ABSTRACT
The need for greener extraction procedures that are quick and efficient has prompted the evolution of pressurized hot water extraction (PHWE). Here, the extraction of flavonoids from Bidens pilosa was demonstrated using PHWE at 50°C, 100°C and 150°C. The extracts were analyzed on UPLC-qTOF-MS/MS and 28 flavonoids of different classes were identified. Further analysis of the data using principal component analysis revealed differential distribution patterns of the identified molecules. In overall, the extraction yield increased proportionately with increasing temperature. It can thus be deduced that PHWE is an excellent extraction method of flavonoids from plant tissues. Again, this study reiterates B. pilosa as a rich source of flavonoids.
RESUMEN
La necesidad de procedimientos de extracción más ecológicos que sean rápidos y eficaces ha impulsado la evolución de la extracción con agua presurizada (PHWE). Se demostró la extracción de flavonoides de Bidens pilosa utilizando PHWE a 50, 100 y 150°C. Los extractos fueron analizados por UPLC-qTOF-MS/MS y se identificaron 28 flavonoides de diferentes clases. Un análisis posterior de los datos utilizando el Análisis de componentes principales reveló los patrones de distribución diferenciales de las moléculas identificadas. En general, el rendimiento de extracción incrementó proporcionalmente con el aumento de la temperatura. Por lo tanto, podría deducirse que PHWE se trata de un excelente método de extracción de flavonoides de tejidos vegetales. De nuevo, este estudio reitera que B. pilosa se trata de una fuente rica en flavonoides.
Introduction
Plants have been known to be essential to life as they are rich in a wide variety of secondary metabolites that perform important biological functions (Atanasov et al., Citation2015). Bidens pilosa is an underutilized plant species widely distributed all over the world (Grombone-Guaratini, Silva-Brandão, Solferini, Semir, & Trigo, Citation2005; Tereza, Mansanares, Semir, & Solferini, Citation2006). It is a rich source of food for humans and animals particularly in the tropics (Bairwa, Kumar, Sharma, & Roy, Citation2010; Bartolome, Villaseñor, & Yang, Citation2013). The plant contains a diversity of interesting metabolites, including hydroxycinnamic acids and flavonoids (Bartolome et al., Citation2013). Aside its use as a source of food, B. pilosa is used in folklore medicine not only in the treatment of more than 40 diseases in man (Borges et al., Citation2013) but also as resistance-modifying agents (Darwish, Aburjai, Al-Khalil, Mahafza, & Al-Abbadi, Citation2002). Some of its important biological activities include antimicrobial (Silva et al., Citation2014), anticancer and antipyretic (Sundararajan et al., Citation2006), anti-oxidative (Yang et al., Citation2006), anti-inflammatory and anti-allergic (Horiuchi & Seyama, Citation2008), antidiabetic (Lai et al., Citation2015) as well as many other beneficial activities as reviewed in other studies (Bairwa et al., Citation2010; Bartolome et al., Citation2013).
These biological functions can be rationalized by the wide spectrum of metabolites detected in this plant (Bartolome et al., Citation2013; Silva et al., Citation2011). Heretofore, a comprehensive list of identified metabolites from this plant have been compiled that constitutes at least 201 compounds including flavonoids, aromatics, terpenoids and other compounds (Chiang et al., Citation2004; Grombone-Guaratini et al., Citation2005; Silva et al., Citation2011). Flavonoids are, however, the predominant class of phenolic metabolites in the Bidens genus (Chiang et al., Citation2004). They are quite a remarkable group of phytonutrients that are bioactive and play several different roles in the health of plants, animals and humans alike (Kris-Etherton et al., Citation2002, Citation2004). Understanding the metabolite composition of various underutilized plants is the key to tapping indigenous knowledge from them and exploiting their potential applications in pharmacology and folklore medicine.
Metabolite fingerprinting is a technological approach for providing information from chromatographic spectra of metabolites (Scholz, Gatzek, Sterling, Fiehn, & Selbig, Citation2004) that has been useful in biochemical and pharmaceutical studies of such plants. Extraction is an empirical and important unit of operation in metabolite fingerprinting of plants as the quality of analytical results has often been directly linked to the extraction technique employed. Extraction has been shown to affect both quantitative and qualitative aspects of data generated (Khoza et al., Citation2014; Song, Pranovich, & Holmbom, Citation2011) as well as the reliability and consistency of such data (Tambellini, Zaremberg, Turner, & Weljie, Citation2013). Selection therefore, of an appropriate extraction technique is crucial particularly when considering the wide array of chemical species present in plants coupled with their individual physicochemical differences such as polarity and chemical stability.
Conventional methods have been applied to extract plant metabolites for analyses of various kinds. However, there are concerns on the potential health (human and environmental) implications associated with them. Essentially, these methods require large volumes of hazardous and environmentally unfriendly organic solvents (Vergara-Salinas et al., Citation2013) and, moreover, they are time consuming and laborious (Herrero, Cifuentes, & Ibañez, Citation2006). These shortcomings have propelled the evolution and adoption of greener routes and more efficacious techniques such as pressurized hot water extraction (PHWE) for the extraction of metabolites from plant tissues. At present, PHWE is the most favored extraction technique with potentials to overcome these drawbacks (King, Citation2000). This technique is environment friendly and promises better selectivity as its solvation power can be manipulated over a wide spectrum of polarities. Moreover, it offers results that are comparable to (or even better than) those obtained when using conventional extraction methods, it is less expensive and requires shorter extraction times (Herrero et al., Citation2006). As an added advantage, PHWE utilizes water as the extraction solvent, which is readily available and compatible with most chromatographic instruments (Liang & Fan, Citation2013; Richter, Toral, & Toledo, Citation2006).
PHWE is increasingly gaining attention in the biochemical and pharmaceutical industry, particularly for the extraction and analysis of plant metabolites (Mushtaq, Choi, Verpoorte, & Wilson, Citation2014). Heretofore, there have been no reports on the application of this technique for the extraction of flavonoids from B. pilosa. Interestingly, the phenomenon of PHWE mimics those of traditional techniques for the preparation of food and herbal portions. In this study, a PHWE, an eco-friendly extraction technique was applied for the extraction and metabolite profiling of 28 flavonoids from B. pilosa using UPLC-qTof-MS/MS.
Materials and methods
Materials: solvents
Solvents used in this study included UPLC/MS grade quality methanol and acetonitrile, purchased from Romil, MicroSep, South Africa. Ultrapure water was purified using a Milli-Q Gradient A10 system (Millipore, Billerica, MA, U.S.A). Analytical grade quality formic acid was purchased from Sigma Aldrich, Germany.
