Abstract
Two studies (storage and challenge tests) investigated the microbial shelf life combined with a semi-quantitative determination of volatile compounds of cloudy apple juices after power ultrasound (US) treatment. The sublethal injury of spoiler yeasts (Candida parapsilosis and Rhodotorula glutinis) was also evaluated. The maximum effect of sonication was observed 14 days post-treatment in both tests, especially on psychrotrophic count. Sublethal injury occurred only in C. parapsilosis. Microbiological data estimated the shelf life of sonicated juices to be around 21 days. The typical aroma compounds of the raw juices decreased quickly in control samples compared with sonicated ones. The flavor stability provided by US treatment seemed to be maintained for at least the first 14 days of cold storage. The US affected the shelf life of raw apple juices, probably due to yeast sublethal stress; some specific aroma compounds could be used as freshness or treatment markers.
Se investigó el desarrollo microbiológico (respectivamente durante 28 y 60 días) a través de dos estudios (storage y challenge test) relacionando con la determinación semicuantitativa de los compuestos volátiles del jugo de manzana luego del tratamiento por ultrasonidos (US). Fueron evaluados los danos subletales de dos levaduras (Candida parapsilosis y Rhodotorula glutinis). En ambos casos fue observado el máximo efecto de la ultrasonificación después de 14 días del tratamiento, especialmente en la cuenta de bacterias sicrotróficas. Fueron observados danos estadísticamente significativos solo con C. parapsilosis. Los datos microbiológicos de los jugos tratados por ultrasonidos permitieron estimar como plazo de conservación en 21 días. La estabilidad del aroma después del tratamiento por ultrasonidos se mantiene constante por lo menos por los primeros 14 días de almacenamiento en frío. El tratamiento por ultrasonidos modifica la fecha de caducidad del jugo de manzana probablemente provocando estrés en las levaduras. Esta investigación también ha destacado que algunos compuestos específicos del aroma podrían ser utilizados como marcadores de frescura o del tratamiento mismo.
Introduction
Apple juice is one of the most popular fruit juices. Due to the acidic pH, this juice was not considered to be a potential vector for foodborne pathogens. However, enhanced acid resistance of Escherichia. coli O157:H7 has led to foodborne outbreaks by means of acidic fruit juices (Patil et al., Citation2010). Conventional thermal pasteurization and sterilization are the most common methods currently used to inactivate microorganisms, enzymes, and spores in food products. Unfortunately, intensities of treatment, time, and process temperature are also proportional to the amount of nutrient loss, the development of undesirable flavors, and the deterioration of functional properties of food products (Chemat, Zill, & Khan, Citation2011). Therefore, non-thermal technologies have received increasing attention in recent years for the preservation of beverages, due to their potential antimicrobial effect coupled with reduced quality loss.
Ultrasound (US)-assisted processes have been proposed for nearly every aspect of food production. Several studies on juice or beverage models reported a 5-log10CFU/ml reduction of some pathogens by using sonication alone or in combination with other preserving methods (Chemat et al., Citation2011; Ugarte-Romero, Feng, & Martin, Citation2007). In addition, sonication can also modify proteins, the structure of the product and inactivate the enzyme (Abid et al., Citation2013; Knorr et al., Citation2011). Moreover, US can improve the phytonutrients present in apple juices (Abid et al., Citation2014). The antimicrobial effects of US are mainly due to the unstable internal cavitation observed at low frequencies of 20–100 kHz. Intracellular acoustic cavitation increases the permeability of membranes and causes the thinning of cell membranes (Sams & Feria, Citation1991), localized heating (Suslick, Citation1989), and the production of free radicals (Butz & Tauscher, Citation2002). Furthermore, sonolysis enhances the inactivation of microorganisms (Suslick, Citation1989). However, less information is available about volatile compounds and off-flavors of sonicated apple juices combined with microbial spoilage (Abid et al., Citation2013; Gabriel, Citation2012; Muñoz et al., Citation2012). After sonication, some liquid matrices (e.g. milk) were unpleasant and rejected by the consumer (Marchesini et al., Citation2012) or the treatment can reduce some sensory traits (e.g. apple juices – Šimunek et al., Citation2013).
This study aimed to evaluate a semi-quantitative determination of the volatile compounds combined with the microbial shelf life of a cloudy apple juice after sonication treatment (storage test). To better understand the yeast development, two spoilers (Rhodotorula glutinis and Candida parapsilosis) were inoculated in the juices (challenge test); moreover, the appearance of sublethally injured cells was also evaluated (Somolinos, Garcia, Condón, Maňas, & Pagán, Citation2007; Jasson, Uyttendaele, Rajkovic, and Debevere (Citation2007)). The strains tested for the spoilage inoculum had been previously isolated and characterized during preliminary experiments as the dominant yeast species that were resistant to weaker sonication treatments (300 s/100 mL and amplitude of 100%; in apple juice (Montemurro et al., Citation2014)). Moreover, R. glutinis (Wirth & Goldani, Citation2012) and C. parapsilosis (Selvarangan, Bui, Limaye, & Cookson, Citation2003; Tavanti et al., Citation2010) are classified as pathogens for susceptible patients. The results should help in understanding the physiology of the resistance of these yeasts to novel preservation technologies.
Materials and methods
Sample processing and experimental design
Golden delicious apples (Melinda protected designation of origin 4021) were washed, portioned without peel, and squeezed using a juicer (Centrika Metal, Ariete, Firenze, I) (Guo, Yue, & Yuan, Citation2012). Two different sets of experiments were carried out to test the quality shelf life of a sonicated cloudy apple juice stored at 4°C for a period of 28 days and after 60 days: a) storage test – the sonicated juice (SJ) and the untreated juice (BJ) were checked on the day of the treatment (T0) and at 7 (T7), 14 (T14), 21 (T21), 28 (T28), and 60 (T60) days post-treatment; and b) a challenge test (same experimental times) was performed by inoculating the juice with two spoilage yeasts, R. glutinis and C. parapsilosis (IBJ, control).
