Abstract
Phytase enzyme was purified from the Pinar melkior (Lactarius piperatus) mushroom using ammonium sulfate precipitation and DEAE-sephadex ion exchange chromatography techniques. First, the purified phytase enzyme was covalently bound to the surface of magnetite-CTS NPs in yield of 87%. Then, optimum pHs of the free and immobilized enzymes were determined as 5.0 and 4.0, respectively. Optimum temperature of free and immobilized enzymes was found as 60°C. Also, the effects of some metal ions on activity of free and immobilized phytase enzymes were investigated. Also, research was undertaken as to whether the purified free and immobilized phytase enzyme could hydrolyze the phytic acid in many cereal products or not. And, it was discovered that the immobilized phytase enzyme hydrolyzed the phytic acid at the highest rate (75.02% rate) in wheat. From the findings obtained, that immobilized enzyme was quite resistant to temperature, pH and metal ions.
Se purificó la enzima fitasa de la seta Pinar melkior (Lactarius piperatus) utilizando precipitación de sulfato de amonio y técnicas cromatográficas de intercambio iónico DEAE con sephadex. Primeramente, la enzima fitasa purificada fue ligada de forma covalente a la superficie de CTS NP con magnetita con una eficiencia del 87%. Después, el pH óptimo de las enzimas libres e inmovilizadas fue determinado como 5,0 y 4,0 respectivamente. La temperatura óptima de las enzimas libres e inmovilizadas se encontró a 60°C. Además, se investigaron los efectos de algunos iones metálicos en la actividad de las enzimas fitasas libres e inmovilizadas. También, se estudió si las enzimas fitasas libres e inmovilizadas purificadas podrían hidrolizar el ácido fítico en varios productos que contengan cereales. Además, se determinó que las enzimas fitasa inmovilizadas, hidrolizaron el ácido fítico con el valor más alto (75,02%) en el trigo. A partir de estos resultados se encontró que las enzimas inmovilizadas eran muy resistentes a la temperatura, pH e iones metálicos.
1. Introduction
Phytase (myo-inositol hexakisphosphate phosphohydrolase, EC 3.1.3.8) is an enzyme which catalyzes the hydrolysis reaction of phytic acid (myo-inositol hexaphosphate) to inorganic monophosphate, myo-inositol phosphate and free myo-inositol. Phytic acid is the main storage of the source of phosphorus of food material including grains, legumes and oil seeds. Taking phytate from food into the body causes adverse effects by creating a negative effect on mineral intake. In blood pH, it causes mineral deficiencies of metabolism by leading to insoluble mineral-phytate complex formation of zinc, iron, calcium, magnesium, manganese and copper metal ions which both act as enzyme cofactors and as different metabolic processes. Also, phytates completely prevent the formation of the phytate-protein complex of protein and amino acids (Kumar, Sinha, Makkar, & Becker, Citation2010; Zhang et al., Citation2010).
Phytase enzyme is located in animals, plants, microorganisms and fungi. First, phytases were identified in rice bran in the early 20th century, and are produced by animals, plants and microorganisms including bacteria, yeast and fungi.
Phytase enzyme was purified from many microorganisms especially Aspergillus niger, Aspergillus terreus, Aspergillus oryzae, Escherichia coli, Emericella nidulans and Thermomyces lanuginosus and characterized (Greiner & Konietzny, Citation2006). However, the first commercial phytase enzyme obtained from Aspergillus niger and was offered for sale in 1991. Also, several yeast species such as Saccharomyces cerevisiae, Candida tropicalis, Kluyveromyces fragilis, Torulopsis candida, Debaryomyces castelli, Schwanniomyces castelli produced phytase enzymes and their phytase enzymes were determined (Nayini & Markakis, Citation1984).
The presence of phytase enzymes in the blood of calves as animal origin were determined (Mccoollum & Hart, Citation1908). Also, resources of phytase produced as endogenous by microflora related to mucosa of the small intestine and large the bowel in humans and animals were available. In general, endogenous phytase activity in humans and animals is less important compared with phytase activity in plants, yeast, fungi and microbial phytase (Weremko et al., Citation1997).
Phytase enzyme was purified and characterized from few types of edible mushrooms. such as Agrocybe pediades, Cenporia sp., Peniophora lycii, Trametes pubenscens and Agaricus bisporus (Collopy & Royse, Citation2004; Lassen et al., Citation2001). Phytases were used in many areas such as animal feed, the food industry, the preparation of myo-inositol phosphate, the paper industry, soil improvement and elimination of environmental pollution. A few studies were being conducted, especially in the food industry, for reducing the amount of phytate in cereal products in recent years.
