Abstract
Polyphenol oxidase (PPO) was extracted from whole peaches and its catecholase activity was studied spectrophotometrically against three substrates: catechol (Cat), 4-methyl catechol (4MC), and chlorogenic acid (CA). Peach PPO specificity was: CA > 4MC ≈ Cat. 4-hexylresorcinol (4HR, 0.5 mM) inhibited oxidation of Cat and CA by 50% or more, at all substrate concentrations, but had no effect on the oxidation of 4MC, suggesting that laccase-like activity was present in the extract. β-cyclodextrin (β-CD, 10 mM) inhibited only CA oxidation and this effect was not abolished when free substrate concentrations were calculated suggesting a mechanism, additional to substrate complexation, in β-CD action. Combination of 4HR and β-CD had only a weak inhibitory effect on CA oxidation. In conclusion, β-CD and specially 4HR could be used as browning inhibitors in peach products and also as tools for studying different phenol oxidases. Combination of both compounds is not recommended for peaches.
Se extrajo la enzima polifenoloxidasa (PPO) a partir de duraznos completos y se estudió su actividad catecolasa, espectrofotométricamente, frente a tres diferentes sustratos:catecol (Cat), 4-metilcatecol (4MC) y ácido clorogénico (CA). La especificidad de la PPO fue: CA > 4MC ≈ Cat. El 4-hexilresorcinol (4HR, 0,5 mM) inhibió, en un 50% o más, la oxidación de Cat y CA, pero no tuvo ningún efecto sobre la oxidación del 4MC, lo cual sugiere que el extracto posee actividad lacasa. La β-ciclodextrina (β-CD, 10 mM) inhibió únicamente la oxidación del CA, aún si se consideran las concentraciones libres del sustrato, lo cual sugiere que la acción de la β-CD depende de un mecanismo adicional a la complejación de sustrato. La combinación de ambos inhibidores tuvo un efecto débil sobre la oxidación del CA. Se concluye que la β-CD y especialmente el 4HR pueden ser usados como inhibidores del oscurecimiento enzimático en productos de durazno, así como para estudiar los diferentes tipos de fenoloxidasas. La combinación de ambos agentes no es recomendable en duraznos.
Palabras clave:
Introduction
Prevention of enzymatic browning is a major challenge for vegetable and fruit processing in food industry. Polyphenol oxidase (PPO) is the enzyme responsible for this deteriorative process. Its inhibition by safe and potent products of natural origin, which do not alter food's sensorial properties is highly desirable, specially for the growing industry of fresh-cut and minimally processed fruits and vegetables. PPO, also known as tyrosinase, is a copper-containing enzyme present in plants, animals, fungi, and bacteria (Mayer, Citation2006). It catalyzes the hydroxylation of monophenols in o-position (cresolase activity, E.C. 1.14. 18.1) and further oxidation of o-diphenols to o-quinones (catecholase acticity, E.C. 1.10.3.2). The active site of PPO can exist in three forms (reviewed by Rescigno, Sollai, Pisu, Rinaldi, & Sanjust, Citation2002; Whitaker & Lee, 1995): met form (CuII) is the resting state which can be reduced by oxidation of a diphenol, converting PPO to the deoxy form (CuI); oxygen binding to this form induces the oxy conformation (CuII) which is the only form with cresolase and catecholase activity (Pérez-Gilabert & García-Carmona, Citation2000).
Traditionally, sulfites had been used as antibrowning agents in the food industry; however, their use became restricted because of concerns about negative effects on human health. For this reason, several studies have been recently carried out, using different kinds of natural inhibitors, either alone or in combination, in order to inhibit PPO activity (Casado-Vela, Selles, & Bru, Citation2006) and consequently browning of fruit products (Guerrero-Beltrán, Swanson, & Barbosa-Cánovas, Citation2005; López-Nicolás, Pérez-López, Carbonell-Barrachina, & García-Carmona, 2007). One of the more promising compounds is 4-Hexylresorcinol (4HR), a resorcinol derivative that has been “generally regarded as safe” (GRAS) by FDA and successfully used to prevent browning of animal and vegetal tissues (Luo & Barbosa-Cánovas, 1995; Monsalve-González, Barbosa-Canovas, Cavalieri, McEvily, & Iyengar, Citation1993). Application of 4HR in several food products has been studied (Chen, Ke, Song, Huang, & Liu, Citation2004; Guerrero-Beltrán et al., Citation2005) and its inhibitory mechanism is the subject of recent studies (Arias, González, Oria, & López-Buesa, Citation2007). 4HR is also a useful tool in differentiating between laccase (p-diphenol oxidase) and PPO (Dawley & Flurkey, Citation1993; Mayer, Citation2006). Despite the effectiveness of 4HR to prevent browning of different fruit tissues, to our knowledge, inhibition of peach PPO by 4HR has not been reported in the literature yet.
