250 ml/kg/day of water in diarrheal discharge), bloody diarrhea (diarrheal discharge with visible blood), and insistent diarrhea (usually last for more than two weeks). Out of these, acute watery diarrhea that leads to dehydration is the major reason of mortality due to diarrhea.[Citation3] Each year, the death of almost 1.7 billion children occurs due to diarrhea and likewise 500,000 deaths of children under five years are also reported.[Citation2] Africa has the highest number of diarrhea prevalence across the world.[Citation3] Although the diarrhea treatment is insufficient due to unproductive, contraindication of various medicines (Sinan et al., 2020) and due to the prevalence of drug resistance, but it still can be prevented and curable. In this context, medicinal plants have significant importance toward treatment of diarrhea and its associated symptoms.[Citation3]
As the medicinal plants are cost-effective, frequently available, and relatively more potent in the treatment of diarrheal disorders, most of the Africans and ayurvedics rely on their use as compared to synthetic medicines.[Citation1] Plants are considered as the dense source of several bioactives enriched with potent antioxidant and therapeutic attributes.[Citation4] Various medicinal plants are reported to possess the antidiarrheal activity that might be linked with the presence of naturally occurring potent plant bioactives such as phenols, flavonoids, alkaloids, saponins, tannins, terpenoids, and steroids.[Citation4] WHO has launched various programs for the eradication of diarrhea by the use of conventional medicinal plants including date palm fruit (Phoenix dactylifera L.), which has been reported to be used for the treatment and control of diarrhea.[Citation5]
The date palm (Phoenix dactylifera L.) has been grown in different countries across the globe since 6000 years ago.[Citation6] With the current situation of world food supply insufficiency and increase in production, the date fruit is likely to remain a low-cost food item. In 1994, date production was 4.60 million metric tons that has been increased to 8.52 million tons in 2018, which indicated that the world date production has increased.[Citation7] The edible portion of fruit contains pit inside, which is usually discarded as a by-product without accounting its health benefits.[Citation8] The antimicrobial potential, high bioactive profile, physicochemical, and rheological aspects of the foods containing dates are improved as compared with the products containing artificial sugar contents.[Citation9] The phytochemicals in date extract have the ability to inhibit the growth of Gram-positive and Gram-negative bacterial by damaging their cell membrane.[Citation10] Date syrup has been found as a substitute of sugar in different conventional dishes.[Citation11] The date pit is also reported to contain high amount of fatty acids including stearic acid, palmitic acid, oleic acid, linoleic acid, and traces of lauric acid.[Citation12]
Keeping in view the health benefits and therapeutic effects of date palm and date palm fruits, the current study was designed to extract the polyphenol and flavonoid-rich extracts of three parts of Ajwa fruit such as whole fruit, pit, and flesh. Moreover, the date parts were also evaluated for their antidiarrheal potential against castor oil-induced diarrheal animal models.
Material and methods
Collection of plant material
Commercially available Ajwa date was purchased from the supermarkets of Al Madinah – Al Monawarah, Saudi Arabia. Ajwa was collected at Tamar stage (fully ripened stage). Samples were selected randomly without preference of size, shape, color, and appearance. Date pits were physically removed from flesh parts and rinsed with double-distilled water. Flesh was refrigerated at 4°C in plastic bags while pit was shade-dried for 4 days and pulverized into fine powder for further analysis.
Extracts preparation
The extract of each part was prepared using two solvents: ethanol and water. The whole date fruit extract was prepared by adding equal quantity of flesh and pit powder (75 g each) on measuring scale. Then, 150 g of whole, pit powder, and flesh part was added in 500 mL of extraction solvent and blended in an electric blender. Different solvent fractions were used such as ethanol 0%, ethanol 20%, ethanol 40%, ethanol 60%, ethanol 80%, and absolute ethanol. After blending, the mixture was transferred into a conical flask and covered with aluminum foil to prevent the evaporation of solvent. The flasks were placed in shaking incubator (Lab Line Instruments, Inc., 26, Melrose Park, IL, USA) for 6 h at 35°C with continuous stirring. Then, the mixture was filtered using Whatman no. 1 filter paper and concentrated on rotary evaporator (Model Heidolph, Germany)) at 3000 rpm. All the extracts were preserved in plastic bottles and stored at −18°C for further analyses.
Antioxidant potential of date extract
Total phenolic contents (TPC)
Total phenolic content of date extracts was estimated by Folin-Ciocalteu method described by Lim with little modifications.[Citation13] 200 µL was taken from stock solution in Al foil-wrapped test tubes and 1.5 ml FC reagent. After 8 min, 1.5 mL NA2CO3 (60 g/L) was added to neutralize the above solution. The solution was kept in dark for 1 h at 40°C, and absorbance was recorded at 765 nm using UV-visible spectrophotometer (Model T70, PG, USA). TPCs were expressed as mg/L gallic acid equivalent (GAE) (Merck, Darmstadt, Germany). Each extract was analyzed in triplicate.
Total flavonoid contents (TFC)
Total flavonoid contents of date parts was evaluated by following the method reported by Park et al.[Citation14]100 mg extract sample was taken in a test tube by adding 1 mL ethanol. After that 150 µl of 0.5 M NaNO2 solution and150 μL of 0.3 M AlCl3 solution (0.725 g of AlCl3.6H2O in 100 mL) were added. For positive control, different dilutions of rutin were prepared to make standard calibration curve. For negative control, ethanol was used instead of sample extract. Absorbance was measured at 506 nm. TFC was expressed as mg/100 g of rutin equivalent.
