Abstract
Microwave phosphorylation was applied to modify soy protein isolates (SPI) to improve their emulsifying properties. A Box–Behnken model was obtained to optimize preparation conditions by response surface methodology. The four factors investigated were SPI content, sodium tripolyphosphate (STP) addition, microwave power, and reaction time with the emulsifying activity index (EAI) of the modified product as evaluation index. The optimized conditions were found to be SPI content of 71 g/L, STP addition of 115 g/kg, microwave power of 480 W, and reaction time of 3 min. Under these conditions, the EAI and emulsifying stability index (ESI) of the modified SPI were 0.878 and 33.9 min, respectively. Compared to the original SPI, the EAI and ESI of the modified SPI increased about 113.1% and 26.6%, respectively. Modification of SPI with STP under microwave irradiation might be a potential way to increase the emulsifying properties of SPI.
Se aplicó fosforilación por microondas para modificar aislados de proteína de soja (SPI) con el fin de mejorar sus propiedades emulsionantes. Se obtuvo un diseño Box-Behnken para optimizar las condiciones de preparación por el método de Superficie de respuesta. Los cuatro factores investigados fueron contenido de SPI, adición de tripolifosfato de sodio (STP), potencia de microondas y tiempo de reacción con el índice de actividad emulsionante (EAI) del producto modificado como índice de evaluación. Las condiciones optimizadas resultaron ser un contenido de SPI de 71 g/L, una adición de STP de 115 g/Kg, una potencia de microondas de 480 W y un tiempo de reacción de tres minutos. Bajo esas condiciones, el índice de actividad emulsionante y el índice de estabilidad emulsionante (ESI) de los aislados de proteína de soja modificados fueron 0,878 y 33,9 min, respectivamente. Comparado con los aislados de proteína de soja originales, el índice de actividad emulsionante e índice de estabilidad emulsionante de los modificados aumentó un 113,1% y 26,6%, respectivamente. La modificación con tripolifosfato de sodio bajo irradiación por microondas puede ser una manera potencial de incrementar las propiedades emulsionantes de aislados de proteína de soja.
Nomenclature
| SPI | = |
soy protein isolates |
| STP | = |
sodium tripolyphosphate |
| EAI | = |
emulsifying activity index |
| ESI | = |
emulsifying stability index |
| RSM | = |
response surface methodology |
| SDS | = |
sodium dodecyl sulfate |
| BBD | = |
Box–Behnken design |
Introduction
Soy protein isolates (SPI) as valuable source of food functional ingredient is widely used in food industry for multiple functional properties and high nutritional value (Alibhai, Mondor, Moresoli, Ippersiel, & Lamarche, Citation2006). The most important properties of SPI are emulsifying and gelling properties (Damodaran, Citation2005; Molina, Papadopoulou, & Ledward, Citation2001). However, native soy globulins have poor functionality because of the compact globular structure, which makes it difficult to be directly utilized in food processing (Lee, Ryu, & Rhee, Citation2003; Roesch & Corredig, Citation2003). Adequate modification on SPI might improve its functional properties and applications in the food industry. Physical (Puppo, Chapleau, Speroni, de Lamballerie, Añón, & Anton, Citation2004), chemical (Mirmoghtadaie, Kadivar, & Shahedi, Citation2009), and enzymatic modification (Surówka & Żmudziński, Citation2004) had been used to improve emulsifying properties of SPI. Chemical modification including acylation, phosphorylation, glycosylation, deaminization, and covalent cross-linking were used mostly (Moure, Sineiro, & Domínguez, Citation2006). Among these chemical modifications, phosphorylation had been proven as an important and efficient method with some advantages including low cost, high efficiency, safe, and practicable. Moreover, phosphorylation had non-significant influence on protein digestibility. Additionally, phosphorylation is an effective method to improve emulsifying properties of SPI (Hirotsuka, Taniguchi, Narita, & Kito, Citation1984; Matheis & Whitaker, Citation1984).
Based on its higher efficiency and energy conservation, application of microwave technique has been increased significantly in the field of food science and food industry. For example, Wang, Zhang, and Mujumdar (Citation2010) studied the effects of three different food ingredients on microwave freeze drying of instant vegetable soup, and the results showed that condiments addition could increase drying rate and shorten drying time. Arimi, Duggan, O'Sullivan, Lyng, and O'Riordan (2011) had used microwave expansion to produce imitation cheese. Izquierdo, Peñasa, Baeza, and Gomez (Citation2008) applied combined microwave and enzymatic treatments on the proteolysis of dairy whey proteins to produce hypoallergenic dairy hydrolysates more efficiently. The effects of microwave treatment on gelling behaviors of defatted soy flour dispersions was also analyzed by Bhattacharya and Jena (Citation2007), who proposed a mechanism of gel formation at macromolecular level. Compared to the traditional water bath heating, the microwave heating had speeded up the graft reactions of SPI with carbohydrate (Guan, Qiu, Liu, Hua, & Ma, Citation2006).
