ABSTRACT
The space environment is extreme for plants growth and survival as gravity (gravitropism modification, water distribution), radiations (mutations enhancers), light spectrum regime and temperature are not optimal. Photosynthetic microorganisms are a foreseen solution for supporting plant development, growth, and stress tolerance in closed environments, like those designed for space colonisation. Indeed, photosynthetic microorganisms are known as secondary metabolites producers (exopolysaccharides, indole alkaloids, fertilisers) able to impact plant stimulation. Studying their abilities, application methodologies and best strains for space agriculture may lead to developing a sustainable and efficient approach for food production. Furthermore, as these microorganisms could also be used to produce oxygen and recycle waste materials increasing their interest in closed loop systems is undeniable. In this review we provide an overview of the current state of knowledge about existing biostimulants, their effects and applications, and the potential brought by photosynthetic microorganisms for life in closed environments.
Highlights
Cyanobacteria's and microalgae's secondary metabolites can act as biostimulants for vascular plants when applied to the roots or the leaves.
Production of secondary metabolites in cyanobacteria can be enhanced in stressful environments.
Cyanobacteria can survive space-like stress by sur-producing secondary metabolites giving an advantage for space farming as a source of biostimulant compounds.
1. Introduction
Plants on Earth are estimated to count approximately 300,000 species. The origins of plants are closely tied to the colonisation of land, with the earliest plant fossils estimated to be from between 490 and 425 million years ago. Moreover, plants are a source for primary production and at the centre of our ecosystem structure, both on land and within the oceans (Kreft and Jetz Citation2007). Amongst this diverse group of plant species, vascular plants, or Tracheophyta clade, which includes approximately 260,000 species, are of particular interest in the study of life on Earth (Lucas et al. Citation2013; Crang et al. Citation2018). Vascular plants can be defined as plants composed of lignified tissues (xylem) used to conduct water and minerals within the plant. In addition, non-lignified tissues (phloem) play a crucial role in plant physiology as they are responsible for conducting products of photosynthesis (Lucas et al. Citation2013; Crang et al. Citation2018). Vascular plants include phylogenetic groups such as Gymnospermae, Angiospermae, Polypodiophytae, and Lycophytae (Lucas et al. Citation2013; Crang et al. Citation2018) corresponding to all macroscopic plants on our planet. By extension, edible plants grown nowadays for human consumption are vascular plants. Hence, interest in vascular plants comes from the necessity for our humanity to (1) understand our environment and origins, and (2) produce enough food for the growing population.
Given the importance of vascular plants for both terrestrial and aquatic ecosystems, there is a growing interest in finding ways to optimise their growth and productivity. A first approach that has gained attention in recent years is the use of biostimulants. Biostimulants are defined as a type of substance or live microorganisms formulated from biological materials and designed to improve the productivity of plants when applied to the plant or the rhizosphere (Yakhin et al. Citation2017; Ricci et al. Citation2019; Rouphael and Colla Citation2020; Kapoore et al. Citation2021). They aim to enhance the nutrition efficiency of the plant, and allow abiotic stress tolerance (du Jardin Citation2015). Positive effects of biostimulants on plant productivity are the result of the complex combination of different compounds within the solution used, and not the sole presence of a single known compound (Yakhin et al. Citation2017). A significant aspect of biostimulants is that the mechanism by which they impact plant growth and development is not entirely understood. Despite this lack of knowledge, it is still important to recognise their potential value in promoting plant growth and health.
Nowadays, a fair number of biostimulants are already in use, but some missing information makes it difficult to prove their use as a reliable solution, and more in-depth studies are needed. The first point of concern is the lack of consensus and standardisation on the definition of the term biostimulant. The European Committee for Standardisation (CEN) has initiated a project to create a set of harmonised European standards for biostimulants. This project includes the standardisation of terminology, product specifications, labelling, testing methods, and safety requirements for biostimulants, as well as the verification of product claims. One challenge in this project is to develop standards that allow for comparison between different biostimulant products, while also taking into account the wide range of products, claims, and contexts in which they are used (Ricci et al. Citation2019; ‘CEN/TC Citation455 – Plant Biostimulants and Agricultural Micro-Organisms,’ Citation2022). Another important point is the limited amount of research on this specific field. According to a search on the Google Scholar, PubMed and Scopus database (), there are only a small number of studies that have examined the use of microorganisms as biostimulants for plants.
Table 1. Search and number of results in the PubMed, Google Scholar and Scopus databases on the 5th of July 2023.
Results obtained are mainly review papers or perspective studies rather than primary research studies. This limited number of studies suggests a need for further investigations on the use of microorganisms as biostimulants to fully understand their potential benefits and risks. Nevertheless, a search for ‘biostimulant’ term returns no less than 2,719 references in the Pubmed database, 29,300 in the Google Scholar database and 2,165 in the Scopus database (as of 5 July 2023), showing a growing interest in this matter. Amongst the studies available, it has been demonstrated that photosynthetic microorganisms, including both cyanobacteria and microalgae, have presented the potential to be effective biostimulants due to their positive impact on the growth of vascular plants (EL Arroussi Citation2016; González-Pérez et al. Citation2021; Kapoore et al. Citation2021; Santini et al. Citation2021).
Cyanobacteria are a very large monophyletic group of photoautotrophic prokaryotes. This group has a wide range of morphology and ecological environments as they successfully colonised almost any sunny environment on Earth and play a prominent role in these environments (Stal Citation2007). Cyanobacteria are known to be an old phylum as the oldest traces of their existence go back to 3,500 million years. They are believed to be responsible for the oxygenation of Earth's atmosphere, thanks to their photosynthesis abilities during the Precambrian supereon, and, therefore, contributed to the appearance of oxygen-dependent life on Earth (Rasmussen et al. Citation2008; Garcia-Pichel Citation2009; Kauff and Büdel Citation2011). Indeed, from unicellular to pluricellular species, the major commonality between all cyanobacteria is their ability to carry out oxygenic photosynthesis (Garcia-Pichel Citation2009; Sánchez-Baracaldo and Cardona Citation2020). As a source of carbon, cyanobacteria fix CO2 primarily using the reductive Pentose Phosphate pathway (Garcia-Pichel Citation2009; Shinde et al. Citation2020). Nevertheless, cyanobacteria's morphological diversity and lifestyle make the establishment of a strong taxonomy difficult. Their lifestyle is close to the one observed in eukaryotic microalgae, as they both share the same habitats and ability to perform oxygenic photosynthesis (Kauff and Büdel Citation2011; Mishra Citation2020). These similarities in morphology, lifestyle and photosynthesis ability have deceived taxonomists for years, leading to false taxonomies. Indeed cyanobacteria have been classified as algae, although it is now demonstrated that these species belong to the bacteria domain (Kauff and Büdel Citation2011).
On the other hand, eukaryotic microalgae, like cyanobacteria, are found in a wide range of aquatic environments, including oceans, rivers, lakes and even damp soil (Litchman et al. Citation2007). They are known to be an important source of primary production in aquatic ecosystems (Litchman et al. Citation2007). Algae are a diverse group of aquatic organisms that can be either unicellular or pluricellular (Litchman et al. Citation2007). Microalgae are photosynthetic organisms: they convert CO2 and water into energy and produce O2 as a by-product of photosynthesis. They are, in association with cyanobacteria, an important source of oxygen in Earth's atmosphere (Häder Citation2022).
Overall, the main difference between microalgae and cyanobacteria remains in their phylogenetic classification and their photosynthetic apparatus. While microalgae are classified as eukaryotes, cyanobacteria are classified as prokaryotes and present morphological traits specific to bacteria. Regarding their photosynthetic system, both microalgae and cyanobacteria possess specific pigments, or in different proportions, to conduct photosynthesis (Häder Citation2022). Microalgae contain chlorophyll, which is a green pigment responsible for capturing light energy used during photosynthesis (Melkozernov et al. Citation2006). They also contain carotenoids, which are yellow, orange, or red pigments that possess the ability to protect themselves against an excess of light energy (Pagels et al. Citation2021). Some microalgae also contain phycobilins, which are pigments found in the thylakoid membranes (Heocha Citation1965). Cyanobacteria also contain chlorophyll and carotenoids (Barton et al. Citation2013), in addition to phycobilins, which are pigments found within phycobiliproteins and are responsible for the pigments’ colours (Heocha Citation1965; Tan et al. Citation2023). Cyanobacteria have unique pigments, such as phycocyanin, a phycobiliprotein, which is a blue or green pigment responsible for their blue–green colour (Patel et al. Citation2005). Pigments contained in both microalgae and cyanobacteria are amongst a large variety of metabolites explaining their ability to thrive in a wide range of environments. These metabolites allow them to withstand extreme conditions, such as high levels of UV radiation (UV-B (280–315 nm) and UV-A (315–400 nm)) (Singh et al. Citation2010) or variable temperatures (from 0 to 30°C) (Konopka and Brock Citation1978). This explains their presence in the most extreme environments on Earth including hot springs, polar regions, and the deep seas (Seckbach Citation2007). Yet, what may be considered an ‘optimal environment’ for one species, can be an ‘extreme environment’ for another. Stress, referred as any perturbation occurring in a steady-state environment, can occur in any kind of environment and affect any species living in the said environment. For bacteria, a stressful environment is considered to provide suboptimal conditions, affecting their survival (Zhang et al. Citation2021). The most common stress factors are related to osmotic and acidic-alkaline perturbances, temperature shocks, drought, light, metal exposure, and nutrient deprivation (Zhang et al. Citation2021). Other stress factors are being sought, especially considering the rise in interest in spatial conditions, in the setting up of life support. Thus, radiation exposure, magnetic field, light intensity, microgravity exposure, and exposure to nitrates, differing from Earth usual conditions, are a new range of stress factors to be considered.
These extreme conditions found in space pose significant challenges for the settlement of a human colony, especially regarding food supply. NASA's GeneLab database, collects and provides access to omics data from biospecimens flown in space or exposed to simulated space stressors as a reference of space biology for future space research and settlements (Berrios et al. Citation2021). The ability of microorganisms to adapt and survive in these conditions makes them an attractive option for on-site food production. Space agriculture refers to the cultivation of plants in space for the purpose of producing food, oxygen, and other resources for space missions or future space settlements with a minimal resupply from Earth (De Pascale et al. Citation2021). This concept has gained increasing attention in recent years as the potential for human exploration and colonisation of other planets and moons in our solar system might occur in a few years, as already planned with NASA's ARTEMIS programme, started with the launch of ARTEMIS-I in November 2022 (Creech et al. Citation2022; Kessler et al. Citation2022). The challenges of growing plants in the harsh and extreme conditions of space are significant and require innovative approaches and technologies to overcome these difficulties (De Pascale et al. Citation2021). One of the key considerations for plant growth in space is the impact of radiation. Even if plants are more tolerant to ionising radiation than animals, radiation can be mutagenic and detrimental to plant DNA and cellular processes leading to morphological and anatomical alterations (Arena et al. Citation2014; Furukawa et al. Citation2020). Additionally, the variation of magnetic field in space (depending on the host planet) is perceived by plants as abiotic stress. Therefore, it has been shown that plants exposed to Earth magnetic field variations presented a higher generation of Reactive Oxygen Species (Mridha et al. Citation2016) and an increase of stress enzymes like APX in seedlings (Shabrangy and Majd Citation2009). On the other hand, plants are known to be extremely sensitive to gravity (Muthert et al. Citation2020; Sathasivam et al. Citation2021). The microgravity or low gravity conditions disrupt the normal gravitropism response in plants, leading to altered development shown by disorientations of the seedlings and a modified distribution pattern of auxin phytohormone (Medina et al. Citation2021). Last, the availability and light spectrum regime in space differ significantly from terrestrial conditions. Using artificial light to mimic Earth light require careful management for optimal photosynthesis and growth (Ouzounis et al. Citation2015; Zhang et al. Citation2020).