In this study, phytase enzyme was purified from the edible, non-toxic Pinar melkior (Lactarius piperatus) mushroom using ammonium precipitation and DEAE-Sephadex ion exchange chromatography It was then immobilized on surface of magnetit-CTS NPs to ensure prolonged use and its industrial use of pure enzyme. To make comparisons and optimization methods, values of optimum pH, optimum temperature, stable pH and stable temperatures of soluble and immobilized enyzme were determined. In order to determine the altered factors of enzyme activity, measurements of activity of soluble and immobilized enzymes were performed in the presence of different metal ions. Finally, their applicability was investigated in the degradation of phytate in foodstuffs originating from cereals, especially widely used canned foods, using free and immobilized phytase enzyme.
2. Materials and methods
2.1. Chemicals
Bovine serum albumin (BSA), Na-phytate, xylan, chitin, starch, gelatin, DEAE-sephedex, and Sephacryl S-200, ethylene diamine tetra acetic acid (EDTA), dithioerythritol, β-mercaptoethanol and agents for SDS-PAGE were purchased from Sigma (USA). Ethanol, sodium acetate (CH3COONa), ammonium sulfate ((NH4)2SO4), sodium chloride (NaCl) and sodium hydrogen phosphate monohydrate (Na2HPO4 × H2O), glutaraldehyde were purchased from Merck (Darmstadt, Germany). All other chemicals were of analytical grade.
2.2. Plant material and storage conditions
Pinar melkior (Lactarius piperatus) mushrooms were collected near the city of Manisa between May and July and they were stored at −40°C before beginning research. They were identified by a botanist.
2.3. Purification of phytase enzyme from Pinar Melkior (Lactarius piperatus) mushroom
Phytase enzyme was purified from the mushrooms in two steps. First, ammonium sulfate precipation was made in homogenate of mushroom in the ranges of 0–20%, 20–40%, 40–60%, 60–80% and 80–100%, respectively. The highest phytase enzyme activity was determined in the range of 40–60% precipitate. Then, the precipitate was dissolved in 20 mM Na-acetate buffer at pH 5.5 and it was dialyzed against the same buffer (Nadaroglu & Tasgin, Citation2013).
In the second step, the homogenate was applied to DEAE-Sephadex column which was previously balanced with 20 mM Na-acetate buffer (pH 5.5) and the column was washed with the same buffer. Phytase enzyme was eluted from the column by increasing the ionic strength gradient of the buffer. The activity of phytase enzyme was determined by taking eluates from DEAE-Sephadex column using Na-phytate substrate. The eluates which showed phytase activity were combined and an enzyme pool was created (Nadaroglu & Tasgin, Citation2013).
2.4. SDS-PAGE electrophoresis
The Laemmli method (Laemmli, Citation1970) was used to determine the number of subunits of purified phytase enzyme from Pinar melkior (Lactarius piperatus) mushroom in two different acrylamide concentrations; with stacking gel 3%, seperation gel 10%, sodium dodecyl sulfate gel electrophoresis (SDS-PAGE).
2.5. Immobilization of purified phytase enzyme
2.5.1. Preparation of chitosan particles
Chitosan was purchased from Sigma-Aldrich Co. LLC in quantities of 1–10 μm. Chiu et al’s. (Chiu, Chung, Giridhar & Wu, Citation2004) method was used in the preparation of chitosan particles. First, Chitosan was stirred with acetic acid for 3 hours. Then, the mixture was neutralized by adding 1 N NaOH of ethyl alcohol medium and then the chitosan (CTS) molecules were washed with distilled water. CTS was treated with 5% glutaraldehyde solution for 4 hours to bind amino groups to it. Finally, CTS molecules were washed with distilled water under vacuum to remove the non-binding glutaraldehyde from the medium.
2.5.2. Preparation of nano-magnetite chitosan particles and immobilization of purified phytase enzyme
The magnetic property of surface CTS particles was able to be treated with dispersed Fe3O4 in distilled water. After CTS was treated with nano particles, it was washed with distilled water to remove the non-binding nano particles. Then, the wet precipitate was dried for 72 hours at 40°C and stored at 4°C for use in experimental studies.
Purified phytase enzyme (205 EU/mL; 0.5 mg protein/mL) from Pinar melkior (Lactarius piperatus) was immobilized to the surface of magnetite-CTS NPs and activated CTS with glutaraldehyde in Na-acetate buffer solution (pH 5.5) containing 25 mM NaCNBH3. The amount of protein were determined in the reaction medium at different time intervals, by using Warburg and Bradford methods and the amount of binding phytase enzyme was determined against time (Bradford, Citation1976).
2.6. Determination of optimum conditions for immobilization of purified phytase enzyme onto magnetite-CTS NPs
To determine the most suitable immobilization conditions of phytase enzyme to support material, the following optimization procedure were performed for all studies of immobilization;
For determination of the most appropriate immobilization, pH were immobilized in a range of pH from 4.0 to 8.0. For determination of the most appropriate immobilization temperature, immobilizations were carried out at 10–60°C in previously determined pH.
