Abstract
This study investigated the effects of storage conditions: cool (15 ± 1°C, 90% relative humidity (RH)), ambient (23 ± 2°C, 60% RH) and higher (38 ± 2°C, 60% RH) on changes in physicochemical quality attributes of two cassava flour cultivars (TME 419 and UMUCASS 36) packaged in paper bags and stored for 12 weeks. Physicochemical and microbial qualities were studied at weeks 0, 4, 8 and 12. Moisture content decreased from 12.0% to 7.1% and 9.8% to 6.8% in cultivars ‘TME 419’ and ‘UMUCASS 36’, respectively. Carotenoid content was higher in cultivar (cv.) ‘UMUCASS 36’ (2.5 ± 0.10 mg/g) compare to cv. ‘TME 419’ (1.8 ± 0.11 mg/g). Colour indices of the cassava flour were significantly influenced by storage duration. A slight decrease in microbial load from 5.4 to 4.8 log CFU/g was observed, with increase in temperature from 15°C to 38°C at the end of storage. The ambient storage condition best maintained nutritional and physicochemical quality.
Este estudio investigó los efectos de las condiciones de almacenamiento: frío (15 ± 1°C, 90% humedad relativa (RH); ambiente (23 ± 2°C, 60% RH); y alta (38 ± 2°C, 60% RH) en los cambios en los atributos cualitativos fisicoquímicos de dos cultivares de harina de mandioca (TME 419 y UMUCASS 36) empaquetados en bolsas de papel y almacenados durante 12 semanas. Se estudió la calidad fisicoquímica y microbiana en las semanas 0, 4, 8 y 12. El contenido de humedad disminuyó de 12,0 a 7,1% y de 9,8 a 6,8% en los cultivares ‘TME 419’ y ‘UMUCASS 36’, respectivamente. El contenido en carotenoides fue mayor en el cv. ‘UMUCASS 36’ (2,5 ± 0,10 mg/g) en comparación con el cv. ‘TME 419’ (1,8 ± 0,11 mg/g). Los índices de color de la harina de mandioca estuvieron influenciados significativamente por la duración del periodo de almacenamiento. Se observó un ligero descenso en la carga microbiana de 5,4 a 4,8 log CFU/g, con un aumento en la temperatura de 15°C a 38°C al final del periodo de almacenamiento. El almacenamiento a temperatura ambiente fue el que mantuvo una mejor calidad nutricional y fisicoquímica.
Introduction
High-quality cassava flour is one of the major processed by-products from cassava root. It is gluten-free flour and beneficial in the treatment of coeliac patients (Biagi et al., Citation2009; Briani, Samaroo, & Alaedini, Citation2008). Cassava flour has high carbohydrate (CHO) content and is a source of high caloric food. Research has shown that the consumption of cassava-based food can help to support the nervous system and relieve stress, anxiety and irritable bowel syndrome (Baffour, Citation2009). This is accredited to the high energy value and CHO content. Flour obtained from improved cassava varieties (biofortified with high carotenoid pigment crops) are rich in carotenoids, which serves as a good and cheap source of vitamin A (Liu, Zheng, Ma, Gadidasu, & Zhang, Citation2011; Nassar & Ortiz, Citation2009). The nutritive importance of carotenoid plants is attributed to its conversion to vitamin A, as in the case of β-carotene, and to its antioxidant property (Sánchez et al., Citation2006). Therefore, optimum postharvest handling process is critical in maintaining the bioactive components of cassava flour (Uchechukwu-Agua, Caleb, & Opara, Citation2015).
Furthermore, cassava flour is a useful supplement in the production of baby food, pastas and glucose syrup (Nwabueze & Anoruoh, Citation2011). As composite flour, studies have shown that cassava flour is a better supplement to wheat flour compared to other root and tuber crops (Eddy, Udofia, & Eyo, Citation2007; Olaoye, Ade-Omowaye, Preedy, Watson, & Patel, Citation2011). Hence, it is used in the production of confectionaries in the food industries. The availability of cassava flour also helps to reduce dependency on wheat flour in developing countries (Gyedu-Akoto & Laryea, Citation2013; Kulchan, Boonsupthip, & Suppakul, Citation2010; Shittu, Dixon, Awonorin, Sanni, & Maziya-Dixon, Citation2008).
In recent years, consumers and food processors have shown considerable interest in the nutritional quality and safety of food (free of any environmental, chemical or microbial contamination) rather than the quantity of the product (Shobha, Kumar, Sreeramasetty, Gowda, & Shivakumar, Citation2012). Thus, optimum packaging and storage conditions are essential in maintaining desired quality attributes in food. Food powders (wheat flour, tea powder and whey permeate) exposed to 20°C and 66% relative humidity (RH) in a previous study were observed to have an increase in moisture content (MC), which resulted in increase in wall friction and caking of the flour (Iqbal & Fitzpatrick, Citation2006). This situation was ascribed to the hygroscopic nature of food powders. However, storage of soybean flour at 40°C and 90% RH had a significant influence on the flour quality, as the decrease in fat content was higher at the higher storage temperature than the ambient (25–35°C) during the 75-day storage duration (Agrahar-Murugkar & Jha, Citation2011). The authors attributed this observation to be the effect of high temperature on the lypolytic enzymes, thus causing a decrease in fat content of the bean flour. Similar studies have been carried out on cassava roots and its by-products (Chávez et al., Citation2007; Rodríguez-Sandoval, Fernández-Quintero, Cuvelier, Relkin, & Bello-Pérez, Citation2008; Sánchez et al., Citation2013), but there is limited information with regard to the influence of storage conditions and duration on the physicochemical properties of cassava flour. Therefore, considering the effect of storage on the physicochemical properties of cassava flour, it is paramount for the food scientist and industries to establish suitable storage conditions for packaged cassava flour. This research was therefore designed to evaluate the effects of storage conditions and duration on the proximate composition, physicochemical properties and microbial stability of cassava flour using two newly bred cultivars.
Materials and methods
Plant materials
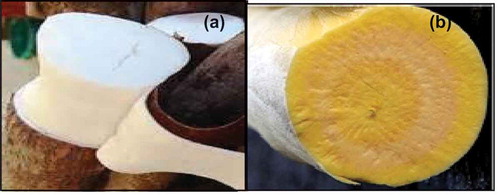
Cassava cultivars ‘TME 419’ and ‘UMUCASS 36’, white and yellow roots, respectively (), were harvested at commercial maturity 12 months after planting from National Root Crops Research Institute, Umudike (5º28′33″N 7º32′56″E), Abia state, Nigeria. The fresh cassava roots were harvested from a randomised complete block design field plot of three replications. These cultivars were selected based on their functional properties (paste stability) which are desirable for use in the food industries (Eleazu & Eleazu, Citation2012; Nwabueze & Anoruoh, Citation2011).
Cassava processing, packaging and storage
Clean cassava root (without breaks/cuts) were sorted, washed, peeled and re-washed with running tap water. The roots were sliced into chips of about 2–3 cm in length using electric stainless steel chips-making machines (YS QS400, Shandong, China) and sun-dried at temperature of about 40 ± 2°C for 3 days to a constant MC of about 13% (Falade & Akingbala, Citation2010). Dried cassava chips obtained from the two cultivars were packaged separately in sterile polyethylene bags and transported at ambient temperature to the Department of Food Science, Stellenbosch University, South Africa. The chips were milled into flour with a Cyclone Laboratory milling machine (Model 3100; Perten Instruments, Hagersten, Sweden) fitted with a sieve size of 0.5 mm. Baseline analysis of cassava flour quality attributes was carried out separately for both cultivars prior to packaging and storage.