Plant materials and metabolite extraction
Leaves and stems of B. pilosa plant used in this study were collected from different sites around the Venda region of Limpopo province (South Africa). Sample preparation and extraction followed procedures described by Khoza et al. (Citation2014). The plant specimens were air-dried at ambient conditions for about 7 days and ground to powder using a mortar and pestle. Extraction of metabolites was achieved by a makeshift laboratory scale PHWE unit described in Khoza, Gbashi, Steenkamp, Njobeh and Madala (Citation2016). PHWE of B. pilosa was conducted at temperatures of 50°C, 100°C and 150°C, a pressure of 1000 ± 200 psi maintained using the back-pressure valve and extraction solvent (pure water) pumped at a flow rate of 5.0 ml/min for approximately 10 min. For the extraction, 4 g of homogenized ground plant materials was mixed with 2 g of diatomaceous earth (Sigma, Munich, Germany), a dispersing agent and placed in an extraction cell located inside the oven with automatically regulated temperature (±1°C). The extracts were collected into sealed falcon tubes up to the 50 mL mark through the outlet coil immersed in a cooling water bath. The extracts were filtered using a 0.22 µm nylon syringe filter into a 2 mL HPLC vial and preserved at −20°C prior to analysis.
Chromatographic separation and mass spectrometry (UHPLC-qTOF-MS)
The chromatographic separation of compounds was performed on a UHPLC hyphenated to a Synapt G1 -qTOF-MS instrument (Waters Corporation, Manchester, U.K.) equipped with a Waters Acquity HSS T3 C18 column (150 mm × 2.1 mm ID and of 1.8 µm particle size) and the column oven temperature maintained at 60°C. The mobile phases used were (A) 0.1% formic acid in deionized water; and (B) 0.1% formic acid in acetonitrile. The chromatographic program began with 2% B for 1 min, ramped to 95% B for 24 min, kept constant for 2 min, and the initial conditions (2% B) were reestablished for 1 min, and the column was re-equilibrated for 2 min for the next run. The total runtime was 30 min with the mobile phases pumped at a flow rate of 0.4 mL/min.
Mass spectrometry was performed using a Waters qTOF-MS instrument (Waters Corporation, Manchester, U.K.) fitted with electrospray ionization (ESI) source operated in both positive and negative ion electrospray modes. The m/z range was 100–1000 Da, scan time 0.2 sec, interscan delay 0.02 sec, with leucine encephalin (556.3 µg/mL) as a lock mass, standard flow rate 0.1 mL/min and mass accuracy window of 5.0 mDa was used for MS data acquisition. Moreover, the instrument was operated on the following settings: collision energy of 3 eV, capillary voltage of 2.5 kV, sample cone voltage of 30 V, detector voltage of 1650 V (1600 V in negative mode), source temperature at 120°C, cone gas flow at 50 (L/h) and desolvation gas flow at 550 (L/h). To achieve metabolite fragmentation patterns necessary for annotation or identification, the collision energy during MS acquisition was experimentally changed in the trap ion optics by acquiring data with various collision energy levels to generate typical MSE fragmentation patterns.
Data analyses and identification of flavonoid compounds
Raw data acquired from UHPLC-qTOF-MS were entered into the MarkerLynx XS application software (Waters Corporation, Manchester, U.K.) for analysis and visualization. For maximum data output, the analysis was carried out using optimized parameters (Khoza et al., Citation2014). Here, only negative data were analyzed using similar optimized parameters, i.e. mass range 100–1000 with mass tolerance of 0.02 Da, retention time (Rt) and Rt window of 1–30 min and 0.2 min, respectively, whereas other parameters were automatically calculated.
Characterization of single components was performed via Rt and accurate molecular masses. Representative single ion monitoring (SIM) chromatograms for target molecules were generated using their m/z values. Moreover, various MS spectra for these molecules were obtained from the chromatograms, their fragmentation patterns observed and molecular formulae calculated on the basis of a 5 mg/kg mass accuracy range. This information was used to confirm the identities of these biomarkers following a search on the Dictionary of Natural Products (DNP) online database (http://dnp.chemnetbase.com/) and the KNApSAcK metabolite information (KMI) database (http://kanaya.naist.jp/knapsack_jsp/top.html). Extraction yields for molecules identified represented the relative peak intensity figures of molecular peaks corresponding to the identified molecules. Relative peak intensity is a dimensionless quantity, and corresponded to the area-under-the-peak values obtained from the peak list. This data file (peak list) is the final output obtained after processing of the MS data using MarkerLynx software (Barbarini & Magni, Citation2010; Khoza et al., Citation2015).
Statistical analysis
The extraction yield patterns of each identified metabolite across the extraction temperature profile were graphically described by the box-and-whiskers plots using IBM SPSS software version 22 (SPSS/IBM, Chicago, Illinois) (Khoza et al., Citation2015). A one-way analysis of variance (ANOVA) was performed to test for differences in the recovery patterns of identified metabolites across the different extraction temperatures using the above mentioned statistical software. Mean values of extraction yields were compared by Tukey’s post hoc test and means were deemed to significantly differ if p ≤ 0.05 as indicated on the box-and-whisker plots (Khoza et al., Citation2015). In order to perform multivariate data analysis, i.e. principal component analysis (PCA), subsequent data matrix obtained from MarkerLynx XS software was exported to SIMCA-P software version 12.0 (Umetrics, Sweden). Unless stated otherwise, all PCA models were pareto scaled. From the PCA loadings plot, metabolites of which the levels were affected by temperature during extraction were also selected.
Results and discussion
provides a list of flavonoids identified in extracts from B. pilosa. It was possible to identify 28 flavonoid metabolites including their respective positional glycosidic isomers (). Characterization of flavonoid metabolites was achieved using the MS fragmentation patterns and order of elution from the UHPLC chromatograms (Madala, Tugizimana, & Steenkamp, Citation2014). Their identities were confirmed using the DNP and KMI databases and various other literature reports in an approach previously reported (Khoza et al., Citation2015; Madala, Steenkamp, Piater, & Dubery, Citation2013). In view of that, Molecule (Mol.) 1 at Rt 15.13 min with m/z 461.0698 [M-H]‾ and MS2 fragment at m/z 285.0370 obtained after loss of 176 amu (glucurone unit) () was tentatively identified as kaempferol-3-O-glucuronide (Kajdžanoska, Gjamovski, & Stefova, Citation2010). Mol. 2 at Rt 15.18 min was tentatively identified as kaempferol-3-O-glucoside with m/z 447.0927 [M-H]‾ and fragment at m/z 285. 0360 obtained due to loss of a hexose moiety (162 amu) (Kajdžanoska et al., Citation2010). Mols. 3 & 4 at Rt of 15.60 and 15.90 min, respectively, also showed similar fragmentation patterns as Mol. 2 and as such, these three molecules were identified as either geometrical or regional isomers of caftaric acid hexose. Mol. 5 at retention 16.47 min was identified to be kaempferol-3-acetyl-glycoside with m/z 489.0989 [M-H]‾ and an MS spectrum showing product ion m/z 285.0350 (after loss of 204 amu: acetyl-hexose) (Kajdžanoska et al., Citation2010; Khoza et al., Citation2015; Ramabulana et al., Citation2015). Mols. 6, 7 & 8 had the same fragmentation patterns as Mol. 5 and thus, they were identified as isomers.
