ABSTRACT
Inhibitory activities of ethanol extracts from seven fermented soybean products, including douchi, sufu, stinky tofu, to acetylcholinesterase were determined, and crude extract from Yongchuan douchi, which showed the strongest inhibitory effect, was further extracted systematically. Highly active anticholinesterase ingredients were mainly found in petroleum ether extracts; after multistep column chromatography, three compounds were purified finally with high-speed countercurrent chromatography. By their spectral data of MS, 1H-NMR, and 13C-NMR, three compounds were identified as genistein, daidzein, and kaempferol, whose further research could lay foundation for research and development of new drugs and foods against Alzheimer’s disease.
RESUMEN
Se determinaron las actividades inhibitorias de extractos de etanol en siete productos de soja fermentados, incluyendo el douchi, el sufu y el tofu maloliente en la acetilcolinesterasa, además de extracto crudo de douchi de Yongchuan, el cual mostró el mayor efecto inhibitorio y posteriormente se extrajo de forma sistemática. Los ingredientes de la anticolinesterasa altamente activos se encontraron mayoritariamente en extractos de éter de petróleo, después de utilizar cromatografía en columna de varias etapas, finalmente se purificaron tres compuestos con cromatografía a contracorriente de alta velocidad. Mediante los datos espectrales de MS, 1H-NMR y 13C-NMR, se identificaron tres compuestos como la genisteína, la daidzeína y el kaempferol, los cuales si se investigaran en más profundidad podrían sentar las bases de investigaciones y desarrollo de nuevos medicamentos y alimentos en contra del AD.
1. Introduction
Alzheimer’s disease (AD), one of the most common types of dementia in the elderly (Adewusi, Moodley, & Steenkamp, Citation2011), is characterized by amnesia, disorientation, cognitive dysfunction, behavioral disorders, and personality changes (Yoon, Ngo, & Kim, Citation2009) and has become the third leading cause of death, after cardiovascular disease and cancer, in developed countries (Ma & Gang, Citation2008).
Acetylcholine (ACh), whose decrease in the hippocampus and cortex of the brain is the most prominent biochemical change of AD patients (Orhan et al., Citation2007), is a neurotransmitter assisting the process of intercellular communication and plays an indispensable role in the excitatory neurotransmission (Aidoo & Ward, Citation2006; Garhyan, Mahecha-Botero, & Elnashaie, Citation2006). It is synthesized by choline acetyltransferase (ChAT) and hydrolyzed by acetylcholinesterase (AChE). Low activity of ChAT or high activity of AChE unbalances the cholinergic system and that is predisposition of AD (Garhyan et al., Citation2006; Rahim et al., Citation2016). Consequently, inhibition to AChE is regarded as a promising remedy against AD (Lenigk et al., Citation2000; Orhan & Üstün, Citation2011).
Currently, AChE inhibitors, which can inhibit AChE from hydrolyzing ACh to augment ACh levels in the brain, are extensively used to alleviate the symptoms of AD patients (Cho, Kim, Ahn, & Je, Citation2011; Zhang et al., Citation2007). However, several AChE inhibitors licensed by the US Food and Drug Administration recently, including tacrine, donepezil, rivastigmine, and galanthamine (Ma & Gang, Citation2008), are reported to have insufficient activity and some side effects, such as hepatotoxicity and gastrointestinal disturbances (Racchi, Mazzucchelli, Porrello, Lanni, & Govoni, Citation2004; Rahim et al., Citation2016; Sancheti, Sancheti, Um, & Seo, Citation2010).
Therefore, search for AChE inhibitors from natural products, especially diets, which are more secure, has been a current research priority (Aremu, Amoo, Ndhlala, Finnie, & Staden, Citation2011; Moyo, Ndhlala, Finnie, & Staden, Citation2010; Sacan & Yanardag, Citation2010; Stasiuk, Bartosiewicz, & Kozubek, Citation2008). Douchi, sufu, and stinky tofu are used traditionally as food condiment and pharmaceutical since ancient times, and today douchi is still added to some traditional Chinese medicines (Chao, Tomii, Watanabe, & Tsai, Citation2008; Han, Cao, Rombouts, & Nout, Citation2004; Moy, Lu, & Chou, Citation2012; Tsai, Kung, Chang, Lee, & Wei, Citation2007; Wang et al., Citation2007). So far, there are many published studies on the angiotensin-I-converting enzyme inhibitory activity (Zhang, Tatsumi, Ding, & Li, Citation2006), anti-α-glucosidase activity (Chen, Cheng, Yamaki, & Li, Citation2007), antioxidant activity (Wang et al., Citation2008) of douchi extracts, antioxidative and antihypertensive effects, anti-AChE activity of sufu extracts (Chen, Quan, Cheng, Sun, & Li, Citation2012) but little information about AChE inhibitory activity of douchi extracts.