2, 2-diphenyl-1-picrylhydrazyl (DPPH)
DPPH radical scavenging assay of date extracts was evaluated by following the mode of Brand-Williams et al.[Citation15]100 µL sample was prepared and DPPH was added in it. The combination was incubated for 30 min at 37°C and antioxidant activity was recorded at 516 nm. For positive control, gallic acid was added in 3.9 mL of DPPH solution. Negative control was prepared by adding 100 μL solvent (ethanol) in 3.9 mL of DPPH solution.
% inhibition of sample was calculated by:
Quantitative phytochemical screening by HPLC
The date extract was subjected to high-performance liquid chromatography (HPLC) for the evaluation of phenolic and flavonoid compounds as described by Qadir et al.[Citation16] Reversed phase HPLC 1200 series Isocratic detector (UV-Vis detector, model: SPD-10 AV, Shimadzu, Japan) Shim-pack CLC-ODS column (4.6 mm × 25 cm, 5 μm) was used with following modifications: nebulizer pressure, capillary temperature, and flow rate of nitrogen were 350°C, 65.0 psi, and 11 L min. The complete scan was done in the range from m/z 100 to m/z 1500. Helium gas was used as collision gas, with voltage ramping cycles from 0.3 to 2 V. Two solvents were used as mobile phase, A (acetic acid: water in 94:6, pH = 2.27) and B (acetonitrile 100%) at a continuous solvent flow rate of 1 ml/min in a linear gradient, initializing with 12% B and reaching 25% B at 30 min, 50% B at 35 min, and 60% B at 37 min. The injection volume was 20 μl. The signs were identified at 280 nm by UV-VIS detector. Eleven phenolic compounds (gallic acid, caffeic acid, benzoic acid, m-coumaric acid, vanillic acid, chlorogenic acid, sinapic acid, p-coumaric acid, ferulic acid, and cinnamic acid) and one flavonoid compound (quercetin) were used separately as a standard reference for the identification of phytochemical compounds. The results were expressed as mg/100 g DW.
Animal study: in vivo trial
Experimental animals
Albino mice (25–30 g) of 8–10 weeks were procured from Animal House Center, Department of zoology, University of Sargodha, Sargodha, Pakistan. Animals were kept in wired cages in animal house and were acclimatized for two weeks before the commencement of experiments.[Citation17] The mice were randomly divided into 12 groups; each group contained eight mice. Each group with their dose levels are mentioned in .
Table 1. Animal study protocol.
Induction of diarrhea
The antidiarrheal potential of Ajwa date fruit extracts was measured by the exposure of castor oil for diarrheal induction following the method described by Fokam et al.[Citation18] The castor oil (0.5 mL) was administered orally to mice to induce diarrhea. Each dose was given according to a standard method, that is, 100 µL/10 g mice. Each group was kept in separate cage with lined transparent paper, during observation period, diarrhea onset time (time of first diarrhea after castor oil administration), number and weight of total and wet stool was measured. Feces of negative group were supposed to be 100% wet (diarrhea). The diarrhea % inhibition was measured according to the following formula.
where ID=inhibition of diarrhea, WFNC=wet feces in the negative control group, WFT=wet feces in the test group, ITFO=Inhibition of total fecal output, TFC=total feces of control, and TFT=total feces of treated.
Enteropooling study
The potential of all solvent fraction extracts in reducing intestinal secretion induced by castor oil was determined according to the method given by Fokam et al.[Citation19] After the administration of castor oil, the mice were sacrificed by cervical dislocation with a surgical blade. The small intestine was taken out and then cut from the pylorus and cecum and ligated at both ends. The weight of intestines was measured before and after removal of intestinal contents and variation was calculated. The content of small intestine was removed in graduate tubes and the volume was measured. Finally, the reduction in weight and volume of intestinal contents compared to negative control was measured by the given formula as follows:
(%) Inhibition = (VDC-VDT)/VDC X 100 (5)
where VDC: volume of the diarrheal control, and VDT: Volume of diarrheal treated
Intestinal transit time
The antimotility test of the extract was determined according to the method described by Fokam et al.[Citation20] After the administration of castor oil, all the mice were treated with 1-mL activated charcoal meal (5% activated charcoal of arabic gum) and after 30 min mice were made unconscious by ketamine injection and dissection of animals was done by cervical dislocation and the abdomens of animals were removed. The length of the intestine was measured with a ruler and the distance traveled by the charcoal meal in the small intestine was calculated and expressed by the peristaltic index (PI), the inhibition percentage was calculated using the following:
IP = Distance traveled by the charcoal marker/total length of the small intestine × 100
The inhibition percentage was calculated by the following formula:
where DCDC: distance traveled by charcoal in the diarrheal control (DC), DCDT: distance traveled by charcoal in the diarrheal treated
Statistical analysis
Results of the three replicates were described as mean values ± standard deviation (SD). Two-way analysis of variance (ANOVA) was employed for estimating the level of significance while Tukeys test was used for comparison of means (statistics 8.1).