The processed cassava flour from both cultivars was packaged (200 g) in separate brown paper bags under aseptic conditions. The packed flour were stored at selected environmental conditions using a test chamber (MLR-351H; Sanyo Electric, Osaka, Japan) as follows: cool condition (15 ± 1°C, 90% RH), ambient condition (23 ± 2°C, 60% RH) and higher temperature (38 ± 2°C, 60% RH) for 12 weeks. Cassava flour was taken from each treatment for analysis every 4 weeks until 12-week duration. All analyses for physicochemical properties, proximate composition and selected bioactive components were performed in triplicates on each sampling day.
Proximate compositions analysis
Moisture and ash contents were carried out according to the relevant Association of Official Analytical Chemist (AOAC, Citation2012) methods for moisture (925.09) and for ash (923.03). Protein content was calculated from % N using a conversion factor of 6.25 (N × 6.25) according to method 920.152 (AOAC, Citation2006). Fat content was determined through the Soxhlet extraction method (AOAC, Citation2006) using 70 mL petroleum ether as the extraction solvent. Percentage CHO content was calculated by difference Equation (1) (Nwabueze & Anoruoh, Citation2011). Energy value was calculated using Atwater factor Equation (2) (Akubor, Adamolekun, Oba, Obari, & Abudu, Citation2003). The dry matter was determined with Equation (3) (Etudaiye, Nwabueze, & Sanni, Citation2009).
Physicochemical analysis
Colour
Colour measurements were taken using a chromameter (CR-400/ 410; Konica Minolta Sensing Inc., Osaka, Japan) according to method described by Rhim and Hong (Citation2011). The instrument was calibrated against a white plate prior to use. The mean of the nine measurements per treatment was calculated. Total colour difference (ΔE) which indicates the magnitude of change in colour parameters between the initial and final colour values during storage was calculated using Equation (4) (Pathare, Opara, & Al-Said, Citation2013):
Furthermore, whiteness index (WI), which describes the total whiteness of food products and indicates the degree of discolouration or deterioration in some products during storage, was calculated (Hsu, Chen, Weng, & Tseng, Citation2003; Lin, Liu, Yu, Lin, & Mau, Citation2009). Whiteness is one of the major attributes the end users of flour desire either for domestic or for industrial uses, and it is often used to express the quality of the flour during storage (Rodriguez-Aguilera, Oliveira, Montanez, & Mahajan, Citation2011). Whiteness is based on the scale of 0–100, with higher values describing brighter colour appearance (Hsu et al., Citation2003). The WI of cassava flour was calculated using Equation (5) (Pathare et al., Citation2013):
Yellowness index (YI) could be used to express the level of yellow colouration especially in products rich in carotenoids (Rodriguez-Amaya, Nutti, & De Carvalho, Citation2011). The yellowness of fresh or minimally processed produce can degrade during processing and exposure to light during storage (Pathare et al., Citation2013). The extent of yellow degradation can be determined with Equation (6):
Water activity
The water activity (aw) of cassava flour was determined using Pawkit water activity meter (Decagon Devices, Inc., Pullman, Washington, DC, USA) at a constant temperature of 21°C. The standard procedure for the instrument was followed. The sample cups were half-filled with cassava flour to ensure accuracy of result. The Pawkit water activity meter was positioned over the cups and the water activity level was measured within 5 min.
Total carotenoids
Spectrophotometric method as described by Opiyo and Ying (Citation2005) with modifications was used to determine the total carotenoid of the cassava flour. According to Rodríguez-Amaya and Kimura (Citation2004), the determination of total carotenoids gives a more accurate estimate for trans-β-carotene; this is because about 90% of the total carotenoids present in cassava root is β-carotene. Sample preparation and readings were done under less intense light in the laboratory because of the sensitivity of carotenoids to light. Total carotenoids content (mg/100 g) was calculated and expressed on dry weight (DW) basis following Equation (7) (Opara & Al-Ani, Citation2010; Opiyo & Ying, Citation2005):
Hydrogen cyanide (HCN) determination
The alkaline picrate method was used with minor modification to quantify the total cyanide content in the cassava flour samples (Onwuka, Citation2005). Standard curve prepared by diluting potassium cyanide (KCN) in water acidified with HCL at different concentrations ranging from (0.01 to 0.05 µg/mL) was used to extrapolate the concentration of hydrogen cyanide (HCN) from y = (2.4x + 0.216) × 10, with R2 = 0.92, where y = concentration, 2.4 = slope of curve, X = absorbance, 0.216 = intercept and 10 is the dilution factor. Results were expressed as mean of triplicate values of each treatment in µg/mg.
Determination of pH
The pH levels of cassava flour were determined with a reference glass electrode pH meter basic 20+ (Crison, 52-01 PI1000, Barcelona, Spain). Cassava flour (10 ± 0.02 g) was dissolved in 10 mL of deionised water (Ogiehor & Ikenebomeh, Citation2006). The pH meter was first calibrated with buffer pH 4 and 7 by placing the electrode in each buffer and rinsing before placing in test samples.
Microbial analysis
Microbial analysis was done to determine the microbial stability of cassava flour over time. A weight of 10 g of cassava flour was aseptically taken from each bag and homogenised in 90 mL of the sterilised physiological solution. Five-fold serial dilution was done and each dilution was plated out in triplicate on a plate count agar for aerobic mesophilic bacteria and potato dextrose agar for mould and yeast using pour plate method. Plates were allowed to cool and then incubated at 37°C for 48 h and 26°C for 72 h for bacteria and yeast and mould count, respectively (Akhtar, Anjum, Rehman, Sheikh, & Farzana, Citation2008). Visible colonies were counted after incubation and the results were reported as log CFU/g. Sterile plates of both agars were open for 10 min around the work area and in the laboratory to ascertain the microbial state of the environmental.
Statistical analysis
Factorial analysis of variance was performed using Statistica software (Statistica 12.0; Statsoft Inc., Tulsa, OK, USA). The significant difference between the mean was determined with Duncan’s multiple range tests at 95% confidence interval. All results obtained for each cultivar and storage conditions were expressed as mean of triplicate values ± standard deviations.
Results and discussion
Proximate composition of fresh cassava flour
The initial compositions of cassava flour cvs. ‘TME 419’ and ‘UMUCASS 36’ showed variations in the cultivars (). Cultivar differences had a significant effect on the proximate composition (p < 0.05). CHO content was 85.5 ± 0.17% for ‘UMUCASS 36’ and 83.6 ± 0.09% for ‘TME 419’. Protein was generally low for both cultivars and significantly higher in ‘UMUCASS 36’ (3.0 ± 0.05%) than in ‘TME 419’ flour (2.0 ± 0.07%). Dry matter content was higher in ‘UMUCASS 36’ (90.2 ± 0.15%) than ‘TME 419’ (88.8 ± 0.11%). The average MC for both cultivars was 10.9%, cultivar (cv.) ‘UMUCASS 36’ was lower (9.78 ± 0.15) compared to ‘TME 419’. However, MC in both cultivars was within the recommended moisture range (12%) for flour products and within the range reported in literature for cassava flour (Charles, Sriroth, & Huang, Citation2005; Falade, Semon, Fadairo, Oladunjoye, & Orou, Citation2014; Shobha et al., Citation2012). Although similar processing and drying techniques were used, MC of the ‘TME 419’ flour was higher compared to ‘UMUCASS 36’ flour in this study. The observed variations in moisture could be attributed to the inherent attributes such as dry matter content of the different cultivars.
Table 1. Comparison of the proximate compositions of cassava flour cultivars ‘TME 419’ and ‘UMUCASS 36’ (dry weight basis).
Tabla 1. Comparativa de las composiciones aproximadas de los cultivares de harina de mandioca ‘TME 419’ y ‘UMUCASS 36’ (peso en seco).