The phytase binding efficiency (E) is defined as follows:
where C1 and Co are the amounts of phytase protein in the solution before and after immobilization, respectively. The amount of activity in the immobilized phytase was calculated as follows:
where A is the activity of the immobilized phytase and A1 and Ao are the activities of the free phytase in solution before and after immobilization, respectively.
2.7. Determination of phytase activity
Phytase activity was determined by measuring the amount of units of inorganic phosphate hydrolyzed from sodium phytate. The reaction mixture consisted of 0.5 mL, 2 mM Na-phytate substrate which was prepared in 20 mM pH 5.0 Na-asetat buffer and 0.2 mL enzyme solution. For the measurement of the immobilized enzyme, reaction mixture consisted of 1 mL 20 mM acetate buffer and 0.5 mL 2 mM Na-phytate substrate which was prepared in 20 mM pH 5.0 Na-asetat buffer and 0.1 g immobilized enzyme. After it was incubated at 50°C for 30 min, the reaction was stopped by adding 0.5 mL 10% (w/v) TCA. The reaction mixture was centrifuged at 5000 rpm for 10 min. Then, the coloring solution was added to the filtrate and the amount of phosphate hydrolyzed in the medium was determined by assaying the changes of absorbance at 700 nm (Iqbal, Lewisi, & Cooper, Citation1994). The one unit of phytase activity (1 EU) was defined as the amount of enzyme that released 1 μmol inorganic phosphate at optimum conditions in 1 min of reaction.
2.8. Protein determination
Protein concentration of samples was determined spectrophotometrically by using the Bradford method (Bradford, Citation1976). Bovine serum albumin (BSA) was used as a standard.
2.9. Determination of the optimal pH for activity, stability and reusability
The pH optimum was determined at 50°C using different buffers: 50 mM citric acid buffer for pH 3.0–5.0, 50 mM MES buffer for pH 5.0–7.0, and 50 mM Tris buffer for pH 7.0–9.0. The immobilized and free-form phytase were suspended in each buffer at different pHs to give an activity of 30 U/mL. pH stability was tested in 100 mM buffer incubated for 4 h at 4 °C.
The various temperatures (20–90°C) were tested in 50 mM Na-acetate buffer (pH 5.0) to determine the optimal temperature for the enzyme reaction. To determine the thermostability of the enzyme, thermal challenges at different temperatures (60°C, 70°C, 80°C, and 90°C) were performed in 50 mM acetate buffer (pH 5.0). Immediately afterwards, the heat-treated enzymes were place on ice. Residual phytase activity was measured at 50°C and pH 5.0, as described previously (Iqbal et al., Citation1994). Each treatment was done a minimum of three times.
For determination of the reusability activity of mannanase enzyme, activity determination was done 20 times at reaction pH and temperature.
2.10. Effect of some metal ions
The influence of various metal ions such as Zn2+, Cu2+, Co2+, Mn2+, Ca2+ and Fe2+ at concentrations of 1 and 5 mM on enzyme activity was investigated by preincubating the phytase with different compounds for 10 min at room temperature. Residual activity was calculated against control.
2.11. Hydrolysis of phytate in some cereals
The legumes such as green lentils, red lentils, peas, pinto beans, beans, brass, corn, dried corn, oat, rye, wheat, broad beans, chickpeas and peanuts were collected at local markets and were used for hydrolyzing the phytate. First of all, the cereals were washed elaborately; then they were kept in air to dry, and they were homogenized. Into the 5 g legume sample homogenate, 2 mL free enzyme (30 U/mL) solution was added. Also, the same experimental mixture was created for immobilized phytase enzyme. At 5.0 pH value, the legume homogenates were processed with free phytase and immobilized phytase enzyme for 4 hour at 50°C. Then, the amount of free phosphorus was determined by assaying the standard activity against a blank sample which was consisted of distilled water (Iqbal et al., Citation1994). The amount of phosphorus was calculated by using a calibration curve. A control was prepared without added enzyme for all the assays.
2.12. Statistical analysis
Data are presented as the mean ± standard deviation of each treatment. Data were analysed for statistical significance using analysis of variance (ANOVA) followed by the Tukey test
(p < 0.05) (SigmaPlot 11.0, SPSS Inc., USA).
3. Results and discussion
Phytases (myo-inositol hexakisphosphate phosphohydrolase) [EC 3.1.3.8] belong to the class of fosfomono esterase of enzymes and they are enzymes which catalyze hydrolysis reaction to phosphate; inositol and phosphate result in sequentially hydrolysis groups of organophosphate of phytic acid (Konietzny & Greiner, Citation2004; Lei & Porres, Citation2003).