US treatment and calorimetry
The juice was sonicated in batches using the sonicator UP 400 S (Hielscher USA, Inc., Ringwood, USA), with the following characteristics: 400 W and 24 kHz. The processor was equipped with a 22 mm diameter sonotrode. Each treatment was applied to the apple juice (1000 mL) in a glass beaker (Duran Group, Mainz, Germany), and the sonotrode was positioned at a fixed depth inside the beaker. The increasing temperature of the juice (final value < 35°C) was controlled by means of an ice/water bath. The ultrasonic process was applied for 360 s/100 mL with an amplitude of 100%. Treatment parameters were extrapolated from previous investigations (data not provided) as the highest level that did not induce a cooked taste and perceived off-flavors. The acoustic power transferred to the liquid sample by US was calculated using the calorimetric method according to Mañas, Pagán, and Raso (Citation2000). After each treatment, the samples of apple juices (three replicates for each thesis and storage time) were stored at 4 ± 1°C in 200 ml sterile polypropylene containers.
Microbiological analysis, challenge test, and determination of sublethal injured cells
Control (fresh squeeze juice – BJ and inoculated juice – IBJ) and their respective sonicated theses (SJ and inoculated sonicated juice (ISJ)) were subjected to the microbiological analyses. The maximum recovery diluent (MRD; Oxoid, Basingstoke, UK) was used for the assessment of 10-fold serial dilutions, each of which was plated on agar medium. In particular, plate count agar (PCA; Oxoid) was used for the mesophilic microflora (30°C, 72 h; TVC = total viable count) (ISO, 4833-1:Citation2013) and psychrotrophic microflora (4°C, 10 days; TPC = total psychrotrophic count) under aerobic conditions, and oxytetracycline glucose yeast extract agar (OGYE; Oxoid) was used for yeast and mold counts (25°C, 3–5 days). Moreover, the pH of each sample was investigated (pH meter Knick 910 Portmess®).
R. glutinis (SU1) and C. parapsilosis (SU5) strains had been isolated and characterized during preliminary experiments. The strains were stored at −80°C in a malt extract broth (Oxoid) and glycerol. Strains were separately plated in OGYE and a single colony was inoculated on the malt extract broth (Oxoid). A dilution of the standardized pre-inoculum (25°C until the stationary growth phase) was used for the challenge test. The raw juice was inoculated with a mix of both strains (3 log10CFU/mL). The evaluation of yeast sublethal injury was conducted according to the plate methods proposed by Somolinos et al. (Citation2007). In order to assess the cell injury, OGYE medium was added with 5% w/v NaCl and 12% w/v NaCl, respectively. These levels were the highest concentrations that did not affect the yeast count under normal growth conditions (non-inhibitory concentration) for R. glutinis (SU1) and C. parapsilosis (SU5). The selective enumeration of both yeasts was performed according to their morphology on normal OGYE plates (without salt) and OGYE plus NaCl (5% and 12%) for IBJ and ISJ thesis. Percentages of sublethal injury were calculated according to Jasson et al. (Citation2007). This percentage elucidates the amount of yeast cells recovered from the OGYE (without salt) that did not grow on OGYE plus NaCl due to the stress induced after sonication.
Analysis of volatile compounds (gas chromatography-mass spectrometry (GC-MS))
Samples of both storage and challenge tests were analyzed. Samples were diluted, supplemented with 1-eptanol (as internal standard), and then centrifuged. The supernatant was subjected to purification using C18 silica-based solid-phase extraction (SPE) cartridges (Waters® Italia, Milano, I) at room temperature. Dichloromethane was the desorbing solvent for free volatile compounds (10 mL, pressure <1 bar). Then, the sample was concentrated with nitrogen (final volume of 200 μL). Analysis of volatile compounds was performed by a GCMSQP2010 (Shimadzu Italia, Milano, I). The GC was equipped with a DB 5 ms (Agilent Technologies, Santa Clara, CA, USA) capillary column (60 m × 0.25 mm × 0.25 μm) and helium as a carrier gas (35 cm/s, linear velocity). On-column injection was performed at 45°C (for 4 min) and the oven temperature increased to 310°C at a rate of 3.5°C/min. The final temperature was maintained for 10 min. The transfer line was held at 250°C. The mass spectrometer was a single quadrupole and the range of mass (m/z) was scanned from 29 to 400. Compounds were identified by matching their mass spectra with mass spectral libraries and by calculation of linear retention indices and comparison with data in the literature. All analyses were performed in duplicate (T14–T28–T60), and the difference from T0 was determined (Δ-values). As a semi-quantitative measure, the peak area of each compound was normalized to 1-eptanol.
Statistical analysis
The data of microbial variables that were distributed normally were analyzed by analysis of variance (ANOVA) in order to estimate the effects of US treatment (control vs. US = BJ vs. SJ and IBJ vs. ISJ), storage time (0, 7, 14, 21, 28, and 60 days), and their interaction. The interaction between treatment and time of storage was evaluated by comparing SJ with BJ and ISJ with IBJ.
Significant differences between pairs of means were evaluated according to the probability of difference adjusted by the Bonferroni test (p < 0.05). The data are reported as least squares mean (LS mean) ± standard error of the mean (SEM). Non-normally distributed variables were analyzed by non-parametric Kruskal–Wallis and Mann–Whitney tests in order to study the US*storage time interaction and effect of US treatment, respectively. The Mann–Whitney test was used again to assess the significance of yeast injury.