Low MC in flour is essential during storage because it favours shelf life stability of flour (Eleazu, Elwazu, Awa, & Chukwuma, Citation2012). Higher MC >12% has been reported to accelerate or enhance microbial growth (Aryee, Oduro, Ellis, & Afuakwa, Citation2006). Therefore, both cultivars used for this study possess the potential of good storage quality although cv. ‘UMUCASS 36’ has a better prospect for longer shelf stability because of the lower moisture and higher dry matter contents.
Dry matter content (88.84 ± 0.11%) of cv. ‘TME 419’ was significantly lower than ‘UMUCASS.36’. This result was consistent with other research done on cassava flour and other cassava products (87.73–71.60%) from other cultivars (Eleazu & Eleazu, Citation2012; Etudaiye et al., Citation2009). Eleazu and Eleazu (Citation2012) in their study on proximate composition of cassava flour also observed that cv. ‘UMUCASS 36’ (the yellow root) had high dry matter content compared to white root (TMS98/0505) suggesting a better functional and cooking quality of the flour during food processing. Therefore, knowledge of dry matter content of flour is essential for the food industries as it could affect cooking time, quality of cooked food and shelf life stability of products.
Protein, fat and ash contents were lower in cv. ‘TME 419’ (2.0 ± 0.07%, 1.0 ± 0.07% and 1.2 ± 0.03%, respectively) compared to the ‘UMUCASS 36’ cultivar (3.0 ± 0.05%, 1.2 ± 0.05% and 1.6 ± 0.03%, respectively). This result was expected because cassava root is generally low in protein, fat and ash components. Previous studies on cassava flour by other researchers also observed low values for protein, fat and ash. Charles et al. (Citation2005) reported mean values of protein (1.5 ± 0.2%), fat (0.2 ± 0.1%) and ash (1.8 ± 0.6%) for five cassava flour cultivars from north Thailand. Similarly, Nwabueze and Anoruoh (Citation2011) reported low protein (2.6 ± 0.12%), fat (0.62 ± 0.1%) and ash (1.48 ± 0.07%) contents for cassava flour cv. ‘TME 419’. In addition, the disparity observed in these components was due to the differences in cassava variety. Aryee et al. (Citation2006) reported cultivar differences in proximate composition of 31 different cultivars of cassava flour; this in turn influenced the utilisation of the flour cultivars for food and industrial purposes.
The energy values for the both cassava flour cultivars were 1487.8 ± 0.05 kJ/kg (355.6 kcal/kg) and 1508.8 ± 0.34 kJ/kg (360.5 kcal/kg) for cultivars ‘TME 419’ and ‘UMUCASS 36’, respectively. Cassava flour from cv. ‘UMUCASS 36’ had higher caloric value consistent with the higher CHO content compared to the other cassava flour cultivars with substitution potential and cassava–wheat composite blend previously studied in literature (Jisha, Sheriff, & Padmaja, Citation2010). These findings highlight the need for critical evaluation of proximate compositions of different cultivars of cassava flour to assess their suitability in the food industries.
Physicochemical analysis
The pH range for the both cultivars was from 5.7 to 6.0, with cv. ‘TME 419’ having higher pH values than cv. ‘UMUCASS 36’. Similar ranges of pH (5.3 to 6.5) have been reported in literature for cassava flour (Aryee et al., Citation2006) and for other flour product from other root crops (Falade & Okafor, Citation2013). The pH value of flour is essential. For instance, it provides a guide for the ratio of cassava flour that could be substituted when mixing composite flour for baking (Aryee et al., Citation2006).
Colour attributes of both cultivars before storage were as follows: L* (lightness) value for cv. ‘TME 419’ was 90.2 ± 0.73 while cv. ‘UMUCASS 36’ was 87.7 ± 0.60. Values were significantly different in both cultivars because of their genetic make-up, ‘TME 419’ (white cultivar) and ‘UMUCASS 36’ (yellow cultivar). Falade et al. (Citation2014) reported similar range in L* value (86.54–92.13) for Africa rice flour, thus confirming considerable lightness of flour products for the food industries. The colour parameters of two traditionally processed Burundian cassava flour showed similar range of L* value (97–74) which was attributed to the long fermentation period (Aloys & Hui Ming, Citation2006). The authors reported that fermentation just like the other processing method (drying) could have a profound influence on the colour attributes of cassava flour.
The initial WIs of both cassava flours were 85.1 ± 0.72 for cv. ‘TME 419’ and 78.8 ± 1.86 for cv. ‘UMUCASS 36’. The higher WI of cultivar ‘TME 419’ flour is not surprising given its natural white colour compared with the WI of cv. ‘UMUCASS 36’. Similarly, the YI of cv. ‘UMUCASS 36’ was higher (28.19 ± 2.31) due to the carotenoid pigment compared to the cv. ‘TME 419’ (17.80 ± 0.86) which is white colour. The values obtained in this present study were consistent with those reported in literature for white yam flour processed under different drying methods (45.8 ± 0.15 to 85.7 ± 0.07) (Hsu et al., Citation2003). Additionally, the authors established that colour and carotenoid retention can be influenced by the drying method employ during processing. Thus, the distinction in WI and YI observed in our study could be attributed to the differences in cultivars, carotenoid content and the effect of sun-drying during chips processing. Carotenoid degradation of both flour cultivars resulted in reductions in the YIs because carotenoid pigments are denoted with yellow colour in food.
Effects of storage conditions on the proximate composition of cassava flour
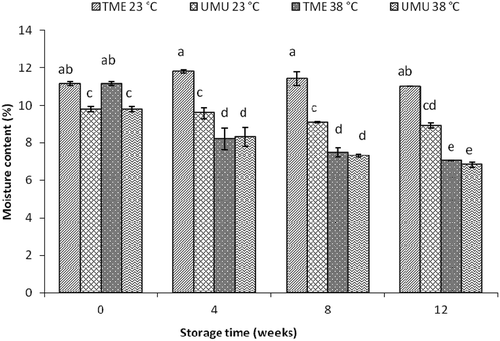
summarises the effects of storage conditions and durations on proximate composition of the two cassava flour cultivars (‘TME 419’ and ‘UMUNCASS 36’). MC in both flour cultivars decreased with increase in storage time. The lowest value in MC (6.83 ± 0.13%) was observed in cv. ‘UMUCASS 36’ under higher condition because of its high dry matter content. Further analysis showed that storage conditions (temperature and RH) had a significant impact on the MC of cassava flour in both cultivars. The reduced trend followed for MC during the storage period in both cultivars was consistent with literature findings for wheat flour and other food powders (Iqbal & Fitzpatrick, Citation2006). Iqbal and Fitzpatrick (Citation2006) evaluated the effect of temperatures (5°C, 15°C and 30°C) on the MC of three food powders and observed the lowest value (12.5%) at 30°C. The authors also noticed an increase in MC at higher RH (60%) which was similar with the observation in this study at 15°C, 90% RH. According to Charles et al. (Citation2005), the observed moisture range in this study after 12-week storage (6.8 ± 0.13 to 11.9 ± 0.08) shows that both cassava flour cultivars are moderately hygroscopic. The low moisture level reported after the storage of both cassava flour cultivars () suggests longer shelf-life stability; however, processing methods, storage conditions and type of packaging materials should be considered during storage for better quality.
Table 2. Changes in proximate compositions of cassava flour under different storage conditions (dry weight basis).
Tabla 2. Cambios en las composiciones aproximadas de harina de mandioca bajo diferentes condiciones de almacenamiento (peso en seco).
