Abstract
Dynamic light scattering (DLS) was applied to the analysis of the hydrodynamic diameter distribution of proteins in extract of germinated cacao. The interactions among divalent cations and their relation with the activity of Xaa-prolyl dipeptidyl aminopeptidase 2 (Xaa-Pro-DAP2) enzyme were evaluated. The peptidases were isolated by 80% saturation of ammonium sulfate from acetone extract of germinated cacao seeds. The results showed a higher activity of Xaa-Pro-DAP2, besides aminopeptidase (APE) and carboxypeptidase (CP) enzymes. Using DLS analysis the size distribution was found to be multi-modal; however with Cu2+ and Cd2+ at 1 mM, the size distribution was found to be monomodal with a hydrodynamic diameter of 158.5 and 119 nm, respectively. The distribution remained constant from 60 to 80°C, suggesting that thermal stability is related to increase in Xaa-Pro-DAP2 activity an lower protein aggregation. APE activity was slightly activated by Co2+ at 1 mM, whereas no significant effect was observed on CP activity.
La dispersión dinámica de luz (DLS) fue aplicada para el análisis de la distribución de diámetros hidrodinámicos de proteínas en un extracto germinado de cacao. Se evaluaron las interacciones con cationes divalentes y su relación con la actividad de la enzima Xaa-prolyl dipeptidyl aminopeptidasa 2 (Xaa-Pro-DAP2). Las peptidasas fueron aisladas de los extractos cetónicos de semillas de cacao germinadas, por precipitación con sulfato de amonio al 80% de saturación. Los resultados mostraron una alta actividad de Xaa-Pro-DAP2, además de las enzimas de aminopeptidasa (APE) y carboxipeptidasa (CP). De acuerdo con el análisis DLS, la distribución de tamaños es multi-modal con diámetros hidrodinámicos de 158.5 y 119 nm, respectivamente. La distribución permaneció constante desde 60°C hasta 80°C, sugiriendo estabilidad térmica relacionada con el incremento de la actividad de Xaa-Pro-DAP2 y la baja agregación de proteínas. La actividad de APE fue ligeramente activada por Co2+ a 1 mM, mientras que no se observó efecto importante sobre la actividad de CP.
Palabras clave:
1. Introduction
Theobroma cacao L. has been described as a plant whose seed contains vicilin-type globulin, but not legumin, as a vacuolar storage protein (Voigt, Biehl & Wazir, Citation1993). Proteolysis is initiated by an endopeptidase with high specificity for seed reserve proteins, which are degraded partially as a consequence of the rupture of specific peptide bonds. The resulting polypeptide chains would are then susceptible to the action of previously inactive endopeptidases and, eventually, to that of carboxypeptidases (CP) (Abecia-Soria, Pezoa-García & Amaya-Farfan, Citation2005; Dunaevsky, Sarbakanova & Belozersky, Citation1989). Among cacao proteins, proline (0.72–1.97 g/100 g of cacao) predominates, and because of its specific conformation it poses many restrictions on the structural aspects of peptides and proteins, and grants particular biological properties to a large range of physiologically important biomolecules (Kalvatchev, Garzaro & Guerra, Citation1998; Kratzer et al., Citation2009). In contrast to the plethora of reports regarding Xaa-prolyl dipeptidyl aminopeptidase (Xaa-Pro-DAP2, EC 3.4.14.5) in lactic acid bacteria, there are few reports on this enzyme in plants (Besanova, Kovacs, Psenak & Barth, Citation1987; Davy et al., Citation2000; . Stano et al., Citation1997, Stano et al., Citation1994a; Stano, Kovács, Nemec & Neubert, Citation1994b), and given the above-mentioned proline content in cacao seeds, the study of this enzyme is relevant.
Many enzymes incorporate divalent cations (Mg2+, Ca2+, Zn2+, Ba2+, Cd2+) within their structure to stabilize the folded conformation of the protein or to facilitate possible direct participation in chemical reactions catalysed by these enzymes. According to Atanasov et al. (Citation2013), the presence of metal ions leads to conformational changes in enzymes and the release of hydrolysis products, using natural or artificial substrates. Chan, Ho, Law and Yuen (Citation2002) found that higher enzyme activity in the presence of metal ions in low-substrate concentrations led to a more stable enzyme–substrate complex and higher release rate of phosphate from the phosphor-enzyme complex. Metals commonly bind with the protein portion of the enzyme by the formation of coordinate bonds with certain amino acid chains. Histidine residues are always found in association with transition metal-binding sites on proteins and are normally associated also with divalent metal ion binding (Copeland, Citation2000).
Dynamic light scattering (DLS) can be used to study various applications in the protein field (Kaszuba, Connah, McNeil-Watson & Nobbmann, Citation2007). As such, DLS is very sensitive to the onset of protein aggregation arising from subtle changes in solution conditions. When a protein denatures, the hydrophobic residues buried within the interior of the folded structure are exposed to the solvent. This entropically unfavourable state is soon replaced, however, by one wherein the hydrophobic residues on one protein associate with those on another protein chain. This non-specific aggregation of denatured proteins is easily monitored with the Zetasizer Nano because of the molar mass dependence of the scattering intensity. Because of its high sensitivity to particles of high molecular weight, DLS is also a useful tool for monitoring the effects of salts on protein aggregation.
The aim of this study was to evaluate the effect of different metal ions on the distribution of hydrodynamic diameters of peptidases under different temperatures in a crude extract of cacao using the technique of DLS, and their relationship with exopeptidase activity, thereby contributing to characterization of the proteolytic system of T. cacao L.
2. Materials and methods
2.1. Seed material and growth conditions
We used cacao (Theobroma cacao L.) seeds of the white almond ‘criollo’ genotype, cultivated in the municipality of Cunduacan, Tabasco State, Mexico. Seeds were germinated for 10 days, during which the highest levels of Xaa-Pro-DAP activity were obtained (Sánchez-Mundo, Bautista-Muñoz & Jaramillo-Flores, Citation2010) before enzymatic extraction. Mucilage was removed from seeds and these were placed to germinate in wet agrolite at 30°C. For the analysis and preparation, only cotyledons were used.