Concerning the volatile compounds, the dataset was studied by principal component analysis (PCA). This analysis was conducted using 13 variables selected as the main attributes of fresh and fermented apple juice aroma (Dixon & Hewett, Citation2000; Gigot et al., Citation2010; Riekstina-Dolge, Kruma, Karlina, & Seglina, Citation2011) ().
Table 1. Substances selected as primary attributes and treatment markers of fresh and fermented apple juice aroma according to Dixon and Hewett (Citation2000), Gigot et al. (Citation2010), and Riekstina-Dolge et al. (Citation2011).
Tabla 1. Moléculas seleccionada como características primarias y marcadores del tratamiento de frescura y del aroma del jugo de manzana fermentado según Dixon y Hewett (Citation2000), Gigot et al. (Citation2010), y Riekstina-Dolge et al. (Citation2011).
Linear discriminant analysis (LDA) was performed to identify specific variables (volatile compounds) able to distinguish between treated (SJ and ISJ) and control (BJ and IBJ) juices. All variables identified were first transformed into natural logarithms. Stepwise selection of variables, which combines forward selection and backward elimination using the minimization of Wilks’ lambda, was adopted. Wilks’ lambda indicated the significance of the discriminant function and provided the proportion of total variability that was not explained. A leaving-one-out cross validation procedure was performed to assess the accuracy of the classification rule. This procedure was repeated a number of times, equal to the number of samples. Consequently, each sample was classified by discriminant functions that were estimated without its contribution. All statistical analyses were performed by IBM® SPSS® Statistics 20 Core System and SAS (SAS 9.3, Institute Inc., Cary, NC).
Results and discussion
Variable distribution
The ANOVA F-statistics and p-values for the normally distributed variables of the storage test and challenge test are reported in . US treatment did not affect the mold count in the challenge test. The storage time affected all variables (p < 0.001), whereas the interactions between storage time and US treatment were found to be significant for all of the variables. The non-parametric tests were applied for the not normally distributed variables ().
Table 2. ANOVA and F-statistics for storage test and challenge test dependent variables.
Tabla 2. Análisis de la varianza y valor F para storage-test y challenge test en dependencia de las variables.
Table 3. Total viable count for challenge test variables not normally distributed (Mann–Whitney test) according to US treatment.
Tabla 3. Cuenta viable total para las variables challenge test con distribución no normal (Mann–Whitney test) según el tratamiento US.
During sonication, the juice showed an increase in temperature due to the energy released by the sonotrode. The average acoustic power measured by the calorimetric method was 160.4 J/s.
Storage test
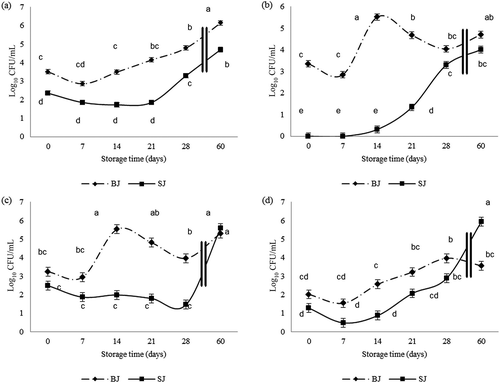
The quality shelf life of food is commonly defined by the number of microorganisms able to impact on product quality or could be described in terms of organoleptic changes and biochemical features (Man, Citation2011). The US treatment affected the growth of all microbial targets (–). In sonicated juices (SJ), the TVC was stable during the first 21 days (around 1.9 log10CFU/mL), while the control samples (BJ) highlighted an increase of TVC after 7 days. Moreover, the microbial level of the SJ was always lower than 2 log10CFU/mL of the BJ levels (). The most relevant effect of sonication was on psychrotrophic counts, especially at 14 days, with the difference between BJ and SJ of 5.2 ± 0.2 log10CFU/mL (). Probably, the endogenous microbial population of the apple juice was mainly composed of psychrotrophic yeasts with slow growth for 28 days. However, there was no significant difference in yeast count between sonicated and untreated juices up to 2 weeks (). shows the lowering effect of US on mold counts. Sonication exerted its maximum effect on psychrotrophic yeasts in a typical microbial population of apple juices. According to TVC and yeast counts, the initial reduction after treatment was very close to the data reported by Gómez-López, Orsolani, Martínez-Yépez, and Tapia (Citation2010) on orange juice. However, the US did not induce a complete decontamination of raw juices, for this reason additional treatments are required to prevent safety problems (Abid et al., Citation2013). The mesophilic bacteria counts remained significantly lower than 4 log10CFU/mL well beyond 28 days, which complies with the Italian regulations to assure microbiological safety of fruit juices and vegetables (Accordo 28 novembre 2002). On the other hand, even yeasts and molds of the sonicated juice showed values that, up to 28 and 21 days, respectively, were consistent with the microbiological criteria for unpasteurized juices and nectars of fruits or vegetables that are ready for consumption (guiding values of 1000 and 100 CFU/mL for yeasts and molds, respectively) (Regione Piemonte, Citation2009). Gao and Vasantha Rupasinghe (Citation2012) suggested around 107 CFU/mL as the threshold level of the aerobic mesophilic count for raw carrot/apple juices. This level was never reached and only the control juices showed a level of 6 log10CFU/mL after 60 days of storage. In general, the maximum effect of sonication was observed after 2 weeks of refrigeration. A similar result was reported for carrot/apple juices, where the decrease in TVC was observed 14 days after sonication; however, it also seemed to be related to pH and juice composition (Gao and Vasantha Rupasinghe (Citation2012). The pH progressively decreased during the storage time (from 3.6 to 3.2) but the differences were only significant after 28 days (Figure S1).