The highest moisture value (11.8 ± 0.08%) was observed in cv. ‘TME 419’ flour at week 4 of storage at ambient conditions (23°C, 60% RH), which later declined with storage duration (). However, the reduction in MC observed for both cvs. ‘TME 419’ (12.0 ± 0.11 to 7.1 ± 0.01%) and ‘UMUCASS 36’ (9.8 ± 9.15 to 6.8 ± 0.13%) was consistent at higher condition. This suggests that storage at 38°C could help maintain the MC of cassava flour below critical level (12%) during storage. However, storage under higher temperature (38–45°C) has been shown to negatively impact nutritional contents in flour products during long storage (Agrahar-Murugkar & Jha, Citation2011). Ambient storage condition (23 ± 2°C, 60% RH) would best retain quality attributes and maintain shelf-life stability of cassava flour for more than 12 weeks of storage. Cool storage condition (15°C, 90% RH) was not effective, as flour absorbed moisture from the environment which led to mould growth on the paper bag before the 4th week of storage.
Figure 2. Changes in moisture content in cassava flour during storage at 23°C and 38°C, 60% RH. Similar letters in superscript are not significantly different between data points (p < 0.05).
Figura 2. Cambios en el contenido de humedad en la harina de mandioca durante almacenamiento a 23 y 38 °C, 60% RH. Las letras de superíndice similares no muestran diferencias significativas entre los datos (p < 0,05).
Ash content gives a quantitative estimation of the minerals available in a given food product (Eleazu et al., Citation2012). Knowledge of the ash content in flour is essential because it allows the milling industries to estimate the expected flour yield as well as identify the milling functionality of flour (Park & Henneberry, Citation2010). Ash content of ‘TME 419’ flour (1.60 ± 0.03%) was not significantly higher than ‘UMUCASS 36’ (1.37 ± 0.34%) at the end of storage. This result falls within the literature range of 1.3–2.8% ash content of different cassava flour cultivars (bitter and sweet) (Charles et al., Citation2005). From this study, we can deduce that high ash content in white flour could influence the brightness and quality appearance of the final product such as white bread. However, for products like brown bread and whole wheat snack, high ash content is recommended.
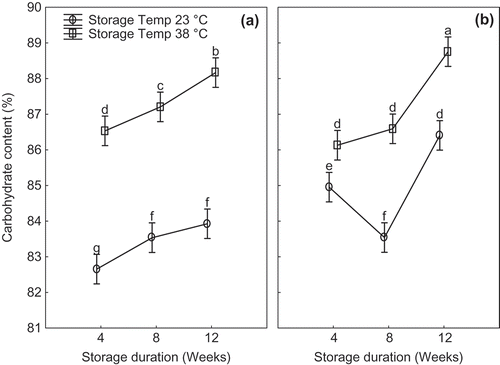
At the end of the 12-week storage, the protein content in both flour cultivars decreased by 34.0% for ‘TME 419’ and 25.1% for cv. ‘UMUCASS 36’. The observed decrease could be attributed to the decrease in MC, which resulted in the decreased protein concentration on DW basis. This trend agrees with literature on the influence of MC on the protein content of soybean flour as increase in moisture led to increase in protein concentration (Agrahar-Murugkar & Jha, Citation2011). In addition, the decline in protein could be attributed to the decrease in the activities of the microorganism as moisture reduced (Butt, Nasir, Akhtar, & Sharif, Citation2004). The fat content decreased with storage duration across treatments. The decrease in fat could be attributed to the lipolytic activities of the enzyme lipase and lipoxidase (Agrahar-Murugkar & Jha, Citation2011), which resulted in the decline in fat content. CHO content ranged from 83.6% to 88.0% in cv. ‘TME 419’ and from 85.45% to 88.76% in cv. ‘UMUCASS 36’. Variation in CHO content could be attributed to the cultivar differences, as well as the level of MC of flour (Charles et al., Citation2005). It is expected that cultivar with lower MC will have higher CHO, because of the differences in the percentage of other proximate compositions (Nwabueze & Anoruoh, Citation2011). Values of CHO obtained at the end of 12-week storage (88.17% and 88.76%) for cvs. ‘TME 419’ and ‘UMUCASS 36’, respectively, were higher than the base line. This outcome was consistent with reports in literature on CHO content of five different cultivars of cassava flour (Charles et al., Citation2005). In addition, the interaction between cultivars, storage conditions and duration had significant effect on percentage CHO (p = 0.004) ().
Figure 3. Interactions between cultivar, storage conditions and duration on the change in percentage carbohydrate content for (a) ‘TME 419’, (b) ‘UMUCASS 36’ cassava. Data points with similar letters are not significantly different according to Duncan LSD test (p < 0.05).
Figura 3. Interacciones entre cultivares, condiciones y duración de almacenamiento en el cambio de porcentaje del contenido en carbohidratos para (a) ‘TME 419’, (b) ‘UMUCASS 36’, mandioca. Los datos con letras similares no muestran diferencias significativas según el test LSD de Duncan (p < 0,05).
The energy values of cassava flour during the storage period were generally high in the range of 1514.2–1553.3 kJ/kg (361.9–371.2 equivalents in kcal/kg). Etudaiye et al. (Citation2009) also reported high energy value for fermented cassava flour ‘fufu’. Therefore, cassava root and by-products are a good source of high caloric food. Storage conditions and duration had no significant effect on the energy values of cassava flour.
Effects of storage on the physicochemical properties of cassava flour
Colour parameters
The measured Commission International de l’Eclairage colour parameters (L* a* b*) changed significantly over the duration of storage and both cultivars were significantly different. gives a summary on the colour parameter. The L* and b* values for both cultivars after storage ranged from 84.6 ± 0.28 to 91.2 ± 2.30. Both cassava flour cultivars had appreciable lightness in flour after storage. However, cv. ‘TME 419’ had the lowest L* value (84.6 ± 0.28) under higher condition, which could be attributed to the degradation in whiteness enhanced at higher temperature. Similar result range of L* (84.64–90.64) and b* (10.74–14.29) was reported in literature for cocoyam, taro, corn and potato flour (Falade & Okafor, Citation2013; Kaur, Kaushal, & Sandhu, Citation2013). The L* value was highest in cultivar ‘UMUCASS 36’ (91.2 ± 2.30) under higher condition after storage because of the level of the loss in yellow pigment and consequently a lighter colour. Yellowness denoted by b* was observed to be higher in cv. ‘UMUCASS 36’ (12.55 ± 1.02) than in cv. ‘TME 419’ (10.74 ± 0.53). Variation in b* value of flour was reported to be a function of the level of protein and the CHO values in the different flour cultivars (Kaur et al., Citation2013). Thus, the decrease observed in b* value could possibly be due to degradation of yellow pigment, cultivar differences and the significant variations in CHO values.
Table 3. Colour analysis of cassava flour stored at different storage conditions (23°C and 38°C, 60% RH).
Tabla 3. Análisis del color de la harina de mandioca almacenada bajo diferentes condiciones de almacenamiento (23 y 38°C, 60% RH).
The total colour difference (ΔE) of cassava flour during the storage period ranged from 2.3 to 5.6 in cv. ‘TME 419’ and from 5.0 to 6.4 in cv. ‘UMUCASS 36’. Colour change increased across the storage duration, and the highest change in colour was observed on flours stored at 38°C. This highlights on the impact of high temperature storage conditions on the colour quality of cassava flour. The percentage colour difference after the storage period was about 59.5% for cv. ‘TME 419’ and 21.9% for cv. ‘UMUCASS 36’. This observation showed that colour degradation was faster in the white cv. ‘TME 419’ than yellow cv. ‘UMUCASS 36’, which implies that ‘UMUCASS 36’ flour cultivar best maintained cassava flour colour. Degradation of colour during storage could also indicate loss of nutritional values due to the auto-oxidation reaction of the anthocyanin and β-carotene (Kaur et al., Citation2013).