In this study, inhibitory activities of ethanol extracts from seven fermented soybean products, including douchi, sufu, and stinky tofu, to acetylcholinesterase were investigated, highly active anticholinesterase ingredients were isolated and purified from the sample that showed the strongest inhibitory effect, and identified by their spectral data.
2. Materials and methods
2.1. Chemicals and adsorbents
Magnesium chloride, sodium dihydrogen phosphate, sodium dihydrogen phosphate, n-hexane, methanol, ethanol, petroleum ether, dichloromethane, ethyl acetate, and n-butanol were analytical reagents purchased from Sinopharm Chemical Reagent Co., Ltd. Acetylcholinesterase (AChE), acetylthiocholine iodide, 5,5ʹ-dithio-bis-(2-nitrobenzoic acid) (DTNB), galantamine, sodium dodecyl sulfate (SDS), and Sephadex LH-20 were purchased from Sigma-Aldrich, Co. HZ-818 macroporous resin was purchased from Shanghai Huazhen Sci. & Tech. Co., Ltd. Column chromatography silica gel was purchased from Qingdao Haiyang Chemical Co., Ltd.
2.2. Fermented soybean products and pretreatment
Seven samples, including Yongchuan douchi, Yangjiang douchi, Buerjia red oil sufu, Buerjia white sufu, Wangzhihe red oil sufu, Wangzhihe white sufu, and Wangzhihe stinky tofu, were bought from Trust-Mart supermarket in Shanghai. The oil and pepper on the samples’ surface were removed by petroleum ether. After vacuum freeze-drying, the samples were sieved with 60-mesh sieve and preserved sealed.
2.3. Sample screening
Each dried sample (10 g) was added into conical flask (250 mL) and extracted with 120 mL ethanol (80%, v/v) under reflux at 70°C for 1 h. Extract was vacuum filtered while hot, filtrate was centrifuged at 4000 r/min for 20 min for obtaining supernatant, which was condensed into extractum applying a rotary evaporator. Extractum was dissolved by ethanol (10%, v/v) into solution (1.0 mg/mL), which was prepared for the determination of inhibition ratio to AChE.
2.4. Determination of inhibition ratio to AchE
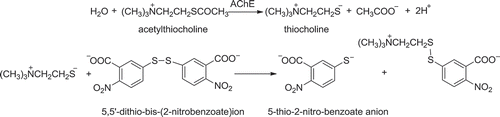
Inhibition ratio to AChE was determined by slightly modifying the spectrophotometric method of Ellman, Courtney, Jr, and Featherstone (Citation1961). The principle of the method was based on two reactions as illustrated in . With the catalysis of AChE, the substrate acetylthiocholine was hydrolyzed into thiocholine, which reacted with 5,5ʹ-dithio-bis-(2-nitrobenzoate) ion to produce the yellow 5-thio-2-nitro-benzoate anion, whose production could be measured at 412 nm by the spectrophotometer .
Figure 1. Reactions about determination of inhibition ratio to acetylcholine.
Figura 1. Reacciones de la determinación de la proporción inhibitoria de la acetilcolina.
About 2900 μL of phosphate buffer solution, 20 μL of magnesium chloride (0.75 M), 20 μL of AChE (0.75 U/mL), and 100 μL of extractum solution were mixed and preheated for 10 min at 37°C. With the addition of 50 μL of DTNB (15 mM) and 50 μL of acetylthiocholine iodide (15 mM), the reaction was initiated and maintained at 37°C water bath for 5 h, then 1000 μL of SDS (4%, w/w) was added to terminate the reaction, and absorbance A1 of solution t5 was determined rapidly. About 20 μL of Phosphate buffer solution was substituted for 20 μL of AChE; absorbance A2 of solution was determined after the reaction’s termination. Ethanol (10%, v/v) and phosphate buffer solution were substituted for extractum solution successively, and absorbances A3 and A0 were determined. All the reactions were performed in triplicate. The inhibition rate was calculated by the following formula.
Galantamine was used as the positive control.