Figure 1. Storage test. Microbial population (log10CFU/mL) of sonicated (SJ) and untreated (BJ) juices stored at 4°C. Variables selected: (a) TVC; (b) TPC; (c) yeasts; (d) molds.Note: TVC: total viable count; TPC: total psychrotrophic count; bar = standard error (±SEM) of the mean (LSmean). Means with different superscripts are different for p < 0.05.
Figura 1. Storage test. Población microbiana (Log10CFU/mL) del jugo tratado por ultrasonidos (SJ) y no tratado (BJ) almacenados a 4°C. Variables seleccionadas: (a) TVC; (b) TPC; (c) levaduras; (d) mohos.
Challenge test
Wareing and Davenport (Citation2005) categorized all spoiler yeasts of the beverage industry into four classes. C. parapsilosis was ascribed to spoilage and hygiene markers that are able to cause severe problems of spoilage, strictly related to good manufacturing practices. R. glutinis was mainly an indicator of poor hygiene.
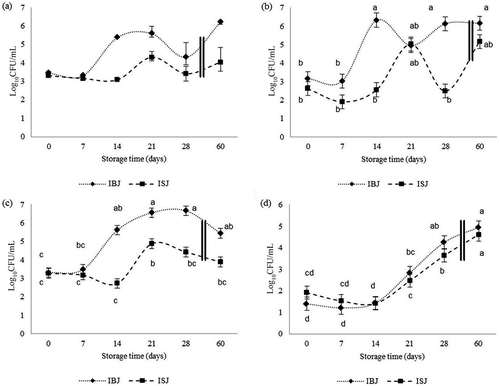
Apple juice inoculated with R. glutinis and C. parapsilosis showed a quite different behavior in the storage test. The inoculated juice was characterized by an accelerated trend of growth curves (–). The interaction between fixed effects strongly influenced TPC and yeast performance. The growth curves were clearly affected by power US, with a shift from T14 to T21 ( and ). The effects of US were not observed immediately after sonication, and the maximum effect was observed after 14 days of refrigeration (). With regard to molds (), the data showed that time (p ≤ 0.001) influenced mold growth more than US*storage time (p ≤ 0.05; ).The total mesophilic counts were statistically significant in both Mann–Whitney and Kruskal–Wallis () tests (p = 0.0008 and p = 0.0013, respectively). The effect of US treatment was confirmed in both sets of experiments (storage and challenge) with a significant reduction in growth of several targets (yeasts, TVC, and TPC), stressing the impact of treatment on independent trials.
Figure 2. Challenge test. Microbial population (log10CFU/mL) of inoculated sonicated (ISJ) and inoculated untreated (IBJ) juices stored at 4°C. Variables selected: (a) TVC; (b) TPC; (c) yeasts; (d) molds.Note: TVC: total viable count; TPC: total psychrotrophic count; TVC: mean values from raw data, bar = min; max. TPC, yeasts and molds: bar = standard error (±SEM) of the mean (LSmean). Means with different superscripts are different for p < 0.05.
Figura 2. Challenge test. Población microbiana (Log10CFU/mL) de la muestra tratada inoculada (ISJ) y del jugo de control inoculado (IBJ) almacenados a 4°C. Variables seleccionadas: (a) TVC; (b) TPC; (c) levaduras; (d) mohos.
The pH (Figure S2) decreases as described for the storage test down to the level of 3.1.
According to Wareing and Davenport (Citation2005), the metabolic activity of yeasts at a cell concentration of up to 10,000/mL is insufficient to produce any appreciable difference in food. Studies by Stratford (Citation2006) using orange juice demonstrated that detectable spoilage required a concentration of yeast that was greater than 10,000/mL, approximately 5–6 log10CFU/mL, which was evaluated at around 14 and 21 days in IBJ and ISJ, respectively (). However, molds also influenced the shelf life of apple juice, since the treatment level did not strongly affect their count (). Molds induced off-flavors and texture changes that are able to limit US-treated juice shelf life for 21 days at 4°C.
Sublethal injury evaluation in R. glutinis and C. parapsilosis
The results of the challenge test could be explained by the presence of sublethal stress on the yeasts. Cell stress due to US treatment emerged in the period T0–T14. Both yeasts presented an initial count (T0) of 3 log10CFU/mL, which verified the concentration of the inoculum. Different levels of injuries could be developed when a preservation treatment was applied. In the present study, a partial inactivation (lethal injury) is described by an overall reduction in yeast counts in both media (OGYE and OGYE plus salt). In contrast, if the amount of recovered yeast was higher in OGYE than OGYE plus salt, the US treatment was considered to have induced a sublethal injury (Jasson et al., Citation2007).
According to the statements, lethal and sublethal injury (Jasson et al., Citation2007) occurred in R. glutinis during the first week, while only sublethal injury occurred in C. parapsilosis during the same period. The percentage of sublethally-injured cells was compared between US-treated juice (ISJ) and the inoculated untreated one (IBJ) during the first two weeks (Mann–Whitney test, Figure S3). The highest values of sublethally-injured cells occurred at 7 days for both yeasts (43% for R. glutinis and 17.5% for C. parapsilosis). However, only C. parapsilosis showed a significant level of sublethally-injured cells in sonicated juices (Mann–Whitney test: p = 0.01; Figure S3). In general, sublethally-injured cells became sensitive to many selective compounds due to the damage of their membranes and modification of their permeability (Jasson et al., Citation2007; Somolinos et al., Citation2007). Furthermore, other studies demonstrated the presence of sublethally-injured yeasts after non-thermal processing, such as pulsed electric fields (Somolinos et al., Citation2007). This evaluation is fundamental, since sublethally-injured cells are able to repair and grow under the given favorable conditions.