Results and discussion
Antioxidant potential of date extract
The total phenolic and flavonoid contents were determined as expressed in . The results revealed that among all solvent fraction TPC was significantly higher (120 ± 2 g/100 g GAE) in 60% ethanol followed by ethanol 100% (27 ± 7 mg/g GAE), ethanol 40% (71 ± 2 mg/g GAE), ethanol 20% (56 ± 2 mg/g GAE), ethanol 80% (29 ± 2 mg/g GAE), and ethanol 0% (11 ± 2 mg/g GAE). Wherein the variation in the parts revealed that greater value of TPC (36 ± 16 mg/g GAE) was observed in whole part, pit (26 ± 12 mg/g GAE), and flesh (17 ± 8 mg/g GAE).
Table 2. Total phenolic and total flavonoid contents of Ajwa date extracts.
The results regarding TFC showed the higher TFC value (867 ± 1 mg/g RE) in 60% ethanol followed by 40% ethanol (798 ± 1 mg/g RE), 20% ethanol (594 ± 3 mg/g RE), 80% ethanol (296 ± 1 mg/g RE), ethanol 100% (186 ± 2 mg/g RE), and ethanol 0% (146 ± 1 mg/g RE) (). The results also revealed that whole part had higher amount of TFC (360 ± 135 mg/g RE) followed by pit (312 ± 115 mg/g RE) and flesh (243 ± 106 mg/g RE).
In the present study, among others, maximum quantity of phenolic acids such as gallic acid (57 ± 1 ppm), caffeic acid (6 ± 1 ppm), benzoic acid (221 ± 0.57 ppm), ferulic acid (14 ± 1 ppm), and cinnamic acid (9 ± 0.56 ppm) were offered by 60% ethanolic extract of whole fruit parts (). The flavonoid such as quercetin was found in extracts of all parts with higher concentration (2 ± 0.1 ppm) in whole part (E 60%), followed by pit extract absolute ethanol (2 ± 0.08 ppm) while flesh part exhibited the larger quantities (1 ± 0.01 ppm) of quercetin in E 0% extract. Pit extracts showed the highest phytochemicals in absolute ethanol while flesh extracts exhibited higher quantities in aqueous extracts.
Table 3. HPLC analysis of Ajwa date extracts and solvent fractions (mg/100 g DW).
W= whole part; P=pit part; F=flesh part
It is evident from the current study that the effect of date palm extracts on DPPH % inhibition () demonstrated that among solvent fractions the ethanol 60% showed maximum inhibition percentage (78 ± 2%). Results revealed that among others, the whole part exhibited the higher % inhibition (60 ± 7%) relative to pit (51 ± 9%) and flesh part (42 ± 10%).
Table 4. DPPH % inhibition of Ajwa parts and solvent fractions (%).
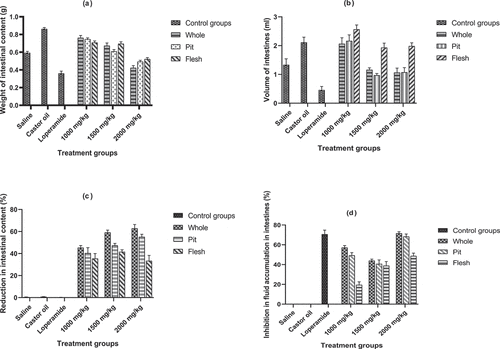
The results pertaining to effect of extracts in decreasing intestinal volume accumulation revealed () that loperamide group significantly lowered the volume (0.44 ± 0.01 mL) followed by saline group (1 ± 0.04 mL), 2000 mg/kg dose group (1.24 ± 0.18 mL), 1500 mg/kg dose (1 ± 0.24 mL), and 1000 mg/kg dose (2 ± 0.01 mL). It was also observed that high dose (2000 mg/kg) of extract exhibited the maximum % inhibition (47 ± 5) in fluid accumulation in small intestines. The whole part was highly effective in reducing the intestinal volume (1 ± 0.33 mL) and % inhibition (35 ± 17) of fluid accumulation as compared to pit and flesh.
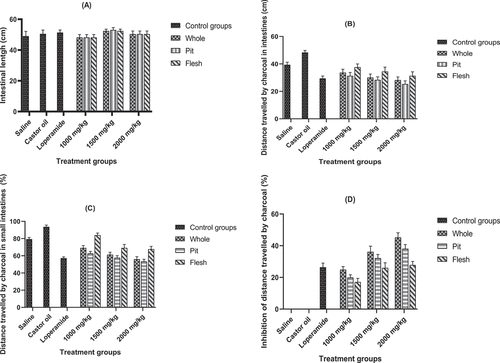
The effect of treatments on distance traveled by charcoal is depicted in . In loperamide group, less distance (29 ± 3 cm) was traveled by charcoal meal in small intestines followed by 2000 mg/kg (31 ± 1 cm), 1500 mg/kg (34 ± 1 cm), 1000 mg/kg (37 ± 1 cm), saline group (39 ± 2 cm), and castor oil (negative control) group (46 ± 3 cm). Similarly, the whole extract of date exhibited less (35 ± 1 cm) distance traveling by charcoal as compared with pit (34 ± 1 cm) and flesh parts (36 ± 1 cm). Current data further revealed that high % inhibition (20 ± 1%) was observed in whole part followed by pit (21 ± 1%) flesh extracts (16 ± 1%). The high dose concentration 2000 mg/kg of date extract exhibited the highest % inhibition (32 ± 3) as compared to low doses ().