One additive approach for space agriculture is the use of photosynthetic microorganisms as a source of food, oxygen and fertilisers for space missions (Mapstone et al. Citation2022), as well as a source of biostimulant production for plant growth (Kapoore et al. Citation2021; Yakhin et al. Citation2017). These microorganisms have several specific advantages for space agriculture, including their small size and low mass, which makes them easy to transport and store in space. In fact, cyanobacteria and microalgae have already been used in several space experiments to study their growth and photosynthetic capabilities under simulated microgravity conditions (Mapstone et al. Citation2022), and their use as food or oxygen suppliers (Gòdia et al. Citation2002; Lasseur et al. Citation2005). Indeed, Limnospira indica, a photosynthetic cyanobacteria has already been flown in order to demonstrate the feasibility of a functional photobioreactor within the International Space Station (Poughon et al. Citation2020; Fahrion et al. Citation2021). Indeed, the MELiSSA project (an ESA project) develops a fully closed loop system. As part of this project, Poughon et al. has work to the integration of a photobioreactor composed of 4 chambers in the ISS Biolab facility. Through this work they have shown that Limnospira indica PCC 8005 was the first successful dynamic culture experiment in space and allows direct measurement and calculation of reliable growth and oxygen production rates. Biostimulant effects from cyanobacteria and algae could be particularly useful for space missions where it is important to maximise the efficiency and productivity of plants’ growth on board (Kapoore et al. Citation2021; Yakhin et al. Citation2017). Nevertheless, many challenges and uncertainties need to be addressed to make space agriculture a viable and sustainable option for future space missions and settlements (Wheeler Citation2017; Mapstone et al. Citation2022). These challenges include the need to better understand the growth and physiological responses of plants and microorganisms to the extreme conditions of space, and their metabolic response to extreme environments (Santomartino et al. Citation2020; Mapstone et al. Citation2022)
In this review, we aim to discuss the diverse applications of biostimulants in plant growth, with a particular focus on edible vascular plants. We will specifically explore the potential of photosynthetic microorganisms as biostimulants and their role in enhancing plant development and stress tolerance. We will dig into their ability to produce secondary metabolites and impact plant stimulation, their potential application methodologies, and optimal strains to lead to sustainable and efficient food production in closed environments. Furthermore, we will delve into the application of biostimulants in indoor farming systems, highlighting their benefits and challenges. Finally, we will explore the perspectives for space farming, examining how biostimulants and photosynthetic microorganisms can contribute to sustainable food production in extraterrestrial environments.
2. The use of biostimulants on edible vascular plants
Defining the biological basis of biostimulants as a class of compounds is complex due to their diverse sources present in the current market. These compounds include stimulants based on bacteria, fungi, seaweeds, vascular plants, animals and humic acids materials (Yakhin et al. Citation2017) and can be produced from whole microorganisms or natural compounds extracted from them (Yakhin et al. Citation2017; Ricci et al. Citation2019; Kapoore et al. Citation2021) before being applied to the plant.
Understanding extreme environments conditions and their impact on plant growth and survival is a necessity to develop effective biostimulants, as they are a source of resilient solutions to a fundamentally unsuitable environment for the plant. These environments include volcanic zones and hot or cold desertic regions (such as Sahara Desert or Antarctic Polar Desert). Besides, space exploration brings a new range of extreme environment for plant cultivation where the use of biostimulant would be crucial. As plants are sessile organisms, they must adapt to their environment through physiological adaptations and microbial associations (Yang Citation2016; Alsharif et al. Citation2020). Amidst these harsh conditions, desertic plants have shown promising resilience in adverse environment by engaging beneficial interactions with endophytes or symbiotic soil bacteria aiding in maintaining plant growth under abiotic stress conditions (Li et al. Citation2019). Local adaptation plays a vital role in the successful colonisation of plants across extreme environments. Plants gradually develop genetic adaptations that allow them to cope with the specific challenges of their surroundings (Shah et al. Citation2020; Znój et al. Citation2022). In addition, plants interactions with microorganisms such as biocontrol agents colonise the plants rhizosphere and act as biopesticides (Alsharif et al. Citation2020; Francis et al. Citation2020; Torracchi C. et al., Citation2020). As an example, Torracchi C. et al. have shown the significant role of cold-adapted microorganisms (psychrotolerants) in protecting plants from pathogens, and their potential for developing environmentally friendly biopesticides. On the other hand, diversity and ecological interactions of microorganisms living in high-altitude volcanic hot springs has been shown to be influenced by abiotic factors (Wang and Pecoraro Citation2021) and present positive effect on the growth and development of plants (Rincón-Molina et al. Citation2022). Therefore, plants leaving in an extreme environment contribute to identify a suitable pool of potentially beneficial microbes to maintain plant growth under abiotic stress conditions (Saad et al. Citation2020). Therefore, plants engage naturally in microbial interaction to protect themselves, a principle that is used to develop biostimulants. Additionally, plants leaving in an extreme environment could serve as a valuable resource for identifying and utilising microbial agents that enhance plant resilience and develop biostimulants.
To further improve our understanding of the use of biostimulants in agriculture, it is important to not only consider the type of biostimulants available and currently in use but also the various methods of application. Some biostimulants are applied via foliar applications, while others are applied directly to the soil or growing medium, and some are used as a seed treatment (coating) before planting () (Ricci et al. Citation2019). The foliar application involves spraying biostimulants directly onto the leaves, allowing for quick absorption and enhanced nutrient uptake (Saa et al. Citation2015). This application aims to increase plant growth stimulation and average leaf size. The foliar application also presents interesting results in plant stress mitigation (Saa et al. Citation2015). Direct application to the soil involves applying a biostimulant solution directly to the growing medium to reach the roots and rhizosphere, the layer of soil influenced by a plant's roots that is richer in root-associated bacteria (rhizobacteria) than the surrounding soil () (Lugtenberg and Kamilova Citation2009). The interaction between these bacteria and the plants is commensal, as bacteria benefit from the metabolites secreted by the plant using them as nutrients (Lugtenberg and Kamilova Citation2009; Stegelmeier et al. Citation2022). These bacteria represent an important biocontrol around the roots of the plant, allowing the reduction or the elimination of pathogens around the plants’ roots. Therefore, they enable better plant growth by reducing the harm caused by pathogens (Lugtenberg and Kamilova Citation2009). Finally, seed treatments are a method of coating or treating the seeds using biostimulants before planting. This methodology allows the biostimulant to be in contact with the emerging seedling, promoting early growth and development (Pedrini et al. Citation2017). It enhances the germination stage, by improving the nutrient and growth hormone uptake of the seedling while increasing stress tolerance (Pedrini et al. Citation2017; Campobenedetto et al. Citation2020). Nevertheless, biostimulant efficiency can be affected by factors such as the type of crop, the stage of crop growth, and environmental conditions (Yakhin et al. Citation2017).
Figure 1. Biostimulant application impact on the plant depending on their nature and their application method. PGPB = Plant Growth Promoting Bacteria; PGPR = Plant Growth Promoting Rhizobacteria.
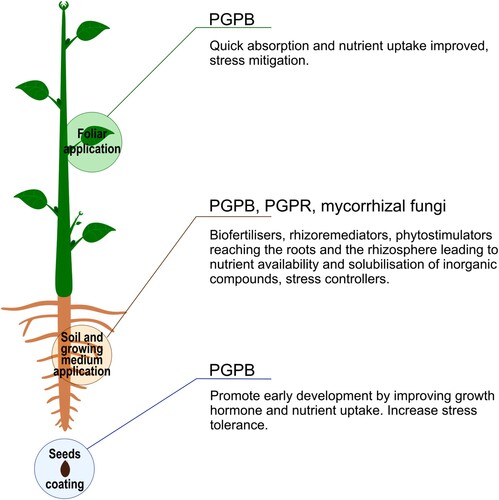
Biostimulants and associated bacteria actions can be classified into several categories based on the mechanism used to promote plant growth. These include (1) Biofertilisers, (2) Rhizoremediators, (3) Phytostimulators, and (4) Stress-controllers. (1) Biofertilisers utilise rhizobacteria to enhance plant growth in the absence of pathogens and supply the plant with nutrients such as ammonia (van Rhijn and Vanderleyden Citation1995; Lugtenberg and Kamilova Citation2009). Nitrogen-fixing bacteria can form nodules on the roots of leguminous plants in which they convert N2 into ammonia used as a nitrogen source, whilst other Plant Growth-Promoting Bacteria can solubilise phosphate from fertilisers for plant use (Vassilev et al. Citation2006; Lugtenberg and Kamilova Citation2009; Stegelmeier et al. Citation2022). (2) Rhizoremediators use rhizobacteria to degrade pollutants in soil (van Rhijn and Vanderleyden Citation1995; Kuiper et al. Citation2001). (3) Phytostimulators utilise rhizobacteria to produce volatile metabolites and the cofactor pyrroloquinoline quinone (PQQ) to supplement plant hormones, such as auxin, gibberellins, and cytokinins (Altman et al. Citation1997; Lugtenberg and Kamilova Citation2009; Stegelmeier et al. Citation2022). (4) Stress controllers use bacteria containing the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase to promote plant growth and development (Glick et al. Citation2007; Lugtenberg and Kamilova Citation2009; Stegelmeier et al. Citation2022).
These biostimulant induce the production of antioxidants, pigments, organic acids, and amino-acids, as well as hormones (auxin-like and gibberellin-like) and allow nitrogen fixation in the plant (Zhang and Schmidt Citation2000; du Jardin Citation2015; Yakhin et al. Citation2017; Vetrano et al. Citation2020). Moreover, while the adverse effects of biotic and abiotic stresses on crop systems are well known, knowledge about mechanisms involved in mitigating these effects is currently limited. Common approaches to prevent abiotic stresses include optimising plant growth conditions, providing water and nutrients, and using plant growth regulators (PGRs – auxins, cytokinins, gibberellins, strigolactones, brassinosteroids) (Yakhin et al. Citation2017; Dubey et al. Citation2020). Regarding their impact on plants, it has been shown that biostimulants can increase growth, yield, and stress tolerance in a wide range of crops, including cereals, fruits, and vegetables, making them promising for all kinds of farming (du Jardin Citation2015; Yakhin et al. Citation2017; Shahrajabian et al. Citation2021).
Biostimulants based on microorganisms include Plant Growth-Promoting Bacteria (PGPB) (Handy et al. Citation2021), Plant Growth-Promoting Rhizobacteria (PGPR) (Lugtenberg and Kamilova Citation2009) and mycorrhizal fungi (Bonfante and Anca Citation2009). PGPB, PGPR and mycorrhizal fungi have several ways to positively impact plants’ environments, as they can solubilise inorganic phosphorus (example of mechanism (1) ‘Biofertilisers’) and fix atmospheric nitrogen (example of mechanism (1) ‘Biofertilisers’) (Lugtenberg and Kamilova Citation2009). Both mechanisms are necessary for many essential processes as plants require phosphorus (i.e. for energy transfer, photosynthesis, and cell division) and nitrogen (for protein synthesis, chlorophyll and enzyme production). However, on the one hand, phosphorus in soils is inorganic and not readily available for plant uptakes and needs to be solubilised before being used by the plant. To this end, plants release acids, and enzymes to solubilise inorganic phosphorus. On the other hand, the atmospheric nitrogen molecule (N2) is relatively inert and needs to be fixed to be available for plant growth and development. PGPR and mycorrhizal fungi, through a symbiotic relationship with the plant, can help to solubilise inorganic phosphorus and fix nitrogen to make it available for absorption by the plant (Bonfante and Anca Citation2009; Lugtenberg and Kamilova Citation2009). In addition, Plant Growth-Promoting Bacteria (PGPB) have shown potential for yield productivity by reducing plant stress (example of mechanism (4) ‘Stress control’), increase nutrient uptake (example of mechanism (1) ‘Biofertilisers’), manage plant hormone modulation (example of mechanism (3) ‘Phytostimulators’) and biocontrol especially in hydroponic growth conditions (Handy et al. Citation2021; Stegelmeier et al. Citation2022). Finally, some rhizobacteria promote plant growth by releasing volatile compounds in the near environment of the plant (Glick et al. Citation2007; Lugtenberg and Kamilova Citation2009). Several forms of stress, such as phytopathogenic bacteria aggression, polyaromatic hydrocarbons presence, or occurrence of heavy metals, are relieved by these volatile compounds, increasing plants’ stress tolerance (example of mechanism (4) ‘Stress control’) (Glick et al. Citation2007; Lugtenberg and Kamilova Citation2009).