Sublethal injury evaluation suggested that there was a synergistic effect between sonication and cold stress due to refrigerated storage. However, the presence of sublethal stress should also be investigated under other conditions such as thermal abuse during the conservation. The higher number of sublethally-injured cells in sonicated juices compared with control juices after 1 week confirmed this hypothesis. The US effects are not only direct and immediate (lethal effect or all-or-nothing event) but produce different levels of cell damage. Moreover, the presence of stressed cells could be a critical point for the quality and safety of food, and therefore also needs to be taken into account in other situations such as thermal abuse during storage.
Volatile compounds
The GC/MS analysis identified 56 main variables, which are reported in Table S5 (Supplemental data). The chemicals recorded were 17 alcohols (30.4%), 8 ketones (14.3%), 1 ketal (1.8%), 1 amide (1.8%), 12 esters (21.4%), 6 carboxylic acids (10.7%), 6 aldehydes (10.7%), and 5 hydrocarbons (8.9% including a monoterpene and a sesquiterpene).
The volatile compounds detected were substantially similar to those reported by Muñoz et al. (Citation2012), Guo et al. (Citation2012), Dixon and Hewett (Citation2000), Gigot et al. (Citation2010), and Riekstina-Dolge et al. (Citation2011). About 80 volatile compounds are routinely detected and considered to contribute to overall apple juice aroma (Muñoz et al., Citation2012). They come from raw materials (primary aroma) and technological processes and storage (secondary aroma) Riekstina-Dolge et al. (Citation2011). Some compounds have proved to be important, e.g. 2-hexenal, 2-hexenol, hexanal, ethyl-2-methylbutanoate, ethyl butanoate, 1-butanol, 1-hexanol, butyl acetate, and β-damascenone (Muñoz et al., Citation2012). Most of the aroma compounds showed the same behaviors in challenge and storage tests; however, some molecules such as 2-methylbutyl acetate and hexyl acetate seemed to be reduced in SJ compared to BJ, while the same trend was not found in the challenge test. The 1,3-octanediol level was higher in SJ than BJ, but lower in ISJ than IBJ. A few alcohols, like 3-methyl-1-butanol and phenylethyl alcohol, as well as unpleasant carboxylic acids that are strictly linked to yeast metabolism (O’Neil, Heckelman, Roman, & Kenny, Citation2013; Riekstina-Dolge et al., Citation2011; Rolls, Kringelbach, & De Araujo, Citation2003), showed increased levels in BJ and IBJ, while they were stable in SJ and ISJ. Specifically, during the first 14 days of storage, the effect of sonication on yeast metabolism was particularly evident. Among alcohols, 1-hexanol is considered to be a characteristic volatile compound of fruits other than apples and is produced during the enzymatic oxidation process of linoleic acid (Fan, Xu, & Han, Citation2011; Nikfardjam & Maier, Citation2011). In the sonicated juice, its abundance, at least in the first 14 days, was lower than the values observed for untreated juice (Figure S6).
Principal component analysis
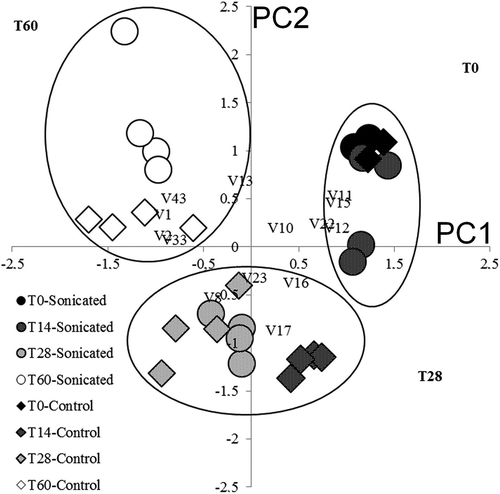
The aroma profile of sonicated juices at 14 days was closely similar to but not the same as the initial juice profiles. On the other hand, at 28 and 60 days, it had deteriorated in all cases. PCA conducted on 14 chemicals confirmed the efficacy of the US treatment on aroma preservation or in the genesis and maintenance of fresh-like molecules. The first two components (PCs) explained the majority of the dataset variability (63%), shows the PC1 and PC2 scores and each selected variable. Three main clusters were found according to the time of storage. The T0 cluster included all initial samples and sonicated samples up to 14 days. This cluster was characterized by fruity, apple-like, grassy, and pleasant flavors since it was composed of esters and aldehydes (e.g. hexanal), which satisfy the demand of the customer for freshness (Fan et al., Citation2011; Patil et al., Citation2010).
Figure 3. PCA 2D chart. Relationship between apple juice attributes and score plot of samples according to treatment and storage time effects.Note: Control (IBJ + BJ) vs. sonicated (ISJ + SJ)
Figura 3. PCA gráfico 2D. Relación entre las características del jugo de manzana y score plot de las muestras según los efectos de tratamiento y de conservación.