2.2. Acetone dry powder
Dry cacao powder (DCP) was obtained according to the method described by Hansen, Del Olmo and Burri (Citation1998). Fat was removed from the powder by the addition of 10 ml/g 100% hexane at 4°C and stirring constantly for 2 h. Finally, the solvent was eliminated by vacuum rotary evaporation from the sample and was dried at 30°C. To remove polyphenols, the defatted seed powder was extracted five times with 80% (v/v) aqueous acetone and then three times with 100% acetone. The suspensions were centrifuged at 4°C for 5 min at 5000 g and 20,000 g for the final three extractions. Finally, the solvent was evaporated from the resulting paste at room temperature and ground until a homogeneous appearance was achieved. The dry powder, which was yellowish-white, was stored at −20°C and served as an enzyme source for the determination of enzyme activities.
2.3. Enzyme extract
The enzyme extract was obtained from 15 mg of DCP and 30 mg of polyvinylpolypyrrolidone (PVPP) in 900 µl of 0.1 M phosphate buffer (pH 7.0) containing 1% Triton X-100. The mixture was stirred for 30 min at 37°C and then centrifuged at 20,000 g for 10 min at 4°C.
2.4. Ammonium sulfate fractionation
The supernatant obtained (in 2.3) was precipitated at 40% saturation of ammonium sulfate. The precipitate was separated by centrifugation at 17,000 g for 20 min at 4°C, the supernatant obtained was subjected again to precipitation at 80% saturation of ammonium and the pellet obtained was dissolved in 20 mM sodium phosphate buffer (pH 7.0) and dialysed for 24 h at 4°C with the same buffer, and then concentrated by ultrafiltration with a 10 kDa molecular weight cut-off membrane (Amicon® Millipore, Inc., Beverly, MA). The protein concentration and peptidase activity were then determined.
2.5. Protein determination
The protein concentration was determined by the method of Folin–Lowry as modified by Markwell, Haas, Bieber and Tolbert (Citation1978), using bovine serum albumin as standard.
2.6. Peptidase activity measurement
Xaa-Pro-DAP2, aminopeptidase (APE) and carboxypeptidase (CP) activity was assayed by measuring the hydrolysis of p-nitroanilide substrates (Bachem, Bubendorf, Switzerland and Sigma-Aldrich, St Louis, MO). Thus, arginine-proline-p-nitroanilide (Arg-Pro-pNA), glycine-proline-p-nitroanilide (Gly-Pro-pNA), alanine-alanine-proline-p-nitroanilide (Ala-Ala-Pro-pNA) and alanine-p-nitroanilide (Ala-pNA) at concentrations of 1 and 10 mM were used to analyse Xaa-Pro-DAP2 activity. APE activity was tested with Ala-pNA, lysine-p-nitroanilide (Lys-pNA) and leucine-p-nitroanilide (Leu-pNA), CP-type activity was tested with benzoyl-tirosine-p-nitroanilide (Bz-Tyr-pNA). The incubation mixture consisted of 30 µl substrate (1 mM and 10 mM), 250 µl of 0.1 M sodium phosphate (pH 7.0) containing 1% Triton X-100, 120 µl distilled water and 100 µl of enzyme extract. After incubation at 37°C for 30 min, the reaction was stopped by the addition of 400 µl ZnSO4 (5%) and 100 µl Ba (OH)2 (7.5%). The mixture was centrifuged (15,000 g for 10 min), and absorbance of the released p-nitroaniline in the clear supernatant was determined at 405 nm. One unit of enzyme (U) was defined as the amount of enzyme producing 1 µmol of p-nitroaniline per minute at 37°C under assay conditions.
2.7. Effect of inhibitors and metal ions on protease activity
The effects of enzyme inhibitors on protease activity were studied using bestatine, pefabloc, leupeptin, pepstatin, diprotine, 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), phenylmethylsulfonyl fluoride (PMSF), ethylenediaminetetraacetic acid (EDTA), p-chloromercuribenzoate (PCMB), dithiothreitol (DTT), β-mercaptoethanol and cysteine. The enzyme solution was incubated with inhibitors for 30 min at 37°C, and then the enzyme assay was applied using Ala-Pro-pNA as substrate. The reaction was stopped by the addition of 400 µl ZnSO4 (5%) and 100 µl Ba (OH)2 (7.5%). The mixture was centrifuged (15,000 g for 10 min), and absorbance of the released p-nitroaniline in the clear supernatant was determined at 405 nm. Activity was expressed as a percentage of the activity obtained in the absence of the added inhibitor.
The effect of various metal ions (0.1 mM and 1.0 mM) on Xaa-Pro-2 activity was investigated by adding the monovalent (Na+) or divalent metal ions (Ba2+, Zn2+, Cu2+, Co2+, Cd2+, Mg2+ and Ca2) to the reaction mixture. The activity of the enzyme without metallic ions was considered as 100% (García-Alvarez, Bordallo, Gascón & Suárez-Rendueles, Citation1985; Haddar, Bougatef, Agrebi, Sellami-Kamoun & Nasri, Citation2009).
2.8. DLS
The DLS technique was applied for analysis of the hydrodynamic diameter distribution of proteins in the enzymatic extract and proteins in the enzymatic extract + ions (Na+, Ba2+, Zn2+, Cu2+, Co2+, Cd2+, Mg2+ and Ca2+) at 25, 30, 40, 50, 60, 70 and 80°C in a Malvern Zetasizer Nano Series S-90 (Malvern Instruments, Malvern, UK). The protein concentration utilized was 0.017 mg/ml according to ISO 13321 (International Standard ISO 13321; Jachimska, Wasilewska & Adamczyk, Citation2008; Kaszuba et al., Citation2007).
2.9. Effect of temperature and Cd2+ and Co2+ ions on peptidases activity
Peptidase activity in the presence of Cd2+ and Co2+ions was determined by incubating of enzymatic extract and ions at 30–80°C for 60 min, after which it was assayed according to Sánchez-Mundo et al. (Citation2010).
2.10. Statistical analyses
Statistical tests and analysis of variance were performed for all substrates, inhibitors and metal ions to determine whether there was a significant difference among chemical substances. Data obtained were analysed using SPSS package software v. 18.0 (IBM Corp, Armonk, NY). Statistical significance was assessed by analyses of variance and significant differences were detected using Duncan´s multiple range test using a 5% significance level.