The usual phosphorylation treatment to modify soy proteins had got good results (Zhang, Li, & Ren, Citation2007). However, the reaction usually took a long time (about 3 h). The phosphorylation of SPI with sodium tripolyphosphate (STP) under the microwave radiation is an interesting issue, as microwave technique might shorten the reaction time or enhance the reaction efficiency. In the present work, the phosphorylation of SPI with STP was carried out in a new approach by using microwave technique. In order to improve the functional properties of SPI, Box–Behnken model by response surface methodology (RSM) was used to optimize phosphorylation conditions. The aim of the work was to find a suitable method to produce functional SPI with better emulsifying properties.
Materials and methods
Materials
SPI and soy oil were purchased from Harbin High-Tech Soy Food Co., Ltd. (Heilongjiang, China). Crude protein contents of SPI were determined by the Kjeldahl method (AACC No. 46-12) with a converting factor of 6.25. STP, sodium dodecyl sulfate (SDS), and other chemicals were of analytical grade.
Preparation of phosphorylated SPI
According to a bibliographic method (Zhang et al., Citation2007), SPI was dissolved in phosphate buffer (pH 6.8) to obtain a dispersion of 40 g/L, and STP was added to the dispersion at a level of 90 g/kg proteins. The mixture was continuously stirred for 3 h, and the pH was kept at 9.0 constant during phosphorylation by 1.0 mol/L NaOH addition. After reaction, the pH of the solution was adjusted to 7.0 adding a few drops of 1 mol/L HCl. The product was dialyzed for 12 h. Phosphorylated SPI was obtained by freeze drying of the product, and served as a control.
In the microwave phosphorylation treatments, SPI was dissolved as above, and STP was added according to the experimental design (). The reaction mixture was placed in a microwave reactor, and the reaction was carried out at the selected microwave power and reaction time. The later treatments were the same as above. The product was microwave-phosphorylated SPI. A lot of microwave-phosphorylated SPI was prepared in the optimized experimental conditions for further determinations.
Table 1. Factors and their levels used in Box–Behnken design.
Tabla 1. Factores y sus niveles usados en el diseño Box-Behnken.
The original and phosphorylated SPI, used as two controls, and microwave-phosphorylated SPI obtained under the optimized experimental conditions were subjected to further evaluation as described below.
Measurement of emulsifying properties
The emulsifying property of these samples was evaluated by a classic turbidimetric method of Pearce and Kinsella (Citation1978) with some modifications to obtain emulsifying activity index (EAI) and emulsion stability index (ESI). To prepare the emulsion, 10 mL of refined soy oil and 30 mL of the sample solution (1 g/L) in 0.1 mol/L sodium phosphate buffer (pH 7.0) were shaken together in a plastic beaker and homogenized with a high speed homogenizer (FA25, Fluko Equipment Shanghai Co., Ltd., China) at 10,000 rpm for 1 min. Then, 100 μL of freshly prepared emulsion was taken from the bottom of the beaker and dispersed into 10 mL of 1 g/kg SDS solution. The absorbance of the solution was measured at 500 nm against 1 g/kg SDS blank in the spectrophotometer (Shimadzu Corporation, Japan), being the EAI value. The prepared emulsion was kept undisturbed for 10 min. Then, 100 μL was taken from the bottom of the beaker and dispersed into 10 mL of 1 g/kg SDS. The absorbance of the sample was measured at 500 nm against 1 g/kg SDS as described above. ESI of the protein samples were calculated by using Equation (1).
Where, A 0 and A 10 represent absorbance of the sample at 500 nm after emulsion formation at 0 and 10 min, respectively, t represents time (10 min).
Evaluation of degree of phosphorylation
The evaluation was carried out with a reference method of Sung, Chen, Liu, and Su (Citation1983) with some modifications. Aliquots (5 mL) of the reaction mixture were taken from reaction system just after the end of phosphorylation. After removal of the protein fractions by precipitating with 100 g/L trichloroacetic acid, the pyrophosphate in the supernatant was precipitated as zinc pyrophosphate by adding 2 mL of 1 mol/L zinc acetate at a pH of about 3.8. Zinc pyrophosphate was then dissolved in an ammonium buffer prior to the titration of zinc ions with EDTA, with Solochrome Black T as indicator.