Table 1. Tentatively identified flavonoid metabolites, extracted from Bidens pilosa using PHWE.
Tabla 1. Metabolitos flavonoides identificados provisionalmente, extraídos de Bidens pilosa utilizando PHWE.
Mol. 9 at Rt 11.36 had m/z 653.0947 [M-H]‾ and fragment ions at m/z 477.0663 (due to loss of a glucuronyl unit, 176 amu) and m/z 301.0303, which indicates the aglycone quercetin and results from a further loss of 176 as indicative of a quercetin diglucuronide with the glucuronyl moieties attached at different positions on the flavonol ring. Furthermore, if the two glucuronyl moiety had been attached to the same position, the formation of an M-17 fragment at m/z 477 would have been improbable, as it has been observed that when anthocyanin disaccharide conjugates fragment, they do so with the loss of an intact disaccharide unit (Giusti, Rodriguez-Saona, Griffin, & Wrolstad, Citation1999). Hence, the molecule was tentatively identified as quercetin 3,7-diglucuronide (Mullen, Citation2009).
Mols. 10 & 11 at Rt 14.59 and 14.70 min, respectively, were annotated as quercetin-3-rhamnosylhexoside with a precursor ion at m/z 609 [M-H]‾ and a product ions at m/z 300.0235 and 300.0212, respectively, a quercetin aglycone [H-H-309] following the loss of a rutinodide sugar (Abu-Reidah, Arráez-Román, Lozano-Sánchez, Segura-Carretero, & Fernández-Gutiérrez, Citation2013; Berger, Küchler, Maaßen, Busch-Stockfisch, & Steinhart, Citation2007). To further deduce the sequence of the sugar, the positive ionization data were referenced since it provided results with more information. The MS spectrum in the positive mode showed a precursor ion at 611 [H + H]+ and fragment ions at 465 [H + H]+ and 303[H + H]+, and an adduct ion at m/z 633 [H + H]+ (Martucci, De Vos, Carollo, & Gobbo-Neto, Citation2014; Ramabulana et al., Citation2015). Mol. 12 at Rt 14.80 min was identified as quercetin monoglucuronide with a precursor ion at m/z 477.0621 [M-H]‾ and a fragment ion at m/z 301.0311 (M-176 amu) (Mullen, Citation2009). Mol. 13 at Rt 14.93 had a similar fragmentation pattern as Mol. 12 and as such, they could be regarded as isomers.
Mol. 14 at Rt 14.88 was the parent compound quercetin-3-glycoside with a precursor ion at m/z 463.0847 [M-H]‾ and MS2 ion at m/z 301.0327 corresponding to the loss of a hexose molecule (Abu-Reidah et al., Citation2013; Khoza et al., Citation2015). Mol. 15 at Rt 15.03 min had a similar fragmentation pattern as Mol. 14 and as such it is considered an isomer of quercetin-3-glycoside. Mols. 16–21 were considered isobaric species as they contained a similar m/z value (575 [M-H]‾), close Rts, and almost analogous fragmentation patterns (). However, due to the efficiency of our extraction method and its compatibility with highly advance chromatographic separation instrumentations (that have high MS1 and MS2 resolution precursor ion selection capabilities), it was possible to unambiguously distinguish between these molecules on SIM chromatograms and subsequently annotate them accordingly. Consequently, Mols. 16, 17 & 18 at Rts 18.85, 19.13, 19.24, respectively, were considered isomers of okanin triacetylglucoside with a precursor ion at m/z 575 [M-H]‾ and MS2 ions at m/z 135, 150, and 287, respectively. These molecules have previously been isolated from B. pilosa (Harborne & Baxter, Citation1999; Hoffmann & Hölzl, Citation1988). Molecules 19, 20 & 21 at Rts 19.92, 20.12 and 20.25 min, respectively, had similar fragmentation patterns with m/z 575 [M-H]‾ and product ions, respectively, at m/z 135, 150, and 285. As such, they were considered isomers of tetrahydroxyflavanone triacetylglucoside (Wang, Yang, Lin, & Sun, Citation1997).
Mols. 22–28 had the same m/z value of 533 [M-H]‾ and similar fragmentation patterns with fragment ions at m/z 135, 150 and 287. A closer look at the SIM chromatograms indicated that these molecules could be isobaric species. Hence, Mols. 22–26 at Rts 16.88, 17.01, 17.10, 17.34 and 17.45 min, respectively, were identified as isomers of okanin- di-O-acetylglucoside). This molecule has been previously described in B. pilosa (Harborne & Baxter, Citation1999; Hoffmann & Hölzl, Citation1988). Mols. 27 and 28 at Rts 18.39 and 18.52 min, respectively, are considered isomers of tetrahydroxyflavanone diacetylglucopyranoside. These molecules have been previously isolated from a Bidens specie, i.e. Bidens bipinnata Linn. (Li, Kuang, Okada, & Okuyama, Citation2005; Yang et al., Citation2012).