The WI of the cassava flour varied from 81.3 ± 0.47 to 85.1 ± 0.02 in cv. ‘TME 419’ while cv. ‘UMUCASS 36’ ranged from 78.7 ± 0.47 to 84.5 ± .0.53 (). The WI for cv. ‘TME 419’ decreased during storage, while about 6% increase in whiteness was observed in cv. ‘UMUCASS 36’ after the 12-week storage period. The decrease in whiteness in cv. ‘TME 419’ would be attributed to the oxidation reactions during storage while the increase in whiteness in cv. ‘UMUCASS 36’ was as a result of oxidation and degradation of carotenoid pigment in the flour. The lowest decrease in whiteness was observed at ambient condition at the last storage week compared to the higher condition and the baseline value prior to storage. Hence, ambient storage condition (23ºC, 60% RH) could be suggested for better stability for whiteness of stored flour. In addition, the variation in whiteness in both cultivars of cassava flour during the storage period could be accredited to the genotypic difference of the cultivar. However, change in yellowness of flour of cv. ‘UMUCASS 36’ could be attributed to the degradation of the yellow pigment (Lin et al., Citation2009). Storage duration had an impact on the whiteness of cassava flours in this study and there was significant difference between the two cultivars. This finding agrees with the observation reported by Hsu et al. (Citation2003). The authors observed that drying had an impact on the WI of white yam flour. This observation should be monitored during storage because higher WI of the flour influences consumers’ acceptability.
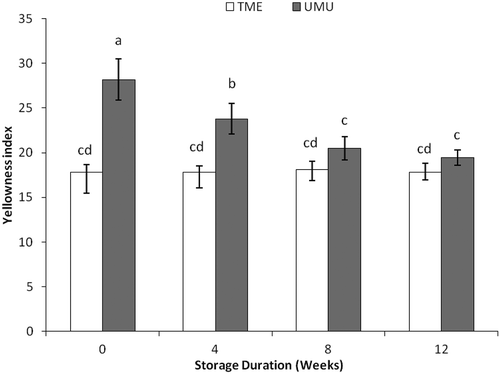
The YI of cassava flour ranged from 17.8 to 18.1 in cv. ‘TME 419’ and from 28.2 to 19.3 in cv. ‘UMUCASS 36’. Decrease in yellowness over time was significant in cv. ‘UMUCASS 36’ at ambient condition (). This decrease resulted in the corresponding increase in the whiteness observed in cv. ‘UMUCASS 36’, which infers corresponding degradation of carotenoids in the flour. Therefore, from this study, yellow colour degradation could be as a result of the intensity of exposure to light during processing or storage duration; thus, the need to reduce light intensity during processing is essential.
Figure 4. Changes in yellowness index of cassava flour cvs. ‘TME 419’ and ‘UMUCASS 36’ stored at 23°C. Similar letters are not significantly different (p < 0.05).
Figura 4. Los cambios en el índice de color amarillento de la harina de mandioca cv. ‘TME 419’ y ‘UMUCASS 36’ almacenados a 23°C. Las letras similares no muestran diferencias significativas (p < 0,05).
Total carotenoids content
Carotenoid-rich plants contain antioxidant components which offer various health benefits, such as reducing the risk of cardiovascular diseases, cancer and other degenerating diseases (Eleazu et al., Citation2012). Prior to flour packaging and storage, the total carotenoid content of both cassava flour cultivars was 1.8 ± 0.11 and 2.5 ± 0.10 mg/100 g, for cv. ‘TME 419’ and ‘UMUCASS 36’, respectively. The yellow cv. ‘UMUCASS 36’ had higher carotenoid content than the white cv. ‘TME 419’. The levels of carotenoids in both cassava flour cultivars were lower when compared to the cassava root (Chávez et al., Citation2007). The ranges were from 1.8 ± 0.11 to 0.84 ± 0.01 mg/100 g for cv. ‘TME 419’ and from 2.5 ± 0.10 to 1.1 ± 0.04 mg/100 g for cv. ‘UMUCASS 36’. The highest retention was observed under ambient condition in both cultivars ().
Figure 5. Effects of storage temperature and duration on carotenoid contents of two cassava flour cultivars (‘TME 419’ and ‘UMUCASS 36’). Data points with similar letters are not significantly different.
Figura 5. Efectos de la temperatura de almacenamiento y la duración en los contenidos en carotenoides de los dos cultivares de harina de mandioca (‘TME 419’ y ‘UMUCASS 36’). Los datos con letras similares no muestran diferencias significativas.
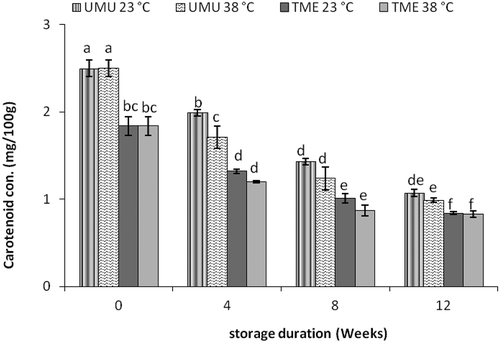
The lower carotenoid content in cassava flour could be attributed to degradation during processing and exposure to light and oxygen during postharvest handling and storage (Chavasit, Pisaphab, Sungpuag, Jittinandana, & Wasantwisut, Citation2002; Chávez et al., Citation2007; Oliveira, Carvalho, Nutti, Carvalho, & Fukuda, Citation2010; Opara & Al-Ani, Citation2010). Other researchers reported that carotenoid pigments especially β-carotene which is predominant in cassava are sensitive to light and can be affected by processing methods like sun-drying (Bechoff et al., Citation2009). A study investigating the loss of carotenoids during drying of orange-fleshed sweet potato and cassava chips also reported highest percentage loss of carotenoid in sun-drying in comparison to other drying methods (Rodriguez-Amaya et al., Citation2011). Furthermore, an evaluation study on the degradation of carotenoid during storage of Einkorn and bread wheat flours deduced that the retention of carotenoids in flour is a function of storage temperature and duration (Hidalgo, Brandolini, & Pompei, Citation2009). The authors also observed degradation of carotenoid with increase in storage time.
Therefore, we can attribute the decrease in concentration of carotenoids in the flour especially on the yellow cultivar to be degradation caused through the processing methods employed (chipping, sun-drying and milling). In addition, the higher temperature (38°C) had highest drop in carotenoid concentration than the lower temperature (23°C). From this result, we can conclude that carotenoid pigment would best be maintained at ambient temperature (23 ± 2°C).
Hydrogen cyanide content
After processing the cassava flour, the amount of cyanide retained in the flour was about 3.9 ± 0.06 µg/mL in cv. ‘TME 419’ and 4.9 ± 0.21 µg/mL in cv. ‘UMUCASS 36’. The yellow cultivar ‘UMUCASS 36’ was slightly higher in HCN retention than the white cultivar ‘TME 419’. The range of total cyanide retained in both cultivars was lower than the reported concentration for fresh cassava root parenchyma (1–1550 mg/kg) and (900–2000 mg/kg) in the root cortex (Cardoso et al., Citation2005). Both cultivars were significantly lower in cyanide concentration compared to the reported lethal dose for cyanide in human (50–300 mg/kg) body weight (Akiyama et al., Citation2006). The reduction in HCN could be credited to the processing methods which include chipping and sun-drying as both methods had been reported in literature to quicken the rate of linamarin breakdown and cyanogen reduction (Eleazu & Eleazu, Citation2012).