3. Results and discussion
3.1. Enzymatic activity in crude extract
The results of enzymatic activity on the assayed substrates are shown in . The degree of hydrolysis at a substrate concentration of 10 mM was: Gly-Pro-pNA > Ala-Pro-pNA and Leu-pNA > Lys-pNA and Ala-pNA > Arg-Pro-pNA, Ala-Ala-Pro-pNA and Bz-Tyr-pNA. The highest enzyme activity was found at 10 mM concentration for Gly-Pro-pNA substrate, with a value 0.796 ± 0.006 U/mg protein, and the lowest enzyme activity was detected at 10 mM concentration using Bz-Tyr-pNA as substrate, with a value 0.254 ± 0.003 U/mg protein. The difference was significant between substrates. However, no significant differences were found between Bz-Tyr-pNA and Ala-Ala-Pro-pNA and Arg-Pro-pNA.
Table 1. Exopeptidase activity of Theobroma cacao towards various chromogenic substrates1.
Tabla 1. Actividad exopeptidasa de Theobroma cacao frente a varios sustratos cromogénicos1.
Of the substrates with proline at the N-penultimate position, the highest degree of hydrolysis was obtained with Gly-Pro-pNA at 10 mM, followed by Ala-Pro-pNA and Arg-Pro-pNA, which shows the preference of Xaa-Pro-DAP2 for the Gly amino acid adjacent to proline, followed by alanine and, finally, by arginine.
With increase in substrate concentration activity increased, so that with Gly-Pro-pNA 8.7-fold higher activity was obtained with 10 mM than with 1 mM, whereas with Leu-pNA and Ala-Pro-pNA the increase was 2.2- and 1.7-fold, respectively; for the remaining substrates, the variations were very small or absent. The highest Xaa-Pro-DAP2 activity in lactic bacteria reported was for substrates with a non-charged N-terminal (Ala or Gly) or basic residues (Arg) (Sanz & Toldrá, Citation2001). Given the presence of these enzymes during germination of the cacao seed and its specificity for proline (an amino acid representing 0.72–1.97% of cacao proteins), their role, in conjunction with other peptidases, is to participate in the hydrolysis of reserve proteins.
According to the activity towards Leu-pNA, a substrate for aminopeptidases, and by the inhibitory action shown by bestatine, as identified APE activity, the highest degree of hydrolysis was with Leu-pNA followed by Lys-pNA at 10 mM (). Likewise, we assessed the Ala-pNA substrate, finding a lower degree of hydrolysis as compared with Leu-pNA and Lys-pNA at 10 mM. The non-specific Type N APE identified in a large number of lactic bacteria demonstrated Lys-pNA, Leu-pNA (Gómez De La Cruz, Citation1996) and Ala-pNA (Magboul & McSweeney, Citation2000) activities. In fermented cacao, the aminopeptidase activity is considered as the second most important enzyme after aspartyl peptidase (AP) (Hansen et al., Citation1998). Because of hydrolysis of the substrate Bz-Tyr-pNA, reported as specific for carboxypeptidase-type enzymes with preference for hydrophobic amino acids in the carboxyl terminal, CP activity was confirmed.
3.2. Effect of peptidase inhibitors and reducing agents
shows the effect of different inhibitors on the activity of the Xaa-Pro-DAP2 enzyme, revealing that at 0.1 mM the optimal inhibitors were bestatine, PCMB, cysteine, diprotine A and pepstatin A, with 52, 48, 47, 47 and 45% inhibition, respectively; whereas at 1 mM, the best inhibitors were pepstatin, diprotine A, AEBSF and pefabloc. Diprotine A (Ile-Pro-Ile) has been identified as a competitive inhibitor of peptidases with dipeptidyl peptidase IV (DPP IV) activity (Rigolet, Xi., Rety & Chich, Citation2005). In barley, a relative activity of 3% was reported with diprotine A at 0.1 mM (Davy et al., Citation2000) and, in Lactobacillus delbrueckii ssp. bulgaricus LBU-147, 15% relative activity was reported with the Ile-Pro-Ile sequence of diprotine A (Miyakawa, Kobayashi, Shimamura & Tomita, Citation1991). A reduction of 48% in Xaa-Pro-DAP2 activity in the presence of PCMB at 0.1 mM, as well as the recovery of enzymatic activity by reducing agents (β-mercapto-ethanol and cysteine), suggests the presence of a cysteine peptidase. Circular dichroism (CD) studies (data not shown) demonstrated a low secondary structure or ‘random coil’ type, which suggests the presence of cysteine peptidases in cacao extract. In Serratia marcescens the activity of endonuclease, containing SH-groups, was affected by PCMB, showing secondary structure changes in the CD spectrum (Filimonova et al., Citation2001).
Table 2. Effect of inhibitors on the exopeptidase activity of Theobroma cacao L1.
Tabla 2. Efecto de inhibidores sobre la actividad exopeptidase de Theobroma cacao L1.
The metallo-peptidase inhibitors, bestatine and EDTA, reduced Xaa-Pro-DAP2 activity at 0.1 mM by 52 and 29%, respectively. These inhibition levels indicate the presence of a metallo-protein. Inhibitors such as PMSF, bestatine and EDTA at 1.0 mM had a lower inhibitory effect on Xaa-Pro-DAP2 activity. This is due to the presence of different peptidases in the enzymatic extract, which are also inhibited by various compounds, reflecting lower proteolytic action, increasing indirectly the Xaa-Pro-DAP2 activity under study; this is the case for the aminopeptidase enzyme reported as serine peptidase (Haddar et al., Citation2009).
The serine peptidase blockers, AEBSF and pefabloc, at higher concentrations, inhibited 52 and 56% of enzymatic activity, respectively, suggesting that serine residues may participate in enzyme catalysis, as occurs with other microbial Xaa-Pro-DAPs (Magboul & McSweeney, Citation2000). However, another serine peptidase inhibitor, PMSF (at 0.1 mM) reduced Xaa-Pro-DAP2 activity by only 24%; leupeptin, which also inhibits cysteine peptidases, had the same effect on enzymatic activity. In contrast to Xaa-Pro-DAP1 obtained at 40% saturation (Sánchez-Mundo et al., Citation2010), in that obtained at 80% saturation the differences shown by the diverse serine peptidase inhibitors revealed that the inhibition mechanism of acylation of the active site of the enzyme is more effective than sulfonation. Results revealed the presence of at least three structural characteristics of Xaa-Pro-DAP peptidases in the enzymatic extract of T. cacao: (1) serine peptidase type; (2) cysteine peptidase type; and (3) a metallic character, apart from a CP and an APE. APE and CP enzymes have been reported as metallopeptidases (Biehl et al., Citation1991; Pérez-Guzmán, Cruz y Victoria, Cruz-Camarillo & Hernández-Sánchez, Citation2006, Citation2004b, Ramírez-Zavala, Mercado-Flores, Hernández-Rodríguez & Vila-Tanaca, Citation2004b).