As can be seen from the fragmentation patterns of these compounds, they are structurally diverse with possibly different physicochemical properties. This reveals that PHWE is efficacious for extracting diverse flavonoids from B. pilosa which is in agreement with the data obtained by Khoza et al. (Citation2014), demonstrating the successful extraction of flavonoids from Momordica foetida using PHWE. The polarity of pressurized hot water (PHW) can easily be manipulated to vary over an extended temperature range just by varying temperature thereof (Chemat, Vian, & Cravotto, Citation2012). However, in order to ensure efficient extraction of the assorted flavonoids in B. pilosa using PHWE, a previously optimized temperature profile, i.e. range of 50–150°C was adopted (Khoza et al., Citation2014). Water at ambient temperature and pressure is more suitable in extracting polar compounds due to its relatively high dielectric constant (i.e. ε = 80 at 25°C at 105 Pa) (Cabane & Vuilleumier, Citation2005; Kruse & Dinjus, Citation2007). However, as the temperature of water increases, its polarity which directly links to its dielectric constant decreases (from ɛ = 53 at 110°C to ɛ = 36.5 at 190°C) to the ranges of that of organic solvents such as methanol (e = 32.6 at 25°C), thus dissolving a wide range of low and medium polarity analytes (Anekpankul, Goto, Sasaki, Pavasant, & Shotipruk, Citation2007; Teo, Tan, Yong, Hew, & Ong, Citation2010). Moreover, the selective extractability of some of the flavonoid molecules during PHWE can be linked to their different structural and physicochemical properties. Elsewhere, it has been shown that there is a relationship between the structure of flavonoid molecules and temperature conditions during PHWE otherwise known as subcritical water extraction (SWE) (Ko, Cheigh, & Chung, Citation2014). Structural configuration such as the presence of double bonds, sugar, polarity of side and the number of carbon atoms in the side groups can ultimately determine the extractability of PHWE (Carr, Mammucari, & Foster, Citation2011; Ko et al., Citation2014).
and show the distribution patterns of some of the identified molecules on a box-and-whiskers plot. Although, all of these molecules were clearly identified by the peak-picking software, it became difficult to accurately annotate some of them (Mol. 17, 21, 24, 25 & 28) on the peak list obtained after processing the data matrix using MarkerLynx XS software, despite following previously optimized and established parameters (see Supplementary Figure 1). The reason for this phenomenon is unclear, however, it seems possible that our robust and sensitive tandem MS approach (UHPLC-qTOF-MS) is a step ahead when making use of our chemometric data analysis software responsible for processing (involving preprocessing, peak selection, peak deisotoping and deconvolution) the data matrix. However, some of the omitted ions share similar precursor ion and fragmentation patterns suggesting that they are isomers of one another.
Figure 1. Box-and-whiskers plots showing the distribution patterns for some of the identified flavonoids (Mols. 1-12).
Figura 1. Diagramas de Caja-Bigotes que muestran los patrones de distribución de algunos de los flavonoides identificados (Mols. 1-12).
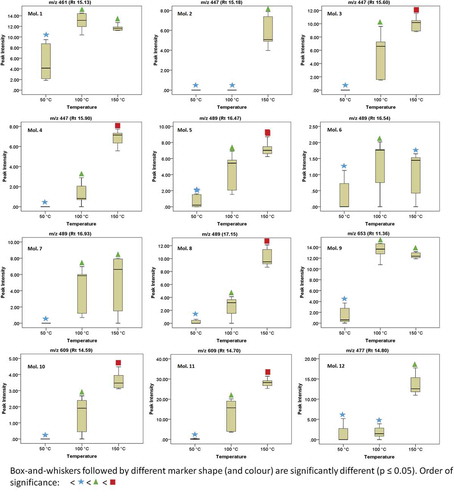
Figure 2. Box-and-whiskers plots showing the distribution patterns for some of the identified flavonoids (Mols. 13-27).
Figura 2. Diagramas de Caja-Bigotes que muestran los patrones de distribución de algunos de los flavonoides identificados (Mols. 13-27).
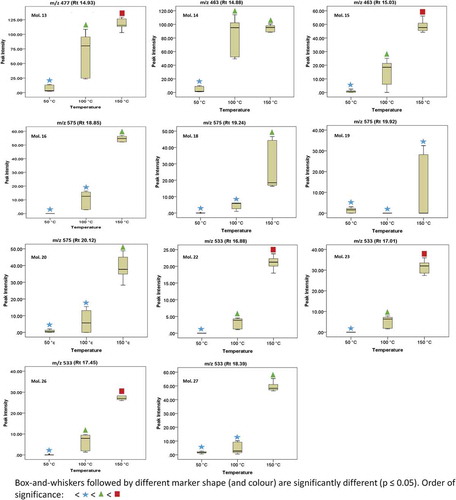
In any case, following ANOVA results, it was possible to indicate on the plots temperature conditions that resulted in significantly different (p ≤ 0.05) yield patterns for each molecule. It can be seen that the yields of these molecules are strongly influenced by temperature. Generally, flavonoid yield increased with increase in extraction temperature. The highest extraction yields were obtained at temperatures of 150°C and included the following molecules in decreasing order; Mol. 13 (Quercetin-3-O-gluconoride isomer 2), Mol. 15 (Quercetin-3-glycoside isomer 2), Mol. 27 (Tetrahydroxyflavanone diacetylglucoside isomer 1), Mol. 16 (Okanin triacetylglucoside) and Mol. 14 (Quercetin-3-glycoside). It was thus evident that quercetin and okanin were the most abundant among the identified aglycones in this plant. In a previous study by Ko, Cheigh, Cho and Chung (Citation2011), it was observed that the highest yield of flavonoids (quercetin) was obtained at a temperature of 165°C during SWE of onion skin. Elsewhere, Cheigh, Chung and Chung (Citation2012) reported maximum yields of flavonoids (up to 99% of the total amount originally present) at an extraction temperature of 160°C when SWE was performed on citrus peels.
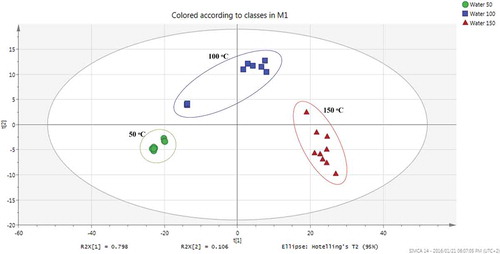
In order to further comprehend the effect of temperature on the relative flavonoid content of B. pilosa plant interpret the patterns within the data obtained from PHWE, we adopted a metabolite fingerprinting approach coupled to the chemometric PCA. This technique is used to emphasize variations that bring out strong patterns in high-dimensional dataset. It identifies patterns in the data and expresses the data by highlighting their similarities and differences (Jolliffe, Citation2002). Data generated in chemometric fingerprinting studies are usually large and highly dimensional, and since finding patterns in such data is hard using ordinary statistical models, PCA is an appropriate tool for analyzing this kind of data (Khoza et al., Citation2014, Citation2015). The PCA score plot indicates significantly different flavonoid distribution patterns amongst extracts based on extraction temperature profile (), which reiterates prior observations ( and ) that temperature is crucial during PHWE of flavonoids.
Figure 3. PCA score plots based on UPLC-qTOF-MS/MS chromatograms from negative ionization data showing various clustering patterns of B. pilosa extracted at different temperatures using PHWE.