3. Photosynthetic microoragnisms metabolites as biostimulants
Amongst biostimulants used on vascular plants, those produced from microorganisms are of great interest. Indeed, these microorganisms have unique properties and mechanisms that can enhance plant growth and improve crop yields. These properties come from their ability to produce metabolites, among which secondary metabolites are of particular interest. Secondary metabolites are compounds produced by the organisms as a by-product of their metabolism, which are not directly required for the organism's primary metabolism, and are derived from mixed biosynthetic pathways (Nunnery et al. Citation2010; Kultschar et al. Citation2018). They are often unique to a particular organism and are not always produced in all environmental conditions. Photosynthetic microorganisms produce secondary metabolites in response to biotic or abiotic stresses in their environment (Kultschar et al. Citation2018) and can be used by plants. The microbial enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase can help plants to cope with abiotic stress, such as high temperatures and drought (Naing et al. Citation2021). Similarly, polysaccharides and exopolysaccharides produced by cyanobacteria allow plants to increase their drought tolerance (Adessi et al. Citation2018; Santini et al., Citation2021). In addition, compounds such as flavonoids, phytoalexins, phenylpropanoids, and carotenoids have been documented in stressed plants inoculated with microorganisms and help plants tolerate abiotic stress by acting as antioxidants that scavenge ROS (Bonini et al. Citation2020; Koza et al. Citation2022). It is suggested that these diverse chemical compounds and enzymes activate plant defense responses, and improve nutrient mobilisation at the seed stage (Poveda Citation2020; Sharma et al. Citation2020). These metabolites possess powerful biological activities that can provide protection and aid in plant survival, giving them an advantage over other species (Gademann and Portmann Citation2008; Kultschar et al. Citation2018).
Given their ability to thrive in a variety of environments, cyanobacteria are a valuable producer of secondary metabolites (Kultschar et al. Citation2018). Several of these compounds exhibit a distinctive combination structural characteristics of peptide with lipid sections, which gain function through unusual oxidations, methylations, and halogenations (Nunnery et al. Citation2010). In addition, metabolites serving a primary function in cyanobacteria, such as hormones, present interesting properties when used as biostimulant for plants as well (Dobbelaere et al. Citation1999; Rodríguez et al. Citation2006; Santini et al. Citation2021). Therefore, the varieties of these metabolites, presented in , are broad and include toxins (microcystin, anatoxin and saxitoxin, displaying hepatotoxicity and neurotoxicity), hormones, vitamins, photoprotective metabolites (Mycosporine-like amino acids and scytonemin), antioxidants (exopolysaccharides), indole alkaloids (ergot, iboga, pyrroloindole, carboline, aspidosperma and strychnos alkaloids), as well as iron chelators (schizokinen, synechobactin and anachelin), and Volatile Organic Compounds (VOC) (Gademann and Portmann Citation2008; du Jardin Citation2015; Kultschar et al. Citation2018; Liu et al. Citation2021). All bioactive secondary metabolites produced by cyanobacteria represent a massive source of compounds for biotechnologies. Already used for their food supplementation, pharmaceutical and cosmetic properties, they bring a new interest as a source of biostimulants (Gademann and Portmann Citation2008; Kultschar et al. Citation2018; Macário et al. Citation2022). Secondary metabolites are produced through different metabolic pathways, in cyanobacteria (Fincheira and Quiroz Citation2018; González-Pérez et al. Citation2021) and their actions may differ from rhizobacteria normally found in the near roots’ environment (Gademann and Portmann Citation2008). The following sections will present the secondary metabolites variety and their actions on plants.
Table 2. Effects of different cyanobacterial metabolites on crop productions, from their cellular targets in plants to whole plant physiological functions.
3.1. Phytohormones
Cyanobacteria produce phytohormones like auxin, cytokinins and gibberellins, which can promote plant growth and development shown by an increase in fresh weight and root size (Santini et al., Citation2021). Furthermore, cyanobacteria, through their metabolite production, can stimulate endogenous hormone synthesis, such as auxin, well known for its implication in plant growth in treated plants (Santini et al., Citation2021).
3.2. Volatile organic compounds (VOCs)
Some microalgae and bacteria, such as Microcystis aeruginosa and Anabaena sp., have been found to induce the emission of VOCs (i.e. dimethyl-sulfide, alkanes, alkenes, alcohols, aldehydes, ketones, furans, sulfides, and halocarbons) which also promote plant growth by rearranging roots architecture making nutrient uptake more efficient (Santos et al. Citation2016; Liu et al. Citation2021; Shanab and Shalaby Citation2021; Gámez-Arcas et al. Citation2022). Other VOCs, such as 3-hydroxy-2-butanone (acetoin), 2,3-butanediol, 2-pentylfuran, or dimethylhexadecylmine, released from diverse microorganisms, have shown their ability to stimulate growth at root and leaf level (Fincheira and Quiroz Citation2018). VOCs can act as signalling molecules between plants and microorganisms and stimulate a range of physiological processes in plants through the activation of a series of signals regulating physiological processes and promoting plant growth and development (Fincheira and Quiroz Citation2018).
3.3. Vitamins
Vitamins produced by cyanobacteria in the environment of plants have the ability to work as signalling molecules to promote plant growth by reducing stress and increasing the plant immunity (Rodríguez et al. Citation2006; Havaux et al. Citation2009; Goyer Citation2010; Singh Citation2014).
3.4. Pigments
Microalgae and cyanobacteria have been shown to produce compounds such as beta-carotene and phycobiliproteins. These compounds present an interesting ability to stimulate tillering and/or root development and/or the yield of a plant impacting positively nutrient uptakes and assimilation (Yvin et al. Citation2019).
3.5. Exopolysaccharides
Polysaccharides and exopolysaccharides are produced by a variety of microorganisms, including microalgae and cyanobacteria (i.e. Chlorella sp., Limnospira indica, Dunaliella salina and Prophorydium sp., Gloeothece verrucosa). Exopolysaccharides are high molecular weight polymers, in which monosaccharides are linked by glycosidic bonds and present various biological activities and physico-chemical properties showing promising abilities in biostimulation (Chaiklahan et al. Citation2013; Laroche Citation2022; Van Camp et al. Citation2022). Indeed, extracted polysaccharides from Limnospira indica has been proven to induce metabolic changes in plants (Cabrera and Wattiez, Citation2018). Amongst their activities observed on plants, they mitigate Reactive Oxygen Species (ROS) toxicity. ROS constitute a category of toxic molecules as they injure cells by oxidating them. Biotic and abiotic stresses, resulting in ROS production, lead to cell injury. Crude polysaccharides from Limnospira indica contain phenolic compounds leading to antioxidant activity (Chaiklahan et al. Citation2013). Phenolic compounds are major contributors to antioxidant capacity and may be linked to various cell component carbohydrates (Chaiklahan et al. Citation2013). As such, cyanobacteria exopolysaccharides enhance plants’ production of ROS antioxidant enzyme activities. This is possible due to their interaction with specific receptors (such as leucine-rich receptors) structurally related to TLR4 y TLR2 with protein kinase activity that activates regulatory mechanisms involved in defence mechanisms (González-Pérez et al. Citation2021). This mechanism leads to a toxicity mitigation and, therefore, stress tolerance is increased (González-Pérez et al. Citation2021).
Another ability from cyanobacteria’ polysaccharides is to increase inter-species interactions. Polysaccharides present in the nearby soil of plants allow other microorganisms, fungi and nitrogen-fixing bacteria to grow efficiently, conducting to enhance beneficial interactions between plant and surrounding microorganisms as growth promoters (Kehr and Dittmann Citation2015).
Cyanobacteria’ exopolysaccharides in the near root's environment can be involved in tillering, roots growth, and plants hormone production. The production and release of phytohormones by cyanobacteria living in symbiotic or parasitic environments have significant ecological consequences for other organisms living in the said environment (Gademann and Portmann Citation2008). Indeed, increasing both tillering and roots growth result in an improved nutrient and water uptake.
Last, EL Arroussi has demonstrated the effect of polysaccharides extracted from Spirulina platensis (currently being named Limnospira indica) on plants growth. It is shown that polysaccharides induce a better growth efficiency in term of plant weight, plant size, and leaves number and size on two plant species Capsicum annum and Solanum lycopersicum (EL Arroussi Citation2016).
3.6. Indole alkaloids
Indole alkaloids such as the tjipanazoles isolated from Tolypothrix tjipanasensis are toxins produced by cyanobacteria. Therefore, they present interesting antifungal properties, in particular against phytopathogenic fungi causing plant infections (Bonjouklian et al. Citation1991; Kulik, Citation1995; Gademann and Portmann Citation2008; Walton and Berry Citation2016).
3.7. Fertilisors
Photosynthetic microorganisms also act as biostimulants through their ability to fix atmospheric compounds. Indeed, photosynthetic microorganisms, such as Nostocales sp., also make essential nutrients, such as Nitrogen, Phosphorus, and Potassium (NPK), available for use by plants by fixing these compounds in an absorbable form (Lugtenberg and Kamilova Citation2009). Cyanobacteria, for instance, can fix nitrogen through the process of nitrogen fixation, which can improve soil fertility leading to better plant growth (Lugtenberg and Kamilova Citation2009). Moreover, photosynthetic microorganisms can also help to solubilise inorganic phosphorus, a key nutrient for plant growth, through the production of organic acids within the soil (Lugtenberg and Kamilova Citation2009). Whilst a proper symbiotic association seems unlikely, as the crop plant environment does not match the natural environment of cyanobacteria, such an enrichment of the environment remains beneficial for plant growth.
Although the production of metabolites is part of photosynthetic microorganisms’ normal metabolism, biotic and abiotic stresses can enhance their production (Kultschar et al. Citation2018). Some environment present high abiotic stress. Extremophiles organisms have the ability to tolerate at least one extreme condition in their environment (Waditee-Sirisattha and Kageyama Citation2022). Cyanobacteria are amongst the group best adapted to various hostile conditions and have developed unique survival strategies to thrive under extreme environmental conditions. Five extremophilic cyanobacteria groups are known: (1) psychrophiles, cope with low temperatures and includes Synechococcales, Nostocales, and Oscillatoriales (Kageyama and Waditee-Sirisattha Citation2022; Kashyap et al. Citation2022), (2) thermophiles, tolerate high temperature and includes Gloeomargarita, Synechococcus and Leptococcus (Kees et al. Citation2022), (3) halophiles, live in high saline conditions and includes Aphanothece halophytica, Halospirulina tapeticola and Coleofasciculus chthonoplastes (Oren Citation2015), and (4) alkaliphiles, thrive in an alkaline environment (pH above 9) and includes Anaerobranca horikoshii. Finally, some polyextremophile cyanobacteria such as Chroococcidiopsis thermalis have the ability to survive to an association of several extreme conditions (Aguiló-Nicolau et al. Citation2023). These extremophile cyanobacteria have shown the ability to produce biologically active compounds (Drobac-Čik et al. Citation2007; Malavasi et al. Citation2020). V. Drobac-Čik et al. demonstrated that cyanobacteria were able to produce bioactive compounds presenting antibacterial and antifungal properties. In accordance to these research, Rajashekhar has proved the ability of four species of cyanobacteria isolated from a sulfur spring to produce antioxidant compounds (total antioxidant capacity, DPPH free radical scavenging and ferric ion reducing assays) (Rajashekhar Citation2013). Further research has highlighted the importance of the medium composition (mimicking the environment of the strain) for bioactive compounds production. Indeed, a medium modification induces a change in the bioactive compounds production (shift from eugenol to 4-tert-butylcyclohexanol), inducing an increase in antimicrobial activity (Semary Citation2012). Last, high levels of UV radiation can lead to the production of UV-absorbing pigments, such as mycosporine-like amino acids, by cyanobacteria (Fuentes-Tristan et al. Citation2019; Geraldes and Pinto Citation2021). Similarly, high salinity can lead to the production of compatible solutes, such as glycine betaine and proline, by cyanobacteria and microalgae (Mutale-joan et al. Citation2021).