shows the specificity of each of the tested inhibitors, as well as the inhibitory effect on the enzymes identified in this study, including the carboxypeptidase- and aminopeptidase-type metallopeptidases of different plant and microorganism sources. In general, the Xaa-Pro-DAPs of different species of Lactobacillus and Lactococcus have been identified as serine and cysteine peptidases due to their inhibition by PMSF and PMCB, respectively (Magboul & McSweeney, Citation2000; Pérez-Guzmán et al., Citation2006), while Xaa-Pro-DAP1 has been identified in cacao (Sánchez-Mundo et al., Citation2010). However, in this study Xaa-Pro-DAP2 revealed a different profile of inhibition. In Hordem vulgare, one of the few reports in this field on plants (Davy et al., Citation2000), DPP IV showed inhibition profiles similar to those found for cacao Xaa-Pro-DAP2 identified in this study, sensitive to AEBSF and diprotine, inhibitors of serine peptidases specific for proline. On the other hand, bestatine, EDTA and leupeptin also showed inhibitory effects on APE and CP from different sources (Biehl et al., Citation1991; Ramírez-Zavala, Mercado-Flores, Hernández-Rodríguez & Vila-Tanaca, et al., Citation2004a), as detected in this study.
Table 3. Effect of various inhibitors on peptidase activity of different sources.
Tabla 3. Efecto de diversos inhibidores sobre la actividad peptidasa de diferentes orígenes.
Data obtained in this work represent one of the few reports on plant Xaa-Pro-DAP, aside from one study on barley, in contrast to studies on acid-lactic bacteria, which use proline-rich substrates for their growth. These stud results share characteristics with the cacao Xaa-Pro-DAP2 of this study: sensitivity to serine peptidase inhibitors (PMSF, AEBSF), inhibition by diprotine A and PCMB and, in some cases, reduction in activity by chelating agents.
3.3. Effect of divalent cations and sodium on Xaa-Pro-DAP2 activity
depicts the effect of divalent cations and sodium on the enzymatic activity. The presence of Cu2+ at 0.1 mM decreased activity by 46%, cations Ba2+, Mg2+ and Ca2+ at 0.1 mM decreased it by 38, 34 and 30%, respectively, while it was decreased by 27 and 20%, respectively, with Cd2+ and Co2+. Ca2+ and Mg2+ caused 46% inhibition at 1 mM and Zn2+ 30%, whereas only a slight effect (10–14%) was observed in the presence of Ba2+ and Na+. Increasing the concentration of Ba2+, Cd2+, Cu2+, Na+ and Co2+ to 1 mM, the inhibitory effect was lower, with Co2+ resulting in a full recovery of the enzymatic activity of Xaa-Pro-DAP2. These findings are probably due to interactions among peptidases with lower specificity for substrates such as AP, APE, and CP, in such a way that the activity of Xaa-Pro-DAP2 is indirectly favoured. For the APE of Ustilago maydis, the presence of Cu2+, Hg2+ and Zn2+ at 1 mM induced complete inhibition of the purified enzyme. Co2+, Ni2+ and Mn2+ had a strong inhibitory effect on APE (Mercado-Flores, Noriega-Reyes, Ramírez-Zavala, Hernández-Rodríguez & Villa-Tanaca, Citation2004). Cu2+, Hg2+ and Zn2+ are reported as strong inhibitors of the Xaa-Pro-DAP2 activity of Lactobacillus helveticus ITG LH1 and L. delbreckii subsp. bulgaricus. In Lactobacillus sakei, Cu2+and Hg2+ resulted in 53 and 78% inhibition, respectively, whereas other cations had no significant inhibitory effect on activity (Sanz & Toldra, Citation2001). The effect of the ionic strength of sodium chloride revealed that activity increases when the former increases in the medium. Monovalent cation coordination plays an influential role in many enzyme-catalysed reactions (Di Cera, Citation2006; Page & Di Cera, Citation2006). Due to limited electrostatic properties, Na+ and K+ are optimal reagents for stabilization of the active conformational state of an enzyme or for facilitating electrostatic interactions between enzyme and substrate (Page & Di Cera, Citation2008).
Table 4. Effect of divalent cations and sodium ion on exopeptidase activity of Theobroma cacao L1.
Tabla 4. Efecto de cationes divalentes e ion sodio sobre la actividad exopeptidasa de Theobroma cacao L1.
3.4. Analysis of protein–cation interaction by DLS
The effect of salts on the aggregation of the protein was monitored by DLS due to its high sensitivity, which makes it a useful tool for this type of study. The intensity-averaged diameter (in nanometres) and polydispersity index (PdI) values (an estimate of distribution width) were determinate as defined in ISO 13321 Standard (Citation1996). Regarding the enzymatic extract, the size distribution intensity at 25°C revealed three protein groups of around 8.281, 151.4 and 4987 nm in diameter, showing a major association at the interval of 151.4 nm with an intensity of 73.1%. Interactions (protein–cation) of the enzymatic extract with the tested cations at both 0.1 and 1.0 mM at 25°C, according to the distribution of hydrodynamic diameters, showed three aggregation groups (≤10 nm, 10 ≤ 100 nm and 100 ≤ 4000 nm). The observation of a higher intensity in the signal demonstrates that the most important protein–cation interactions were for CdCl2 and CuCl2 at 0.1 mM; CdCl2 has three groups of hydrodynamic diameter: 12.7 nm (20%), 141 nm (76%) and 5021 nm (4%), while CuCl2 has 9.9 nm (8%), 104.9 nm (90%) and 4801 nm (2%). Noteworthy are the interactions with CaCl2 at 0.1 mM and 1 mM, which reached their highest intensity among the cations tested with a hydrodynamic diameter of 153.1–131.6 nm. This size distribution is multi-modal (i.e. it reveals the presence of different protein groups, indicating protein aggregation that is reflected in loss of enzymatic activity of Xaa-Pro-DAP2 ()). Incrementing the ionic strength in the medium by adding salts at 1 mM () induces a lower variation in size distribution, but with the same multi-modal behaviour, indicating that the aggregation induced by cations at 1 mM could be due to binding of a metallic ion at a specific site of the protein. There is growing evidence that metal ions can accelerate the aggregation process of several proteins, as occurs in the denaturation and aggregation process of β-lactoglobulin A (BLG-A) in the presence of copper and zinc ions (Stirpe et al., Citation2008). However, interactions involving CuCl2 and CdCl2 are prominent, in which the size distribution is monomodal, with hydrodynamic diameters of 158.5 and 119 nm with PdI = 0.242 and 0.211, respectively, indicating a tendency towards homogeneous molecular size or polydispersed medium; this is related to increase in the enzymatic activity of Xaa-Pro-DAP2 resulting from lower protein aggregation and leading to denaturation induced by the ions. CoCl2 favours the aggregation of several groups of varying molecular size, in contrast to its effect on activity, which is re-established at 1 mM.