3.1. Purification and characterization of phytase from Pınar melkior (Lactarius piperatus)
The phytase enzyme was purified in three steps including ammonium sulfate precipitation, DEAE-sephadex ion exchance chromatography and Sephacryl S200 gel filtration chromatography from Pinar melkior (Lactarius piperatus). All purification steps were summarized in .
Table 1. The purification process of purified fitaz enzyme from Pinar melkior (Lactarius piperatus).
Tabla 1. Proceso de purificación de las enzimas fitasa purificadas de Pinar melkior (Lactarius piperatus).
In the first stage, the crude extract was precipitated between the range of 0–90% ammonium sulfate saturation. These results showed that phytase enzyme activity was determined as the highest rate in the range of 40–60% saturation by precipitation. The purified phytase withy 81.7% yield and 28.13-fold purification was applied to a DEAE-sephadex ion exchange column.
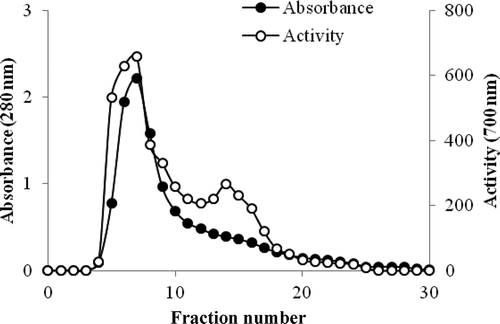
In the second step, amonyum sulfate fraction of 40–60% was applied to the DEAE-sephadex ion exchange column. The phytase was purified more than 70.9 times with a yield of 52.4%, and its specific activity was equivalent to 1423.6 EU/mg protein (). Phytase enzyme from Pinar melkior (Lactarius piperatus) mushroom was purified with anion exchange chromatography and presented in .
Figure 1. Purification of phytase by ion exchange chromatography using DEAE-sephadex.
Figura 1. Purificación de la fitasa mediante cromatografía de intercambio iónico utilizando DEAE con sephadex.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)’s results were presented for purified the phytase enzyme, 65 kDa () consisting of one subunit has been identified (). To find the molecular weights of the active form of the purified enzyme, Sephadex G-100 gel filtration chromatography was performed and the same results were obtained.
Figure 2. SDS-PAGE electrophoretic pattern of phytase [purified phytase enzyme from Pinar melkisi (Lactarius piperatus)].
Figura 2. Patrón electroforético SDS-PAGE de fitasa [encima de fitasa purificada de Pinar melkisi (Lactarius piperatus)].
![Figure 2. SDS-PAGE electrophoretic pattern of phytase [purified phytase enzyme from Pinar melkisi (Lactarius piperatus)].Figura 2. Patrón electroforético SDS-PAGE de fitasa [encima de fitasa purificada de Pinar melkisi (Lactarius piperatus)].](/cms/asset/08e93f44-5db8-4e8f-9972-c8f7233416e0/tcyt_a_1045942_f0002_b.gif)
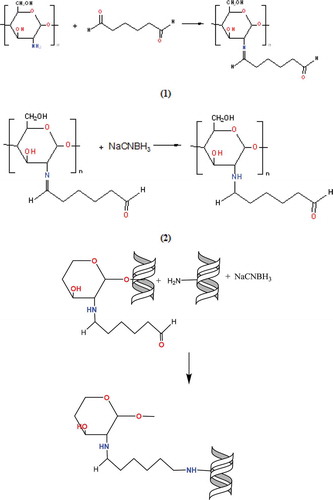
First, chitosan was activated by binding the glutaraldehyde to the amino group (-NH2) located chitosan for connecting the purified phytase enzyme to the magnetite-CTS NPs. Then binding of purified phytase was provided by creating a schift base to glutaraldehyde. NaCNBH3 was used for reducing the schift base formed in both of two steps. After synthesization of the support material, its surface was coated with Fe3O4 nanoparticles to gain the magnetic feature of chitosan. Reactions are shown in .
Figure 3. Reaction mechanisms of chitosan modified with glutaraldehyde and immobilization of phytase enzyme to this support material.
Figura 3. Mecanismos de reacción del chitosán modificado con glutaraldehído e inmovilización de la enzima fitasa en este material de soporte.
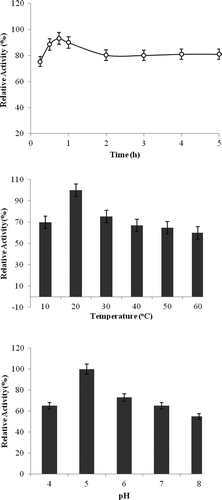
pH and temperature were primarily determined in order to decide the optimum conditions of covalent immobilization to the surface of magnetized citosan with nano Fe3O4 of pure phytase enzyme. Pure phytase enzymes were immobilized by using appropriate buffers among pH 4–8, and relative activity(%) of immobilized phytase was calculated. Accordingly, it was determined that the pure phytase enzyme was connected to support material at the high level at pH 5 and 20°C. Then, the immobilization was monitored for 6 hours to determine the most appropriate immobilization time at pH 5 and 20°C. It was determined that purified phytase enzyme was connected to magnetized citosan with nano Fe3O4 support material after 1 hour at the rate of 82.5% ().