Those descriptors, in particular hexanal and 2-hexenal, were not oxidized to carboxylic acids in US-treated juices. The oxidative process, which was strongly delayed in sonicated juices, is related to the metabolism of the endogenous microbial population, which is mainly composed of psychrotrophic yeasts. Juice is a fermentable product and target compounds due to yeast activity can be identified, such as acetic acid, phenylethyl alcohol, and 3-methyl-1-butanol. According to Riekstina-Dolge et al. (Citation2011), 3-methyl-1-butanol and 2-methyl-1-butanol play a major role in apple juice quality. Both substances are not primary constituents of the apple fruit, but are unavoidably formed during apple juice production, probably through the transamination and decarboxylation of the amino acids leucine and isoleucine, respectively (Riekstina-Dolge et al., Citation2011).
In support of this observation, other chemicals that were not considered in the PCA showed the same trend. β-Damascone, characterized by its pleasant odor (Isoe, Katsumura, & Sakan, Citation1973), was always present at higher levels in the treated juice than in the control one, while diethyl acetic acid, as a product of yeast metabolism, increased in concentration as long as the juice was deteriorating (Table S5).
Otherwise, all control juices at 14 days were grouped in the T28 cluster. The T28 cluster was mainly characterized by pentanoic (V8) and hexanoic (V23) acids as principal variables (especially linked to IBJ and BJ at 14 days). Moreover, 2-hexen-1-ol (V16) and 1-hexanol (V17) also characterized this group of samples and linked all of the control juices from 14 to 28 days and sonicated juices at 28 days. Moreover, the dynamically developed pentanoic acid, 1-hexanol, 3-methyl-1-butanol, and phenylethylalcohol could be considered to be descriptors of different yeast behaviors according to the studied theses (Table S5; Figures S6 and S7). At 60 days, all samples were grouped in the third cluster (T60), represented by alcohols. Furfural (V13), a characteristic compound of heated products, was recorded at a mean value of 1.1 ± 2.2 ppb in all samples.
Linear discriminant analysis
Three different LDAs were performed using 56 aroma profile descriptors in order to identify specific variables that are able to distinguish between US-treated and untreated juices. The first stepwise LDA (LDA1) revealed furfural and 1-nonen-4-ol as the most important contributors in discriminating between sonicated and untreated juices. However, complete separation between the two groups was not achieved with 78.6% of correctly classified samples upon cross-validation ().
Table 4. Discriminant variables of each LDA and their canonical discriminant function coefficients (sonicated vs. control).
Tabla 4. Discriminación por las variables de cada LDA y su función discriminante (tratados vs. control).
Furfural was recorded as a discriminant of US treatment; Suárez-Jacobo, Gervilla, Guamis, Roig-Sagués, and Saldo (Citation2010) considered both 2-furaldehyde (FUR) and 5-hydroxymethyl-2-furaldehyde (HMF) as indicators of quality deterioration (e.g. flavor changes) of fruit juices during the heating process, i.e. concentration, pasteurization, or storage. Furfural was detected in industrial single-strength apple juices at a mean value of 1.6 mg/L (range: 0–25 mg/L) by HRGC-MS. Moreover, it was found at 12 mg/L (range: 2.4–56 mg/L) in apple juice concentrates. The present results (furfural, 1.1 ± 2.2 ppb) confirmed the applied US treatment as a non-thermal technology. Furfural and 1-nonen-4-ol were excluded from the dataset and a new LDA (LDA2) was performed ().
Hexanal (green apple, grass-like), 2-hexen-1-ol (fruity, green, leafy), and hexanoic acid (sour, fatty, sweat), which have been already demonstrated to be fundamental with PCA, were statistically discriminant attributes. Others were 3-methyl-3-pentanol (powerful fruity, green, leafy), 6-methyl-5-hepten-2-one (fruity), hexyl 2-methylbutanoate (apple, grapefruit), α-farnesene (citrus, herbal, bergamot), and isopropyl laurate.
Fruity and natural attributes (limonene: lemongrass; 2,4-dimethyl-benzaldehyde: cherry, almond, vanilla; Blanco Gomis, Gutiérrez Alvarez, Sopeña Naredo, & Mangas Alonso, Citation1991) were detected as statistical discriminants when the storage time considered was T0–T28 in the LDA3 (). These descriptors were related to sonicated juices. On the other hand, α-farnesene, a typical terpene of raw apple, was reduced after treatment. This compound usually decreased during heat treatments. Also, sonication probably enhanced the production of secondary compounds such as 6-methyl-5-hepten-2-one from this terpene (Belitz, Grosch, & Schieberle, Citation2009). The new model did not fully represent all dataset variability (Wilks’ lambda 0.382).
Therefore, LDA2 illustrated the importance of yeast metabolism in the aroma evolution, while LDA3 confirmed the relationship between sonicated juices and the T0 cluster that was described by PCA. These data suggested that for a sonicated juice under refrigeration, the shelf life of the aroma could be taken to be 14 days. The link between organoleptic deterioration and microbial activity was also suggested for sonicated orange juices (with calcium citrate), where sonication prolonged the shelf life of 4 days (Gao & Vasantha Rupasinghe, Citation2012).
The sonication treatment applied was the highest tested before sensory change (e.g. cooked flavors). However, the flavor of sonicated juice matches closely with the freshly-squeezed juice according to specific aroma compounds.
The shelf life of S-treated juices has been determined to be 14 days, and can probably be extended to 21 days according to microbiological outcomes.
Conclusions
These preliminary observations suggested an interesting application of US for the quality maintenance of apple juice.
The main results highlighted that ultrasounds can preserve some volatile compounds or induce a neo-genesis fresh-like aroma. Sonicated juices could be considered to be products whose flavor closely matches the new trend in consumer demand.
The observed microbial stability indicated certain preservation to spoilage; however, the present results were detected under the optimal storage conditions. A combination of US and low temperature enhanced the shelf life of the raw juice.