Figure 1. Effect of temperature on size distribution of interactions (protein–cation) by DLS in the enzymatic extract with Ba2+, Zn2+, Cu2+, Co2+, Cd2+, Mg2+, Ca2+ and Na1+ at 1 mM: (a) 25°C, (b) 40°C, (c) 60°C + (insert: CuCl2 1 mM), (d) 80°C + (insert: CdCl2 1 mM).
Figura 1. Análisis mediante DLS del efecto de la temperatura sobre la distribución de tamaño de interacciones (proteína-catión) en el extracto enzimático con Ba2+, Zn2+, Cu2+, Co2+, Cd2+, Mg2+, Ca2+ y Na1+ at 1 mM (a) 25°C,(b) 40°C, (c) 60°C + (inserto: CuCl2 1 mM), (d) 80°C + (inserto: CdCl2 1 mM).
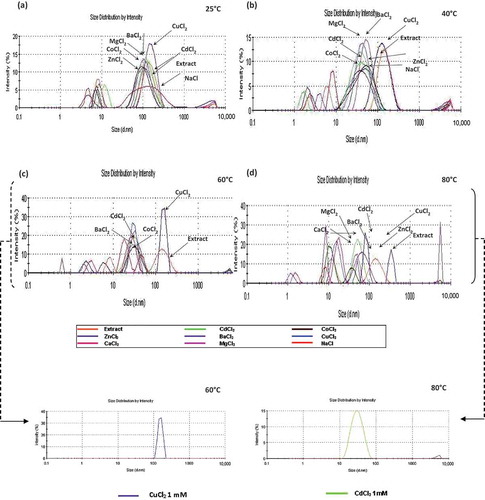
Increments of temperature revealed a higher variation in the distribution of hydrodynamic diameter generated in the interaction with the salts studied; however, the formation of molecular aggregates (protein–cation interaction) was observed between 10 and 70 nm (), this being very evident at 0.1 mM at both 40 and 50°C.
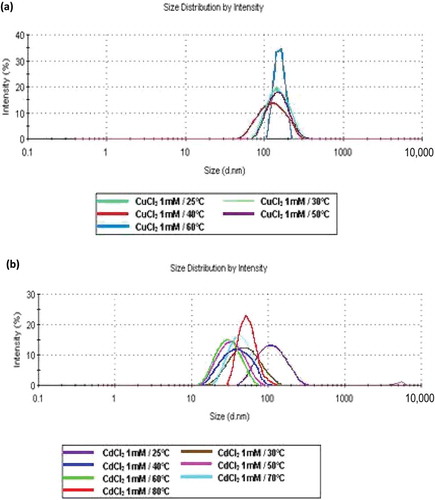
At 40°C, with 0.1 and 1.0 mM, aggregates larger than 5000 nm were observed () that became denatured at temperatures higher than 50°C (), generating large aggregates, except those formed with MgCl2, indicating that this probably results in polymer stability. For CuCl2 at 1 mM, the monomodal behaviour was maintained up to 60°C, with an average size of 154.9 nm and PdI = 0.224 (insert, , ). A similar distribution was obtained with CdCl2 at 1 mM (insert, , ), but was maintained up to 80°C (i.e. these cations favour protein stabilization, since there was no evident increase in protein sizes after denaturation.
Figure 2. Effect of temperature on size distribution of interactions (protein–cation) by DLS in the enzymatic extract: (a) Cu2+ at 25, 30, 40, 50 and 60°C; (b) Cd2+ at 25, 30, 40, 50, 60, 70 and 80°C.
Figura 2. Análisis mediante DLS del efecto de la temperatura sobre la distribución de tamaño de interacciones (proteína-catión) en el extracto enzimático (a) Cu2+at 25, 30, 40, 50 y 60°C (b) Cd2+ at 25, 30, 40, 50, 60, 70 y 80°C.
3.5. Effect of temperature and Cd2+ and Co2+ ions on peptidase activity
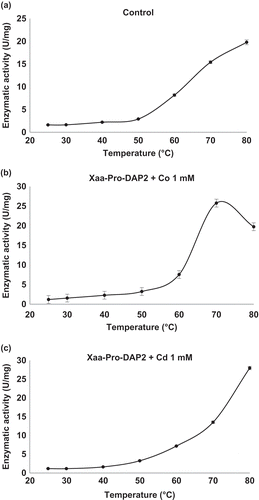
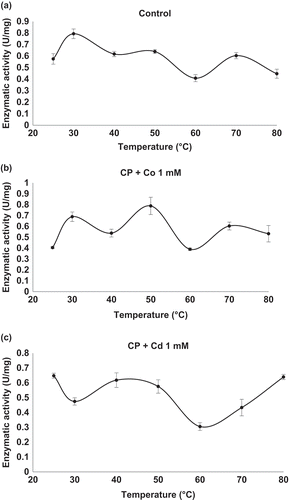
The effect of temperature and the ions Cd2+ and Co2+ on peptidase activity was examined at various temperatures. Xaa-Pro-DAP2 showed 1.6 U/mg protein at 25°C, which increased to 2.9 and 19.7 U/mg at 50 and 80°C, respectively, indicating activation by increasing temperature (). In the presence of CoCl2 at 0.1 mM, activity showed the same behaviour, while at 1 mM a maximal activity of 25.7 U/mg protein was reached at 70°C, decreasing to 19.74 U/mg at the end of the assay (80°C) (). However, the addition of CdCl2 at 1.0 mM achieved higher thermal stability of the Xaa-Pro-DAP2 enzyme at 80°C, reaching 28 U/mg protein (). At 0.1 mM, the CdCl2 effect was very similar. Accordingly, Xaa-Pro-DAP-type enzymes can be ascribed to the only protein groups detected with DLS in the presence of CdCl2, corresponding to 34.87 nm at 50°C until 80°C with 53.48 nm.