Box–Behnken experimental design
According to the principle of Box–Behnken design (BBD) and the results from single factor experiments, four factors were selected as variables as shown in .
A second-order polynomial model corresponding to the BBD was fitted to correlate the relationship between the independent variables and the response (Y, EAI) to predict the optimized conditions. The computer-generated quadratic model is given in the format as Equation (2).
Where, Y is the predicted response, X i and X j are the coded independent variables, β 0 is the intercept coefficient, β i is the linear coefficient, β ii is the squared coefficient, and β ij is the interaction coefficient.
The quality of the fit of the polynomial model equation was expressed by the coefficient of determination R 2, and the values of adjusted-R 2 of models were evaluated to examine the model adequacies. The analysis of variance tables were generated, and the effect and regression coefficients of individual linear, quadratic and interaction terms were determined. The p-values less than 0.05 were considered to be statistically significant. The regression coefficients were then used to make statistical calculation to generate contour and dimensional maps from the regression models.
Statistical analysis
All experiments were performed in triplicate independently. The results from Box–Behnken experimental design were analyzed by using Design-Expert software, Version 7.1.3 (Stat-Ease, Inc., MN, US). The differences between the means of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan's multiple range tests.
Results and discussion
Optimal conditions of microwave-phosphorylated SPI
In order to optimize the preparation conditions of microwave-phosphorylated SPI, a 29-run BBD experiment was carried out as shown in . ANOVA for the response surface quadratic model adequacy was presented in , which showed that the regression model was highly significant (p < 0.01) with F-value of 42.37.
Table 2. BBD and the experimental values of EAI.
Tabla 2. Diseño Box-Behnken y los valores experimentales del índice de actividad emulsionante.
Table 3. Analysis of variance for the response surface quadratic model.
Tabla 3. Análisis de varianza de la superficie de respuesta de modelo cuadrático.
Based on these results, an optimized second-order polynomial model was obtained as below (EquationEquation (3)), which could be applied to predict the EAI of the microwave-phosphorylated SPI.
The regression of all the linear terms and quadratic coefficients of X1 2, X2 2, X3 2, and X4 2 were significant and two cross-products (X2X3 and X2X4) were also significant. So the polynomial model for EAI was regressed by considering only the significant terms and a new ANOVA was recalculated to obtain the coefficients of the final equation, which was shown as below:
The interaction between the variables is graphically presented in , and the optimal levels of variables to obtain the maximum response value could be selected thereafter. The detailed discussion about interaction between these variables was not treated here. With the model (EquationEquation (4)) and calculation by the Design-Expert software, the optimal preparation conditions of microwave-phosphorylated SPI were SPI of 71 g/L, STP of 115 g/kg, microwave power of 480 W, and reaction time of 3 min. To confirm the applicability of the model, confirmation runs were carried out using the calculated levels of the variables. The predicted value for the response was 0.880 while the practical experimental value was 0.878 (mean of three independent trails), which shows high consistency between predicted and practical results.
Figure 1. Effects of the studied variables on EAI of microwave-phosphorylated soy protein isolates. In every representation, the levels for other variables were fixed in their zero levels..
Figura 1. Efectos de las variables estudiadas en el índice de actividad emulsionante de aislados de proteína de soja modificados con fosforilación por microondas. En cada representación los niveles para las otras variables se fijaron en sus niveles cero.
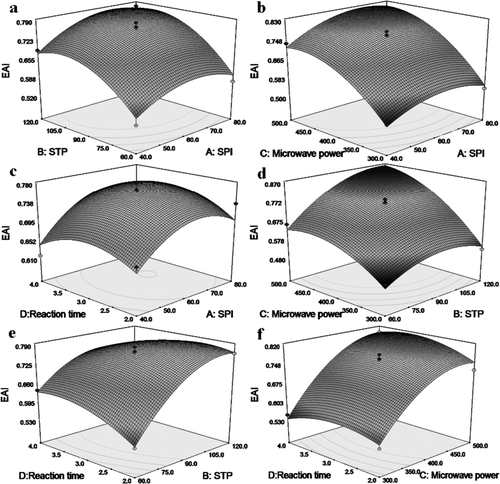
The optimal preparation conditions of phosphorylated SPI revealed by Zhang et al. (2007) were as follow: SPI of 40 g/L, STP of 90 g/kg, pH 9.0, and reaction time of 3 h. In another study reported by Lu, Chen, and Chen (1993), the suitable conditions were SPI of 30 g/L, STP of 30 g/kg, reaction time of 3.5 h, and pH 8.0. In a study of Yao, Yang, and Zhang (2001), the SPI was modified with the selected reaction conditions of SPI 90 g/L, pH 7.0–8.5, STP addition 1–30 g/kg and reaction time of 2.5–3.5 h. Compared to these reported results, the preparation of microwave-phosphorylated SPI in the present work was different in heating method (microwave irradiation vs. heating in water bath) and a much shorter reaction time (3 min vs. 3–3.5 h).