3.2. Structural characterization of support
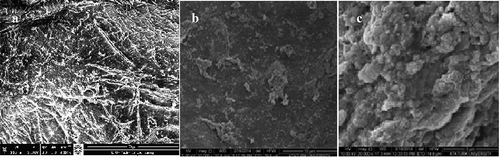
The structure of chitosan, magnetite-CTS NPs; III: immobilized phytase on magnetite-CTS NPs at SEM images are shown in –c. The thickness of the wall is about 20 ~ 30 nm, the pore size is about 0.5 ~ 1 μm. From the SEM image, it was observed that the support material was fine-pore structure and its structure was appropriated to modify with nanomaterials and to immobilization with phytase enzyme. In addition, it had considerable support and structural stability.
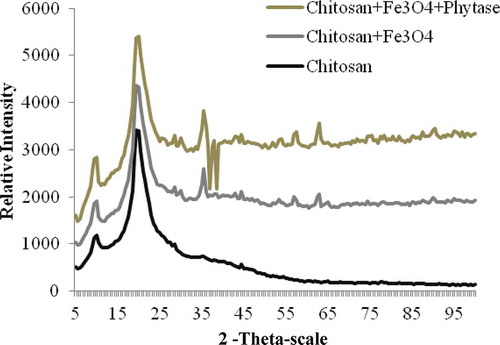
3.3. X-ray diffraction (XRD) analysis
shows XRD patterns of natural chitosan; magnetite-CTS NPs; purified phytase immobilized magnetite-CTS NPs. XRD patterns chitosans are illustrated in . The XRD pattern of chitosan exhibits broad diffraction peaks at 2θ = 9,5° and 19,5° which are typical fingerprints of crystal chitosan (Ogawa, Hirano, Miyanishi, Yui, & Watanabe, Citation1984). In the XRD pattern of prepared Fe3O4 modified chitosan NPs and purified phytase immobilized modified chitosan with nano magnetite, the peak corresponding to chitosan could be seen at around 2θ = 20.0°, but it became much lower and wider. Also, five diffraction peaks were observed at 2θ = 35.5°, 44.5, 54.5, 57.5°, 63.0°. These peaks belong to typical fingerprints of Fe3O4 NPs (Cao et al., Citation2014).
Figure 6. XRD photographs for nature chitosan; modified chitosan with nano magnetite; purified phytase immobilized modified chitosan with nano magnetite.
Figura 6. Imágenes XRD de chitosán natural; chitosán modificado con nanomagnetita; chitosán modificado inmovilizado con fitasa purificada con nanomagnetita.
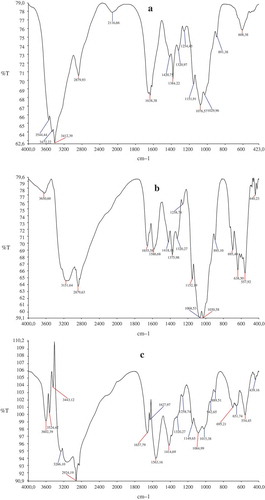
3.4. FTIR analysis
The structure of the support material of natural chitosan (a), modified chitosan with nano magnetite (b), immobilized magnetic Fe3O4-chitosan nanoparticles with purified phytase from Pinar melkior (Lactarius piperatus) (c) was confirmed by FTIR analysis. The spectra of chitosan showed a broad absorbance at 3650.60–2879 cm−1 (O-H, hydroxyls and NH stretching vibrations of free amino groups), at 2879.93 cm−1 and 2116 cm−1 (-CH3 and -CH2 in chitosan structure), at 1638.38–1029.96 cm−1 (-C = O stretching of amide, -C = O secondary amide, -N-H bounds stretching vibrations) and at 891.38 cm−1 and 608.38 cm−1 (-C-H bending of amide bonds stretching vibrations) in curve in . In and , the peaks at 557.92 cm−1 and 554.45 cm−1 related to the Fe-O group of Fe3O4 nanoparticles. When was compared with , a new sharp peak appeared at 1627 cm−1. It indicated that at the 2116.66 cm−1 peak of N–H bending, vibration shifted to 1589.68 cm−1 and chitosan reacted with glutaraldehyde to form Schiff base (Guo, Diao, & Cai, Citation2005; Li, Jiang, Huang, Ding, & Chen, Citation2008). It was shown that there was no significant change in the functional biomass groups of magnetic Fe3O4-chitosan nanoparticles after immobilization of phytase enzyme. The results of FT-IR spectra indicated that chitosan was coated with the magnetic Fe3O4 nanoparticles and phytase enzyme was immobilized onto the magnetic Fe3O4-chitosan nanoparticles successfully.