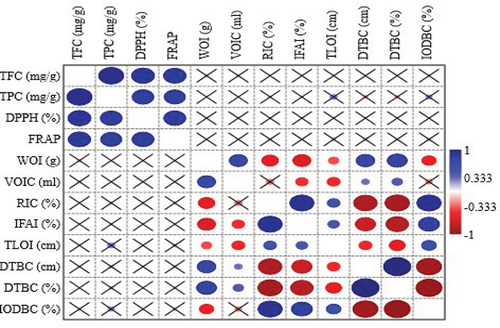
Principle component analysis (PCA) was used to find out the correlation between phytochemical components, antioxidant potential, and antidiarrheal activity of under studied extracts of Ajwa date. PCA bi-plot indicating the relationship among phytochemicals, antioxidant potential and antidiarrheal activity is given in . PC 1 (44.92%) and PC 2 (27.75%) elucidated 72.67% of the total variance (). The PCA results indicated a clear separation among various parameters of antidiarrheal activity, namely, inhibition of distance traveled by charcoal (%), reduction in intestinal contents (%), and inhibition of fluid accumulation in intestines (%), weight of intestines, distance traveled by charcoal (%), distance traveled by charcoal (cm), and antioxidant activities, (DPPH, FRAP, TPC, and TFC) along the PC1. The variation in PC2 was associated with the inhibition of distance traveled by charcoal (%), reduction in intestinal contents (%), and inhibition of fluid accumulation in intestines (%). The results showed that mostly solvent fractions of flesh part are positioned far from the TPC, TFC, DPPH, and FRAP; however, solvent fractions of whole and pit parts are closely grouped to the inhibition of distance traveled by charcoal (%), reduction in intestinal content (%), and inhibition in fluid accumulation in intestines (%). Extracts of selected parts such as EoH 40% and EoH 80% of flesh, EoH 100%; EoH 40%, EoH 40% of pit, and EoH 100% of whole exhibited a negative correlation with that of TPC, TFC, DPPH, and FRAP along the PC2 axis, while on this axis, EoH60% and EoH40% of whole and EoH 100%, 80%, and 60% of pit were strongly correlated with each other and showed a positive correlation with that of TPC, TFC, DPPH, and FRAP. EoH 100%, EoH 20% of flesh, EoH 20% and 40% of whole indicated a negative correlation with that of antidiarrheal activity, such as IODBC (%), RIC (%), and IFAI (%), while EoH 40% of pit and EoH 100% and 80% exhibited strong close correlation with IODBC (%), RIC (%), and IFAI (%).
Figure 3. Principle component analysis (PCA) bi-plot shows the relationship between phytochemicals, antioxidant potential, and antidiarrheal activity aqueous, and ethanolic extracts of Ajwa date parts.
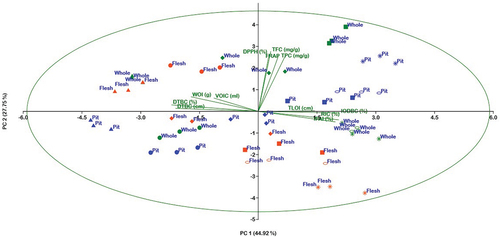
Considering the data in , Ajwa whole parts showed the highest antioxidant activity evaluated by the above-mentioned method in comparison with pit and flesh parts; this might be due to the fact that higher concentration of TPC and TFC is in whole part as noted in the current study. Furthermore, the antioxidant activity of whole part positively correlates with its antidiarrheal activity, which clearly indicates that with the increase in phytochemical contents, the antidiarrheal activity increases.
Discussion
In the current study, the effect of solvent fractions and selected parts revealed that the highest amount of TPC and TFC was observed in ethanol 60% solvent fraction of whole part that might be due to the fact that the whole part is a combination of pit (hydrophobic phytochemical) and flesh parts (hydrophilic phytochemicals). The hydrophobic phytochemicals are more soluble in organic solvents while hydrophilic showed more solubility in aqueous solvent fractions.[Citation21] It was also observed that single solvent cannot be selected for maximum extraction of polyphenols from whole part; hence, the highest extraction of phenolics/flavonoids from whole part was noted in ethanol 60% solvent fraction. This might be due to the fact that phenolics attained equilibrium at this concentration and their solubility increases in extracting power of solvent fraction. The range of TFC in the current study is in line with the findings of Afifi et al.[Citation22] who reported the maximum extraction of TFC (455.77 mg CEQ/100 g) from date seed extracts using various aqueous-organic solvent concentration. Present results are also in agreement with the studies of Abou-Elella and Mourad,[Citation23] who found the highest TFC value in date pit ethanol extracts of ten date varieties by using organic solvent. The results are also in line with the results of Al-Jasass et al.[Citation24] who studied the phenolic contents of six date varieties using aqueous and organic solvents. The combination of organic-aqueous solvent fractions makes excellent medium for maximum extraction of phytochemicals from plant materials because of its ability to extract phenolics from protein matrix.[Citation25] The TFC values of date extracts in the current study are in line with the values observed by Bano et al.[Citation26] in four different varieties. Among pit extracts, higher quantity of TPC and TFC was detected in absolute ethanol than other solvent fractions which might be due to the fact that date pits contained significant amount of aromatic hydrophobic phytochemicals, which can only be soluble in organic solvent than other combinations.[Citation21] These results are also in agreement with the previous data reported by Abou-Elella and Mourad,[Citation23] who demonstrated that ethanol is the best solvent for phenolic extraction from date pit. Extracts of flesh exhibited high values of TPC and TFC in aqueous solvent that could possibly be due to the presence of high amount of aliphatic hydrophilic phytochemicals in date flesh, which are more likely to be soluble in aqueous medium than organic solvents.[Citation21] Present findings are similar with the previous study and that the aqueous solvent extracted the highest amount (14.8 mg/g GAE) of TPC in date flesh than organic solvents (10.31 mg/g GAE).[Citation3]
According to the data in current studies, Ajwa date exhibited high concentration of phytochemicals which is in close agreement with the findings of Eid et al.[Citation27] who reported the results of three date varieties including Ajwa. Main phenolics identified were protocatechuic acid, benzoic acid, vanillic acid, gallic acid, chlorogenic acid, ferulic acid, caffeic acid, cinnamic acid, and chlorogenic acid. Later, Hamad et al.[Citation28] also mentioned the presence of gallic acid and ferulic acid in Ajwa flesh extracts among phenolics of 12 date varieties using HPLC technique. Current results are also in line with previous studies conducted by Bouhlali et al.[Citation29]who reported the presence of phenolics in different date cultivars used.