Figura 3. Gráficos de resultados de PCA basados en cromatogramas por UPLC-qTOF-MS/MS de datos de ionización negativa que muestran diferentes patrones de agrupación de B. pilosa extraída a distintas temperaturas utilizando PHWE.
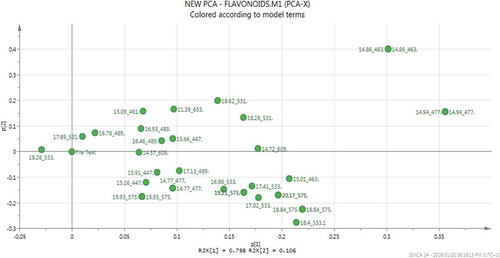
The PCA loadings plot () was used to identify the relationship between the temperature profile and the extracted metabolites in order to comprehend the tight grouping patterns observed in the PCA score plots. Hence, it was observed that the differential clustering into distinct groups on the PCA scores plot was due to the unique effect each extraction temperature had on the extractability of each molecule and that molecules with similar physicochemical characteristics had analogous distribution patterns. During PHWE of Momordica foetida, Khoza et al. (Citation2015) also observed that different temperature conditions resulted in distinctive extraction patterns for different types of flavonoids. Overall, the PCA model provided symbolic representations from which the variations in metabolite profile due to temperature change could be conveniently visualized and interpreted.
Figure 4. PCA loadings plots based on UPLC-qTOF-MS/MS chromatograms from negative ionization data showing the dimensional subspatial orientation of identified flavonoid molecules relative to the different extraction temperatures during PHWE of B. pilosa.
Figura 4. Gráficos de carga de PCA basados en cromatogramas por UPLC-qTOF-MS/MS de datos de ionización negativa que muestran la orientación subespacial dimensional de las moléculas de flavonoides identificados relativas a distintas temperaturas de extracción durante PHWE de B. pilosa.
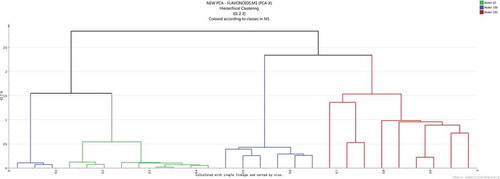
Furthermore, to visualize any potential outliers, hierarchical clustering analysis (HCA) was also generated. From the HCA dendrogram (), it can be seen that at least one sample from 100°C extracts was an outlier, with characteristics similar to extracts obtained at both 50 and 100°C. This could have been caused by the sample being the first one to be extracted just after extraction at 50°C when the temperature was still not sufficiently distributed across the entire heating system. Moreover, orthogonal partial least squares discriminant analysis (OPLS-DA) was also performed on the dataset (Supplementary Figure 2), however, this added very little additional information but supported the clustering patterns as seen on the PCA score plot () and also reaffirms the distribution pattern of some of the metabolites across extracts obtained at various temperature conditions, a phenomenon which is currently well depicted on the box-and-whiskers plots ( and ).
Figure 5. HCA dendrogram showing the degree of similarity/dissimilarity amongst extracts obtained at different extraction temperatures using the data’s full dimensionality as obtained from the UPLC-qTOF-MS/MS chromatograms.
Figura 5. Dendrograma HCA que muestra el grado de similitud/disparidad entre los extractos obtenidos a distintas temperaturas de extracción utilizando la dimensionalidad completa de los datos obtenidos de los cromatogramas por UPLC-qTOF-MS/MS.
The dependency of flavonoid yield on temperature is due to their increased solubility in PHW as water temperature increases (Khoza et al., Citation2015). Moreover, the supplied heat energy increases the rate of diffusion of the molecules, as well as weakens the intermolecular forces within plant tissues, thus lowering the activation energy needed for the desorption process (Teo et al., Citation2010). High pressures aid in extraction by disrupting tissue configuration and forcing water to permeate matrix areas (pores) where water at lower pressures may not normally reach (Richter et al., Citation1996).
Though, extraction efficiency increases with an increase in temperature, extreme temperatures could result in degradation of flavonoids and similar polycyclic aromatic hydrocarbons (Andersson, Hartonen, Hyotylainen, & Riekkola, Citation2003; Khoza et al., Citation2014; Yang & Hildebrand, Citation2006) as well as possible oxidation of metabolites (Ko et al., Citation2014), hence temperature of 150°C was not exceeded. For example, we observed that 3 of the 28 identified metabolites (Molecules 1, 6, and 9) were best extracted at a temperature of 100°C rather than at 150°C. This could be as a result of thermal degradation of these metabolites. In a study on the effect of temperature on PHWE of pharmacologically important metabolites from Moringa oleifera, Khoza et al. (Citation2014) observed that yields of some flavonoid molecules were adversely affected by increase in temperature, such that extraction temperature optimization was essential for efficiency and ‘pharmacological potency’ of the extracts.
A number of studies have linked different flavonoids with various medicinal and pharmacological activities (Kumar, Gupta, & Pandey, Citation2013; Kumar & Pandey, Citation2013; Pandey, Citation2007). These activities have been suggested to be dependent on the structural configuration of the flavonoid molecule (Heim, Tagliaferro, & Bobilya, Citation2002; Kumar & Pandey, Citation2013). Interestingly, some of these flavonoids (i.e. quercetin) have already been used in clinical trials (Hirpara, Aggarwal, Mukherjee, Joshi, & Burman, Citation2009). Quercetin, the most abundant flavonoid aglycone identified in our extracts have been suggested to be hapatoprotective (Kumar & Pandey, Citation2013; Tapas, Sakarkar, & Kakde, Citation2008) as well as regulate cell death pathways and proliferation of cancerous cells (Hirpara et al., Citation2009). Okanins, a dominant chalcone flavonoid in the genus Bidens has been reported to possess potent anticancer and antibacterial properties (Cushnie & Lamb, Citation2005; Makita et al., Citation1996). Various biological functions such as anticancer activity in several human cancer cell lines and inhibition of oxidative stress in animal and plants cells have been directly linked with kaempferol (Berger et al., Citation2013; Leung et al., Citation2007; Marfe et al., Citation2009). Essentially, we were able to extract various groups of important flavonoids from a highly potent medicinal plant (B. pilosa) using a green and efficient extraction method (PHWE). This flavonoid-rich herb has previously been linked with various folk medications in Korea, China and Southern Africa (Arthur, Naidoo, & Coopoosamy, Citation2012; Kil et al., Citation2011).