The impact of environmental stress on the production of secondary metabolites in cyanobacteria is clear. However, the pathways involved in these biostimulating mechanisms are poorly studied. Further research may highlight important molecular and metabolic processes, either regarding their production, their secretion in the environment or the mode of action for plant biostimulation. Additionally, the production and extraction of metabolites of interest could be used as a supplement to watering solutions for plants disregarding the farming methodology in use.
4. Using microorganism biostimulants in indoor farming systems, applications and methodologies
Biostimulant use in diverse agricultural systems has gained significant interest in recent years. They can be applied as a treatment in a variety of forms, such as soil amendments, foliar sprays, and seed coatings, and can be used in various farming systems. These farming methodologies can be broadly classified into two categories: outdoor farming and indoor farming.
Outdoor farming, also known as field farming or in-ground farming, corresponds to traditional in-ground crop management, such as row crops (annual crops) and perennial crops. These systems rely on natural sunlight and weather patterns to grow crops and can include various types of soil-based or soil-less systems. Crops are grown in the ground or a growing medium, such as soil or peat moss, in fields outside. As already seen, the use of biostimulants in in-ground agriculture can improve soil health and fertility, allowing for better growth and development of plants, increased nutrient, mineral and water uptake, stress tolerance in crops, and solubilisation of nutrients to improve soil fertility (Lugtenberg and Kamilova Citation2009; Altomare and Tringovska Citation2011). Despite the potential benefits of using biostimulants, traditional in-ground agriculture presents several challenges, such as the need for specific management and precise monitoring of the soil's humidity and fertility to ensure optimal crop growth. Adding biostimulants requires good management of the field to adapt the treatment.
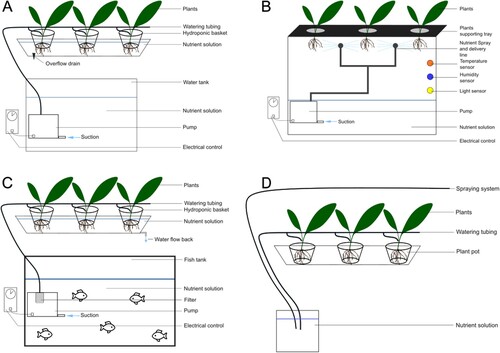
In contrast, indoor farming () can be divided into different methodologies: (1) Hydroponic systems, (2) Aeroponic systems, and (3) Aquaponic systems.
Hydroponic systems are indoor farming systems in which plants are grown in nutrient-rich water, rather than in soil. These systems can be used to grow crops with highly efficient water usage and space utilisation. It can be set up in various places, such as in greenhouses, warehouses, or containers. Hydroponic systems require precise control of temperature, humidity, and nutrients, as well as regular maintenance and monitoring (Son et al. Citation2020).
Aeroponic systems are another kind of indoor farming system in which plants are grown with roots suspended in the air and misted with nutrient-rich water. This method is considered the most efficient water usage, while also providing a high yield per square foot (Nir Citation1982; Lakhiar et al. Citation2018).
Aquaponic systems are a combination of hydroponic and aquaculture systems, where fishes and plants are grown together in a symbiotic relationship. Fishes provide nutrients for the plants, while the plants help to purify water for the fishes. Aquaponic systems can be set up in many places, such as in greenhouses, warehouses, or even outdoors (Yep and Zheng Citation2019).
Figure 2. Schematic view of different types of indoor farming. (A) Hydroponics, (B) Aeroponics, (C) Aquaponics, (D) In soil growth with watering system on the soil or by spraying the leaves.
Although outdoor farming systems are less expensive to set up and maintain than indoor systems, the latter present several advantages. Firstly, indoor farming systems allow for full control over growing conditions with precise control over temperature, humidity, lighting, and nutrient levels (Despommier Citation2019). One other benefit of using them is that they enable crops to avoid the negative effects of extreme conditions, such as drought, severe weather, and frost. These elements enable the optimisation of crop growth and yield, as well as easy protection against pests and diseases, reducing the need for pesticides (Hall Citation2021). Secondly, indoor farming allows for year-round production with the cultivation of crops in any climate and at any time of the year. Additionally, resource management is enhanced in indoor farming systems, as water consumption is optimised through recycling and water reuse. The space is optimised as well as crops are grown in stages.
In addition to the advantages of allowing full control over the growing conditions, indoor farming systems also offer the ability to easily apply biostimulants to crops (Azizoglu et al. Citation2021; Chiaranunt and White Citation2023). Depending on the type of biostimulants and the crops being grown, there are several methods for application in an indoor farm. A foliar spray application can be performed by using a misting system, which helps nutrients to be absorbed through the leaves and roots (Lakhiar et al. Citation2018). If plants are grown on a substrate, biostimulants can be added to the soil around plant roots to improve their health and nutrient availability in the near environment of the roots. For hydroponic systems, biostimulant solutions can be incorporated into the nutrient solution that is applied to the roots and leaves of the plants, improving the nutrient uptake as observed with foliar spray and soil drench methods (Son et al. Citation2020). Lastly, biostimulant solutions can be used to treat seeds before planting, to improve their germination and early plant growth.
By using various application methods, indoor farming allows for precise and targeted biostimulation. The key advantage of using biostimulants in indoor farming systems is the ability to precisely control their application, allowing for targeted application at specific growth stages and optimisation of biostimulants used based on the specific needs of the crop. Furthermore, biostimulants can help to reduce dependence on synthetic stimulants and pesticides in indoor and outdoor farming systems (bio-label farming), which can be harmful to both the environment and human health (du Jardin Citation2015). Indoor farming systems present a holistic approach to plant nutrition, health, and production management, which is critical for sustainable and reliable agriculture.
The use of indoor farming systems and biostimulants can help to address the challenges of conventional outdoor farming. Nevertheless, farming in extreme environments represents one of the biggest challenges for agriculture. As we look towards the possibility of space farming and food as well as oxygen (Shao et al. Citation2021) production beyond our planet, there are many unique challenges to consider. However, the precision control offered by indoor farming and the benefits of biostimulant applications may prove to be valuable tools in meeting the challenges of space farming and ensuring sustainable food production for future missions when associated to sustainable energy production and management.
4.1. Perspectives for space farming
As agriculture practices on Earth have evolved over thousands of years, they must adapt to meet the challenges induced by space conditions to be applied to space-based agriculture. The unique challenges presented by the space environment mean that there are high-reliability requirements during a space mission, as resource availability is limited, and extreme environmental stress is high (Wheeler Citation2017). Indeed, in comparison to the Earth's environment, a space settlement environment present various influencing parameters affecting plant development (). One of the most significant variables in space is gravity. Indeed, it can differ greatly depending on the location in the universe, such as planets, moons, spacecraft orbiting Earth, or orbital transfer vehicles subject to microgravity (Brian Yager Citationn.d.). Gravity, a fundamental interaction leading to a mutual attraction between bodies, to which an object is subjected will vary depending on several factors. On a planet or moon, the mass and the distance from its centre will impact the observed gravity value, measured as a force. The object's location (whether on a surface, in the atmosphere, or in space) directly affects the gravity it experiences (David and Carney Citationn.d.). Additionally, the acceleration of a vehicle, which contains the object, also alters the perceived gravity of the object. Indeed, the law of physics driving this concept is the equivalence principle, which is a fundamental notion in Albert Einstein's theory of general relativity. According to the equivalence principle, the effects of gravity and acceleration are indistinguishable. Then, when an object is in a vehicle that undergoes acceleration, the object experiences a force that is equivalent to the force of gravity. This leads to a change in the perceived gravity of the object. The lowest gravity, called microgravity, is reached when a spacecraft is outside an atmosphere and under no acceleration and objects subjected to the same forces as the spacecraft appear weightless (MSFC Citation2015). Nevertheless, all elements included in a space station (space settlement or space transport systems) are subject to this physical force. Therefore, the movement of heat, water vapour, CO2, and O2 between plant surfaces and their environment is also affected by gravity. As described by Charles Darwin in 1880, the root tip has the power to direct plant movements led by gravity attraction (Darwin and Darwin Citation1880). Since, 4 roots zones (meristematic zone, transition zone, elongation zone, and differentiation zone) has been identified to play a role in the roots elongations, each playing a role in responses to trophic signals (Verbelen et al. Citation2006). In microgravity, these processes, necessary for the well-being of the plant, may have an impact on its growth efficiency. Reduced mass transport and thicker boundary layers around plant organs may be the cause of this phenomenon and may result from the absence (zero-gravity) or reduction (microgravity) of buoyancy-dependent convective transport. Previous studies suggest that microgravity affects plant physiology by altering growth and development through gravitropism dynamics modifications (Shimazu et al. Citation2001; Medina et al. Citation2021; Chin and Blancaflor Citation2022). Shimazu et al. showed that plant growth under simulated microgravity conditions seems to be controlled by the mechanical properties of cell walls and the osmotic properties influenced by auxin and/or other plant hormones rather than gravity influenced mechanisms. In addition, microgravity has been identified as an affecting factor in regulatory Ca2+ messenger system who is known to play a crucial role in stimulus-response coupling for many plants cellular signalling pathways, many cellular functions are altered. This includes cytoskeleton, carbohydrate and lipid metabolism, enzyme activity, protein expression, and chloroplast structures (Kordyum Citation2003) and alters the primary functions of plants cells. These limitations on basic physiological and biochemical processes implies that under long-term microgravity influence, plants adapt their anatomical features as a way of adapting to the stress condition (Akomolafe et al. Citation2017).
Figure 3. Impact of influencing parameters on plants on Earth and other planetary bodies. Impact is represented by a range of colour: green = positive impact; orange = mid negative impact; red = negative impact; grey = neutral, impact not applicable in this case (Space Settlement environment image from SpaceX (FECHT Citation2023)).
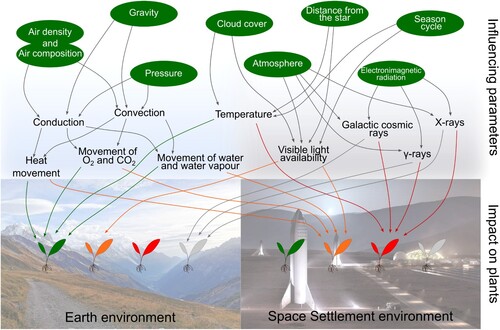
Furthermore, in addition to the effects of microgravity, plants in space also face the detrimental impacts of radiation, which pose additional challenges to their growth and survival. Radiation is energy emitted in the form of rays, electromagnetic waves or particles (Perez Citation2017). A variability of radiations is present in space: (1) Electromagnetic radiations coming from the magnetic field, (2) infrared, and ultraviolet radiation (UV) caused by the Sun, (3) x-rays, γ-rays, and streams of protons and electrons caused by solar flares, (4) galactic cosmic rays (high energy protons and ions), α-particles, β-particles coming from the outer space (Perez Citation2017). Electromagnetic radiation has an impact on plants depending on the strength and exposure period. While a certain combination of magnetic field and duration of the exposure might be a source of stress, it has been shown its efficacity, when induced at a specific level (low frequency, magnetic flux density, time of exposure), in enhancing growth characteristics (Bilalis et al. Citation2012; Rostami zadeh et al. Citation2014; Martinez et al. Citation2017; Nyakane et al. Citation2019; Tirono et al. Citation2021). Studies suggest that plants cope with space radiations through various mechanisms such as reducing light interception, and utilising multiple pathways to handle radiation-induced stress such as the antioxidant systems (Hoeck et al. Citation2015; Gudkov et al. Citation2019; Grinberg et al. Citation2021). The effects of ionising radiation on photosynthesis, respiration, long-distance transport, hormonal systems, and biosynthetic processes in plants have been studied extensively. Ionising radiation can cause changes in the content and ratio of photosynthetic pigments, as well as affect various components of the photosynthetic apparatus. It can also influence the intensity of respiration, with high doses of ionising radiation leading to an increase in respiration activity (Gudkov et al. Citation2019). Long-distance transport in plants can be disrupted by ionising radiation, leading to changes in the activity of physiological processes (Gudkov et al. Citation2019; Grinberg et al. Citation2023). Ionising radiation can also affect hormonal systems and biosynthetic processes in plants, although the specific mechanisms are not fully understood (Gudkov et al. Citation2019). Plants (Lemna minor) growth inhibition has been observed while exposed to high dose rate radiations (from 27 mGy h−1 to 1500 mGy h−1) (Hoeck et al. Citation2015). In addition, biochemical changes, all part of the antioxidative defence system, has been observed with a significant enhancement of the catalase activity, guaiacol peroxidase, syringaldazine peroxidase and superoxide dismutase after high radiation dose exposure (Hoeck et al. Citation2015). However, plants that have the ability to reduce light interception at midday, thereby reducing light and temperature stress effects, may be better prepared to cope with the additional effects of UV-B radiation on photosynthesis, stomatal conductance, plant morphology, and growth (Rosa and Forseth Citation1996).