The observed differences in HCN levels could also be attributed to the difference in pH values of both cultivars. For instance, Cumbana, Mirione, Cliff, and Bradbury (Citation2007) evaluated the effect of pH on the HCN retention in cassava flour and concluded that near neutral pH value (6.2–6.7) of flour will lead to corresponding decrease in HCN concentration. Thus, the HCN concentration was lower in cv. ‘TME 419’ which had the highest pH value after 12 weeks of storage. The reduction in cyanide level of flour confirms previous reports that processing cassava root helps to reduce the level of cyanide in the product and improve palatability of cassava products (Cumbana et al., Citation2007; Nwabueze & Anoruoh, Citation2011). The milling process of flour from cassava chips created more surface area, thus enhancing the breakdown of the cyanognic compound in the flour.
Total cyanide retained in both cultivars after processing falls within the safe level (0–50 mg/kg) for human consumption as justified in literature (Akiyama et al., Citation2006). This comparison of both cultivars in this study showed that cultivar differences as well as pH had an influence on the cyanide level of cassava flour. A slight decrease in HCN was observed during the storage duration and the rate of decrease was higher in flours stored at 38°C than at ambient temperature (23°C). This observation correlates with reports in literature on the effect of storage temperature and duration on HCN level in cassava flour and other flour products (Burns, Bradbury, Cavagnaro, & Gleadow, Citation2012; Eleazu & Eleazu, Citation2012). The total HCN content retained in both cassava flour cultivars at the end of 12-week storage period ranged from 3.3 ± 0.14 to 4.4 ± 0.05 µg/mL, with the highest values observed under ambient condition in cv. ‘UMUCASS 36’. Therefore, from the result of this study, the concentration of cyanide retained in the flour before and after 12-week storage may not confer any toxic effect to the users as it falls below the safe limit for cyanide in human foods.
Microbial stability of cassava flour during storage
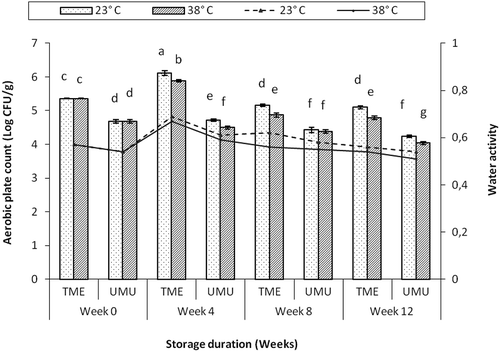
Effects of storage condition (temperature and RH) and duration on the microbial stability of cassava flour were monitored for 12 weeks. To determine the microbial stability of cassava flour stored in the cool storage conditions (15°C, 90% RH), a weekly evaluation was conducted on weeks 0, 1 and 2, in order to monitor the accelerated microbial growth prior to spoilage and visible decay. The observed colonies at the first week were higher than the microbial level on the initial week (fresh/unpacked) samples (5.4 ± 0.0–5.6 ± 0.04 log CFU/g) for total aerobic mesophilic bacteria and (5.0 ± 0.04–5.2 ± 0.02 log CFU/g) for yeast and mould. Similar trends were also observed in the cultivar ‘UMUCASS 36’. By week 2, significant increase in microbial load was observed in both cultivars (6.3 ± 0.05 log CFU/g) for total aerobic mesophilic bacteria and (5.6 ± 0.03 log CFU/g) for yeast and mould. And the flour had slight discolouration. The values were already higher than the acceptable microbial limits (250 g), 2.4 log CFU/g for South Africa (DOH, Citation2001; HPA, Citation2009). Visual observation of mould growth and decay was first noticed on the packages stored under cool condition after the 3rd week of storage. The observed decay could be attributed to the effect of high RH, which contributed to increase in water activity and percentage MC of the flour (). This condition consequently enhanced the growth of microorganisms. Therefore, paper bag storage at cool condition will result in shelf-life instability and unwholesome flour because of the high microbial growth. Hence, this situation clearly indicates loss of flour quality and generally unacceptable for domestic and industrial uses.
The total aerobic mesophilic bacteria load in cassava flours stored at 23°C and 38°C were significantly influenced by aw level of the flour (). At week 4 of storage, the colony forming unit of cv. ‘TME 419’ increased significantly from 5.4 ± 0.01 to 6.1 ± 0.07 log CFU/g as the water activity level increased from 0.57 ± 0.01 to 0.68 ± 0.00. Subsequently, the microbial load decreased with the decrease in aw of flour to 5.1 ± 0.04 and 4.8 ± 0.05 log CFU/g under ambient and higher storage temperature, respectively, at the end of week 12.
Figure 6. Effects of water activity and storage duration on the growth of aerobic mesophilic bacteria in cassava flour cultivars ‘TME419’ and ‘UMUCASS 36’. Similar letters are not significantly different (p < 0.05).
Figura 6. Efectos de la actividad del agua y duración del periodo de almacenamiento en la aparición de bacterias mesófilas aerobias en los cultivares de harina de mandioca ‘TME419’ y ‘UMUCASS 36’. Las letras similares no muestran diferencias significativas (p < 0,05).
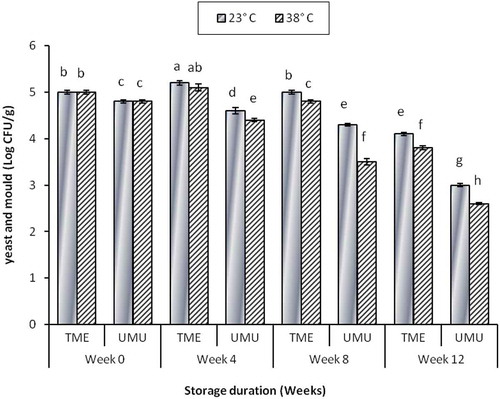
Yeast and mould also were observed to decrease with the increase in storage duration at higher temperature. Similar trend was observed in both cultivars, but cv. ‘TME 419’ had higher yeast and mould counts at the last storage period (4.1 ± 0.04 log CFU/g) at 23°C and (3.8 ± 0.04 log CFU/g) at 38°C (). Yeast and mould count observed at 23°C agrees with the evaluation of flour stored at ambient conditions (Berghofer, Hocking, Miskelly, & Jansson, Citation2003). In addition, the yeast and mould count at the end of storage was within the acceptable limit of 4.0 log CFU/g (HPA, Citation2009). Microbial load decreased during weeks 8 and 12 of storage. Storage temperature and cultivar differences had significant effect on the microbial load. This result was consistent with observation in literature. Reduced moisture level and low aw have been reported to retard microbial growth (Padonou, Hounhouigan, & Nago, Citation2009).
Figure 7. Effects of storage temperature and duration on yeast and mould count of cassava flour cultivars ‘TME419’ and ‘UMUCASS 36’. Letters that are similar are not significantly different (p < 0.05).
Figura 7. Efectos de la temperatura y la duración de almacenamiento en el recuento de levaduras y hongos en los cultivares de harina de mandioca ‘TME419’ y ‘UMUCASS 36’. Las letras que son similares no muestran diferencias significativas (p < 0,05).
Conclusion
Based on the outcome of this study, quality attributes of cassava flour can be significantly influenced by proper cultivar selection, storage conditions and duration. Storage conditions (temperature and RH) affected the physicochemical properties of cassava flour during the 12-week storage period. Total carotenoid and HCN concentration significantly decreased with the length of storage. This reduction in carotenoid concentration during storage is a concern for enjoying the health benefits of carotenoid as vitamin A precursor. This is attributed to the sensitivity of carotenoid to light and oxygen. Therefore, food processors and industries should adopt appropriate measures during processing and storage to ensure adequate retention of carotenoid in cassava flour. Reduction in HCN content in the cassava flour at the end of 12-week storage corroborate with other research findings that processing cassava root into flour and other products helps to reduce the HCN content.