Figure 7. FT-IR spectrum of nature chitosan (a), modified chitosan with nano magnetite (b), immobilized chitosan with purified phytase from Pinar melkior (Lactarius piperatus) (c).
Figura 7. Espectro FT-IR de chitosán natural (a), chitosán modificado con nanomagnetita (b), chitosán inmovilizado con fitasa purificada de Pinar melkior (Lactarius piperatus) (c).
3.5. Biochemical properties of free and ımmobilized phytase
3.5.1. Loading amount and activity of immobilized phytase
The loading amount and activity of Immobilized phytase were summarized in . The average loading amount of modified support is about ng/g. The phytase enzyme was bound to the surface as covalent. It was also bound there with weak bonds with wander walls bonds and binding rate was increased. In a study, it was observed that the enzyme was connected to modified support using nanostructure at most amount and powerfully. These obtained results seem to support our work (Shang, Li, & Zhang, Citation2014).
Table 2. The effect of some chemical compounds on free and immobilized phytase activity.
Tabla 2. Efecto de algunos compuestos químicos en la actividad de fitasa libre e inmovilizada.
3.6. Effect of incubation time, pH and temperature
3.6.1. Effect of reaction temperature
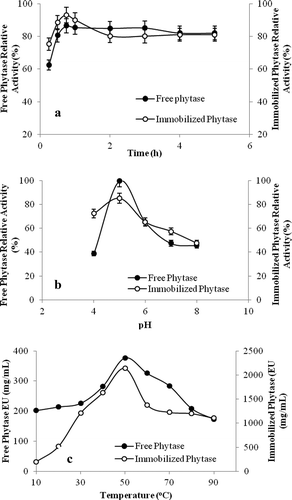
The reaction was carried out from 10 to 90°C using Na-fitat as a substrate at pH 5.0 for 30 min incubation time for free and immobilized phytase enzyme. The maximum phytase activity for free and immobilized phytase was found at 50°C. But, it was determined that both their activities decreased in higher temperatures ().
Figure 8. Optimization of purified and immobilized phytase enzyme activity.
Figura 8. Optimización de la actividad de enzimas fitasa purificadas e inmovilizadas.
3.6.2. Effect of pH
Enzyme substrate reaction was done at 50°C for 30 min varying the pH from 4.0 to 8.0. Optimum pH was found as 5.0 for free and immobilized phytase enzyme ().
3.7. Effect of reaction time
Enzyme substrate reaction was done at 50°C and pH 5.0 during 5 h. For free and immobilized phytase, maximum enzyme activity was found to be at 45 min incubation time and reaction was reached to balance at 1 hour ().
3.8. pH stability, thermal stability and reusability
3.8.1. Thermal stability
Enzymes are composed of protein. They are often sensitive to heat and can be inhibited. Immobilized enzymes were resistant to high temperatures, so there were many advantages for use in applications. The thermostability of the immobilized phytase was measured in comparison with that of free phytase as shown in . The rates of thermal inactivation of the soluble and immobilized enzyme were studied at the temperature range of 20–90°C at pH 5.0 in the 50 mM Na-acetate buffer.
Figure 9. (a) Thermal stability, (b) pH stability for free and immobilized phytase.
Figura 9. (a) Estabilidad térmica, (b) estabilidad del pH para la fitasa libre e inmovilizada.
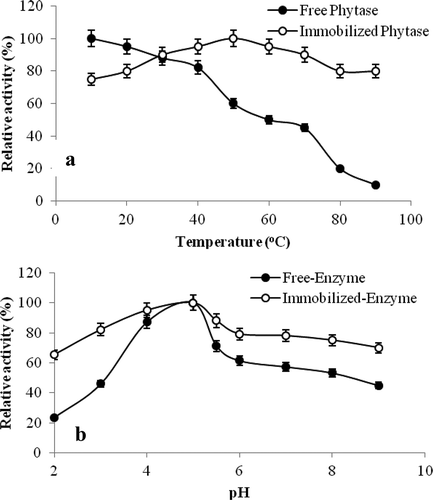
While the free enzyme was losing approximately 80% rate of its activity at 80°C after heat treatment for 1 hour, immobilized phytase was only losing 20% rate of its activity. Consequently, covalently linked immobilized phytase onto Fe3O4 modified chitosan NPs was more resistant to temperature than soluble phytase. When the enzyme was covalently bounded onto magnetite-CTS NPs, it could be thought that phytase enzyme was protected from environmental factors which caused conformational changes.