Figure 3. Effect of temperature on enzymatic activity of Xaa-Prolyl dipeptidyl aminopeptidase (Xaa-Pro-DAP2). (a) Control: Xaa-Pro-DAP2 activity without cations in the enzyme extract. (b) Xaa-Pro-DAP2 activity in the enzyme extract in the presence of CoCl2 at a concentration of 1 mM. (c) Xaa-Pro-DAP2 activity in the enzyme extract in the presence of CdCl2 at concentration of 1.0 mM. Mean values are connected by a line, bars represent standard deviations (n = 3).
Figura 3. Efecto de la temperatura sobre la actividad enzimática de Xaa-Prolyl dipeptidyl aminopeptidase (Xaa-Pro-DAP2). (a) Control: Actividad de Xaa-Pro-DAP2 en el extracto enzimático en ausencia de cationes (b) Actividad de Xaa-Pro-DAP2 en el extracto enzimático en presencia de CoCl2 a 1 mM y (c) Actividad de Xaa-Pro-DAP2 en el extracto enzimático en presencia de CdCl2 a 1.0 mM. Los valores de las medias están unidos por una línea, las barras representan la desviación standard (n = 3).
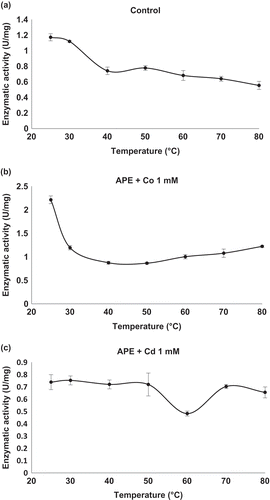
APE activity showed a decreasing trend following thermal denaturation, from 0.7 U/mg protein at 40°C to 0.5 U/mg protein at 80°C (). The activity levels of APE in the enzymatic extract were low as compared with Xaa-Pro-DAP2. However, in the presence of CoCl2 at 1 mM, APE activity became stabilized, with 1.0 and 1.2 U/mg protein at 60 and 80°C, respectively (). Increases of 1.7- to 50-fold the original activity of CP (Cheng, Ramakrishnan & Chan, Citation1999; Kishimura, Hayashi & Ando, Citation2006) and of APE enzymes (Bolumar, Sanz, Aristoy, & Toldrá, Citation2003; Dong et al., Citation2005), activated by cobalt, were attained using 4 µM to 1 mM CoCl2. Nevertheless, inhibition of APE by Co2+ at 10 mM has also been detected (Mercado-Flores et al., Citation2004; Mohamed et al., Citation2009). The effect of CdCl2 on APE activity was not significant, although the lowest enzymatic activity was detected at 60°C (0.483 U/mg protein), a value below that obtained in the absence of the cation ().
Figure 4. Effect of temperature on enzymatic activity of aminopeptidase (APE) in the enzymatic extract. (a) Control: APE activity without cations. (b) APE activity in the presence of CoCl2 at a concentration of 1 mM. (c) APE activity in the presence of CdCl2 at a concentration of 1.0 mM. Mean values are connected by a line, bars represent standard deviations (n = 3).
Figura 4. Efecto de la temperatura sobre la actividad enzimática de aminopeptidasa (APE) en el extracto enzimático. (a) Control: Actividad APE en ausencia de cationes; (b) Actividad APE activity en presencia de CoCl2 a 1 mM y (c) Actividad APE en presencia de CdCl2 a 1.0 mM. Los valores de las medias están unidos por una línea, las barras representan la desviación standard (n = 3).
Finally, CP-type activity depicted a random behaviour, recording 0.6 U/mg protein at 40°C to 0.4 U/mg protein at 80°C (). The presence of CoCl2 at 0.1 mM and 1 mM did not exert any evident effect on CP activity (), whereas the addition of CdCl2 at both concentrations was able to slightly recover slightly CP activity, from 0.34–0.30 U/mg protein at 60°C to 0.64–0.63 U/mg protein at the end of the assay ().
Figure 5. Effect of temperature on enzymatic activity of carboxypeptidase (CP). (a) Control: CP activity without cations in the enzyme extract. (b) CP activity in the enzyme extract in the presence of CoCl2 at a concentration of 1 mM. (c) CP activity in the enzyme extract in the presence of CdCl2 at a concentration of 1.0 mM. Mean values are connected by a line, bars represent standard deviations (n = 3).
Figura 5. Efecto de la temperatura sobre la actividad enzimática de carboxipeptidasa (CP) en el extracto enzimático. (a) Control: Actividad de CP en ausencia de cationes; (b) actividad de CP en presencia de CoCl2 a 1 mM and (c) actividad CP en presencia de CdCl2 a 1.0 mM. Los valores de las medias están unidos por una línea, las barras representan la desviación standard (n = 3).
4. Conclusions
In the enzymatic extract of germinated T. cacao, Xaa-Pro-DAP2 activity with a preference for Gly-Pro-pNA and Ala-Pro-pNA was identified. Also detected was lysine and leucine aminopeptidase activity, as well as carboxypeptidase-type activity. The Xaa-Pro-DAP2 enzyme was inhibited by the divalent ions Cu2+ (0.1 mM) and Ca2+ and Mg2+ (1 mM); in the absence of divalent ions, activity was increased by the effect of temperature with values of 2.9 U/mg protein at 50°C and 19.7 U/mg protein at 80°C. The addition of Cd2+ ions favoured enzymatic stability, with 28 U/mg protein detected at the end of the assay (80°C). According to DLS analysis, in the presence of Cd2+ (1 mM) only one protein group of 53.48 nm diameter at 80°C was detected, which can be attributed to a Xaa-Pro-DAP-type enzyme, indicating thermostability. APE activity was stabilized by 1 mM Co2+, whereas Co2+ had no significant effect on CP activity. Despite APE and CP activity levels being lower than those detected for Xaa-Pro-DAP2, the three enzymes were able to maintain residual activity up to 80°C, indicating low thermal denaturation.