Antioxidant activity by DPPH % inhibition revealed that the whole part was more effective with maximum inhibition percentage fraction which is in line with the findings of Bensaci et al.[Citation30] who investigated the DPPH antioxidant activity of date fruit using aqueous-organic solvent combination. Present results are also in agreement with Jasass et al.[Citation24] who investigated the DPPH inhibition percentage from flesh of six date varieties using aqueous-organic solvents. The antioxidant activity (DPPH) is directly associated with the concentration of TPC and TFC in date extracts as observed in the current study; the reason behind the higher inhibition percentage of whole part extracts in ethanol 60% was based on phytochemical compounds in whole part (hydrophobic and hydrophilic) because ethanol 60% fraction extracted the maximum amount of TPC and TFC in whole part of all date varieties in preliminary analysis. Current findings are in line with Hussain et al.[Citation31] who mentioned the close association between phytochemical compounds and antioxidant activities of date extracts. The results of present research were also in agreement with Al-Farsi et al.[Citation6] who observed the highest concentration of hydrophilic phytochemicals in Ajwa flesh, which was associated with its high antioxidant activity.
In the current study, strong antidiarrheal activity of Ajwa whole at high dose of 2000 mg/kg extract could be due to several mechanisms including increased reabsorption of water and NaCl, reduction in intestinal mucosa and inhibition of prostaglandins secreted from intestinal mucosa. These results would be justified by the presence of high amount of minerals and vitamins in Ajwa whole extract, which helped in the restoration of electrolyte imbalance, occurred during diarrheal alterations in the body. It has been reported that anti-diarrheal and anti-secretory mechanisms of date extracts could be due to the high concentration of flavonoids.[Citation32] Current findings of phytochemicals in extracts are in line with previously reported phytochemical compounds such as phenolic acids (benzoic, ferulic, cinnamic, p-coumaric, and caffeic) in date extracts.[Citation6] Present results are in agreement with the findings of Al Juhaimi et al.[Citation33] who reported that flavonoid (quercetin) produces an inhibitory action on gastro-intestinal motility and secretions in a dose-dependent manner. These antioxidants compounds help in the reduction of intestinal peristalsis and also activate the Na+/K+ ATPase activity.[Citation34] Date fruit contains high concentration of macro-/micro-nutrients especially minerals and vitamins.[Citation35] Date seeds are considered as a secondary part after separation of flesh part and are being utilized for animal feed along with food industries because they are good source of antioxidants, minerals, and vitamin.[Citation36]
Ricinoleic acid discharged as a result of castor oil induction and is poorly absorbed in small intestines, which stimulates the peristaltic activity in small intestines leading to the changes in permeability of intestinal mucosa and finally resulted in hyper-excretion of diarrhea. Furthermore, the motility of smooth muscles is changed due to the metabolites of castor oil.[Citation37]
Phytochemical screening of extracts indicated the presence of wide range of flavonoids and alkaloids which are well known for inhibiting the release of prostaglandins by preventing the secretions resulting after castor oil induction.[Citation38] These components are also responsible for antidiarrheal activity of date extracts in mice. Present antidiarrheal activities of date extracts are in line with the previous data that flavonoids, triterpenoids, and saponins have ability to prevent intestinal motility and hydroelectrolytic secretions, which are known to be altered in diarrheal conditions.[Citation39] The antidiarrheal properties of flavonoids are associated with their ability to inhibit peristaltic activity and hydroelectrolyte secretions, which were increased during diarrhea.[Citation40]
The combination of tannins and flavonoids presumably resulted in synergistic antidiarrheal activity of date extracts in albino mice. Flavonoids are the second most abundant phytochemicals found in date extracts with significant biological properties.[Citation41] Current antidiarrheal studies of date extracts on the basis of phytochemical screening are closely linked with the findings of Ndukuiet al.[Citation42]who studied the antidiarrheal activity of guava aqueous-ethanol extract in castor-oil-induced rats on the basis of phytochemical compounds especially tannins and flavonoids.