Furthermore, the influence of storage conditions (temperature and RH) on microbial stability, MC and water activity level of cassava flour were significant. The decrease observed in microbial load during the storage duration is favourable for shelf-life stability of cassava flour. Highest decrease in moisture and water activity level was noticed under higher storage condition (38°C, 60% RH), which also resulted in low microbial count in cassava flour at the end of 12-week storage. This observation confirms that hot storage condition will be best to stabilise MC and extend shelf-life stability except that it causes significant loss of essential nutrients. Based on this study, it can be concluded that ambient storage (23°C, 60% RH) could best maintain flour quality of the cassava cultivars investigated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agrahar-Murugkar, D., & Jha, K. (2011). Influence of storage and packaging conditions on the quality of soy flour from sprouted soybean. Journal of Food Science and Technology, 48, 325–328. doi:10.1007/s13197-011-0242-2
- Akhtar, S., Anjum, F. M., Rehman, S.-U., Sheikh, M. A., & Farzana, K. (2008). Effect of fortification on physico-chemical and microbiological stability of whole wheat flour. Food Chemistry, 110, 113–119. doi:10.1016/j.foodchem.2008.01.065
- Akiyama, H., Toida, T., Sakai, S., Amakura, Y., Kondo, K., Sugita-Konishi, Y., & Maitani, T. (2006). Determination of cyanide and thiocyanate in sugihiratake mushroom using hplc method with fluorometric detection. Journal of Health Science, 52, 73–77. doi:10.1248/jhs.52.73
- Akubor, P. I., Adamolekun, F. O., Oba, C. A., Obari, H., & Abudu, I. O. (2003). Chemical composition and functional properties of cowpea and plantain flour blends for cookie production. Plant Foods for Human Nutrition, 58, 1–9. doi:10.1023/B:QUAL.0000041160.25384.f6
- Aloys, N., & Hui Ming, Z. (2006). Traditional cassava foods in burundi—A review. Food Reviews International, 22, 1–27. doi:10.1080/87559120500379761
- AOAC. (2006). Official methods of analysis (18th ed.; W. Horwitz, Ed.). Gaithersberg, MD: Association of Official Analytical Chemists.
- AOAC. (2012). Official methods of analysis (18th ed.; W. Horwitz, Ed.). Gaithersberg, MD: Association of Official Analytical Chemists.
- Aryee, F. N. A., Oduro, I., Ellis, W. O., & Afuakwa, J. J. (2006). The physicochemical properties of flour samples from the roots of 31 varieties of cassava. Food Control, 17, 916–922. doi:10.1016/j.foodcont.2005.06.013
- Baffour, T. (2009, December 30). Health news of Wednesday. Retrieved from http://www.ghanaweb.com
- Bechoff, A., Dufour, D., Dhuique-Mayer, C., Marouzé, C., Reynes, M., & Westby, A. (2009). Effect of hot air, solar and sun drying treatments on provitamin a retention in orange-fleshed sweet potato. Journal of Food Engineering, 92, 164–171. doi:10.1016/j.jfoodeng.2008.10.034
- Berghofer, L. K., Hocking, A. D., Miskelly, D., & Jansson, E. (2003). Microbiology of wheat and flour milling in Australia. International Journal of Food Microbiology, 85, 137–149. doi:10.1016/S0168-1605(02)00507-X
- Biagi, F., Andrealli, A., Bianchi, P. I., Marchese, A., Klersy, C., & Corazza, G. R. (2009). A gluten-free diet score to evaluate dietary compliance in patients with coeliac. British Journal of Nutrition, 102, 882–887.
- Briani, C., Samaroo, D., & Alaedini, A. (2008). Celiac disease: From gluten to autoimmunity. Autoimmunity Reviews, 7, 644–650. doi:10.1016/j.autrev.2008.05.006
- Burns, A. E., Bradbury, J. H., Cavagnaro, T. R., & Gleadow, R. M. (2012). Total cyanide content of cassava food products in Australia. Journal of Food Composition and Analysis, 25, 79–82. doi:10.1016/j.jfca.2011.06.005
- Butt, M. S., Nasir, M., Akhtar, S., & Sharif, K. (2004). Effect of moisture and packaging on the shelf life of wheat flour. Internet Journal of Food Safety, 4, 1–6.
- Cardoso, A. P., Mirione, E., Ernesto, M., Massaza, F., Cliff, J., Rezaul, H. M., & Bradbury, J. H. (2005). Processing of cassava roots to remove cyanogens. Journal of Food Composition and Analysis, 18, 451–460. doi:10.1016/j.jfca.2004.04.002
- Charles, A., Sriroth, K., & Huang, T. (2005). Proximate composition, mineral contents, hydrogen cyanide and phytic acid of 5 cassava genotypes. Food Chemistry, 92, 615–620. doi:10.1016/j.foodchem.2004.08.024
- Chavasit, V., Pisaphab, R., Sungpuag, P., Jittinandana, S., & Wasantwisut, E. (2002). Changes in β-carotene and vitamin a contents of vitamin a-rich foods in Thailand during preservation and storage. Journal of Food Science, 67, 375–379. doi:10.1111/j.1365-2621.2002.tb11413.x
- Chávez, A. L., Sánchez, T., Ceballos, H., Rodriguez-Amaya, D. B., Nestel, P., Tohme, J., & Ishitani, M. (2007). Retention of carotenoids in cassava roots submitted to different processing methods. Journal of the Science of Food and Agriculture, 87, 388–393. doi:10.1002/(ISSN)1097-0010
- Cumbana, A., Mirione, E., Cliff, J., & Bradbury, J. H. (2007). Reduction of cyanide content of cassava flour in Mozambique by the wetting method. Food Chemistry, 101, 894–897. doi:10.1016/j.foodchem.2006.02.062
- Department of Health (DOH). (2001). Guideline for environmental health officers on the interpretation of microbiological analysis data of food. South Africa: Department of Health Directorate. Food control.
- Eddy, N., Udofia, P., & Eyo, D. (2007). Sensory evaluation of wheat/cassava composite bread and effect of label information on acceptance and preference. African Journal of Biotechnology, 6, 2415–2418.
- Eleazu, C., & Eleazu, K. (2012). Determination of the proximate composition, total carotenoid, reducing sugars and residual cyanide levels of flours of 6 new yellow and white cassava (Manihot esculenta Crantz) varieties. Varieties American Journal of Food Technology, 7, 642–649. doi:10.3923/ajft.2012.642.649
- Eleazu, C., Eleazu, K., Awa, E., & Chukwuma, S. (2012). Comparative study of the phytochemical composition of the leaves of five Nigerian medicinal plants. Journal of Biotechnology and Pharmaceutical Research, 3, 42–46.
- Etudaiye, H., Nwabueze, T., & Sanni, L. (2009). Quality of fufu processed from cassava mosaic disease (CMD) resistant varieties. African. Journal of. Food Science, 3, 061–067.
- Falade, K. O., & Akingbala, J. O. (2010). Utilization of cassava for food. Food Reviews International, 27, 51–83. doi:10.1080/87559129.2010.518296
- Falade, K. O., & Okafor, C. A. (2013). Physicochemical properties of five cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) starches. Food Hydrocolloids, 30, 173–181. doi:10.1016/j.foodhyd.2012.05.006
- Falade, K. O., Semon, M., Fadairo, O. S., Oladunjoye, A. O., & Orou, K. K. (2014). Functional and physico-chemical properties of flours and starches of African rice cultivars. Food Hydrocolloids, 39, 41–50. doi:10.1016/j.foodhyd.2013.11.002
- Gyedu-Akoto, E., & Laryea, D. (2013). Evaluation of cassava flour in the production of cocoa powder-based biscuits. Nutrition & Food Science, 43, 55–59. doi:10.1108/00346651311295914
- Health Protection Agency (HPA). (2009). Guideline for assessing the microbiological safety of ready-to-eat food. Health Protection Agency London: UK Printers.