2.5. High-speed countercurrent chromatography (HSCCC) separation
The solvent system dichloromethane–methanol–water (4:3:2, v/v/v) was thoroughly equilibrated in a separatory funnel, and upper phase (stationary phase) and lower phase (mobile phase) were separated before use.
First, the multilayer coiled column was entirely filled with the stationary phase. The mobile phase was then pumped into the head end of the column at the flow rate of 2.0 mL/min while the apparatus was rotated at 900 r/min. After hydrodynamic equilibrium, which was indicated by a clear mobile phase eluting at the tail outlet, the sample solution was injected into the injection valve. The effluent from the tail end of the column was continuously monitored with a UV detector at 254 nm, and the chromatogram was recorded, and peak fractions were collected manually according to the chromatographic profile and prepared for structure identification.
2.6. Chemical structure identification
Chemical structure identification was completed in Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The structure of compounds separated from sample had been identified by MS, 1H-NMR, and 13 C-NMR.
3. Results and discussion
3.1. Inhibition ratio of fermented soybean products to AchE
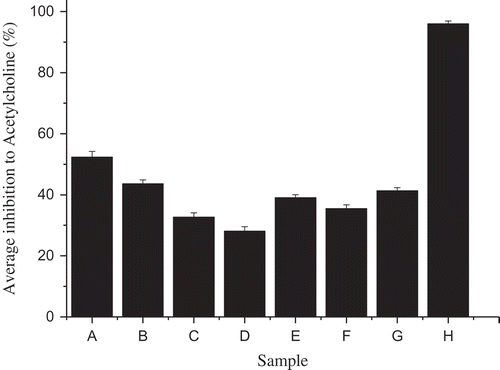
As illustrated in , samples were varied in inhibition ratio to AchE, because douchi, sufu, and stinky tofu were naturally fermented soybean products, whose qualities were influenced by technological processes, production seasons, and produce districts. Since ethanol extracts from Yongchuan douchi, whose inhibition ratio reached 52.36%, showed the strongest inhibitory effect, further isolation and purification of acetylcholinesterase inhibitor was focused on ethanol extracts from Yongchuan douchi. Galantamine was used as a positive control (standard drug) in this article (inhibition ratio 96.04%). Galantamine showed a stronger AChE inhibitory activity than fermented soybean products in this work.
Figure 2. Inhibition ratios of samples to acetylcholine. A, Yongchuan douchi; B, Yangjiang douchi; C, Buerjia red oil sufu; D, Buerjia white sufu; E, Wangzhihe red oil sufu; F, Wangzhihe white sufu; G, Wangzhihe stinky tofu; H, Galantamine.
Figura 2. Proporciones inhibitorias de muestras de acetilcolina. A, douche de Yongchuan; B, douchi de Yangjiang; C, sufu en aceite rojo de Buerjia; D, sufu blanco de Buerjia; E, sufu en aceite rojo de Wangzhihe; F, sufu blanco de Wangzhihe; G, tofu maloliente de Wangzhihe; H,Galantamina.
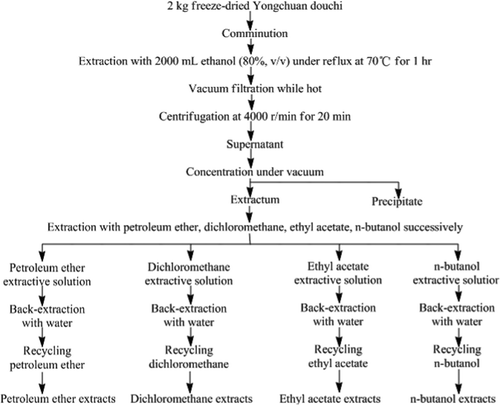
3.2. Systematical extraction of Yongchuan douchi crude extract
As shown in , extractum of freeze-dried Yongchuan douchi was prepared following the steps in the sample screening. Then extractum was dispersed in a little distilled water and extracted three times with petroleum ether, dichloromethane, ethyl acetate, and n-butanol respectively and successively. Extractive solutions were combined and back-extracted with the same volume of water; organic solvents were recycled from organic phase to obtain petroleum ether extracts (80.111 g), dichloromethane extracts (40.134 g), ethyl acetate extracts (9.136 g), and n-butanol extracts (17.345 g). After diluted to 1.0 mg/mL by ethanol (80%, v/v), organic solvents extracts were prepared for the determination of inhibition ratio to AChE, respectively.