3.8.2. pH stability
Free and immobilized phytase enzymes were incubated for 1 hour at 4°C by using a buffer of acetate from pH 3 to 5, phosphate from pH 6 to 7 and Tris/HCI from pH 8 to 9 and their activity was measured after 1 hour and the results are shown in . It was determined that free enzyme maintained 46% rate of its activity while immobilized enzyme was maintaining 82% rate of its activity at pH 3. Also, free phytase maintained 45% rate of its activity while immobilized phytase maintained 70% rate of its activity at pH 9.0. As seen from the results, immobilized phytase enzyme activity was more stable against different pH values according to free enzyme.
3.8.3. Reusability
To determine the reusability activity of phytase enzyme, the enzyme activity determination was done 20 times at reaction pH and temperature. Immobilized phytase enzyme did not show noticeable change in its activity up to 9 times, but it lost 55% of its activity after 20 times.
3.9. The effects of Fe2+, Cu2+, Hg22+, Co2+, Mg2+ and Zn2+ on the activity of free and immobilized phytase enzyme
Effects of Fe2+, Cu2+, Hg22+, Co2+, Mg2+ and Zn2+ on activity of free and immobilized purified phytase enzyme from Pinar melkior (Lactarius piperatus) mushroom onto magnetite-CTS NPs were investigated and all results were given in as relative activity%. While all metal ions were highly inhibited to the activity of free phytase enzyme, only Cu2+ and Hg22+ were inhibited to the activity of immobilized phytase enzyme. While Fe2+, Co2+, Mg2+ ions were increased to the activity of immobilized phytase, Zn2+ had no effect on it. It was thought that immobilized mannanase already had iron nanoparticules, so Fe2+ was activated by this enzyme activity. From the results, it was concluded that immobilized phytase enzyme was more resistant to metal ions. because of this feature of the enzyme. Immobilized phytase could be found to be highly suitable for industrial application.
Table 3. The hydrolsis of phytate from some legumes using free and immobilized phytase enzymes.
Tabla 3. Hidrólisis de fitasa de algunas legumbres utilizando enzimas fitasa libres e inmovilizadas.
3.10. Hydrolysis of phytate in some cereals
The form of complex phytic acid with calcium is referred to as phytate and is capable of making strong chelating. They form insoluble complex salts by combining with metal cations (Zn2+, Cu2+, Co2+, Mn2+, Ca2+ ve Fe2+) and they prevent the absorption of metal ions from the intestine. Phytates decrease digestibility of protein and amino acid and they lead to negative effect in mineral intake as a result of forming phytate-protein complexes which are harder to distintegrate by proteolytic enzymes binding endogenous proteases especially trypsin and chemotrypsin. (Konietzny & Greiner, Citation2003; Lopez et al., Citation2000). Therefore, this could cause iron, calcium and zinc deficiencies especially in communities adopting this type of vegetative feeding (Ferguson, Gibson, Thomson, & Ounpuu, Citation1989).
Because of this, phytic acid which was located in the outer shell of grains and legumes was hydrolyzed using free and immobilized phytase enzyme and the results are given in . From the results, it was observed that immobilized phytase enzyme was more effective at the hydrolysis of phytate in dry beans and it demonstrated the highest effective on wheat and peanuts at the end of 4th at a rate of 75%. Immobilized phytase enzyme could be used as additive material in bread making, preparation of vegetable protein hydrolyzate products and breakdown of bran in cereals.
4. Conclusions
The phytase was first purified and characterized from Pinar melkior (Lactarius piperatus) and immobilized onto magnetite-CTS NPs. This research was focused on the food industry. It was found that purified and immobilized phytase enzyme had high catalytic activity and it was resistant to metal ions, high temperature and different pHs. Also, it was observed that immobilized enzyme was effective on phytate in dry beans. Due to this reason, the immobilized phytase enzyme onto surface of magnetite-CTS NPs could also be used in different industrial areas. In this context, there could be large potential for use in the fields of food, animal feed and fertilizers.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3
- Cao, C., Xiao, L., Chen, C., Shi, X., Cao, Q., & Gao, L. (2014). In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technology, 260, 90–97. doi:10.1016/j.powtec.2014.03.025
- Chiu, S.-H., Chung, T.-W., Giridhar, R., & Wu, W.-T. (2004). Immobilization of β-cyclodextrin in chitosan beads for separation of cholesterol from egg yolk. Food Research International, 37, 217–223. doi:10.1016/j.foodres.2003.12.001
- Collopy, P. D., & Royse, D. J. (2004). Characterization of phytase activity from cultivated edible mushrooms and their production substrates. Journal of Agricultural and Food Chemistry, 52, 7518–7524. doi:10.1021/jf040220m
- Ferguson, E. L., Gibson, R. S., Thomson, L. U., & Ounpuu, S. (1989). Dietary calcium, phytate, and zinc intakes and the calcium, phytate, and zinc molar ratios of the diets of a selected group of East African children. The American Journal of Clinical Nutrition, 50, 1450–1456. Retrieved from http://ajcn.nutrition.org/content/50/6/1450
- Greiner, R., & Konietzny, U. (2006). Phytase for food application. Food Technology and Biotechnology, 44(2), 125–140.