Further investigations are required to couple the US process with other treatments in order to enhance microbial stability and flavor attributes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/19476337.2015.1045432.
TCYT-2015-0023_Supplementary_REVISED.docx
Download MS Word (301.9 KB)Acknowledgments
The authors want to thank Robertino Barcarolo for his skilled assistance in chemical analysis and Laura Scandolara for the scientific Spanish translation.
Additional information
Funding
References
- Abid, M., Jabbar, S., Wu, T., Hashim, M. M., Hu, B., Lei, S., … Zeng, X. (2013). Effect of ultrasound on different quality parameters of apple juice. Ultrasonics Sonochemistry, 20, 1182–1187. doi:10.1016/j.ultsonch.2013.02.010
- Abid, M., Jabbar, S., Wu, T., Hashim, M. M., Hu, B., Lei, S. C., & Zeng, X. (2014). Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrasonics Sonochemistry, 21, 93–97. doi:10.1016/j.ultsonch.2013.06.002
- Accordo 28 novembre 2002. Attuazione della raccomandazione della Commissione europea del 25 gennaio 2002, n. 2002/66/CE). Retrieved January 7, 2015, from http://www.governo.it/backoffice/allegati/17761-1110.pdf
- Belitz, H. D., Grosch, W., & Schieberle, P. (2009). Aroma compounds. In H. D. Belitz, W. Grosch, & P. Schieberle (Eds.), Food chemistry (pp. 340–400). Berlin Heidelberg, DE: Springer-Verlag.
- Blanco Gomis, D., Gutiérrez Alvarez, M. D., Sopeña Naredo, L., & Mangas Alonso, J. J. (1991). High-Performance liquid chromatographic determination of furfural and hydroxymethylfurfural in apple juices and concentrates. Chromatographia, 32, 45–48. doi:10.1007/BF02262465
- Butz, P., & Tauscher, B. (2002). Emerging technologies: Chemical aspects. Food Research International, 35, 279–284. doi:10.1016/S0963-9969(01)00197-1
- Chemat, F., Zill, H., & Khan, M. K. (2011). Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry, 18, 813–835. doi:10.1016/j.ultsonch.2010.11.023
- Dixon, J., & Hewett, E. W. (2000). Factors affecting apple aroma/flavour volatile concentration: A review. New Zealand Journal of Crop and Horticultural Science, 28, 155–173. doi:10.1080/01140671.2000.9514136
- Fan, W., Xu, Y., & Han, Y. (2011). Quantification of volatile compounds in Chinese ciders by stir bar sorptive extraction (SBSE) and gas-chromatography-mass spectrometry (GC-MS). Journal of the Institute of Brewing, 117, 61–66. doi:10.1002/j.2050-0416.2011.tb00444.x
- Gabriel, A. A. (2012). Microbial inactivation in cloudy apple juice by multi-frequency dynashock power ultrasound. Ultrasonics Sonochemistry, 19, 346–351. doi:10.1016/j.ultsonch.2011.06.003
- Gao, J., & Vasantha Rupasinghe, H. P. (2012). Nutritional, physicochemical and microbial quality of ultrasound-treated apple-carrot juice blends. Food and Nutrition Sciences, 3, 212–218. doi:10.4236/fns.2012.32031
- Gigot, C., Ongena, M., Fauconier, M. L., Wathelet, J. P., Du Jardin, P., & Thonart, P. (2010). The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnologie, Agronomie, Société et Environnement, 14, 451–460. http://popups.ulg.ac.be/1780-4507/
- Gómez-López, V. M., Orsolani, L., Martínez-Yépez, A., & Tapia, M. S. (2010). Microbiological and sensory quality of sonicated calcium-added orange juice. LWT - Food Science and Technology, 43, 808–813. doi:10.1016/j.lwt.2010.01.008
- Guo, J., Yue, T., & Yuan, Y. (2012). Feature selection and recognition from nonspecific volatile profiles for discrimination of apple juices according to variety and geographical origin. Journal of Food Science, 77, C1090-1096. doi:10.1111/j.1750-3841.2012.02914.x
- ISO. (4833-1:2013). Microbiology of the food chain — horizontal method for the enumeration of microorganisms — Part 1: Colony count at 30 degrees C by the pour plate technique. Geneva, CH: International Organization for Standardization.
- Isoe, S., Katsumura, S., & Sakan, T. (1973). The synthesis of Damascenone and beta-Damascone and the possible mechanism of their formation from carotenoids. Helvetica Chimica Acta, 56(5), 1514–1516. doi:10.1002/(ISSN)1522-2675
- Jasson, V., Uyttendaele, M., Rajkovic, A., & Debevere, J. (2007). Establishment of procedures provoking sub-lethal injury of Lysteria monocytogenes, Campylobacter jejuni and Escherichia coli O157 to serve method performance testing. International Journal of Food Microbiology, 118, 241–249. doi:10.1016/j.ijfoodmicro.2007.07.016
- Knorr, D., Froehling, A., Jaeger, H., Reineke, K., Schlueter, O., & Schoessler, K. (2011). Emerging technologies in food processing. Annual Review of Food Science and Technology, 2, 203–235. doi:10.1146/annurev.food.102308.124129
- Man, C. M. D. (2011). Food storage trials: An introduction. In D. Kilcast & P. Subramaniam (Eds.), Food and beverage stability and shelf life (pp. 325–349). Cambridge: Woodhead Publishing Limited.