Therefore, it is understood that environment impact plants with a variable range of impact on them (). However, like farmers on Earth who adapted their farming practices to accommodate various geographical zones (e.g. climatic, or geological), future space farmers must adapt to the various conditions of space to ensure optimal yield production (Hessel et al. Citation2022). This results in the need to adapt farming methodologies to the various gravities observed. To this end, in addition to programmable levels of light and temperature, farming chambers must provide relative humidity and manage carbon dioxide according to the needs of the settlement's gravity (Hall Citation2021). Moreover, adequate ventilation and automated water/nutrient delivery systems to sustain plant growth are also of great importance (Hall Citation2021). Regardless of all constraints brought by space conditions, hydroponic systems seem promising. They are feasible in space due to gravity-driven drainage capabilities, which undergo problems, such as degassing of fluids in microgravity, the need for multiple containment of water in space vehicles, and better moisture distribution in the roots area (Mungin et al. Citation2019). One of the major sources of stress observed in space agriculture while running the VEGGIE experiment is the behaviour of water in microgravity which can limit nutrient uptake, suffocate roots, and allow to potentially pathogenic microorganisms, such as fungi, better access to host tissues as water builds up on leaves and around roots (Massa et al. Citation2017; Khodadad et al. Citation2020; Handy et al. Citation2021). Moreover, variable pressure can be used in a farming system, but low pressures used in the GreenHab area would not only affect plants but also human safety by inducing hypoxia, or even body fluid boiling. This effect appears when reaching ‘Armstrong's limit’ named after an American aerospace medicine physician, Harry G. Armstrong (Tarver et al. Citation2022). This limit has been delimited in high altitude on Earth and corresponds, depending on the atmospheric conditions, to an altitude of 60,000 ft, equivalent to a pressure of about 60–70 millibars (as a reference, mean atmospheric pressure on Mars is 5.7 millibars (Barth Citation1974; Haberle Citation2015)). Then, human access to the low-pressure plant growing areas would require protective space suits as used for EVAs (Extravehicular Activities). While using low pressure presents economic advantages regarding functioning and setup, it also presents challenges. Indeed, maintaining adequate oxygen levels for plants at a reduced pressure may be problematic, and plants need to adapt for nutrient and oxygen absorption (Paul Citation2002; Davis Citation2017). On the other hand, the tendency for higher evaporation rates and a reduced ability to convectively transfer heat in a low-pressure environment may pose challenges in maintaining humidity control (Carey et al. Citation2014; Liu et al. Citation2019).
The nature of indoor farming systems asks farmers to rely on artificially introduced elements (e.g. microorganisms, mineral elements) to maintain plant health. This constitutes the main aspect of closed-loop systems and the future of space farming, as natural elements needed by the plants are not fully accessible in the near environment. To this end, the growth environment must be artificially created. However, planetary settlements must still take advantage of local resources. Regardless of the absence of natural life on other planets, some local elements can be found and used for farming systems. Amongst them, regolith, water, and CO2 are available and can help defray some of the energy and mass costs required for material transportation for farming in space. Space farming must accommodate the mass and volume constraints imposed on current flight experiments and technologies for its set-up.
The use of microorganisms, especially Plant Growth-Promoting Bacteria (PGPB), for crop yield increase is a common practice in farming systems on Earth (Stegelmeier et al. Citation2022). However, the use of microorganisms, and more precisely photosynthetic microorganisms, for space farming systems presents a unique set of challenges and opportunities. On the one hand, the space environment presents some limitations and constraints on plants’ and microorganisms’ growth. These constraints prevent the presence of natural resources that can be used for agriculture as, until now, no life has been demonstrated to be able to survive in such a combination of highly hostile conditions. On the other hand, the use of photosynthetic biostimulants in space agriculture offers potential benefits considering the impact of environmental stress on valuable biostimulant metabolites production. Indeed, it has been demonstrated that environmental stress can induce and enhance the production of secondary metabolites with a beneficial impact on plants’ development, growth and health, as well as direct human food supplement (Kultschar et al. Citation2018). The production of these metabolites can be linked to the modulation of various metabolic pathways in response to the external environment as a way to adapt, and could therefore be highly impacted during a mission in space conditions (Kultschar et al. Citation2018). In addition to this ability to produce valuable metabolites, photosynthetic microorganisms have been shown to efficiently produce oxygen under low light conditions (7 µmol of photons m−2 s−1) (Macário et al. Citation2022). Lastly, photosynthetic microorganisms also present interest in aquaculture as they are an interesting nutritive source for fish (Przybyla Citation2021). These different food production methodologies, in addition to oxygen production, offer the creation of an interesting ecosystem in a space station. These elements make photosynthetic microorganisms attractive for space applications both in farming systems and life support systems for humans from a holistic point of view.
Moreover, cyanobacteria are highly resistant to radiation on Earth (UV range) and are able to repair DNA damage. This ability might be associated with antioxidant production, protein resynthesis, programmed cell death, and UV-absorbing/screening compounds synthesis, such as mycosporine-like amino acids (MAAs) and scytonemin (Rastogi et al. Citation2014). The limited resource availability in space makes photosynthetic microorganisms like cyanobacteria well-suited for space agriculture because of their ability to use light as an energy source and carbon dioxide as a carbon source. Additionally, they are capable of growing in a wide variety of environments and nutrient conditions while producing oxygen and fixing atmospheric nitrogen, which is essential for the support of human life in space (Stal Citation2007; Lugtenberg and Kamilova Citation2009; Mapstone et al. Citation2022). Moreover, these systems aim to achieve food security for future crew and, therefore, are intended to be highly reliable.
5. Conclusion
The exciting field of agriculture adapted to space conditions is at its beginning. So far, photosynthetic microorganisms have shown unique properties and mechanisms proving their ability to enhance plant growth and improve crop yields in various environments by means of secondary metabolite and fertilisers production. This production of secondary metabolites is enhanced in a stressful environment, such as in space conditions. Indeed, the space radiations (i.e. Gamma-ray, X-ray, protons), light conditions (direct light from the star or full darkness), microgravity (variable depending on the environment) and temperature (from 3 K to the theoretical Planck temperature of 1032 K) occurring in space environments present an important source of stress for microorganisms. If correctly managed, these stressful conditions present an advantage in the quest for valuable components for biostimulation. They can be used as a watering solution in hydroponic systems, soil amendment in in-soil systems, or seed coatings both on Earth and in space settlements. One of them seems particularly interesting. Indeed, exoployssacharides found in the culture medium (after growth) or the polyssacharides associated with the biomass could be used in crops and particularly in hydroponics systems such as the one presented in the MELiSSA loop. Understanding extreme environments conditions pathways involved to improve our cultivation methodologies accordingly. It would nevertheless appear that an association of bacteria (cyanobacteria, PGPB, PGPR) seems important to consider for better stimulation. A variety of microorganisms would produce a wider range of beneficial secondary metabolites to be used by the plant. Nevertheless, photosynthetic microorganisms have advantages regarding space settlements, as they offer a variety of uses and a wide range of biostimulating metabolites as a by-product of their normal metabolism. Furthermore, photosynthetic microorganisms present not only interesting properties for biostimulation in a space station but could also support a crew through oxygen and food supplement production, when produced in food grade methods, as well as part of an aquaculture system for fish nutrition.
As we continue to explore the possibilities of crewed space missions, space-based agriculture seems undeniable. Incorporating biostimulants from photosynthetic cyanobacteria may be key to ensuring successful plant growth and resource production in space.
Acknowledgment
This article has been made possible through the author’s involvement in the MELiSSA project, ESA’s life support system program (https://www.esa.int/Enabling_Support/Space_Engineering_Technology/Melissa) and Belspo through the ARTEMiSS Prodex contrat which pays for Cécile Renaud's PhD grant.
Figures were created using Inkscape.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Cécile Renaud
Cécile Renaud is a PhD student at the University of Mons under the supervision of Prof. Ruddy Wattiez. She has completed a master of science in integrative biology at Sorbonne University and a master's in Innovation at AgroParisTech. She has studied biostimulation on plant related to the space environment as an analog astronaut at the Mars Desert Research Station and is pursuing this goal.
Natalie Leys
Natalie Leys is a doctor in science, leading the Microbiology Research team and the coordinator of the Space Life Science Research program at SCK CEN. She specialized in Research and Development (R&D), Life Sciences, Microbiology, Genetics, and Space Microbiology. She is a strong professional with expertise in people, program and project management, and science communication. She studies the biological impact of ionizing radiation and radionuclides, and the role of microbes, in nuclear installations, in the environment, in health, and space.
Ruddy Wattiez
Ruddy Wattiez obtained a Ph.D. in chemistry from the University of Mons (UMONS). Ruddy Wattiez was promoted in 2000 to FNRS qualified researcher and in 2011 full professor at the same University. He became scientific director of the Materia Nova research center and, in 2016, Vice-Rector for Research, Innovation and Entrepreneurship at UMONS. He has been involved in the MELiSSA project of the Space European Agency for over 20 years. Drawing on its expertise in the functional analysis of microorganisms and the study of their adaptive capacity, it has been involved from the outset, in studying the adaptation of the microorganisms used in the MELiSSA loop, including the microalga Arthrospira platensis and the purple bacterium Rhodospirullum Rubrum, to space conditions. In parallel, he is developing, among other things, the production of so-called alternative proteins for feed and food applications from microorganisms and the development of new bio-stimulant molecules for agriculture.
References
- Adessi A, Cruz de Carvalho R, De Philippis R, Branquinho C, Marques da Silva J. 2018. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol Biochem. 116:67–69. doi:10.1016/j.soilbio.2017.10.002.
- Aguiló-Nicolau P, Galmés J, Fais G, Capó-Bauçà S, Cao G, Iñiguez C. 2023. Singular adaptations in the carbon assimilation mechanism of the polyextremophile cyanobacterium Chroococcidiopsis thermalis. Photosynth Res. 156:231–245. doi:10.1007/s11120-023-01008-y.
- Akomolafe G, Omojola J, Joshua E, Adediwura SC, Adesuji ET, Odey M, Dedeke OA, Labulo AH. 2017. Growth and anatomical responses of lycopersicon esculentum (tomatoes) under microgravity and normal gravity conditions. World Acad Sci, Eng Technol, Int J Biol Biomol Agric Food Biotechnol Eng. 11(5): 335–338.
- Alsharif W, Saad MM, Hirt H. 2020. Desert microbes for boosting sustainable agriculture in extreme environments. Front Microbiol. 11. doi:10.3389/fmicb.2020.01666.
- Altman A, Lugtenberg B, Bloemberg G, Okon Y. 1997. Biotechnology of biofertilization and phytostimulation. In: Books in soils, plants, and the environment. CRC Press; p. 327–349. doi:10.1201/9781420049275.pt2a.
- Altomare C, Tringovska I. 2011. Beneficial soil microorganisms, an ecological alternative for soil fertility management. In: Lichtfouse E., editor. Genetics, biofuels and local farming systems, sustainable agriculture reviews. Dordrecht: Springer Netherlands; p. 161–214. doi:10.1007/978-94-007-1521-9_6.
- Arena C, De Micco V, Macaeva E, Quintens R. 2014. Space radiation effects on plant and mammalian cells. Acta Astronaut. 104:419–431. doi:10.1016/j.actaastro.2014.05.005.