Cyclodextrins (CD) are natural cyclic oligomers built up from 6 (α-CD), 7 (β-CD), or 8 (γ-CD) glucopyranose units. It has been reported that β-CD and derivatives, in combination with phosphate salts and antioxidants, inhibited browning of apple juices (Billaud, Regaudie, Fayad, Richard Forget, & Nicolas, 1995; Gacche, Zore, & Ghole, Citation2003; Hicks et al., Citation1996) and have been found to inhibit PPO of different sources (Casado-Vela et al., Citation2006), including sodium dodecyl sulphate (SDS)-activated peach PPO (Laveda, Núñez-Delicado, García-Carmona, & Sánchez-Ferrer, Citation2000). Recently, cyclodextrin derivatives have been shown to effectively inhibit browning of apple and peach juices (López-Nicolás, Núñez-Delicado, Pérez-López, Sánchez-Ferrer, & García-Carmona, Citation2007; López-Nicolás et al., Citation2007). Cyclodextrins posses a hydrophobic inner cavity, in which PPO polyphenolic substrates can be trapped, forming inclusion complexes which render the polyphenols unavailable to PPO oxidation. Therefore, cyclodextrins inhibit PPO activity by an indirect mechanism: reduction of initial substrate concentration (Billaud et al., Citation1995). However, additional mechanisms for β-CD inhibition of PPO have been described, including SDS complexation (Laveda et al., Citation2000); non-competitive inhibition (Gacche et al., Citation2003) and a mixed inhibitory pattern, in which β-CD is suggested to interact also with hydrophobic portions of apple PPO (Alvarez-Parrilla et al., Citation2007).
Several isoforms of peach PPO have been characterized from different peach varieties (Flurkey & Jen, Citation1980; Wong, Luh, & Whitaker, Citation1971) with diphenolase activity towards a wide range of diphenols, including flavonoids (Flurkey & Jen, Citation1980; Jiménez-Atiénzar, Cabanes, Gandía-Herrero, & García-Carmona, Citation2004). Peach PPO has been inhibited by non-specific inhibitors (Flurkey & Jen, Citation1980; Wong et al., Citation1971), cyclodextrins (Laveda et al., Citation2000), and tropolone (Jiménez-Atiénzar, et al., Citation2004; Jiménez-Atiénzar, Pedreño, Caballero, Cabanes, & García-Carmona, Citation2007) and early studies found a laccase-like (p-diphenol oxidase) peach PPO (Mayer & Harel, Citation1968). However, there is no information about the type of PPO activity from Mexican peach varieties. For this reason, the aim of this study was to evaluate the PPO activity of “Prisco” Mexican peaches, which are an economically relevant product of the state of Chihuahua. Enzyme activity was measured spectrophotometrically using different diphenolic substrates and inhibition by 4HR and β-CD (Figure 1) was studied at different substrate concentrations for the three diphenols studied.
Materials and methods
Fruits and reagents
Fruits were obtained from “Frutas Paquimé” (Ciudad Juárez, México). Peaches (Prisco variety) were uniformly selected, washed with chlorinated water and cut in pieces, frozen at −80 °C, lyophilized (Labconco Freeze dry/shell freeze system), vacuum sealed, and stored at −80 °C until used. β-cyclodextrin (β-CD, CAVAMAX C-7®) was kindly supplied by Wacker Biochem, México. Chlorogenic acid (CA) (1,3,4,5-tetrahydroxycyclohexane carboxylic acid 3-[3,4- dihydroxycinamate]), catechol (1,2-dihydroxybenzene), 4-methyl catechol (4MC), 4-hexyl resorcinol (4HR), polyvinylpolypirrolidone (PVPP), citric acid, Bradford reagent, and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO). Phosphate salts were A.C.S. grade from Jalmek (San Nicolás de los Garza, México).