Emulsifying properties of microwave-phosphorylated SPI
Based on the optimal conditions obtained, a microwave-phosphorylated SPI product was prepared with a determined degree of phosphorylation of 82.1 g/kg. Its EAI and ESI were evaluated and given in . The original SPI and phosphorylated SPI were used here as two controls to show the impact of microwave-phosphorylation treatment on the EAI or ESI of SPI. The evaluation results in indicate that both EAI and ESI of microwave-phosphorylated SPI were much superior to the original SPI, but not different from that of the phosphorylated SPI. As the microwave-phosphorylated SPI was prepared at a short reaction time (3 min) while the control phosphorylated SPI was prepared with much longer time (3 h), the beneficial application of microwave phosphorylation to modify SPI exhibits clearly here.
Table 4. Emulsifying properties of original soy protein isolates (SPI) and two phosphorylated SPI products.
Tabla 4. Propiedades emulsionantes de los aislados de proteína de soja originales y dos modificados por fosforilación.
The prepared microwave-phosphorylated SPI had an increase in EAI or ESI about 113.1% or 26.6%. It was suggested that the stronger repulsion between phosphorylated SPI molecules and the exposure of hydrophobic groups in the phosphorylated SPI molecules advanced the diffusion of SPI in the oil–water interface (Waniska, Shetty, & Kinsella, Citation1981), which lead to an improved EAI or ESI. The emulsifying properties (EAI and ESI) of phosphorylated SPI product prepared by Zhang et al. (Citation2007) were increased significantly, sharing similarity to the results in the present work. In a study of Yao et al. (2001), the SPI was modified to a degree of phosphorylation of 53 g/kg, compared with the original SPI, the emulsifying capacity of the modified SPI they prepared had an increase of about 25%. Liet al. (2010) phosphorylated whey soy proteins with pyrophosphate, and the modified product had a degree of phosphorylation of 85 g/kg and an enhanced EAI than the original proteins (1.69 vs. 1.10).
Conclusions
The preparation conditions of microwave-phosphorylated SPI were optimized with a BBD. With the optimal conditions, a microwave-phosphorylated SPI was prepared with the degree of phosphorylation of 82.1 g/kg, which shows better or similar emulsifying properties than original SPI or a phosphorylated SPI prepared as a reference method. The preparation time applied in microwave phosphorylation was much shorter than that applied in the reference work, revealing that microwave phosphorylation is an efficient and better way to prepare modified SPI with better emulsifying properties.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 30871954) and the Earmarked Fund for China Modern Agriculture Research System. The authors thank anonymous reviewers and the editors for their constructive and valuable work on this article.
References
- Alibhai , Z. , Mondor , M. , Moresoli , C. , Ippersiel , D. and Lamarche , F. 2006 . Production of soy protein concentrates/isolates: Traditional and membrane technologies . Desalination , 191 : 351 – 358 .
- Arimi , J.M. , Duggan , E. , O'Sullivan , M. , Lyng , J.G. and O'Riordan , E.D. 2011 . Effect of protein:starch ratio on microwave expansion of imitation cheese-based product . Food Hydrocolloids , 25 : 1069 – 1076 .
- Bhattacharya , S. and Jena , R. 2007 . Gelling behavior of defatted soybean flour dispersions due to microwave treatment: Textural, oscillatory, microstructural and sensory properties . Journal of Food Engineering , 78 : 1305 – 1314 .
- Damodaran , S. 2005 . Protein stabilization of emulsions and foams . Journal of Food Science , 70 : 54 – 66 .
- Guan , J.J. , Qiu , A.Y. , Liu , X.Y. , Hua , Y.F. and Ma , Y.H. 2006 . Microwave improvement of soy protein isolate–saccharide graft reactions . Food Chemistry , 97 : 577 – 585 .