Present research indicated that extracts offered relaxation of intestinal smooth muscles in diarrheal mice in dose-dependent manner. The contractions of smooth muscles are associated with the concentration of intracellular Ca+2.[Citation43] Plant extracts caused inhibition of contractions of ileum; this implies that date extracts reduced the cytosolic calcium, either by inhibiting Ca+2 influx or by inhibiting Ca+2 release from intracellular storage. The relaxant effect might also be due to other mechanisms such as reduction in sensitivity of contractile muscles to existing Ca+2 concentrations or inhibition of Ca-binding to contractile proteins.[Citation44]
Conclusion
Phytochemical screening of date extracts indicated the presence of wide range of flavonoids and phenolics that are well known for inhibiting the release of prostaglandins by preventing the secretions resulted after castor oil induction. Present antidiarrheal activities of date extracts were based on the presence of phytochemicals especially flavonoids, and phenolics that have the ability to prevent intestinal motility and hydro-electrolytic secretions. The antidiarrheal properties of flavonoids and phenolics may be associated with their ability to inhibit peristaltic activity and hydro-electrolyte secretions which are increased during diarrhea. These components are also responsible for antidiarrheal activity of date extracts in mice. It is suggested that Ajwa whole fruit part exhibited maximum protective effect at high dose 2000 mg/kg in castor-oil-induced enteropooling study in albino mice with significant reduction in intra-luminal fluid accumulation and high inhibition percentage in the intestinal tract of diarrheal mice. Further studies are required to find out the exact mechanism of compounds in date extracts responsible for antidiarrheal action.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sinan, K. I.; Chiavaroli, A.; Orlando, G.; Bene, K.; Zengin, G.; Cziáky, Z.; Jekő, J.; Fawzi Mahomoodally, M.; Picot-Allain, M. C. N.; Menghini, L. Biopotential of Bersamaabyssinicafresen Stem Bark Extracts: UHPLC Profiles, Antioxidant, Enzyme Inhibitory, and Antiproliferative Propensities. Antioxidants. 2020, 9(2), 163. DOI: 10.3390/antiox9020163.
- Anza, M.; Worku, F.; Libsu, S.; Mamo, F.; Endale, M. Phytochemical Screening and Antibacterial Activity of Leaves Extract of Bersamaabyssinica. J. Adv. Bot. Zool. 2015, 3(2), 1–5.
- Sinan, K. I.; Bene, K.; Zengin, G.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Picot-Allain, C. M. N.; Mollica, A.; Rengasamy, K. R.; Mahomoodally, M. F. A Comparative Study of the HPLC-MS Profiles and Biological Efficiency of Different Solvent Leaf Extracts of Two African Plants: Bersama Abyssinica and Scoparia Dulcis. Int. J. Env. Heal. Res. 2019, 31(3), 285–297. DOI: 10.1080/09603123.2019.1652885.
- Ekiert, H. M.; Szopa, A. Biological Activities of Natural Products. Molecules. 2020, 25(23), 5769. DOI: 10.3390/molecules25235769.
- Agunu, A.; y, S.; Gabriel, O. A.; Zezi, A. U.; Abdurrahman, E. M. Evaluation of Five Nigerian Medicinal Plants Used in Treatment of Diarrhea in Nigeria. J. Ethnopharmacol. 2005, 61, 209–213.
- Al-Farsi, M.; Lee, C. Y. Optimization of Phenolics and Dietary Fiber Extraction from Date Seeds. Food Chem. 2008, 108(3), 977–985. DOI: 10.1016/j.foodchem.2007.12.009.
- FAO. (2020). FAOSTAT Statistical Database. Livestock Primary. http://www.fao. org/faostat/en/#data/QL. (Accessed Aug 26, 2021).
- Besbes, S.; Blecker, C.; Deroanne, C.; Drira, N.; Attia, H. Date Seeds: Chemical Composition and Characteristic Profiles of the Lipid Fraction. J. Food Chem. 2004, 84(4), 577–584. DOI: 10.1016/S0308-8146(03)00281-4.
- Djaoud, K.; Boulekbache‐Makhlouf, L.; Yahia, M.; Mansouri, H.; Mansouri, N.; Madani, K.; Romero, A. Dairy Dessert Processing: Effect of Sugar Substitution by Date Syrup and Powder on Its Quality Characteristics. J. Food Pro. Pres. 2020, 44(5), 14414. DOI: 10.1111/jfpp.14414.
- Halabi, A. A.; Elwakil, B. H.; Hagar, M.; Olama, Z. A. Date Fruit (Phoenix Dactylifera L.) Cultivar Extracts: Nanoparticle Synthesis, Antimicrobial and Antioxidant Activities. Molecules. 2022, 27(16), 5165. DOI: 10.3390/molecules27165165.
- Manickvasagan, A.; Kumar, C.; Al-Attabi, Z. Effect of Sugar Replacement with Date Paste and Date Syrup on Texture and Sensory Quality of Kesari (Traditional Indian Dessert). J Agri. Mar. Sci. 2018, 22(1), 67. DOI: 10.24200/jams.vol22iss1pp67-74.
- Al-Khayri, J.; Naik, P. Date Palm Micropropagation: Advances and Applications. Ciênc. Agrotec. 2017, 41(4), 347–358. DOI: 10.1590/1413-70542017414000217.