Enzyme extraction
One gram of lyophilized peach was blended for 5 min with 10 mL modified McIlvaine buffer (30 mM citric acid, 140 mM K2HPO4) pH 6.5, 4 °C, containing 2.5% (w/v) polyvinyl polypirrolidone (PVPP). The homogenate was centrifuged for 30 min at 1000 g, 4 °C (IEC NH-SII centrifuge). The supernatant was recovered, filtered through a Whatman No. 1 paper, and total protein determined by Bradford assay, using BSA as standard, in a BioRad Benchmark Plus microplate reader. Extracts were frozen at −20 °C until used. PPO activity was always determined within the next 48 h after extraction.
Enzyme activity and substrate specificity
Enzymatic activity was determined spectroscopically (Agilent 8453 spectrophotometer) by measuring the increase in absorbance due to formation of oxidation products. For each substrate, the variation in the absorption spectra, in the presence of PPO extract was measured, in order to find the optimum wavelength to detect the corresponding oxidation product (data not shown). For all the studied o-diphenols: catechol (Cat), 4MC and CA, a wavelength of 400 nm was used. Enzyme activity was measured at different substrate concentrations (0.5–8.5 mM) by mixing different volumes of substrate stock solutions (11 mM in citrate buffer 0.1 M, pH 5), with different volumes of citrate buffer (0.1 M, pH 5) in a 1 mL quartz cuvette. Protein concentration was determined in the extracts and dilutions were made to have a protein concentration of 120 μg/mL in all extracts. One-hundred microliters of enzyme extract were added (12 μg/mL final protein concentration) and the cuvette was rapidly mixed and placed in the spectrophotometer at 25 °C. Absorbance was monitored every 15 s for 5 min and enzyme activity (V o) was determined at each substrate concentration by plotting absorbance vs. time and calculating the slope of the linear region (which lasted less than 2 min). To obtain kinetic constants (K M and V Max) for each substrate, V o was plotted against substrate concentration and data fitted non-linearly to a Michaellis-Menten model (SigmaPlot 9.01).
Inhibition studies
Stock solutions of 10 mM β-CD, 0.5 mM 4HR and a mixture of both were prepared in citrate buffer (0.1 M, pH 5). These inhibitor-containing solutions were used to prepare substrate stocks as described in the previous section, and mixed with inhibitor-containing citrate buffer to measure V o at different substrate concentrations and a constant protein and inhibitor concentration. Data were taken and analyzed as described in the previous section.
Free substrate concentration
The free substrate concentrations (FSC) of Cat and CA in the presence of 10 mM β-CD were calculated as previously described (Alvarez-Parrilla et al., Citation2007), using a stability constant of 465 M−1 for CA (Alvarez-Parrilla, de la Rosa, Torres-Rivas, Rodrigo-García, & González-Aguilar, Citation2005) and 20.4 M−1 for Cat (Meijide et al., Citation2001).
Statistical analysis
Values represent the mean ± SEM of at least four replicates. T-test for independent samples, ANOVA and LSD analyses were performed to determine differences in V o among treatments, using the commercial software SPSS 13.0 (SPSS, Chicago, Illinois).
Results and discussion
Cat, 4MC, and CA were used as o-diphenolic substrates for a peach PPO extract, and their apparent kinetic constants (K M and V Max) were calculated by linear and non-linear fitting. Kinetic data are summarized in . PPO activity expressed in terms of ΔAbs of product formation may be misleading because different quinones may have different extinction coefficients (Casado-Vela et al., Citation2006; Muñoz et al., Citation2006); therefore we used ε values calculated by the former authors to calculate V Max values as mmol/L min. The following ε values were used: 1300 M−1 cm−1 for 4MC, 600 M−1 cm−1 for CA (Casado-Vela et al., Citation2006), and 1600 M−1 cm−1 for 4MC. Next, to compare substrate specificity, the V Max/K M ratio was calculated for all substrates, showing that Prisco peach PPO substrate specificity is about 10 times higher for CA (≈ 0.3) than for 4MC and Cat (4MC ≈ Cat ≈ 0.03). CA is one of the main phenols, and the main hydroxycinnamate in peaches (Chang, Tan, Frankel, & Barrett, Citation2000; Tomás-Barberán et al., Citation2001), it could also be one of the main PPO substrates in vivo, together with the flavonoid catechin (Jiménez-Atiénzar et al., Citation2004).
Table 1. Substrate specificity of peach PPO.
Tabla 1. Especificidad de la PPO de durazno.