- Guo, M., Diao, P., & Cai, S. (2005). Hydrothermal growth of well aligned ZnO nanorodarrays: Dependence of morphology and alignment ordering upon preparing conditions. Journal of Solid State Chemistry, 178(6), 1864–1873. doi:10.1016/j.jssc.2005.03.031
- Iqbal, T. H., Lewisi, K. O., & Cooper, B. T. (1994). Phytase activity in the human and rat small intestine. Gut, 35, 1233–1236. doi:10.1136/gut.35.9.1233.
- Konietzny, U., & Greiner, R. (2003). Phytic acid: Nutritional ımpact. In B. Caballero, L. Trugo, & P. Finglas (Eds.), Encyclopedia of food science and nutrition (pp. 4555–4563). London: Elsevier. doi:10.1016/B0-12-227055-X/00923-8
- Konietzny, U., & Greiner, R. (2004). Bacterial phytase: Potential application, in vivo function and regulation of its synthesis. Brazilian Journal of Microbiology, 35, 12–18. doi:10.1590/S1517-83822004000100002
- Kumar, V., Sinha, A. K., Makkar, H. P. S., & Becker, K. (2010). Dietary roles of phytate and phytase in human nutrition: A review. Food Chemistry, 120(4), 945–959. doi:10.1016/j.foodchem.2009.11.052
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. doi:10.1038/227680a0.
- Lassen, S. F., Breinholt, J., Ostergaard, P. R., Brugger, R., Bischoff, A., Wyss, M., & Fuglsang, C. C. (2001). Expression, gene cloning, and characterization of five novel phytases from four basidiomycete fungi: Peniophora lycii, Agrocybe pediades, a Ceriporia sp., and Trametes pubescens. Applied and Environmental Microbiology, 67, 4701–4707. doi:10.1128/AEM.67.10.4701-4707.2001
- Lei, X. G., & Porres, J. M. (2003). Phytase enzymology, applications, and biotechnology. Biotechnology Letters, 25, 1787–1794. doi:10.1023/A:1026224101580
- Li, G., Jiang, Y., Huang, K., Ding, P., & Chen, J. (2008). Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. Journal of Alloys and Compounds, 466, 451–456. doi:10.1016/j.jallcom.2007.11.100
- Lopez, H. W., Vallery, F., Levrat-Verny, M. A., Coudray, C., Deminge, C., & Emesy, C. (2000). Dietary phytic acid and wheat bran enhance mucosal phytase activity in rat small intestine. Journal of Nutrition, 130, 2020–2025. Retrieved from http://jn.nutrition.org/content/130/8/2020.full
- Mccoollum, E. V., & Hart, E. B. (1908). On the occurrence of a phytin-splitting enzyme in animal tissues. Journal of Biological Chemistry, 4(6), 497–500. doi:10.1016/S0014-827X(01)01084-9.
- Nadaroglu, H., & Tasgin, E. (2013). Purification and characterisation of laccase from Lactarius volemus and its application of removal phenolic compounds from some fruit juices. International Journal of Food, Agriculture & Environment, 11(3&4), 109–114. doi:10.12691/wjar-2-3-4
- Nayini, N. R., & Markakis, P. (1984). The phytase of yeast. Lebensmittel-Wissenschaft & Technologie, 17, 24–26. doi:10.1021/jf0730486
- Ogawa, K., Hirano, S., Miyanishi, T., Yui, T., & Watanabe, T. (1984). A new polymorph of chitosan. Macromolecules, 17, 973–975. doi:10.1021/ma00134a076
- Shang, C.-Y., Li, W.-X., & Zhang, R.-F. (2014). Immobilized Candida antarctica lipase B on ZnO nanowires/macroporous silica composites for catalyzing chiral resolution of (R, S)-2-octanol. Enzyme Microbial Technology, 61–62, 28–34. doi:10.1016/j.enzmictec.2014.04.014
- Weremko, D., Fandrejewski, H., Zebrowska, T., Han, K., Kim, J. H., & Cho, W. T. (1997). Bioavailability of phosphorus in feeds of plant origin for pigs – Review. Asian-Australasian Journal of Animal Sciences, 10, 551–566. doi:10.1080/00071667308416053
- Zhang, G. Q., Dong, X. F., Wang, Z. H., Zhang, Q., Wang, H. X., & Tong, J. M. (2010). Purification, characterization, and cloning of a novel phytase with low pH optimum and strong proteolysis resistance from Aspergillus ficuum NTG-23. Bioresource Technology, 101(11), 4125–4131. doi:10.1016/j.biortech.2010.01.001