Conclusion
This study demonstrated PHWE of 28 flavonoid molecules from B. pilosa, an underutilized herbal and food plant found abundantly in South Africa and some tropical regions of the world. The results reported herein show that PHWE is a feasible green technique for extracting different flavonoids with diverse structural and physicochemical properties. This simple technique is cheap, easy to adopt and utilizes water as the extraction solvent. Moreover, the dynamics of PHWE tends to parallel common food processing operations such as blanching, as well as other hydrothermal processes associated with preparation of herbal concoctions and food rations for consumption. The essence is to depict a true-as-possible reflection of the ethnopharmacological exposure of the layman (user of traditional medicine) who does not have access to the sophisticated methods for metabolite extraction scientists usually employ in the laboratory. Lastly, the results from this study reiterates B. pilosa as a rich source of flavonoids and that UPLC-qTOF-MS instrumentation, in combination with PCA, is a suitable omics profiling approach for the analysis of flavonoids and other metabolites in plants.
Supplementary Figures 1 and 2
Download MS Word (376.5 KB)Acknowledgments
This work was financially supported via the Global Excellence and Stature (GES) Fellowship of the University of Johannesburg granted to the main author (S. Gbashi). The article was supported in part via the Centre of Excellence in Food Security cohosted by the University of Pretoria and the University of the Western Cape in South Africa.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Abu-Reidah, I.M., Arráez-Román, D., Lozano-Sánchez, J., Segura-Carretero, A., & Fernández-Gutiérrez, A. (2013). Phytochemical characterization of green beans (Phaseolus vulgaris L.) by using high-performance liquid chromatography coupled with time-of-flight mass spectrometry. Phytochemical Analysis, 24(2), 105–116. doi:10.1002/pca.2385
- Andersson, T., Hartonen, K., Hyotylainen, T., & Riekkola, M. (2003). Stability of polycyclic aromatic hydrocarbons in pressurized hot water. The Analyst, 128, 150–155. doi:10.1039/b211447j
- Anekpankul, T., Goto, M., Sasaki, M., Pavasant, P., & Shotipruk, A. (2007). Extraction of anti-cancer damnacanthal from roots of Morinda citrifolia by subcritical water. Separation and Purification Technology, 55(3), 343–349. doi:10.1016/j.seppur.2007.01.004
- Arthur, G.D., Naidoo, K.K., & Coopoosamy, R.M. (2012). Bidens pilosa L.: Agricultural and pharmaceutical importance. Journal of Medicinal Plants Research, 6(17), 3282–3287. doi:10.5897/JMPR12.195
- Atanasov, A., Waltenberger, B., Pferschy-Wenzig, E.M., Linder, T., Wawrosch, C., Uhrin, P., … Stuppner, H. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology Advances, 33(8), 1582–1614. doi:10.1016/j.biotechadv.2015.08.001
- Bairwa, K., Kumar, R., Sharma, R.J., & Roy, R.K. (2010). An updated review on Bidens Pilosa L. Der Pharma Chemica, 2(3), 325–337.
- Barbarini, N., & Magni, P. (2010). Accurate peak list extraction from proteomic mass spectra for identification and profiling studies. BMC Bioinformatics, 11(1), 1–14. doi:10.1186/1471-2105-11-518
- Bartolome, A.P., Villaseñor, I.M., & Yang, W.C. (2013). Bidens pilosa L. (Asteraceae): Botanical properties, traditional uses, phytochemistry, and pharmacology. Evidence-Based Complementary and Alternative Medicine, 2013 (Article ID 340215), 1–51. doi:10.1155/2013/340215
- Berger, A., Venturelli, S., Kallnischkies, M., Böcker, A., Busch, C., Weiland, T., … Bitzer, M. (2013). Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. The Journal of Nutritional Biochemistry, 24(6), 977–985. doi:10.1016/j.jnutbio.2012.07.001
- Berger, M., Küchler, T., Maaßen, A., Busch-Stockfisch, M., & Steinhart, H. (2007). Correlations of ingredients with sensory attributes in green beans and peas under different storage conditions. Food Chemistry, 103, 875–884. doi:10.1016/j.foodchem.2006.09.039
- Borges, C.C., Matos, T.F., Moreira, J., Rossato, A.E., Zanette, V.C., & Amaral, P.A. (2013). Bidens pilosa L. (Asteraceae): Traditional use in a community of southern Brazil. Revista Brasileira de Plantas Medicinais, 15(1), 34–40. doi:10.1590/S1516-05722013000100004
- Cabane, B., & Vuilleumier, R. (2005). The physics of liquid water. Comptes Rendus Geoscience, Elsevier, 337, 1–23.
- Carr, A., Mammucari, R., & Foster, N. (2011). A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chemical Engineering Journal, 172(1), 1–17. doi:10.1016/j.cej.2011.06.007
- Cheigh, C.I., Chung, E.Y., & Chung, M.S. (2012). Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. Journal of Food Engineering, 110, 472–477. doi:10.1016/j.jfoodeng.2011.12.019
- Chemat, F., Vian, M., & Cravotto, G. (2012). Green extraction of natural products: Concept and principles. International Journal of Molecular Sciences, 13, 8615–8627. doi:10.3390/ijms13078615
- Chiang, Y.M., Chuang, D.Y., Wang, S.Y., Kuo, Y.H., Tsai, P.W., & Shyur, L.F. (2004). Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens Pilosa. Journal of Ethnopharmacology, 95(2–3), 409–419. doi:10.1016/j.jep.2004.08.010
- Cushnie, T.T., & Lamb, A.J. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26(5), 343–356. doi:10.1016/j.ijantimicag.2005.09.002
- Darwish, R.M., Aburjai, T., Al-Khalil, S., Mahafza, A., & Al-Abbadi, A. (2002). Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Staphylococcus aureus. Journal of Ethnopharmacology, 79, 359–364. doi:10.1016/S0378-8741(01)00411-1
- Dictionary of Natural Products (Online). Retrieved December 29, 2015, from http://dnp.chemnetbase.com/dictionary-search.do;jsessionid=A9890698699B3BE33D7EEAB7ECEBF3B7?method=view&id=11497129&si=
- Giusti, M.M., Rodriguez-Saona, L.E., Griffin, D., & Wrolstad, R.E. (1999). Electrospray and tandem mass spectroscopy as tools for anthocyanin characterization. Journal of Agriculture and Food Chemistry, 47, 4657–4664.
- Grombone-Guaratini, M.T., Silva-Brandão, K.L., Solferini, V.N., Semir, J., & Trigo, J.R. (2005). Sesquiterpene and polyacetylene profile of the Bidens pilosa complex (Asteraceae: Heliantheae) from Southeast of Brazil. Biochemical Systematics and Ecology, 33(5), 479–486.