Substrate specificity of PPO isozymes from Redskin peaches has been studied, showing PPO dependent oxidation of Cat and 4MC was similar and slower than catechin oxidation (Flurkey & Jen, Citation1980). In the same study, K M for Cat was found to be 2 mM, comparable to results found in the present study (4 mM). K M of peach PPO for CA was 0.61 mM (), this value is comparable with K M reported for catechin, 0.2 mM (Jiménez-Atiénzar et al., Citation2004) and lower than apple PPO K M for CA, 3 mM (Alvarez-Parrilla et al., Citation2007). Specific activity of peach PPO was also lower than specific activity of apple PPO (0.01 vs. 0.03 ΔAbs/min μg protein), using CA as substrate (Alvarez-Parrilla et al., Citation2007). It can be concluded that Prisco peach PPO has kinetic properties similar to other peach PPOs and natural-occurring polyphenols are better substrates for these enzymes.
Inhibition studies were carried out using 4HR, as a potent and specific inhibitor of animal, vegetal and mushroom PPOs (Dawley & Flurkey, Citation1993; Jiménez & García-Carmona, Citation1997). A concentration of 0.5 mM was used which is just higher than the K I calculated previously by our group for the inhibition of apple PPO (0.26 mM) and to the concentration used by Dawley and Flurkey (Citation1993) to differentiate laccase and PPO activity in mushrooms (0.1 mM). As expected, Cat and CA oxidation were highly inhibited by 4HR (B and 2C) surprisingly the inhibitor had no effect on 4MC oxidation (A).
Figure 1. Chemical structures of substrates and inhibitors used in this study. (a) catechol; (b) 4-methyl catechol; (c) chlorogenic acid; (d) 4-hexylresorcinol; (e) β-cyclodextrin.
Figura 1. Estructuras químicas de sustratos e inhibidores utilizados en este trabajo. (a) catecol; (b) 4-metil catecol; (c) ácido clorogénico; (d) 4-hexilresorcinol; (e) β-ciclodextrina.
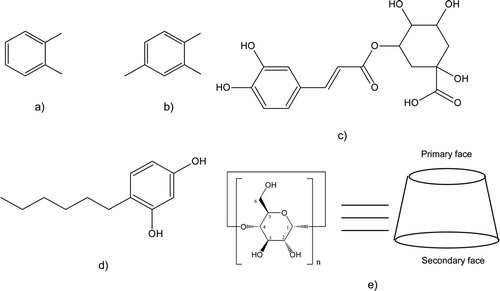
Figure 2. Inhibition of peach PPO by 4-hexylresorcinol (0.5 mM). Enzyme activity was measured spectroscopically (λ = 400 nm, pH 5, 25 °C, 12 μg/mL protein) in the presence of different substrate concentrations and a fixed 4HR concentration. (A) Inhibition of catechol oxidation. (B) Inhibition of 4-methyl catechol oxidation. (C) Inhibition of chlorogenic acid oxidation. Symbols are mean ± SEM of four determinations, lines are non-linear fits to experimental data. *indicates statistical differences from control (T-test, p < 0.05).
Figura 2. Inhibición de la PPO de durazno por 4-hexilresorcinol (0,5 mM). La actividad enzimática se determinó espectrofotométricamente (λ = 400 nm, pH 5, 25 °C, 12 μg/mL de proteína) en presencia de diferentes concentraciones de sustrato y una concentración constante de 4HR. (A) Inhibición de la oxidación del catecol. (B) Inhibición de la oxidación del 4-metil catecol. (C) Inhibición de la oxidación del ácido clorogénico. Los símbolos representan la media ± ESM de 4 experimentos, las líneas son ajustes no lineales a los datos experimentales. *indica diferencias significativas con respecto al control (prueba T, p < 0,05).
These results suggest oxidation of 4-MC is not due to a traditional PPO. A peach laccase, which oxidizes 4MC, was described by early studies of Mayer's group (Lehman, Harel, & Mayer, Citation1974; Mayer & Harel, Citation1968) in peaches of different varieties, although more recent studies have found no such type of activity in Babygold peaches (Jiménez-Atiénzar, Escribano, Cabanes, Gandía-Herrero, & García-Carmona, Citation2005). However, since 4HR does not inhibit laccase-dependent phenolic oxidation and has been used as a tool for discriminating tyrosinase and laccase in mushroom extracts and partially purified mushroom enzymes (Dawley & Flurkey, Citation1993), the present results support the presence of a laccase in peach extracts. Laccases are p-diphenol oxidases that are widely distributed in fungi, while they have been characterized less frequently in higher plants (Mayer & Staples, Citation2002), nevertheless, several laccase-like enzymes have been described in higher plants, involved in biosynthesis of hydrolysable tannins (Niemetz & Gross, Citation2005). In contrast to 4-MC, oxidation of Cat and CA was strongly inhibited by 4HR suggesting these diphenols are not good substrates to peach laccase, and their oxidation is PPO-dependent.