- Lim, Y.; Murtijaya, J. Antioxidant Properties of Phyllanthusamarus Extracts as Affected by Different Drying Methods. LWT - Food Sci. Tech. 2007, 40(9), 1664–1669. DOI: 10.1016/j.lwt.2006.12.013.
- Park, Y.; Jung, S.; Kang, S.; Heo, B.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and Proteins in Ethylene-Treated Kiwifruits. Food Chem. 2008, 107(2), 640–648. DOI: 10.1016/j.foodchem.2007.08.070.
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Sci. Tech. 1995, 28(1), 25–30. DOI: 10.1016/S0023-6438(95)80008-5.
- Qadir, R.; Anwar, F.; Naseem, K.; Tahir, M. H.; Alhumade, H. Enzyme-Assisted Extraction of Phenolics from Capparis Spinosa Fruit: Modeling and Optimization of the Process by RSM and ANN. ACS Omega. 2022, 7(37), 33031–33038. DOI: 10.1021/acsomega.2c02850.
- Kamgang, R.; Youmbi Mboumi, R.; FoyetFondjo, A.; FokamTagne, M.; Mengue N’dillé, G.; NgogangYonkeu, J. Antihyperglycaemic Potential of the Water–Ethanol Extract of Kalanchoecrenata (Crassulaceae). J. Nat. Med. 2008, 62(1), 34–40. DOI: 10.1007/s11418-007-0179-y.
- FokamTagne, M.; Noubissi, P.; Fankem, G.; Kamgang, R. Effects of Oxalis Barrelieri L. (Oxalidaceae) Aqueous Extract on Diarrhea Induced by Shigelladysenteriae Type 1 in Rats. Heal. Sci. Rep. 2017, 1(2), e20. DOI: 10.1002/hsr2.20.
- Ezekwesili, C. N.; Obiora, K. A.; Ugwu, O. P. Evaluation of Anti-Diarrhoeal Property of Crude Aqueous Extract of Ocimumgratissimum L. (Labiatae) in Rats. Biokemistr. 2004, 16(2), 122–131. DOI: 10.4314/biokem.v16i2.32580.
- FokamTagne, M.; Akaou, H.; Noubissi, P.; FoyetFondjo, A.; Rékabi, Y.; Wambe, H.; Kamgang, R.; Essame Oyono, J.-L. Effect of the Hydroethanolic Extract of Bixaorellana Linn (Bixaceae) Leaves on Castor Oil-Induced Diarrhea in Swiss Albino Mice. Gastroent. Res. Pract. 2019, 2019, 1–8. DOI: 10.1155/2019/6963548.
- Saleh, E.; Tawfik, M.; Abu-Tarboush, H. Phenolic Contents and Antioxidant Activity of Various Date Palm (Phoenixdactylifera L.) Fruits from Saudi Arabia. Food Nut. Sci. 2011, 02(10), 1134–1141. DOI: 10.4236/fns.2011.210152.
- Afifi, F.; Kasabri, V.; Al-Jaber, H.; Abu-Irmaileh, B.; Al-Qudah, M.; Abaza, I. Composition and Biological Activity of Volatile Oil from Salvia Judaica and S. Multicaulis from Jordan. Nat. Prod. Comm. 2016, 11(4), 1934578X1601100. DOI: 10.1177/1934578X1601100429.
- Abou-Elella, F.; Mourad, R. Anticancer and Anti-Oxidant Potentials of Ethanolic Extracts of Phoenix Dactylifera, Musa Acuminata and Cucurbita Maxima. Res. J. Pharm. Bio. Chem. Sci. 2015, 6(1), 710–720.
- Al-Jasass, F.; Siddiq, M.; Sogi, D. Antioxidants Activity and Color Evaluation of Date Fruit of Selected Cultivars Commercially Available in the United States. Adv. Chem. 2015, 2015, 1–5. DOI: 10.1155/2015/567203.
- Lu, Y.; Yeap Foo, L. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68(1), 81–85. DOI: 10.1016/S0308-8146(99)00167-3.
- Bano, Y.; Rakha, A.; Issa Khan, M.; Asgher, M. Chemical Composition and Antioxidant Activity of Date (Phoenix Dactylifera L.) Varieties at Various Maturity Stages. Food Sci. Technol. Campinas. 2022, 42, e29022. DOI: 10.1590/fst.29022.
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J. P. E. The Impact of Date Palm Fruits and Their Component Polyphenols, on Gut Microbial Ecology, Bacterial Metabolites and Colon Cancer Cell Proliferation. J. Nut. Sci. 2014, 3, e46. DOI: 10.1017/jns.2014.16.
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic Analysis of Various Date Palm Fruit (Phoenix Dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules. 2015, 20(8), 13620–13641. DOI: 10.3390/molecules200813620.
- Bouhlali, E.; Alem, C.; Ennassir, J.; Benlyas, M.; Mbark, A.; Zegzouti, Y. Phytochemical Compositions and Antioxidant Capacity of Three Date (Phoenix Dactylifera L.) Seeds Varieties Grown in the South East Morocco. J. Saudi Soc. Agri. Sci. 2017, 16(4), 350–357. DOI: 10.1016/j.jssas.2015.11.002.