- Azizoglu U, Yilmaz N, Simsek O, Ibal JC, Tagele SB, Shin J-H. 2021. The fate of plant growth-promoting rhizobacteria in soilless agriculture: future perspectives. 3 Biotech. 11:382. doi:10.1007/s13205-021-02941-2.
- Barth CA. 1974. The atmosphere of Mars. Annu Rev Earth Planet Sci. 2:333–367. doi:10.1146/annurev.ea.02.050174.002001.
- Barton AD, Pershing AJ, Litchman E, Record NR, Edwards KF, Finkel ZV, Kiørboe T, Ward BA. 2013. The biogeography of marine plankton traits. Ecol Lett. 16:522–534. doi:10.1111/ele.12063.
- Berrios DC, Galazka J, Grigorev K, Gebre S, Costes SV. 2021. NASA genelab: interfaces for the exploration of space omics data. Nucleic Acids Res. 49:D1515–D1522. doi:10.1093/nar/gkaa887.
- Bilalis DJ, Katsenios N, Efthimiadou A, Karkanis A. 2012. Pulsed electromagnetic field: an organic compatible method to promote plant growth and yield in two corn types. Electromagn Biol Med. 31:333–343. doi:10.3109/15368378.2012.661699.
- Bonfante P, Anca I-A. 2009. Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol. 63:363–383. doi:10.1146/annurev.micro.091208.073504.
- Bonini P, Rouphael Y, Miras-Moreno B, Lee B, Cardarelli M, Erice G, Cirino V, Lucini L, Colla G. 2020. A microbial-based biostimulant enhances sweet pepper performance by metabolic reprogramming of phytohormone profile and secondary metabolism. Front Plant Sci. 11. doi:10.3389/fpls.2020.567388.
- Bonjouklian R, Smitka TA, Doolin LE, Molloy RM, Debono M, Shaffer SA, Moore RE, Stewart JB, Patterson GML. 1991. Tjipanazoles, new antifungal agents from the blue-green alga tolypothrix tjipanasensis. Tetrahedron. 47:7739–7750. doi:10.1016/S0040-4020(01)81932-3.
- Brian Yager. n.d. Gravity [WWW document]. National space society; [accessed 5 Jul 2023]. https://space.nss.org/settlement/nasa/teacher/lessons/bryan/microgravity/gravback.html.
- Campobenedetto C, Grange E, Mannino G, van Arkel J, Beekwilder J, Karlova R, Garabello C, Contartese V, Bertea CM. 2020. A biostimulant seed treatment improved heat stress tolerance during cucumber seed germination by acting on the antioxidant system and glyoxylate cycle. Front Plant Sci. 11. doi:10.3389/fpls.2020.00836.
- Carey EM, Castillo-Rogez J, Scully JEC, Russell CT. 2014. Rate of evaporation of water under low-pressure conditions 2060.
- CEN/TC 455 – Plant Biostimulants and Agricultural Micro-Organisms [WWW Document]. 2022. iTeh standards store; [accessed 12 Dec 2022]. https://standards.iteh.ai/catalog/tc/cen/249041e0-ca8b-4039-a65c-ad232d6e57f0/cen-tc-455.
- Chaiklahan R, Chirasuwan N, Triratana P, Loha V, Tia S, Bunnag B. 2013. Polysaccharide extraction from spirulina sp. and its antioxidant capacity. Int J Biol Macromol. 58:73–78. doi:10.1016/j.ijbiomac.2013.03.046.
- Chiaranunt P, White JF. 2023. Plant beneficial bacteria and their potential applications in vertical farming systems. Plants. 12:400. doi:10.3390/plants12020400.
- Chin S, Blancaflor EB. 2022. Plant gravitropism: from mechanistic insights into plant function on earth to plants colonizing other worlds. In: Blancaflor E.B., editor. Plant gravitropism: methods and protocols, methods in molecular biology. New York, NY: Springer US; p. 1–41. doi:10.1007/978-1-0716-1677-2_1.
- Crang R, Lyons-Sobaski S, Wise R. 2018. Plant anatomy: a concept-based approach to the structure of seed plants. SpringerLink.
- Creech S, Guidi J, Elburn D. 2022. Artemis: an overview of NASA’s activities to return humans to the moon. In: 2022 IEEE aerospace conference (AERO). presented at the 2022 IEEE aerospace conference (AERO). p. 1–7. doi:10.1109/AERO53065.2022.9843277.
- Darwin C, Darwin F. 1880. The power of movement in plants. London, UK: William Clowes and Sons.
- David P, Carney S. n.d. Basics of space flight – Solar system exploration: NASA science [WWW Document]. NASA Solar System Exploration; [accessed 5 Jul 2023]. https://solarsystem.nasa.gov/basics/chapter4-1/.
- Davis F. 2017. The biology of growing plants under low pressure (hypobaric) systems for NASA – Challenges in Lunar and Martian agriculture.
- De Pascale S, Arena C, Aronne G, De Micco V, Pannico A, Paradiso R, Rouphael Y. 2021. Biology and crop production in space environments: challenges and opportunities. Life Sci Space Res (Amst). 29:30–37. doi:10.1016/j.lssr.2021.02.005.
- Despommier D. 2019. Vertical farms, building a viable indoor farming model for cities. Field Actions Sci Rep. J Field Actions. 68–73.
- Dobbelaere S, Croonenborghs A, Thys A, Vande Broek A, Vanderleyden J. 1999. Phytostimulatory effect of azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil. 212:153–162. doi:10.1023/A:1004658000815.
- Drobac-Čik AV, Dulic TI, Stojanović D, Svircev ZB. 2007. The importance of extremophile cyanobacteria in the production of biologically active compounds. Zbornik Matice Srpske za Prirodne Nauke. 2007:57–66. doi:10.2298/ZMSPN0712057D.
- Dubey P, Kumar V, Ponnusamy K, Sonwani R, Singh AK, Suyal DC, Soni R. 2020. Microbe assisted plant stress management. Elsevier, pp. 351–378. doi:10.1016/B978-0-12-821265-3.00015-3.
- du Jardin P. 2015. Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic, Biostimulants in Horticulture. 196:3–14. doi:10.1016/j.scienta.2015.09.021.
- EL Arroussi H. 2016. Microalgae polysaccharides a promising plant growth biostimulant. Journal of Algal Biomass Utilization eISSN: 2229–6905. 7(4):55–63.
- Fahrion J, Mastroleo F, Dussap C-G, Leys N. 2021. Use of photobioreactors in regenerative life support systems for human space exploration. Front Microbiol. 12. doi:10.3389/fmicb.2021.699525.
- Fecht S. 2023. Would Earth laws apply to Mars colonists? Popular Science; [accessed 28 Mar 2023]. https://www.popsci.com/who-would-rule-colony-on-mars/.
- Fincheira P, Quiroz A. 2018. Microbial volatiles as plant growth inducers. Microbiol Res. 208:63–75. doi:10.1016/j.micres.2018.01.002.
- Francis F, Jacquemyn H, Delvigne F, Lievens B. 2020. From diverse origins to specific targets: role of microorganisms in indirect pest biological control. Insects. 11:533. doi:10.3390/insects11080533.
- Fuentes-Tristan S, Parra-Saldivar R, Iqbal HMN, Carrillo-Nieves D. 2019. Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. J Photochem Photobiol B. 201:111684. doi:10.1016/j.jphotobiol.2019.111684.
- Furukawa S, Nagamatsu A, Nenoi M, Fujimori A, Kakinuma S, Katsube T, Wang B, Tsuruoka C, Shirai T, Nakamura AJ, et al. 2020. Space radiation biology for “living in space”. BioMed Res Int. 2020:e4703286. doi:10.1155/2020/4703286.
- Gademann K, Portmann C. 2008. Secondary metabolites from cyanobacteria: complex structures and powerful bioactivities. Curr Org Chem. 12:326–341. doi:10.2174/138527208783743750.
- Gámez-Arcas S, Baroja-Fernández E, García-Gómez P, Muñoz FJ, Almagro G, Bahaji A, Sánchez-López ÁM, Pozueta-Romero J. 2022. Action mechanisms of small microbial volatile compounds in plants. J Exp Bot. 73:498–510. doi:10.1093/jxb/erab463.
- Garcia-Pichel F. 2009. Cyanobacteria. In: Encyclopedia of microbiology. Elsevier Inc.; p. 107–124. doi:10.1016/B978-012373944-5.00250-9.
- Geraldes V, Pinto E. 2021. Mycosporine-like amino acids (MAAs): biology, chemistry and identification features. Pharmaceuticals (Basel). 14:63. doi:10.3390/ph14010063.
- Glick BR, Cheng Z, Czarny J, Duan J. 2007. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 119:329–339. doi:10.1007/s10658-007-9162-4.
- Gòdia F, Albiol J, Montesinos JL, Pérez J, Creus N, Cabello F, Mengual X, Montras A, Lasseur C. 2002. MELISSA: a loop of interconnected bioreactors to develop life support in space. J Biotechnol. 99:319–330. doi:10.1016/S0168-1656(02)00222-5.
- González-Pérez BK, Rivas-Castillo AM, Valdez-Calderón A, Gayosso-Morales MA. 2021. Microalgae as biostimulants: a new approach in agriculture. World J Microbiol Biotechnol. 38:4. doi:10.1007/s11274-021-03192-2.
- Goyer A. 2010. Thiamine in plants: aspects of its metabolism and functions. Phytochemistry. 71:1615–1624. doi:10.1016/j.phytochem.2010.06.022.
- Grinberg M, Nemtsova Y, Ageyeva M, Brilkina A, Vodeneev V. 2023. Effect of low-dose ionizing radiation on spatiotemporal parameters of functional responses induced by electrical signals in tobacco plants. Photosynth Res. doi:10.1007/s11120-023-01027-9.
- Grinberg MA, Gudkov SV, Balalaeva IV, Gromova E, Sinitsyna Y, Sukhov V, Vodeneev V. 2021. Effect of chronic β-radiation on long-distance electrical signals in wheat and their role in adaptation to heat stress. Environ Exp Bot. 184:104378. doi:10.1016/j.envexpbot.2021.104378.
- Gudkov SV, Grinberg MA, Sukhov V, Vodeneev V. 2019. Effect of ionizing radiation on physiological and molecular processes in plants. J Environ Radioact. 202:8–24. doi:10.1016/j.jenvrad.2019.02.001.
- Haberle RM. 2015. Solar System/Sun, atmospheres, evolution of atmospheres planetary atmospheres: Mars. In: North G.R., Pyle J., Zhang F, editor. Encyclopedia of atmospheric sciences (2nd ed.). Oxford: Academic Press; p. 168–177. doi:10.1016/B978-0-12-382225-3.00312-1.
- Häder D-P. 2022. Photosynthesis in plants and algae. Anticancer Res. 42:5035–5041. doi:10.21873/anticanres.16012.
- Hall L. 2021. NASA research launches a new generation of indoor farming [WWW Document]. NASA; (accessed 14 Mar 2023). http://www.nasa.gov/directorates/spacetech/spinoff/NASA_Research_Launches_a_New_Generation_of_Indoor_Farming.
- Handy D, Hummerick ME, Dixit AR, Ruby AM, Massa G, Palmer A. 2021. Identification of plant growth promoting bacteria within space crop production systems. Front Astron Space Sci. 8. doi:10.3389/fspas.2021.735834.
- Havaux M, Ksas B, Szewczyk A, Rumeau D, Franck F, Caffarri S, Triantaphylidès C. 2009. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 9:130. doi:10.1186/1471-2229-9-130.
- Heocha CO. 1965. Biliproteins of algae. Annu Rev Plant Physiol. 16:415–434. doi:10.1146/annurev.pp.16.060165.002215.
- Hessel V, Liang S, Tran NN, Escribà-Gelonch M, Zeckovic O, Knowling M, Rebrov E, This H, Westra S, Fisk I, et al. 2022. Eustress in space: opportunities for plant stressors beyond the earth ecosystem. Front Astron Space Sci. 9. doi:10.3389/fspas.2022.841211.
- Hoeck A, Horemans N, Hees M, Nauts R, Knapen D, Vandenhove H, Blust R. 2015. Characterizing dose response relationships: chronic gamma radiation in Lemna minor induces oxidative stress and altered polyploidy level. J Environ Radioact. 150:195–202. doi:10.1016/j.jenvrad.2015.08.017.