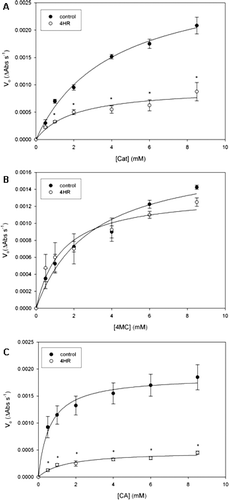
Therefore, we continued to study inhibition of Cat and CA oxidation by β-CD (10 mM). This concentration is near to the highest possible β-CD concentration in water (12–14 mM), and almost three times K I for apple PPO (3.4 mM, Alvarez-Parrilla et al., Citation2007). However, even at this high concentration, this compound had no effect on peach PPO activity towards Cat (A). In fact a slight enhancement of Cat oxidation is observed when its concentration is 2 mM. The effects of β-CD and a mixture of both inhibitors (0.5 mM 4HR + 10 mM β-CD), were tested in the enzymatic oxidation of CA (B). At the studied concentrations, β-CD inhibited enzyme activity in approximately the same degree as 4HR. These results are in agreement with previous studies done in our laboratory, in which inhibition of apple PPO by 4HR and β-CD was described (Alvarez-Parrilla et al., Citation2007), however, contrary to apple PPO, in which a synergic effect of the mixture of HR and β-CD was observed, in the case of peach PPO, combination of both inhibitors decreased their inhibitory effect, showing important differences between apple and peach PPO enzymatic activity.
Figure 3. Inhibition of peach PPO by β-cyclodextrin (10 mM). Enzyme activity was measured spectroscopically (λ = 400 nm, pH 5, 25 °C, 12 μg/mL protein) in the presence of different substrate concentrations and fixed inhibitor concentrations. (A) Inhibition of catechol oxidation. (B) Inhibition of chlorogenic acid oxidation by β-CD and a mixture of β-CD and 4HR. Symbols are mean ± SEM of four determinations, lines are non-linear fits to experimental data. *indicates statistical differences from control; $indicates statistical differences from control and from β-CD + 4HR (LSD, p < 0.05). (C) Inhibition of free-CA oxidation by β-CD; free-CA concentrations were calculated from the stability constant of the β-CD/CA complex and the initial CA concentrations used in the experiment of figure 3B. Initial velocities in the presence of β-CD are the same as in figure 3B; initial control velocities were calculated from the non-linear fit to control data in figure 3B.
Figura 3. Inhibición de la PPO de durazno por β-ciclodextrina (10 mM). La actividad enzimática se determinó espectrofotométricamente (λ = 400 nm, pH 5, 25 °C, 12 μg/mL de proteína) en presencia de diferentes concentraciones de sustrato y una concentración constante de 4HR. (A) Inhibición de la oxidación del catecol. (B) Inhibición de la oxidación del ácido clorogénico oxidation por β-CD y mezcla de β-CD y 4HR. Los símbolos representan la media ± ESM de 4 experimentos, las líneas son ajustes no lineales a los datos experimentales. *indica diferencias significativas con respecto al control; $indica diferencia significativa con respecto al tratamiento β-CD + 4HR (DMS, p < 0,05). (C) Inhibición de la oxidación del CA libre por β-CD; las concentraciones de CA libre se calcularon mediante la constante de estabilidad del complejo β-CD/CA y las concentraciones iniciales de CA utilizadas en el experimento de la figura 3B. Los datos de velocidades iniciales en presencia de β-CD son los mismos que en la figura 3B; los datos de velocidades iniciales control fueron calculados a partir del ajuste no lineal a los datos control de la figura 3B.