- Hidalgo, A., Brandolini, A., & Pompei, C. (2009). Kinetics of tocols degradation during the storage of einkorn (Triticum monococcum L. Ssp. monococcum) and breadwheat (Triticum aestivum L. Ssp. aestivum) flours. Food Chemistry, 116, 821–827. doi:10.1016/j.foodchem.2009.01.075
- Hsu, C. L., Chen, W., Weng, Y. M., & Tseng, C. Y. (2003). Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chemistry, 83, 85–92. doi:10.1016/S0308-8146(03)00053-0
- Iqbal, T., & Fitzpatrick, J. J. (2006). Effect of storage conditions on the wall friction characteristics of three food powders. Journal of Food Engineering, 72, 273–280. doi:10.1016/j.jfoodeng.2004.12.007
- Jisha, S., Sheriff, J. T., & Padmaja, G. (2010). Nutritional, functional and physical properties of extrudates from blends of cassava flour with cereal and legume flours. International Journal of Food Properties, 13, 1002–1011. doi:10.1080/10942910902934090
- Kaur, M., Kaushal, P., & Sandhu, K. S. (2013). Studies on physicochemical and pasting properties of taro (Colocasia esculenta l.) flour in comparison with a cereal, tuber and legume flour. Journal of Food Science and Technology, 50, 94–100. doi:10.1007/s13197-010-0227-6
- Kulchan, R., Boonsupthip, W., & Suppakul, P. (2010). Shelf life prediction of packaged cassava-flour-based baked product by using empirical models and activation energy for water vapor permeability of polyolefin films. Journal of Food Engineering, 100, 461–467. doi:10.1016/j.jfoodeng.2010.04.031
- Lin, L.-Y., Liu, H.-M., Yu, Y.-W., Lin, S.-D., & Mau, J.-L. (2009). Quality and antioxidant property of buckwheat enhanced wheat bread. Food Chemistry, 112, 987–991. doi:10.1016/j.foodchem.2008.07.022
- Liu, J., Zheng, Q., Ma, Q., Gadidasu, K. K., & Zhang, P. (2011). Cassava genetic transformation and its application in breeding. Journal of Integrative Plant Biology, 53, 552–569. doi:10.1111/jipb.2011.53.issue-7
- Nassar, N. M., & Ortiz, R. (2009). Five cassava genetic resources: Manipulation for crop improvement. Plant Breeding Reviews, 31, 247.
- Nwabueze, T. U., & Anoruoh, G. A. (2011). Evaluation of flour and extruded noodles from eight cassava mosaic disease (CMD)-resistant varieties. Food and Bioprocess Technology, 4, 80–91. doi:10.1007/s11947-009-0200-4
- Ogiehor, I., & Ikenebomeh, M. (2006). The effects of different packaging materials on the shelf stability of garri. African Journal of Biotechnology, 5, 741–745.
- Olaoye, O., Ade-Omowaye, B., Preedy, V., Watson, R., & Patel, V. (2011). Composite flours and breads: Potential of local crops in developing countries. In V. R. Preedy, R. R. Watson, & V. B. Patel (Eds.), Flour and breads and their fortification in health and disease prevention (pp. 183–192). London: Academic press.
- Oliveira, A., Carvalho, L., Nutti, R. M., Carvalho, J., & Fukuda, W. G. (2010). Assessment and degradation study of total carotenoids and b-carotene in bitter yellow cassava (Manihot esculenta Crantz) varieties. African Journal of Food Science, 4, 148–155.
- Onwuka, G. (2005). Food analysis and instrumentation: Theory and practice. Food Science Journal, 8, 3–35.
- Opara, U. L., & Al-Ani, M. R. (2010). Effects of cooking methods on carotenoids content of Omani kingfish (Scomeberomorus commerson L.). British Food Journal, 112, 811–820. doi:10.1108/00070701011067433
- Opiyo, A. M., & Ying, T.-J. (2005). The effects of 1-methylcyclopropene treatment on the shelf life and quality of cherry tomato (Lycopersicon esculentum var. Cerasiforme) fruit. International Journal of Food Science and Technology, 40, 665–673. doi:10.1111/j.1365-2621.2005.00977.x
- Padonou, S. W., Hounhouigan, J. D., & Nago, M. C. (2009). Physical, chemical and microbiological characteristics of lafun produced in beninn. African Journal of Biotechnology, 8, 124–129.
- Park, J., & Henneberry, S. R. (2010, February). South Korean millers’ preferences for the quality characteristics of hard white wheat that is used in producing all-purpose flour. 2010 Annual Meeting, Orlando, FL, Southern Agricultural Economics Association.
- Pathare, P. B., Opara, U. L., & Al-Said, F. A. J. (2013). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology, 6, 36–60. doi:10.1007/s11947-012-0867-9
- Rhim, J.-W., & Hong, S.-I. (2011). Effect of water activity and temperature on the color change of red pepper (Capsicum annuum L.) powder. Food Science and Biotechnology, 20, 215–222. doi:10.1007/s10068-011-0029-2
- Rodriguez-Aguilera, R., Oliveira, J. C., Montanez, J. C., & Mahajan, P. V. (2011). Effect of modified atmosphere packaging on quality factors and shelf-life of mould surface-ripened cheese: Part II varying storage temperature. LWT-Food Science and Technology, 44, 337–342. doi:10.1016/j.lwt.2010.06.014
- Rodríguez-Amaya, D. B., & Kimura, M. (2004). Harvestplus handbook for carotenoid analysis. Retrieved from http://www.harvestplus.org/content/harvestplus-handbookcarotenoid-analysis
- Rodriguez-Amaya, D. B., Nutti, M. R., & De Carvalho, J. L. V. (2011). Carotenoids of sweet potato, cassava, and maize and their use in bread and flour fortification. In V. R. Preedy, R. R. Watson, & V. B. Patel (Eds.), Flour and breads and their fortification in health and disease prevention (pp. 301–311). London: Academic press.
- Rodríguez-Sandoval, E., Fernández-Quintero, A., Cuvelier, G., Relkin, P., & Bello-Pérez, L. A. (2008). Starch retrogradation in cassava flour from cooked parenchyma. Starch - Stärke, 60, 174–180. doi:10.1002/(ISSN)1521-379X
- Sánchez, T., Chávez, A. L., Ceballos, H., Rodriguez-Amaya, D. B., Nestel, P., & Ishitani, M. (2006). Reduction or delay of post-harvest physiological deterioration in cassava roots with higher carotenoid content. Journal of the Science of Food and Agriculture, 86, 634–639. doi:10.1002/(ISSN)1097-0010
- Sánchez, T., Dufour, D., Moreno, J., Pizarro, M., Aragón, I., Domínguez, M., & Ceballos, H. (2013). Changes in extended shelf life of cassava roots during storage in ambient conditions. Postharvest Biology and Technology, 86, 520–528. doi:10.1016/j.postharvbio.2013.07.014
- Shittu, T., Dixon, A., Awonorin, S., Sanni, L., & Maziya-Dixon, B. (2008). Bread from composite cassava–wheat flour. II: Effect of cassava genotype and nitrogen fertilizer on bread quality. Food Research International, 41, 569–578. doi:10.1016/j.foodres.2008.03.008
- Shobha, D., Kumar, H. V. D., Sreeramasetty, T. A., Gowda, K. T. P., & Shivakumar, G. B. (2012). Storage influence on the functional, sensory and keeping quality of quality protein maize flour. Journal of Food Science and Technology. doi:10.1007/s13197-012-0788-7
- Uchechukwu-Agua, A. D., Caleb, O. J., & Opara, U. L. (2015). Postharvest handling and storage of fresh cassava root and products: A review. Food and Bioprocess Technology, 8, 729–748. doi:10.1007/s11947-015-1478-z