In the present study, the effect of some metal ions on the protease activities was assayed. The metallic ions Cd2+ and Co2+ at 1 mM may have been responsible for the thermal stability observed during the reaction catalysed for proteases in the cacao extract. Also, the DLS study showed that Cd2+ and Cu2+ at 1 mM induced monomodal distribution, possibly due to conformational changes. Prepurified Xaa-Pro-DAP1 showed increased activity in substrates with Ala-Pro-pNA residues (Sánchez-Mundo et al., Citation2010), whereas the present study showed higher specificity on substrates with Arg-Pro-pNA residues. Moreover, the effect of cations on activity and stability varied since Xaa-Pro-DAP1 had no effect on stability. The results contribute to the study of Xaa-Pro-DAP2, APE and CP enzymes, which are important because of their participation in degrading reserve proteins during the germination of cacao, especially given the scarce information available on these types of vegetable-derived enzymes. Following our findings here, for further research is possible especially in regard to determining which enzyme components link with metal ions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abecia-Soria, L., Pezoa-García, N., & Amaya-Farfan, J. (2005). Soluble albumin and biological value of protein in cocoa (Theobroma cacao L.) beans as a function of roasting time. Journal of Food Science, 70, S294–S298. doi:10.1111/j.1365-2621.2005.tb07205.x
- Atanasov, V., Stoykova, S., Kolev, H., Mitewa, M., Petrova, S., & Pantcheva, I. (2013). Effect of some divalent metal ions on enzymatic activity of secreted phospholipase <sub>A2(sPLA2) isolated from Bulgarian Vipera ammodytes meridionalis. Biotechnology & Biotechnological Equipment, 27, 4181–4185. doi:10.5504/BBEQ.2013.0072
- Besanova, M., Kovacs, P., Psenak, M., & Barth, A. (1987). Dipeptidyl peptidase of poppy seedlings. Biologia, 42, 779–787.
- Biehl, B., Ziegeler-Berghausen, H., Srivastava, S., Xiong, Q., Passern, D., & Heinrichs, H. (1991, September). Cocoa specificity of proteolytic flavor precursors: The cocoa seed proteases. Proceedings of the 1991 International Cocoa Conference: Challenges in the 90s, Kuala Lumpur, Malaysia.
- Bolumar, T., Sanz, Y., Aristoy, M. C., & Todrá, F. (2003). Purification and properties of an arginyl aminopeptidase from Debaryomyces hansenii. International Journal of Food Microbiology, 86, 141–151. doi:10.1016/S0168-1605(03)00069-2
- Chan, K. C., Ho, S., Law, J., & Yuen, V. (2002). The effects of various divalent cations on the enzyme activity of bovine intestinal alkaline phosphatase. Journal of Experimental Microbiology and Immunology, 2, 13–21.
- Cheng, T. C., Ramakrishnan, V., & Chan, S. (1999). Purification and characterization of a cobalt-activated carboxypeptidase from the hyperthermophilic archaeon Pyrococcus furiosus. Protein Science, 8, 2474–2486. doi:10.1110/ps.8.11.2474
- Copeland, R. A. (2000). Enzymes: A practical introduction to structure, mechanism, and data analysis (2nd ed.). New York, NY: Wiley-Vch.
- Davy, A., Thomsen, K., Juliano, M. A., Alves, L. C., Svendsen, I., & Simpson, D. J. (2000). Purification and characterization of barley dipeptidyl peptidase IV. Plant Physiology, 122, 425–432. doi:10.1104/pp.122.2.425
- Di Cera, E. (2006). A structural perspective on enzymes activated by monovalent cations. Journal of Biological Chemistry, 281, 1305–1308. doi:10.1074/jbc.R500023200
- Dong, L., Cheng, N., Wang, M.-W., Zhang, J., Chang, S., & De-XuZhu, Z. (2005). The leucyl aminopeptidase from Helicobacter pylori is an allosteric enzyme. Microbiology, 151, 2017–2023. doi:10.1099/mic.0.27767-0
- Dunaevsky, Y. E., Sarbakanova, S. T., & Belozersky, M. A. (1989). Wheat seed carboxypeptidase and joint action on gliadin of proteases from dry and germinating seeds. Journal of Experimental Botany, 40, 1323–1329. doi:10.1093/jxb/40.12.1323
- Filimonova, M. N., Gubskaya, V. P., Nuretdinov, I. A., Benedik, M. J., Cherepanova, N. A., & Leshchinskaya, I. B. (2001). Study of the mechanism of action of p-chloromercuribenzoate on endonuclease from the bacterium Serratia marcescens. Biochemistry (Moscow), 66, 323–327. doi:10.1023/A:1010212232287
- Gallo, G., De Angelis, M., McSweeney, P. L. H., Corbo, M. R., & Gobbetti, M. (2005). Partial purification and characterization of an X-prolyl dipeptidyl aminopeptidase from Lactobacillus sanfranciscencis CB1. Food Chemistry, 9, 535–544.
- García Alvárez, N., Bordallo, C., Gascón, S., & Suárez-Rendueles, P. (1985). Purification and characterization of a thermosensitive X-prolyl dipeptidyl aminopeptidase (dipeptidyl aminopeptidase yscV) from Saccharomyces cerevisiae. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology, 832, 119–125. doi:10.1016/0167-4838(85)90180-3
- Gómez De La Cruz, M. J. (1996). Degradación de péptidos hidrófobos por bacterias lácticas ( Doctoral Thesis). Aplicación en la eliminación del sabor amargo en queso. Universidad Complutense de Madrid, España. pp. 21–22.
- Guilloteau, M., Laloi, M., Michaux, S., Bucheli, P., & McCarthy, J. (2005). Identification and characterisation of the major aspartic proteinase activity in Theobroma cacao seeds. Journal of the Science of Food and Agriculture, 85, 549–562. doi:10.1002/jsfa.1777
- Haddar, A., Bougatef, A., Agrebi, R., Sellami-Kamoun, A., & Nasri, M. (2009). A novel surfactant-stable alkaline serine-protease from a newly isolated Bacillus mojavensis A21. Purification and characterization. Process Biochemistry, 44, 29–35. doi:10.1016/j.procbio.2008.09.003
- Hansen, C. E., Del Olmo, M., & Burri, C. (1998). Enzyme activities in cocoa beans during fermentation. Journal of the Science of Food and Agriculture, 77, 273–281. doi:10.1002/(SICI)1097-0010(199806)77:2<273::AID-JSFA40>3.0.CO;2-M
- Hook, V. Y. H., & Peng Loh, Y. (1984). Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proceedings of the National Academy of Sciences USA, 81, 2776–2780. doi:10.1073/pnas.81.9.2776
- International Standard ISO 13321. (1996). Particle size analysis-photon correlation spectroscopy. Genéve: International Organization for Standardization (ISO).