It has been demonstrated that β-CD inhibition of PPO depends on the formation of inclusion complexes with polyphenolic substrates. Complexation makes substrates unavailable for enzymatic oxidation, and therefore it actually reduces FSC (Billaud et al., Citation1995). This is in agreement with the analysis of the inhibition of peach PPO by β-CD in the presence of different substrates, in the present study. It is possible to observe that β-CD only inhibits the oxidation of CA but not that of Cat (A and 3B). This could be explained by considering that CA is complexed in a higher degree than Cat. In fact, stability constants for the complexes are 465 M−1 for β-CD/CA (Alvarez-Parrilla et al., Citation2005) and 20.4 M−1 for β-CD/Cat (Meijide et al., Citation2001). Nevertheless, the inhibitory effect of β-CD on CA oxidation by apple PPO could not be fully explained by this mechanism (Alvarez-Parrilla et al., Citation2007). Using the mentioned stability constant for the β-CD/CA complex, we calculated FSC for every initial substrate concentration used experimentally and data of initial velocities were re-plotted against the calculated FSC (C). This figure shows oxidation of CA was still strongly inhibited by β-CD when the FSC were considered, suggesting an additional inhibitory mechanism, apart from complex formation. Inhibition of partially purified peach PPO by a CD derivative was shown to be due to two factors: substrate (4-tert-buthylcatechol) complexation and SDS removal (Laveda et al., Citation2000). Purified PPO of many sources becomes inactive and needs to be activated by SDS or other treatments (Jiménez & García-Carmona, Citation1999; Laveda, Núñez-Delicado, García-Carmona, & Sánchez-Ferrer, Citation2001), in contrast, in our peach extract, PPO was sufficiently active, at our assay conditions (pH 5.0, 25 °C, 12 μg of protein). It may be possible that a SDS-like activator (hydrophobic) exists in this crude extract, therefore in the present study, an additional mechanism of inhibition of peach PPO by β-CD might be related to interaction with hydrophobic regions of the enzyme or with some endogenous enzyme activator present in the crude extract. In vivo, latency of PPO has been observed and the mechanism for its conversion to active forms is still not fully understood (Mayer, Citation2006). Finally, quinones evolve differently depending on the parent compounds (Muñoz et al., Citation2006) changing color and chemical properties after initial enzymatic oxidation, therefore the evolution of CA quinones could also be modified by β-CD. The stability and evolution of β-CD/phenol and β-CD/quinone complexes are currently being studied in our group.
Conclusion
A crude PPO extract from “Prisco” peach showed 4HR-sensitive (CA and Cat) and insensitive (4-MC) diphenolic oxidation suggesting the presence of a laccase-like enzyme in these fruits. In the same extract, β-CD acted as a potent inhibitor of CA oxidation but had no effect on Cat oxidation, possibly due to a weak complexation of the latter. Inhibition of CA oxidation by β-CD cannot be explained only in terms of substrate complexation, therefore an additional mechanism of β-CD action is suggested. Combination of 4HR and β-CD had only a low inhibitory effect on CA oxidation indicating this mixture is not desirable for practical applications. Further studies using 4HR as a browning inhibitor in fresh peach products are desired, since it rapidly inhibited, by 50% or more, PPO catalyzed oxidation of all concentrations of Cat and CA.
Acknowledgements
This study was supported financially from UACJ (Internal projects) and CONACyT-Gobierno del Estado de Chihuahua, México (CHIH-2005-C01-22028) is gratefully acknowledged. G. Mercado-Mercado is a recipient of an undergraduate scholarship from SAGARPA-CONACyT (SAGARPA 2002-C01-0787).
References
- Alvarez-Parrilla , E. , de la Rosa , L. A. , Rodrigo-García , J. , Escobedo-González , G. , Mercado-Mercado , E. Moyers-Montoya , A. 2007 . Dual effect of β-cyclodextrin (β-CD) on the inhibition of apple polyphenol oxidase by 4-hexylresorcinol (HR) and methyl jasmonate (MJ) . Food Chemistry , 101 : 1346 – 1356 .
- Alvarez-Parrilla , E. , de la Rosa , L. A. , Torres-Rivas , F. , Rodrigo-García , J. and González-Aguilar , G. A. 2005 . Complexation of apple antioxidants: chlorogenic acid, quercetin and rutin by β-cyclodextrin (β-CD) . Journal of Inclussion Phenomena and Macrocyclic Chemistry , 53 ( 1 ) : 121 – 129 .
- Arias , E. , González , J. , Oria , R. and López-Buesa , P. 2007 . Ascorbic acid and 4-hexylresorcinol effects on pear PPO and PPO catalyzed browning reaction . Journal of Food Science , 72 ( 8 ) : C422 – C429 .