- Mañas, P., Pagán, R., & Raso, J. (2000). Predicting lethal effect of ultrasonic waves under pressure treatments on Listeria monocytogenes ATCC 15313 by power measurements. Journal of Food Science, 65, 663–667. doi:10.1111/j.1365-2621.2000.tb16069.x
- Marchesini, G., Balzan, S., Montemurro, F., Fasolato, L., Andrighetto, I., Segato, S., & Novelli, E. (2012). Effect of ultrasound alone or ultrasound coupled with CO2 on the chemical composition, cheese-making properties and sensory traits of raw milk. Innovative Food Science & Emerging Technologies, 16, 391–397. doi:10.1016/j.ifset.2012.09.003
- Montemurro, F., Fasolato, L., Balzan, S., De Nardi, R., Marchesini, G., Cardazzo, B., & Novelli, E. (2014). Storage test on apple juice after ultrasound treatment. Italian Journal of Food Safety, 3, 64–68. doi:10.4081/ijfs.2014.955
- Muñoz, A., Caminiti, I. M., Palgan, I., Pataro, G., Noci, F., Morgan, D. J., & Lyng, J. G. (2012). Effects on Escherichia coli inactivation and quality attributes in apple juice treated by combinations of pulsed light and thermosonication. Food Research International, 45, 299–305. doi:10.1016/j.foodres.2011.08.020
- Nikfardjam, M. P., & Maier, D. (2011). Development of a headspace trap HRGC/MS method for the assessment of the relevance of certain aroma compounds on the sensorial characteristics of commercial apple juice. Food Chemistry, 126, 1926–1933. doi:10.1016/j.foodchem.2010.12.021
- O’Neil, M. J., Heckelman, P. E., Roman, K. J., & Kenny, C. M. (2013). The merck index: An encyclopaedia of chemicals, drugs and biological (15th ed.). Cambridge: Royal Society of Chemistry.
- Patil, S., Torres, B., Tiwari, B. K., Wijngaard, H. H., Bourke, P., Cullen, P. J., … Valdramidis, V. P. (2010). safety and quality assessment during the ozonation of cloudy apple juice. Journal of Food Science, 75, M437–443. doi:10.1111/j.1750-3841.2010.01750.x
- Regione Piemonte (2009). Allegato 1 – Protocollo tecnico Rev. 04:2012. Retrieved January 7, 2015, from http://www.regione.piemonte.it/sanita
- Riekstina-Dolge, R., Kruma, Z., Karlina, D., & Seglina, D. (2011). Composition of aroma compounds in fermented apple juice: Effect of apple variety, fermentation temperature and inoculated yeast concentration. Procedia Food Science, 1, 1709–1716. doi:10.1016/j.profoo.2011.09.252
- Rolls, E. T., Kringelbach, M. L., & De Araujo, I. E. T. (2003). Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience, 18, 695–703. doi:10.1046/j.1460-9568.2003.02779.x
- Sams, A. R., & Feria, R. (1991). Microbial effects of ultrasonication of broiler drumstick skin. Journal of Food Science, 56, 247–248. doi:10.1111/jfds.1991.56.issue-1
- Selvarangan, R., Bui, U., Limaye, A. P., & Cookson, B. T. (2003). Rapid identification of commonly encountered Candida species directly from blood culture bottles. Journal of Clinical Microbiology, 41, 5660–5664. doi:10.1128/JCM.41.12.5660-5664.2003
- Šimunek, M., Jambrak, A. R., Petrović, M., Juretić, H., Major, N., Herceg, Z., … Vukušić, T. (2013). Aroma profile and sensory properties of ultrasound-treated apple juice and nectar. Food Technology & Biotechnology, 51(1), 101–111.
- Somolinos, M., Garcia, D., Condón, S., Maňas, P., & Pagán, R. (2007). Relationship between sublethal injury and inactivation of yeast cells by the combination of sorbic acid and pulsed electric fields. Applied and Environmental Microbiology, 73, 3814–3821. doi:10.1128/AEM.00517-07
- Stratford, M. (2006). Food and beverage spoilage yeasts. In A. Querol & G. H. Fleet (Eds.), Yeasts in food and beverages (pp. 335–379). Berlin Heidelberg, DE: Springer-Verlag.
- Suárez-Jacobo, Á., Gervilla, R., Guamis, B., Roig-Sagués, A. X., & Saldo, J. (2010). Effect of UHPH on indigenous microbiota of apple juice. A preliminary study of microbial shelf-life. International Journal of Food Microbiology, 136, 261–267. doi:10.1016/j.ijfoodmicro.2009.11.011
- Suslick, K. S. (1989). The chemical effects of ultrasound. Scientific American, 260, 80–86. doi:10.1038/scientificamerican0289-80
- Tavanti, A., Hensgens, L. A. M., Mogavero, S., Majoros, L., Senesi, S., & Campa M. (2010). Genotypic and phenotypic properties of Candida parapsilosis sensu strictu strains isolated from different geographic regions and body sites. BMC Microbiology, 10, 203–214. doi:10.1186/1471-2180-10-203
- Ugarte-Romero, E., Feng, H., & Martin, S. E. (2007). Inactivation of Shigella boydii 18 IDPH and Listeria monocytogenes Scott A with power ultrasound at different acoustic energy densities and temperatures. Journal of Food Science, 72, M103-107. doi:10.1111/j.1750-3841.2007.00340.x
- Wareing, P., & Davenport, R. R. (2005). Microbiology of soft drinks and fruit juices. In P. R. Ashurst (Ed.), Chemistry and technology of soft drinks and fruit juices (pp. 279–299). Oxford: Blackwell.
- Wirth, F., & Goldani, L. Z. (2012). Epidemiology of Rhodotorula: An emerging pathogen. Interdisciplinary Perspectives on Infectious Diseases, 2012, 1–7. doi:10.1155/2012/465717