- Jachimska, B., Wasilewska, M., & Adamczyk, Z. (2008). Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility and viscosity measurements. Langmuir: the ACS Journal of Surfaces and Colloids, 24, 6866–6872. doi:10.1021/la800548p
- Kalvatchev, Z., Garzaro, D., & Guerra, F. (1998). Theobroma cacao L.: Un nuevo enfoque para nutrición y salud. Agroalimentaria, 4(6), 23–25.
- Kaszuba, M., Connah, M. T., McNeil-Watson, F., & Nobbmann, U. (2007). Resolving concentrated particle size mixtures using dynamic light scattering. Particle & Particle Systems Characterization, 24, 159–162. doi:10.1002/ppsc.200601035
- Kishimura, H., Hayashi, K., & Ando, S. (2006). Characteristics of carboxypeptidase B from pyloricceca of the starfish Asterina pectinifera. Food Chemistry, 95, 264–269. doi:10.1016/j.foodchem.2005.01.001
- Kratzer, U., Frank, R., Kalbacher, H., Biehl, B., Wöstemeyer, J., & Voigt, J. (2009). Subunit structure of the vicilin-like globular storage protein of cocoa seeds and the origin of cocoa- and chocolate-specific aroma precursors. Food Chemistry, 113, 903–913. doi:10.1016/j.foodchem.2008.08.017
- Magboul, A., & McSweeney, P. (2000). Purification and characterization of an X-prolyl-dipeptidyl aminopeptidase from Lactobacillus curvatus DPC2024. Le Lait, 80, 385–396. doi:10.1051/lait:2000133
- Markwell, M. A. K., Haas, S. M., Bieber, L. L., & Tolbert, N. E. (1978). A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Analytical Biochemistry, 87, 206–210. doi:10.1016/0003-2697(78)90586-9
- Mercado-Flores, Y., Noriega-Reyes, Y., RamÃrez-Zavala, B., Hernández-RodrÃguez, C., & Villa-Tanaca, L. (2004). Purification and characterization of aminopeptidase (pumAPE) from Ustilago maydis. FEMS Microbiology Letters, 234, 247–253. doi:10.1111/j.1574-6968.2004.tb09540.x
- Miyakawa, H., Kobayashi, S., Shimamura, S., & Tomita, M. (1991). Purification and characterization of an X-prolyl dipeptidyl aminopeptidase from Lactobacillus delbrueckii ssp. bulgaricus LBU-147. Journal of Dairy Science, 74, 2375–2381. doi:10.3168/jds.S0022-0302(91)78411-7
- Mohamed, S., El-Badry, M., Hamdy, S., Abdel-Ghany, S., Salah, H., & Fahmy, A. (2009). Faschiola gigantica: Purification and characterization of a leucine aminopeptidase. Journal of Applied Sciences Research, 5, 905–913.
- Page, M. J., & Di Cera, E. (2006). Role of Na+ and K+ in enzyme function. Physiological Reviews, 86, 1049–1092. doi:10.1152/physrev.00008.2006
- Page, M. J., & Di Cera, E. (2008). Serine peptidases: Classification, structure and function. Cellular and Molecular Life Sciences, 65, 1220–1236. doi:10.1007/s00018-008-7565-9
- Pérez-Guzmán, A. E., Cruz Y Victoria, T., Cruz-Camarillo, R., & Hernández-Sánchez, H. (2006). Purification and characterization of X-prolyl-dipeptidyl aminopeptidase from Lactococcus lactis subsp. cremoris NRRL 634. Journal World Journal of Microbiology and Biotechnology, 22, 953–958. doi:10.1007/s11274-006-9140-6
- Ramı́rez-Zavala, B., Mercado-Flores, Y., Hernández-Rodrı́guez, C., & Villa-Tanaca, L. (2004a). Purification and characterization of a lysine aminopeptidase from Kluyveromyces marxianus. FEMS Microbiology Letters, 235, 369–375. doi:10.1111/j.1574-6968.2004.tb09612.x
- Ramı́rez-Zavala, B., Mercado-Flores, Y., Hernández-Rodrı́guez, C., & Villa-Tanaca, L. (2004b). Purification and characterization of a serine carboxypeptidase from Kluyveromyces marxianus. International Journal of Food Microbiology, 91, 245–252. doi:10.1016/S0168-1605(03)00409-4
- Rigolet, P., Xi, X. G., Rety, S., & Chich, J. (2005). The structural comparison of the bacterial PepX and human DPP-IV reveals sites for the design of inhibitors of PepX activity. FEBS Journal, 272, 2050–2059. doi:10.1111/j.1742-4658.2005.04631.x
- Sánchez-Mundo, M. L., Bautista-Muñoz, C., & Jaramillo-Flores, M. E. (2010). Characterization of the exopeptidase activity existing in Theobroma cacao L. during germination. Process Biochemistry, 45, 1156–1162. doi:10.1016/j.procbio.2010.04.012
- Sanz, Y., & Toldra, F. (2001). Purification and characterization of an X-prolyl dipeptidyl peptidase from Lactobacillus sakei. Applied and Environmental Microbiology, 67, 1815–1820. doi:10.1128/AEM.67.4.1815-1820.2001
- Stano, J., Kovács, P., Kákoniová, D., Lisková, D., Kirilova, N. D., & Komov, V. (1994a). Activity of dipeptidyl peptidase IV in ginseng callus culture. Biologia, 49, 905–910.
- Stano, J., Kovács, P., Nemec, P., & Neubert, K. (1994b). Dipeptidyl peptidase IV in gherkin seedlings Cucumis sativus L. cv. Pálava. Biologia, 49, 905–910.
- Stano, J., Kovács, P., Psenak, M., Gajdos, J., Erdelsky, K., Kákoniová, D., & Neubert, K. (1997). Distribution of dipeptidyl peptidase IV in organ tissue cultures of poppy plants Papaver somniferum L., cv ‘Amarin’. Die Pharmazie, 52, 319–321.
- Stirpe, A., Rizzuti, B., Pantusa, M., Bartucci, R., Sportelli, L., & Guzzi, R. (2008). Thermally induced denaturation and aggregation of BLG-A: Effect of the Cu2+ and Zn2+ metal ions. European Biophysics Journal, 37, 1351–1360. doi:10.1007/s00249-008-0346-4
- Voigt, J., Biehl, B., & Wazir, S. K. S. (1993). The major seed proteins of Theobroma cacao L. Food Chemistry, 47, 145–151. doi:10.1016/0308-8146(93)90236-9