- Billaud , C. , Regaudie , E. , Fayad , N. , Richard Forget , C. and Nicolas , J. 1995 . “ Effect of cyclodextrin on polyphenol oxidation catalyzed by apple polyphenol oxidase ” . In Enzymatic browning and its prevention , Edited by: Lee , C. Y. and Whitaker , J. R. 295 – 312 . Washington : ACS .
- Casado-Vela , J. , Selles , S. and Bru , R. 2006 . Influence of developmental stage, cultivar, and hexapeptide and cyclodextrin inhibitors on polyphenol oxidase activity from tomato fruits . Journal of Food Biochemistry , 30 : 623 – 640 .
- Chang , S. , Tan , C. , Frankel , E. N. and Barrett , D. M. 2000 . Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol activity in selected clingstone peach cultivars . Journal of Agricultural and Food Chemistry , 48 ( 2 ) : 147 – 151 .
- Chen , Q. X. , Ke , L. N. , Song , K. K. , Huang , H. and Liu , X. D. 2004 . Inhibitory effects of hexylresorcinol and dodecylrersorcinol on mushroom (Agaricus bisporus) tyrosinase . Protein Journal , 23 ( 2 ) : 135 – 141 .
- Dawley , R. M. and Flurkey , W. H. 1993 . Differentiation of tyrosinase and laccase using 4-hexylresorcinol, a tyrosinase inhibitor . Phytochemistry , 33 ( 2 ) : 281 – 284 .
- Flurkey , W. H. and Jen , J. J. 1980 . Purification of peach polyphenol oxidase in the presence of added protease inhibitors . Journal of Food Biochemistry , 4 : 29 – 41 .
- Gacche , R. N. , Zore , G. B. and Ghole , V. S. 2003 . Kinetics of inhibition of polyphenol oxidase mediated browning in apple juice by β-cyclodextrin and l-ascorbate-2-triphosphate . Journal of Enzyme Inhibition and Medicinal Chemistry , 18 ( 1 ) : 1 – 5 .
- Guerrero-Beltrán , J. A. , Swanson , B. G. and Barbosa-Cánovas , G. V. 2005 . Inhibition of polyphenoloxidase in mango puree with 4-hexylresorcinol, cysteine and ascrobic acid . LWT Food Science and Technology , 38 : 625 – 630 .
- Hicks , K. , Haines , R. M. , Tong , C. B.S. , Sapers , G. M. , El-Atawy , Y. Irwin , P. 1996 . Inhibition of enzymatic browning in fresh fruit and vegetable juices by soluble and insoluble forms of β-ciclodextrin alone or in combination with phosphates . Journal of Agricultural and Food Chemistry , 44 ( 9 ) : 2591 – 2594 .
- Jiménez , M. and García-Carmona , F. 1997 . 4-substituted resorcinols (sulfite alternatives) as slow-binding inhibitors of tyrosinase chatecholase activity . Journal of Agricultural and Food Chemistry , 45 ( 6 ) : 2061 – 2065 .
- Jiménez , M. and García-Carmona , F. 1999 . Oxidation of the flavonol quercetin by polyphenol oxidase . Journal of Agricultural and Food Chemistry , 47 ( 1 ) : 56 – 60 .
- Jiménez-Atiénzar , M. , Cabanes , J. , Gandía-Herrero , F. and García-Carmona , F. 2004 . Kinetic analysis of catechin oxidation by polyphenol oxidase at neutral pH . Biochemical and Biophysical Research Communications , 319 : 902 – 910 .
- Jiménez-Atiénzar , M. , Escribano , J. , Cabanes , J. , Gandía-Herrero , F. and García-Carmona , F. 2005 . The flavonoid eriodictyol as substrate of peach polyphenol oxidase . Journal of Food Science , 70 ( 9 ) : C540 – C544 .
- Jiménez-Atiénzar , M. , Pedreño , M. A. , Caballero , N. , Cabanes , J. and García-Carmona , F. 2007 . Characterization of polyphenol oxidase and peroxidase from peach mesocarp (Prunus persica L. cv. Babygold) . Journal of the Science of Food and Agriculture , 87 : 1682 – 1690 .
- Laveda , F. , Núñez-Delicado , E. , García-Carmona , F. and Sánchez-Ferrer , A. 2000 . Reversible sodium dodecyl sulfate activation of latent peach polyphenol oxidase by cyclodextrins . Archives of Biochemistry and Biophysics , 379 ( 1 ) : 1 – 6 .