- Hirotsuka , M. , Taniguchi , H. , Narita , H. and Kito , M. 1984 . Functionality and digestibility of a highly phosphorylated soy protein . Agricultural and Biological Chemistry , 48 : 93 – 100 .
- Izquierdo , F.J. , Peñasa , E. , Baeza , M.L. and Gomez , R. 2008 . Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins . International Dairy Journal , 18 : 918 – 922 .
- Khuri , A.I. and Cornell , J.A. 1987 . Response surfaces: Designs and analyses , 105 – 205 . New York : Marcel Dekker Inc .
- Lee , K.H. , Ryu , H.S. and Rhee , K.C. 2003 . Protein solubility characteristics of commercial soy protein products . Journal of the American Oil Chemists’ Society , 80 : 85 – 90 .
- Li , C.P. , Chen , D. , Peng , J. , Enomoto , H. , Hayashi , Y. , Li , C. … & and Aoki , T. 2010 . Improvement of functional properties of whey soy protein phosphorylated by dry-heating in the presence of pyrophosphate . LWT – Food Science & Technology , 43 : 919 – 925 .
- Lidstrom , P. , Tierney , J. , Wathey , B. and Westman , J. 2001 . Microwave assisted organic synthesis-a review . Tetrahedron , 57 : 9225 – 9283 .
- Lu , Y.Q. , Chen , T.H. and Chen , L.J. 1993 . Functional properties of phosphorylated soybean protein . Food and fermentation industries , 1 : 17 – 24 .
- Matheis , G. and Whitaker , J.R. 1984 . Chemical phosphorylation of food protein: An overview and a prospectus . Journal of Agricultural and Food Chemistry , 32 : 699 – 705 .
- Mirmoghtadaie , L. , Kadivar , M. and Shahedi , M. 2009 . Effects of succinylation and deamidation on functional properties of oat protein isolate . Food Chemistry , 114 : 127 – 131 .
- Molina , E. , Papadopoulou , A. and Ledward , D.A. 2001 . Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins . Food Hydrocolloids , 15 : 263 – 269 .
- Moure , A. , Sineiro , J. and Domínguez , H. 2006 . Functionality of oilseed protein products: A review . Food Research International , 39 : 945 – 963 .
- Pearce , K.N. and Kinsella , J.E. 1978 . Emulsifying properties of proteins: Evaluation of a turbidimetric technique . Journal of Agricultural and Food Chemistry , 26 : 716 – 723 .
- Puppo , N. , Chapleau , F. , Speroni , M. , de Lamballerie , M.C. Añón and Anton , M. 2004 . Physicochemical modifications of high-pressure-treated soybean protein isolates . Journal of Agricultural and Food Chemistry , 52 : 1564 – 1571 .
- Roesch , R.R. and Corredig , M. 2003 . Texture and microstructure of emulsions prepared with soy protein concentrate by high-pressure homogenization . Food Science and Technology , 36 : 113 – 124 .
- Sung , H.Y. , Chen , H.J. , Liu , T.Y. and Su , J.C. 1983 . Improvement of functionalities of soy protein isolate through chemical phosphorylation . Food Science , 48 : 716 – 720 .
- Surówka , K. and Żmudziński , D. 2004 . Functional properties modification of extruded soy protein using neutrase . Czech Journal of Food Sciences , 22 : 163 – 174 .
- Wang , R. , Zhang , M. and Mujumdar , A.S. 2010 . Effect of food ingredient on microwave freeze drying of instant vegetable soup . LWT – Food Science and Technology , 43 : 1144 – 1150 .
- Waniska , R.D. , Shetty , J.K. and Kinsella , J.E. 1981 . Protein-stabilized emulsions: Effects of modification on the emulsifying activity of bovine serum albumin in a model system . Journal of Agricultural and Food Chemistry , 29 : 826 – 830 .
- Xiong , J. , Feng , L.J. and Ye , J. 2006 . The effects on solubility of SPC by microwave radiation . Food and Fermentation Industries , 32 : 107 – 110 .
- Yao , Y.J. , Yang , X.Q. and Zhang , X.H. 2001 . Research on functional properties of phosphorylated soybean protein isolate . Food and fermentation industries , 27 : 5 – 8 .
- Zhang , K. , Li , Y.Y. and Ren , Y.X. 2007 . Research on the phosphorylation of soy protein isolate with sodium tripolyphospate . Journal of Food Engineering , 79 : 1233 – 1237 .