- Harborne, J.B., & Baxter, H. (1999). The handbook of natural flavonoids. Volume 2, the handbook of natural flavonoids (2nd ed.). Chichester: Wiley.
- Heim, K.E., Tagliaferro, A.R., & Bobilya, D.J. (2002). Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry, 13, 572–584. doi:10.1016/S0955-2863(02)00208-5
- Herrero, M., Cifuentes, A., & Ibañez, E. (2006). Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chemistry, 98(1), 136–148. doi:10.1016/j.foodchem.2005.05.058
- Hirpara, K.V., Aggarwal, P., Mukherjee, A.J., Joshi, N., & Burman, A.C. (2009). Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anti-Cancer Agents in Medicinal Chemistry, 9(2), 138–161. doi:10.2174/187152009787313855
- Hoffmann, B., & Hölzl, J. (1988). A methylated chalcone glucoside from Bidens Pilosa. Phytochemistry, 27(11), 3700–3701. doi:10.1016/0031-9422(88)80806-9
- Horiuchi, M., & Seyama, Y. (2008). Improvement of the antiinflammatory and antiallergic activity of Bidens pilosa L. var. radiata SCHERFF treated with enzyme (cellulosine). Journal of Health Science, 54(3), 294–301. doi:10.1248/jhs.54.294
- Jolliffe, I.T. (2002). Principal component analysis Springer Series in Statistics (2nd ed.). Heidelberg: Springer.
- Kajdžanoska, M., Gjamovski, V., & Stefova, M. (2010). HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Macedonian Journal of Chemistry and Chemical Engineering, 29(2), 181–194.
- Khoza, B.S., Chimuka, L., Mukwevho, E., Mukwevho, E., Steenkamp, P.A., & Madala, N.E. (2014). The effect of temperature on pressurized hot water extraction of pharmacologically-important metabolites as analyzed by UPLC-qTOF-MS and PCA. Evidence-Based Complementary and Alternative Medicine, 2014 (Article ID 914759), 1–9. doi:10.1155/2014/914759
- Khoza, B.S., Dubery, I.A., Byth-Illing, H.A., Steenkamp, P.A., Chimuka, L., & Madala, N.E. (2015). Optimization of pressurized hot water extraction of flavonoids from Momordica foetida using UHPLC-qTOF-MS and multivariate chemometric approaches. Food Analytical Methods, 2015, 1–10.
- Khoza, B.S., Gbashi, S., Steenkamp, P.A., Njobeh, P.B., & Madala, N.E. (2016). Identification of hydroxylcinnamoyl tartaric acid esters in Bidens pilosa by UPLC-tandem mass spectrometry. South African Journal of Botany, 2016, 96–100.
- Kil, J.S., Son, Y., Cheong, Y.K., Kim, N.H., Jeong, H.J., Kwon, J.W. … Pae, H.O. (2011). Okanin, a chalcone found in the genus Bidens, and 3-penten-2-one inhibit inducible nitric oxide synthase expression via heme oxygenase-1 induction in RAW264.7 macrophages activated with lipopolysaccharide. Journal of Clinical Biochemistry and Nutrition, 50(1), 53–58. doi:10.3164/jcbn.11-30
- King, J.W. (2000). Advances in critical fluid technology for food processing. Food Science and Technology Today, 14, 186–191.
- KNApSAcK Metabolite Information Database (Online). KNApSAcK: A comprehensive species-metabolite relationship database. Retrieved December 29, 2015, from http://kanaya.naist.jp/knapsack_jsp/top.html
- Ko, M.J., Cheigh, C.I., Cho, S.W., & Chung, M.S. (2011). Subcritical water extraction of flavonol quercetin from onion skin. Journal of Food Engineering, 102, 327–333. doi:10.1016/j.jfoodeng.2010.09.008
- Ko, M.J., Cheigh, C.I., & Chung, M.S. (2014). Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chemistry, 143, 147–155. doi:10.1016/j.foodchem.2013.07.104
- Kris-Etherton, P.M., Hecker, K.D., Bonanome, A., Coval, S.M., Binkoski, A.E., Hilpert, K.F. … Etherton, T.D. (2002). Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. The American Journal of Medicine, 113(9), 71–88. doi:10.1016/S0002-9343(01)00995-0
- Kris-Etherton, P.M., Lefevre, M., Beecher, G.R., Gross, M.D., Keen, C.L., & Etherton, T.D. (2004). Bioactive compounds in nutrition and health-research methodologies for establishing biological function: The antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annual Reviews in Nutrition, 24, 511–538. doi:10.1146/annurev.nutr.23.011702.073237
- Kruse, A., & Dinjus, E. (2007). Hot compressed water as reaction medium and reactant properties and synthesis reactions. The Journal of Supercritical Fluids, 39, 362–380. doi:10.1016/j.supflu.2006.03.016
- Kumar, S., Gupta, A., & Pandey, A.K. (2013). Calotropis procera root extract has the capability to combat free radical mediated damage. ISRN Pharmacology, 2013 (Article ID 691372), 1–8. doi:10.1155/2013/691372
- Kumar, S., & Pandey, A.K. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 2013 (Article ID 162750), 1–16.
- Lai, B.Y., Chen, T.Y., Huang, S.H., Kuo, T.F., Chang, T.H., Chiang, C.K. … Chang, C.L.T. (2015). Bidens pilosa formulation improves blood homeostasis and B-cell function in men: A pilot study. Evidence-Based Complementary and Alternative Medicine, 2015, 1–5.
- Leung, H.W.C., Lin, C.J., Hour, M.J., Yang, W.H., Wang, M.Y., & Lee, H.Z. (2007). Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food and Chemical Toxicology, 45(10), 2005–2013. doi:10.1016/j.fct.2007.04.023
- Li, S., Kuang, H.X., Okada, Y., & Okuyama, T. (2005). New flavanone and chalcone glucosides from Bidens bipinnata Linn. Journal of Asian Natural Products Research, 7(1), 67–70. doi:10.1080/10286020310001617147
- Liang, X., & Fan, Q. (2013). Application of sub-critical water extraction in pharmaceutical industry. Journal of Materials Science and Chemical Engineering, 01, 1–6. doi:10.4236/msce.2013.15001
- Madala, N.E., Steenkamp, P.A., Piater, L.A., & Dubery, I.A. (2013). Metabolomic analysis of isonitrosoacetophenone-induced perturbations in phenolic metabolism of Nicotiana tabacum cells. Phytochemistry, 94, 82–90. doi:10.1016/j.phytochem.2013.05.010
- Madala, N.E., Tugizimana, F., & Steenkamp, P. (2014). Development and optimization of an UPLC-QTOF-MS/MS method based on an in-source collision induced dissociation approach for comprehensive discrimination of chlorogenic acids isomers from Momordica plant species. Journal of Analytical Methods in Chemistry, 2014, 1–7. doi:10.1155/2014/650879
- Makita, H., Tanaka, T., Fujitsuka, H., Tatematsu, N., Satoh, K., Hara, A., & Mori, H. (1996). Chemoprevention of 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis by the dietary flavonoids chalcone, 2-hydroxychalcone, and quercetin. Cancer Research, 56(21), 4904–4909.