- Laveda , F. , Núñez-Delicado , E. , García-Carmona , F. and Sánchez-Ferrer , A. 2001 . Proteolytic activation of latent paraguaya peach PPO. Characterization of monophenolase activity . Journal of Agricultural and Food Chemistry , 49 : 1003 – 1008 .
- Lehman , E. , Harel , E. and Mayer , A. M. 1974 . Copper content and other characteristics of purified peach laccase . Phytochemistry , 13 : 1713 – 1717 .
- López-Nicolás , J. M. , Núñez-Delicado , E. , Pérez-López , A. J. , Sánchez-Ferrer , A. and García-Carmona , F. 2007 . Reaction's mechanism of fresh apple juice enzymatic browning in the presence of maltosyl-β-cyclodextrin . Journal of Inclussion Phenomena and Macrocyclic Chemistry , 57 : 219 – 222 .
- López-Nicolás , J. M. , Pérez-López , A. J. , Carbonell-Barrachina , A. and García-Coarmona , F. 2007 . Use of natural and modified cyclodextrins as inhibiting agents of peach juice enzymatic browning . Journal of Agricultural and Food Chemistry , 55 ( 13 ) : 5312 – 5319 .
- Luo , Y. and Barbosa-Cánovas , G. V. 1995 . “ Inhibition of apple-slice browning by 4-hexylresorcinol ” . In Enzymatic browning and its prevention , Edited by: Lee , C. Y. and Whitaker , J. R. 240 – 250 . Washington : American Chemical Society .
- Mayer , A. M. 2006 . Polyphenol oxidases in plants and fungi: Going places? A review . Phytochemistry , 67 : 2318 – 2331 .
- Mayer , A. M. and Harel , E. 1968 . A laccase-like enzyme in peaches . Phytochemistry , 7 : 1253 – 1256 .
- Mayer , A. M. and Staples , R. C. 2002 . Laccase: New functions for an old enzyme . Phytochemistry , 60 : 551 – 565 .
- Meijide , F. , Pérez , J. , Ramos Cabrer , P. , Seijas , J. A. , Ulloa , H. Fraga , F. 2001 . Thermodynamic interactions of model allelopathic compounds (polyphenols) with α- and β-cyclodextrin . Biological Journal of Armenia , 53 ( special issue ) : 207 – 217 .
- Monsalve-González , A. , Barbosa-Canovas , G. V. , Cavalieri , R. P. , McEvily , A. J. and Iyengar , R. 1993 . Control of browning during storage of apple slices preserved by combined methods. 4-hexylresorcinol as anti-browning agent . Journal of Food Science , 58 ( 4 ) : 797 – 800 .
- Muñoz , J. L. , García.Molina , F. , Varón , R. , Rodriguez-Lopez , J. N. , García-Cánovas , F. and Tudela , J. 2006 . Calculating molar absorptivities for quinones: Application to the measurement of tyrosinase activity . Analytical Biochemistry , 351 : 128 – 138 .
- Niemetz , R. and Gross , G. G. 2005 . Enzymology of gallotannin and allegitannin biosynthesis . Phytochemistry , 66 : 2001 – 2011 .
- Pérez-Gilabert , M. and García-Carmona , F. 2000 . Characterization of catecholase and cresolase activities of eggplant polyphenol oxidase . Journal of Agricultural and Food Chemistry , 48 ( 3 ) : 695 – 700 .
- Rescigno , A. , Sollai , F. , Pisu , B. , Rinaldi , A. and Sanjust , E. 2002 . Tyrosinase inhibition: General and applied aspects . Journal of Enzyme Inhibition and Medicinal Chemistry , 17 ( 4 ) : 207 – 218 .
- Tomás-Barberán , F. A. , Gil , M. I. , Cremin , P. , Waterhouse , A. L. , Hess-Pierce , B. and Kader , A. A. 2001 . HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches and plums . Journal of Agricultural and Food Chemistry , 49 : 4748 – 4760 .
- Whitaker , J. R. and Lee , C. Y. 1995 . “ Recent advances in chemistry of enzymatic browning ” . In Enzymatic browning and its prevention , Edited by: Lee , C. Y. and Whitaker , J. R. 2 – 7 . Washington : American Chemical Society .
- Wong , T. C. , Luh , B. S. and Whitaker , J. R. 1971 . Isolation and characterization of polyphenol oxidase isozymes of clingstone peach . Plant Physiology , 48 : 19 – 23 .