- Juan Carlos CABRERA. 2018. Activators of plant metabolic changes. PCT/EP2018/050147.
- Kageyama H, Waditee-Sirisattha R. 2022. Cyanobacterial physiology: from fundamentals to biotechnology. London: Academic Press.
- Kapoore RV, Wood EE, Llewellyn CA. 2021. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol Adv. 49:107754. doi:10.1016/j.biotechadv.2021.107754.
- Kashyap AK, Dubey SK, Jain BP. 2022. 1 - Cyanobacterial diversity concerning the extreme environment and their bioprospecting. In: Singh P., Fillat M., Kumar A., editor. Cyanobacterial lifestyle and its applications in biotechnology. Academic Press; p. 1–22. doi:10.1016/B978-0-323-90634-0.00008-1.
- Kauff F, Büdel B. 2011. Phylogeny of cyanobacteria: an overview. In: Lüttge U.E., Beyschlag W., Büdel B., Francis D, editor. Progress in botany, Vol. 72, progress in botany. Berlin, Heidelberg: Springer; p. 209–224. doi:10.1007/978-3-642-13145-5_8.
- Kees ED, Murugapiran SK, Bennett AC, Hamilton TL. 2022. Distribution and genomic variation of thermophilic cyanobacteria in diverse microbial mats at the upper temperature limits of photosynthesis. mSystems. 7:e00317–22. doi:10.1128/msystems.00317-22.
- Kehr J-C, Dittmann E. 2015. Biosynthesis and function of extracellular glycans in cyanobacteria. Life (Basel). 5:164–180. doi:10.3390/life5010164.
- Kessler P, Prater T, Nickens T, Harris D. 2022. Artemis deep space habitation: enabling a sustained human presence on the moon and beyond. In: 2022 IEEE aerospace conference (AERO). Presented at the 2022 IEEE aerospace conference (AERO). p. 01–12. doi:10.1109/AERO53065.2022.9843393.
- Khodadad CLM, Hummerick ME, Spencer LE, Dixit AR, Richards JT, Romeyn MW, Smith TM, Wheeler RM, Massa GD. 2020. Microbiological and nutritional analysis of lettuce crops grown on the international space station. Front Plant Sci. 11. doi:10.3389/fpls.2020.00199.
- Konopka A, Brock TD. 1978. Effect of temperature on blue-green algae (cyanobacteria) in lake mendota. Appl Environ Microbiol. 36:572–576. doi:10.1128/aem.36.4.572-576.1978.
- Kordyum E. 2003. Calcium signaling in plant cells in altered gravity. Adv Space Res. 32:1621–1630. doi:10.1016/S0273-1177(03)90403-0.
- Koza N, Adedayo A, Babalola O, Kappo A. 2022. Microorganisms in plant growth and development: roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms. 10:1528. doi:10.3390/microorganisms10081528.
- Kreft H, Jetz W. 2007. Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci USA. 104:5925–5930. doi:10.1073/pnas.0608361104.
- Kuiper I, Bloemberg GV, Lugtenberg BJ. 2001. Selection of a plant-bacterium pair as a novel tool for rhizostimulation of polycyclic aromatic hydrocarbon-degrading bacteria. Mol Plant Microbe Interact. 14:1197–1205. doi:10.1094/MPMI.2001.14.10.1197.
- Kulik MM. 1995. The potential for using cyanobacteria (blue-green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur J Plant Pathol. 101:585–599. doi:10.1007/BF01874863.
- Kultschar B, Llewellyn C, Kultschar B, Llewellyn C. 2018. Secondary metabolites in cyanobacteria, secondary metabolites – sources and applications. IntechOpen. doi:10.5772/intechopen.75648.
- Lakhiar IA, Gao J, Syed TN, Chandio FA, Buttar NA. 2018. Modern plant cultivation technologies in agriculture under controlled environment: a review on aeroponics. J Plant Interact. 13:338–352. doi:10.1080/17429145.2018.1472308.
- Laroche C. 2022. Exopolysaccharides from microalgae and cyanobacteria: diversity of strains, production strategies, and applications. Mar Drugs. 20:336. doi:10.3390/md20050336.
- Lasseur C, Paillé C, Lamaze B, Rebeyre P, Rodriguez A, Ordonez L, Marty F. 2005. MELISSA: overview of the project and perspectives. Presented at the International conference on environmental systems. p. 2005-01–3066. doi:10.4271/2005-01-3066.
- Li F, He X, Sun Y, Zhang X, Tang X, Li Y, Yi Y. 2019. Distinct endophytes are used by diverse plants for adaptation to karst regions. Sci Rep. 9:5246. doi:10.1038/s41598-019-41802-0.
- Litchman E, Klausmeier CA, Schofield OM, Falkowski PG. 2007. The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level. Ecol Lett. 10:1170–1181. doi:10.1111/j.1461-0248.2007.01117.x.
- Liu M, Wu T, Zhao X, Zan F, Yang G, Miao Y. 2021. Cyanobacteria blooms potentially enhance volatile organic compound (VOC) emissions from a eutrophic lake: field and experimental evidence. Environ Res. 202:111664. doi:10.1016/j.envres.2021.111664.
- Liu W, Wang J, Li Y, Zhu Z, Qie D, Ding L. 2019. Natural convection heat transfer at reduced pressures. Exp Heat Transf. 32:14–24. doi:10.1080/08916152.2018.1468833.
- Lucas WJ, Groover A, Lichtenberger R, Furuta K, Yadav S-R, Helariutta Y, He X-Q, Fukuda H, Kang J, Brady SM, et al. 2013. The plant vascular system: evolution, development and functions. J Integr Plant Biol. 55:294–388. doi:10.1111/jipb.12041.
- Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 63:541–556. doi:10.1146/annurev.micro.62.081307.162918.
- Macário IPE, Veloso T, Frankenbach S, Serôdio J, Passos H, Sousa C, Gonçalves FJM, Ventura SPM, Pereira JL. 2022. Cyanobacteria as candidates to support Mars colonization: growth and biofertilization potential using Mars regolith as a resource. Front Microbiol. 13:840098. doi:10.3389/fmicb.2022.840098.
- Malavasi V, Soru S, Cao G. 2020. Extremophile microalgae: the potential for biotechnological application. J Phycol. 56:559–573. doi:10.1111/jpy.12965.
- Mapstone LJ, Leite MN, Purton S, Crawford IA, Dartnell L. 2022. Cyanobacteria and microalgae in supporting human habitation on Mars. Biotechnol Adv. 59:107946. doi:10.1016/j.biotechadv.2022.107946.
- Martinez E, Florez M, Carbonell MV. 2017. Stimulatory effect of the magnetic treatment on the germination of cereal seeds. Int J Environ Agric Biotechnol. 2. doi:10.22161/ijeab/2.1.47.
- Massa GD, Dufour NF, Carver JA, Hummerick ME, Wheeler RM, Morrow RC, Smith TM. 2017. VEG-01: veggie hardware validation testing on the international space station. Open Agric. 2:33–41. doi:10.1515/opag-2017-0003.
- Medina FJ, Manzano A, Villacampa A, Ciska M, Herranz R. 2021. Understanding reduced gravity effects on early plant development before attempting life-support farming in the moon and Mars. Front Astron Space Sci. 8. doi:10.3389/fspas.2021.729154.
- Melkozernov AN, Barber J, Blankenship RE. 2006. Light harvesting in photosystem I supercomplexes. Biochemistry. 45:331–345. doi:10.1021/bi051932o.
- Mishra S. 2020. Cyanobacterial imprints in diversity and phylogeny. Elsevier. 1–15. doi:10.1016/B978-0-12-819311-2.00001-2.
- Mridha N, Chattaraj S, Chakraborty D, Anand A, Aggarwal P, Nagarajan S. 2016. Pre-sowing static magnetic field treatment for improving water and radiation use efficiency in chickpea (Cicer arietinum L.) under soil moisture stress. Bioelectromagnetics. 37:400–408. doi:10.1002/bem.21994.
- Msfc JW. 2015. What is microgravity? [WWW Document]. NASA; [accessed 29 Aug 2022]. http://www.nasa.gov/audience/forstudents/5-8/features/nasa-knows/what-is-microgravity-58.html.
- Mungin R, Weislogel M, Hatch T, McQuillen J. 2019. Omni-gravity hydroponics for space exploration.
- Mutale-joan C, Rachidi F, Mohamed HA, Mernissi NE, Aasfar A, Barakate M, Mohammed D, Sbabou L, Arroussi HE. 2021. Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J Appl Phycol. 33:3779–3795. doi:10.1007/s10811-021-02559-0.
- Muthert LWF, Izzo LG, van Zanten M, Aronne G. 2020. Root tropisms: investigations on earth and in space to unravel plant growth direction. Front Plant Sci. 10. doi:10.3389/fpls.2019.01807.
- Naing AH, Maung T-T, Kim CK. 2021. The ACC deaminase-producing plant growth-promoting bacteria: influences of bacterial strains and ACC deaminase activities in plant tolerance to abiotic stress. Physiol Plant. 173:1992–2012. doi:10.1111/ppl.13545.
- Nir I. 1982. Growing plants in aeroponics growth system. Acta Hortic. 435–448. doi:10.17660/ActaHortic.1982.126.49.
- Nunnery JK, Mevers E, Gerwick WH. 2010. Biologically active secondary metabolites from marine cyanobacteria. Curr Opin Biotechnol, Chem Biotechnol – Pharm Biotechnol. 21:787–793. doi:10.1016/j.copbio.2010.09.019.
- Nyakane NE, Markus ED, Sedibe MM. 2019. The effects of magnetic fields on plants growth: a comprehensive review. ETP Int J Food Eng. 79–87. doi:10.18178/ijfe.5.1.79-87.
- Oren A. 2015. Cyanobacteria in hypersaline environments: biodiversity and physiological properties. Biodivers Conserv. 24:781–798. doi:10.1007/s10531-015-0882-z.
- Ouzounis T, Rosenqvist E, Ottosen C-O. 2015. Spectral effects of artificial light on plant physiology and secondary metabolism: a review. HortScience. 50:1128–1135. doi:10.21273/HORTSCI.50.8.1128.
- Pagels F, Vasconcelos V, Guedes AC. 2021. Carotenoids from cyanobacteria: biotechnological potential and optimization strategies. Biomolecules. 11:735. doi:10.3390/biom11050735.
- Patel A, Mishra S, Pawar R, Ghosh PK. 2005. Purification and characterization of C-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expression Purif. 40:248–255. doi:10.1016/j.pep.2004.10.028.
- Paul A-L. 2002. Plant adaptation to low atmospheric pressures: potential molecular responses. Life Support Biosph Sci. 8:93–101.
- Pedrini S, Merritt DJ, Stevens J, Dixon K. 2017. Seed coating: science or marketing spin? Trends Plant Sci. 22:106–116. doi:10.1016/j.tplants.2016.11.002.
- Perez J. 2017. Why space radiation matters [WWW document]. NASA; [accessed 10 Jul 2023]. http://www.nasa.gov/analogs/nsrl/why-space-radiation-matters.
- Poughon L, Laroche C, Creuly C, Dussap C-G, Paille C, Lasseur C, Monsieurs P, Heylen W, Coninx I, Mastroleo F, Leys N. 2020. Limnospira indica PCC8005 growth in photobioreactor: model and simulation of the ISS and ground experiments. Life Sci Space Res (Amst). 25:53–65. doi:10.1016/j.lssr.2020.03.002.
- Poveda J. 2020. Cyanobacteria in plant health: biological strategy against abiotic and biotic stresses. Crop Prot. 141:105450. doi:10.1016/j.cropro.2020.105450.
- Przybyla C. 2021. Space aquaculture: prospects for raising aquatic vertebrates in a bioregenerative life-support system on a lunar base. Front Astron Space Sci. 8. doi:10.3389/fspas.2021.699097.
- Rajashekhar K. 2013. Antioxidant activity in the four species of cyanobacteria isolated from a sulfur spring in the Western Ghats of Karnataka. Int J Pharma Bio Sci 4:275–285.
- Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR. 2008. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature. 455:1101–1104. doi:10.1038/nature07381.
- Rastogi RP, Sinha RP, Moh SH, Lee TK, Kottuparambil S, Kim Y-J, Rhee J-S, Choi E-M, Brown MT, Häder D-P, Han T. 2014. Ultraviolet radiation and cyanobacteria. J Photochem Photobiol, B. 141:154–169. doi:10.1016/j.jphotobiol.2014.09.020.
- Ricci M, Tilbury L, Daridon B, Sukalac K. 2019. General principles to justify plant biostimulant claims. Front Plant Sci. 10. doi:10.3389/fpls.2019.00494.
- Rincón-Molina CI, Martínez-Romero E, Aguirre-Noyola JL, Manzano-Gómez LA, Zenteno-Rojas A, Rogel MA, Rincón-Molina FA, Ruíz-Valdiviezo VM, Rincón-Rosales R. 2022. Bacterial community with plant growth-promoting potential associated to pioneer plants from an active Mexican volcanic complex. Microorganisms. 10:1568. doi:10.3390/microorganisms10081568.
- Rodríguez A, Stella A, Storni M, Zulpa G, Zaccaro M. 2006. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2:7. doi:10.1186/1746-1448-2-7.
- Rosa LM, Forseth IN. 1996. Diurnal patterns of soybean leaf inclination angles and azimuthal orientation under different levels of ultraviolet-B radiation. Agric For Meteorol. 78:107–119. doi:10.1016/0168-1923(95)02249-X.
- Rostami zadeh E, Majd A, Arbabian S. 2014. Effects of electromagnetic fields on seed germination in Utica Dioica L.
- Rouphael Y, Colla G. 2020. Editorial: biostimulants in agriculture. Front. Plant Sci. 11:40. doi:10.3389/fpls.2020.00040.
- Saa S, Olivos-Del Rio A, Castro S, Brown PH. 2015. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Front Plant Sci. 6. doi:10.3389/fpls.2015.00087.
- Saad MM, Eida AA, Hirt H. 2020. Tailoring plant-associated microbial inoculants in agriculture: a roadmap for successful application. J Exp Bot. 71:3878–3901. doi:10.1093/jxb/eraa111.
- Sánchez-Baracaldo P, Cardona T. 2020. On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol. 225:1440–1446. doi:10.1111/nph.16249.
- Santini G, Biondi N, Rodolfi L, Tredici MR. 2021. Plant biostimulants from cyanobacteria: an emerging strategy to improve yields and sustainability in agriculture. Plants (Basel). 10:643. doi:10.3390/plants10040643.
- Santomartino R, Waajen AC, de Wit W, Nicholson N, Parmitano L, Loudon C-M, Moeller R, Rettberg P, Fuchs FM, Van Houdt R, et al. 2020. No effect of microgravity and simulated Mars gravity on final bacterial cell concentrations on the international space station: applications to space bioproduction. Front Microbiol. 11. doi:10.3389/fmicb.2020.579156.
- Santos A, Vieira K, Nogara G, Wagner R, Jacob-Lopes E, Zepka L. 2016. Biogeneration of volatile organic compounds by microalgae: occurrence, behavior, ecological implications and industrial applications.
- Sathasivam M, Hosamani RK, Swamy B, Kumaran GS. 2021. Plant responses to real and simulated microgravity. Life Sci Space Res (Amst). 28:74–86. doi:10.1016/j.lssr.2020.10.001.
- Seckbach J. 2007. Algae and cyanobacteria in extreme environments. Doordrecht, The Netherlands: Springer Science & Business Media.
- Semary NEE. 2012. The antimicrobial profile of extracts of a Phormidium-like cyanobacterium changes with phosphate levels. World J Microbiol Biotechnol. 28: 585–593. doi:10.1007/s11274-011-0851-y.
- Shabrangy A, Majd A. 2009. Effect of magnetic fields on growth and antioxidant systems in agricultural plants. PIERS Proceedings, Beijing, China. 23–27.
- Shah N, Wakabayashi T, Kawamura Y, Skovbjerg CK, Wang M-Z, Mustamin Y, Isomura Y, Gupta V, Jin H, Mun T, et al. 2020. Extreme genetic signatures of local adaptation during Lotus japonicus colonization of Japan. Nat Commun. 11:253. doi:10.1038/s41467-019-14213-y.
- Shahrajabian MH, Chaski C, Polyzos N, Petropoulos SA. 2021. Biostimulants application: a low input cropping management tool for sustainable farming of vegetables. Biomolecules. 11:698. doi:10.3390/biom11050698.
- Shanab SM, Shalaby EA. 2021. Production of plant hormones from algae and Its relation to plant growth. In: Mohamed H.I., El-Beltagi H.E.-D.S., Abd-Elsalam K.A, editor. Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Cham: Springer International Publishing; p. 395–423. doi:10.1007/978-3-030-66587-6_14.
- Shao Y, Li J, Zhou Z, Zhang F, Cui Y. 2021. The impact of indoor living wall system on air quality: a comparative monitoring test in building corridors. Sustainability. 13:7884. doi:10.3390/su13147884.
- Sharma V, Prasanna R, Hossain F, Muthusamy V, Nain L, Das S, Shivay YS, Kumar A. 2020. Priming maize seeds with cyanobacteria enhances seed vigour and plant growth in elite maize inbreds. 3 Biotech. 10. doi:10.1007/s13205-020-2141-6.
- Shimazu T, Yuda T, Miyamoto K, Yamashita M, Ueda J. 2001. Growth and development in higher plants under simulated microgravity conditions on a 3-dimensional clinostat. Adv Space Res. 27:995–1000. doi:10.1016/S0273-1177(01)00165-X.
- Shinde S, Zhang X, Singapuri SP, Kalra I, Liu X, Morgan-Kiss RM, Wang X. 2020. Glycogen metabolism supports photosynthesis start through the oxidative pentose phosphate pathway in cyanobacteria1 [OPEN]. Plant Physiol. 182:507–517. doi:10.1104/pp.19.01184.
- Singh S. 2014. A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J Appl Microbiol. 117:1221–1244. doi:10.1111/jam.12612.
- Singh SP, Häder D-P, Sinha RP. 2010. Cyanobacteria and ultraviolet radiation (UVR) stress: mitigation strategies. Ageing Res Rev, Microbes Ageing. 9:79–90. doi:10.1016/j.arr.2009.05.004.
- Son JE, Kim HJ, Ahn TI. 2020. Chapter 20 – hydroponic systems. In: Kozai T., Niu G., Takagaki M., editor. Plant factory (2nd ed.). Academic Press; p. 273–283. doi:10.1016/B978-0-12-816691-8.00020-0.
- Stal LJ Ph.D. 2007. Algae and cyanobacteria in extreme environments, cellular origin, life in extreme habitats and astrobiology. Dordrecht: Springer.
- Stegelmeier AA, Rose DM, Joris BR, Glick BR. 2022. The use of PGPB to promote plant hydroponic growth. Plants. 11:2783. doi:10.3390/plants11202783.
- Tan HT, Yusoff FMd, Khaw YS, Nazarudin MF, Noor Mazli NAI, Ahmad SA, Shaharuddin NA, Toda T. 2023. Characterisation and selection of freshwater cyanobacteria for phycobiliprotein contents. Aquacult Int. 31:447–477. doi:10.1007/s10499-022-00985-6.
- Tarver WJ, Volner K, Cooper JS. 2022. Aerospace pressure effects. In: Statpearls. Treasure Island, FL: StatPearls Publishing.
- Tirono M, Hananto FS, Suhariningsih, Aini VQ. 2021. An effective dose of magnetic field to increase sesame plant growth and Its resistance to fusarium oxysporum wilt. Int J Des Nat Ecodyn. 16:285–291. doi:10.18280/ijdne.160306.
- Torracchi C JE, Morel MA, Tapia-Vázquez I, Castro-Sowinski S, Batista-García RA, Yarzábal RLA. 2020. Fighting plant pathogens with cold-active microorganisms: biopesticide development and agriculture intensification in cold climates. Appl Microbiol Biotechnol. 104:8243–8256. doi:10.1007/s00253-020-10812-8.
- Van Camp C, Fraikin C, Claverie E, Onderwater R, Wattiez R. 2022. Capsular polysaccharides and exopolysaccharides from Gloeothece verrucosa under various nitrogen regimes and their potential plant defence stimulation activity. Algal Res. 64:102680. doi:10.1016/j.algal.2022.102680.
- van Rhijn P, Vanderleyden J. 1995. The Rhizobium-plant symbiosis. Microbiol Rev. 59:124–142. doi:10.1128/mr.59.1.124-142.1995.
- Vassilev N, Vassileva M, Nikolaeva I. 2006. Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol. 71:137–144. doi:10.1007/s00253-006-0380-z.
- Verbelen J-P, De Cnodder T, Le J, Vissenberg K, Baluska F. 2006. The root apex of arabidopsis thaliana consists of four distinct zones of growth activities: meristematic zone, transition zone, fast elongation zone and growth terminating zone. Plant Signal Behav. 1:296–304. doi:10.4161/psb.1.6.3511.
- Vetrano F, Moncada A, Miceli A. 2020. Use of gibberellic acid to increase the salt tolerance of leaf lettuce and rocket grown in a floating system. Agronomy. 10:505. doi:10.3390/agronomy10040505.
- Waditee-Sirisattha R, Kageyama H. 2022. Chapter 7 – extremophilic cyanobacteria. In: Kageyama H., Waditee-Sirisattha R., editor. Cyanobacterial physiology. Academic Press; p. 85–99. doi:10.1016/B978-0-323-96106-6.00012-5.
- Walton K, Berry JP. 2016. Indole alkaloids of the stigonematales (cyanophyta): chemical diversity, biosynthesis and biological activity. Mar Drugs. 14:73. doi:10.3390/md14040073.
- Wang X, Pecoraro L. 2021. Diversity and co-occurrence patterns of fungal and bacterial communities from alkaline sediments and water of julong high-altitude hot Springs at Tianchi Volcano, Northeast China. Biology (Basel). 10:894. doi:10.3390/biology10090894.
- Wheeler RM. 2017. Agriculture for space: people and places paving the Way. Open Agric. 2:14–32. doi:10.1515/opag-2017-0002.
- Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH. 2017. Biostimulants in plant science: a global perspective. Front Plant Sci. 7:2049. doi:10.3389/fpls.2016.02049.
- Yang C. 2016. Adaptive plant physiology in extreme environments. J Plant Physiol. 194:1. doi:10.1016/j.jplph.2016.03.003.
- Yep B, Zheng Y. 2019. Aquaponic trends and challenges – a review. J Cleaner Prod. 228:1586–1599. doi:10.1016/j.jclepro.2019.04.290.
- Yvin J-C, Cruz F, Devault MCPV, Villar L. 2019. Use of phycobiliproteins or an extract containing as fertilizer. FR3074801A1.
- Zanin L, Tomasi N, Cesco S, Varanini Z, Pinton R. 2019. Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front Plant Sci. 10. doi:10.3389/fpls.2019.00675.
- Zhang S, Ma J, Zou H, Zhang L, Li S, Wang Y. 2020. The combination of blue and red LED light improves growth and phenolic acid contents in Salvia miltiorrhiza Bunge. Ind Crops Prod. 158:112959. doi:10.1016/j.indcrop.2020.112959.
- Zhang X, Li Z, Pang S, Jiang B, Yang Y, Duan Q, Zhu G. 2021. The impact of cell structure, metabolism and group behavior for the survival of bacteria under stress conditions. Arch Microbiol. 203:431–441. doi:10.1007/s00203-020-02050-3.
- Zhang X, Schmidt RE. 2000. Hormone-Containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 40:1344–1349. doi:10.2135/cropsci2000.4051344x.
- Znój A, Gawor J, Gromadka R, Chwedorzewska KJ, Grzesiak J. 2022. Root-associated bacteria community characteristics of antarctic plants: deschampsia Antarctica and colobanthus quitensis—a comparison. Microb Ecol. 84:808–820. doi:10.1007/s00248-021-01891-9.