- Cheyma, B.; Zineb, G.; Mokhtar, S. Effects of Extraction Solvents on Bound Phenolic Contents and Antioxidant Activities of Tantboucht Dates (Phoenix Dactylifera L.) from Algeria. J. Chem. Pharmaceutical Res. 2015, 7(7), 27–31.
- Hussain, M.; Farooq, M.; Syed, Q. Nutritional and Biological Characteristics of the Date Palm Fruit (Phoenix Dactylifera L.) – a Review. Food Biosci. 2020, 34, 100509. DOI: 10.1016/j.fbio.2019.100509.
- Akuodor, G.; Muazzam, I.; Usman-Idris, M.; Megwas, U.; Akpan, J.; Chilaka, K.; Okoroafor, U.; Osunkwo, U. Evaluation of the Antidiarrheal Activity of Methanol Leaf Extract of Bombaxbuonopozense in Rats. Ibnosina J. Med. Biomedical Sci. 2011, 3(1), 15–20. DOI: 10.4103/1947-489X.210845.
- Al-Juhaimi, F.; Ghafoor, K.; Özcan, M. M.; Jahurul, M. H. A.; Babiker, E. E.; Jinap, S.; Sahena, F.; Sharifudin, M. S.; Zaidul, I. S. M. Effect of Various Food Processing and Handling Methods on Preservation of Natural Antioxidants in Fruits and Vegetables. J. Food Sci. Technol. 2018, 55(10), 3872–3880. DOI: 10.1007/s13197-018-3370-0.
- Tagne, M. A.; Kamgang, R.; Noubissi, P. A.; Oyono, J. L. Activity of Oxalis Barrelieri Aqueous Extract on Rat Secretory Diarrhea and Intestine Transit. J. App. Pharm. Sci. 2015, 5(1), 058–62.
- Kuras, E. R.; Warren, P. S.; Zinda, J. A.; Aronson, M. F.; Cilliers, S.; Goddard, M. A.; Nilon, C. H.; Winkler, R. Urban Socioeconomic Inequality and Biodiversity Often Converge, but Not Always: A Global Meta-Analysis. Lands. Urb. Plan. 2020, 198, 103799. DOI: 10.1016/j.landurbplan.2020.103799.
- Ibrahim, S. A.; Fidan, H.; Aljaloud, S. O.; Stankov, S.; Ivanov, G. Application of Date (Phoenix Dactylifera L.) Fruit in the Composition of a Novel Snack Bar. Foods. 2021, 10(5), 918. DOI: 10.3390/foods10050918.
- Bakare, R. I.; Magbagbeola, O. A.; Akinwande, A. I.; Okunowo, O. W.; Green, M. Antidiarrhoeal Activity of Aqueous Leaf Extract of Momordicacharantia in Rats. J Pharma. Phyt. 2011, 3(1), 1–7. DOI:10.5897/JPP.9000010.
- Veiga, V. F.; Zunino, L.; Calixto, J. B.; Patitucci, M. L.; Pinto, A. C. Phytochemical and Antioedematogenic Studies of Commercial Copaíba Oils Available in Brazil. Phytother. Res. 2001, 15(6), 476–480. DOI: 10.1002/ptr.976.
- Emudainohwo, J.; Erhirhie, E.; Moke, E. Anti-Diarrheal Activity of the Aqueous Leaf Extract of Ageratum Conyzoides in Wistar Rats. J Appl. Sci. Environ. Man. 2015, 19(2), 169–175. DOI: 10.4314/jasem.v19i2.1.
- Derebe, D.; Abdulwuhab, M.; Wubetu, M.; Mohammed, F. Investigation of the Antidiarrheal and Antimicrobial Activities of 80% Methanolic Leaf Extract of Discopodiumpenninervum (Hochst.). Evid. Based Compl. Alter. Med. 2018, 2018, 1360486. DOI: 10.1155/2018/1360486.
- Ghafoor, K.; Sarker, M. Z. I.; Al-Juhaimi, F. Y.; Babiker, E. E.; Alkaltham, M. S.; Almubarak, A. K.; Ahmed, I. A. M. Innovative and Green Extraction Techniques for the Optimal Recovery of Phytochemicals from Saudi Date Fruit Flesh. Processes. 2022, 10(11), 2224. DOI: 10.3390/pr10112224.
- Gakunga, J. N.; Mirianga, B.; Muwonge, H.; Sembajwe, L. F.; Kateregga, J. Antidiarrheal Activity of Ethanolic Fruit Extract of Psidiumguajava (Guava) in Castor Oil Induced Diarrhea in Albino Rats. Nat. J. Phys. Pharm. Pharmac. 1970, 3(2), 191–197. DOI: 10.5455/njppp.2013.3.100620131.
- Ganitkevich, V.; Hasse, V.; Pfitzer, G. Ca2þ-Dependent and Ca2þ-Independent Regulation of Smooth Muscle Contraction. J. Mus. Res. Cell Motil. 2002, 23(1), 47–52. DOI: 10.1023/A:1019956529549.
- Adeniyi, O. S.; Omale, J.; Omeje, S. C.; Edino, V. O. Antidiarrheal Activity of Hexane Extract of Citrus limon Peel in an Experimental Animal Model. J. Integ. Med. 2017, 15(2), 158–164. DOI: 10.1016/S2095-4964(17)60327-3.