- Marfe, G., Tafani, M., Indelicato, M., Sinibaldi-Salimei, P., Reali, V., Pucci, B. … Russo, M.A. (2009). Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. Journal of Cellular Biochemistry, 106(4), 643–650. doi:10.1002/jcb.v106:4
- Martucci, M.E.P., De Vos, R.C.H., Carollo, C.A., & Gobbo-Neto, L. (2014). Metabolomics as a potential chemotaxonomical tool: Application in the genus Vernonia schreb. PloS One, 9, 1–9. doi:10.1371/journal.pone.0093149
- Mullen, W. (2009). Investigation of the fate of dietary flavonols in humans and rats using HPLC-MS2 techniques ( Doctor of Philosophy (PhD) thesis). Faculty of Medicine, University of Glasgow, Glasgow.
- Mushtaq, M.Y., Choi, Y.H., Verpoorte, R., & Wilson, E.G. (2014). Extraction for metabolomics. Phytochemical Analysis, 25(4), 291–306. doi:10.1002/pca.2505
- Pandey, A.K. (2007). Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Parthenium histerophorus: An in vitro study. National Academy Science Letters, 30(11–12), 383–386.
- Ramabulana, T., Mavunda, R.D., Steenkamp, P.A., Piater, L.A., Dubery, I.A., & Madala, N.E. (2015). Secondary metabolite perturbations in Phaseolus vulgaris leaves due to gamma radiation. Plant Physiology and Biochemistry, 97, 287–295. doi:10.1016/j.plaphy.2015.10.018
- Richter, B., Jones, B., Ezzell, J., Porter, N., Avdalovic, N., & Pohl, C. (1996). Accelerated solvent extraction: A technique for sample preparation. Analytical Chemistry, 68, 1033–1039. doi:10.1021/ac9508199
- Richter, P., Toral, M.I., & Toledo, C. (2006). Subcritical water extraction and determination of nifedipine in pharmaceutical formulations. Journal of AOAC International, 89(2), 365–368.
- Scholz, M., Gatzek, S., Sterling, A., Fiehn, O., & Selbig, J. (2004). Metabolite fingerprinting: Detecting biological features by independent component analysis. Bioinformatics, 20(15), 2447–2454. doi:10.1093/bioinformatics/bth270
- Silva, J.J., Cerdeira, C.D., Chavasco, J.M., Cintra, A.B., Silva, C.B., Mendonça, A.N. … Chavasco, J.K. (2014). In vitro screening antibacterial activity of Bidens pilosa Linné and Annona crassiflora Mart. against oxacillin resistant Staphylococcus aureus (ORSA) from the aerial environment at the dental clinic. Revista Do Instituto De Medicina Tropical De Sao Paulo, 56(4), 333–340. doi:10.1590/S0036-46652014000400011
- Silva, F.L., Fischer, D.C.H., Tavares, J.F., Silva, M.S., De Athayde-Filho, P.F., & Barbosa-Filho, J.M. (2011). Compilation of secondary metabolites from Bidens pilosa L. Molecules, 16(2), 1070–1102.
- Song, T., Pranovich, A., & Holmbom, B. (2011). Characterization of Norway spruce hemicelluloses extracted by pressurized hot-water extraction (ASE) in the presence of sodium bicarbonate. Holzforschung, 65(1), 32–45. doi:10.1515/hf.2011.015
- Sundararajan, P., Dey, A., Smith, A., Doss, A.G., Rajappan, M., & Natarajan, S. (2006). Studies of anticancer and antipyretic activity of Bidens pilosa whole plant. African Health Sciences, 6(1), 27–30.
- Tambellini, N.P., Zaremberg, V., Turner, R.J., & Weljie, A.M. (2013). Evaluation of extraction protocols for simultaneous polar and nonpolar yeast metabolite analysis using multivariate projection methods. Metabolites, 3(3), 592–605. doi:10.3390/metabo3030592
- Tapas, A.R., Sakarkar, D.M., & Kakde, R.B. (2008). Flavonoids as nutraceuticals: A review. Tropical Journal of Pharmaceutical Research, 7(3), 1089–1099. doi:10.4314/tjpr.v7i3.14693
- Teo, C.C., Tan, S.N., Yong, J., Hew, C.S., & Ong, E.S. (2010). Pressurized hot water extraction (PHWE). Journal of Chromatography A, 1217, 2484–2494. doi:10.1016/j.chroma.2009.12.050
- Tereza, G.G.M., Mansanares, M.E., Semir, J., & Solferini, V.N. (2006). Chromosomal studies of three species of Bidens (L.) (Asteraceae). Caryologia, 59(1), 14–18. doi:10.1080/00087114.2006.10797892
- Vergara-Salinas, J.R., Bulnes, P., Zúñiga, M.C., Pérez-Jiménez, J., Torres, J.L., Mateos-Martín, M.L. … Pérez-Correa, J.R. (2013). Effect of pressurized hot water extraction on antioxidants from grape pomace before and after enological fermentation. Journal of Agricultural and Food Chemistry, 61(28), 6929–6936. doi:10.1021/jf4010143
- Wang, J., Yang, H., Lin, Z.W., & Sun, H.D. (1997). Flavonoids from Bidens pilosa var. radiata. Phytochemistry, 46(7), 1275–1278. doi:10.1016/S0031-9422(97)80026-X
- Yang, H.L., Chen, S.C., Chang, N.W., Chang, J.M., Lee, M.L., Tsai, P.C. … Hseu, Y.C. (2006). Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food and Chemical Toxicology, 44(9), 1513–1521. doi:10.1016/j.fct.2006.04.006
- Yang, X.W., Huang, M.Z., Jin, Y.S., Sun, L.N., Song, Y., & Chen, H.S. (2012). Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia, 83(7), 1169–1175. doi:10.1016/j.fitote.2012.07.005
- Yang, Y., & Hildebrand, F. (2006). Phenanthrene degradation in subcritical water. Analytica Chimica Acta, 555, 364–369. doi:10.1016/j.aca.2005.08.078