3.3. Organic solvents extracts’ inhibition ratio to AchE
As shown in , petroleum ether extracts exhibited the highest inhibition to AChE, and dichloromethane extracts’ and ethyl acetate extracts’ inhibitions to AChE were relatively high, while n-butanol extracts’ inhibition was low. According to the theory that substances with similarity in polarity have better solubility, the active ingredients, which were isolated and purified from petroleum ether extracts, dichloromethane extracts, and ethyl acetate extracts by column chromatography, were no polar or weakly polar substances. For with the highest inhibition activity to AChE and the heaviest weight, petroleum ether extracts were selected for further investigation.
Table 1. Organic solvent extracts’ inhibition ratio to acetylcholine esterase (AchE).
Tabla 1. Proporción inhibitoria de los extractos solventes orgánicos de la acetilcolinesterasa (AchE).
3.4. Gradient elution
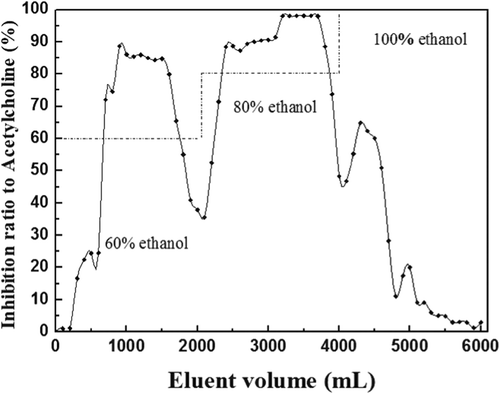
Petroleum ether extracts (80.111 g) were dissolved with 30% ethanol and then filtrated, and filtrate’s inhibition ratio to AChE was 86.31%. HZ-818 macroporous resin was immersed with the filtrate (50 mg/mL) for 3 h and then filtrated, and the remaining solution’s inhibition ratio to AChE was 24.64%. Saturated HZ-818 macroporous resin was desorbed successively by 60%, 80%, 100% ethanol with the flow rate of 1.0 mL/min, and each 100 mL eluent’s ratio to AChE was measured. As shown in , highly active anticholinesterase ingredients were mainly found in the eluent of 60% and 80% ethanol. After rotary evaporation and vacuum freeze-drying, the eluents of 60% and 80% ethanol were dried and yellow powders were obtained as Pr. 1 (14.331 g) and Pr. 2 (38.106 g), respectively.
3.5. Multistep column chromatography
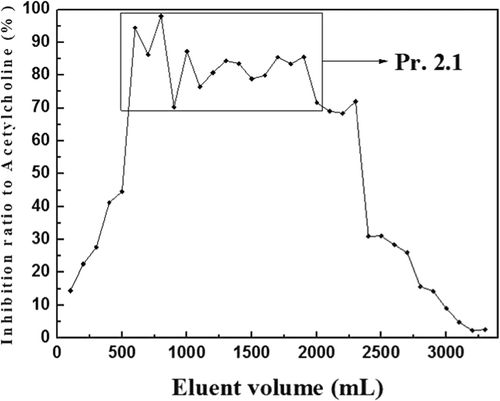
Pr. 2, which had more weight and higher anticholinesterase activity, was dissolved with methylene chloride–methanol (30:1, v/v) and then filtrated and separated with silica gel column chromatography. Every 10 min, 100 mL eluent was collected and prepared for the determination of inhibition ratio to AChE; the results are shown in .
Eluents that had high anticholinesterase activity were merged and concentrated. After vacuum freeze-drying, Pr. 2.1 (10.120 g) was obtained.
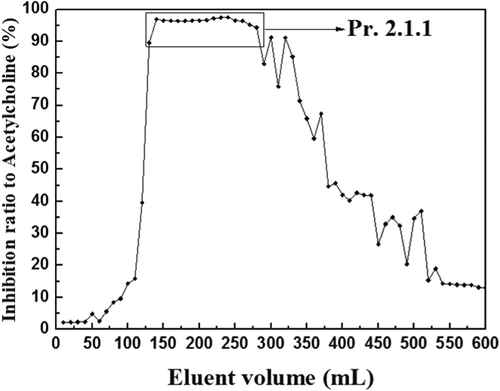
Pr. 2.1 was dissolved with methylene chloride–methanol (60:1, v/v) and then filtrated and separated with silica gel column chromatography. Every 5 min, 10 mL eluent was collected and prepared for the determination of inhibition ratio to AChE, and the result is shown in .
Eluents that had highly anticholinesterase activity were merged and concentrated, and Pr. 2.1.1 (3.077 g) was obtained after vacuum freeze-drying.
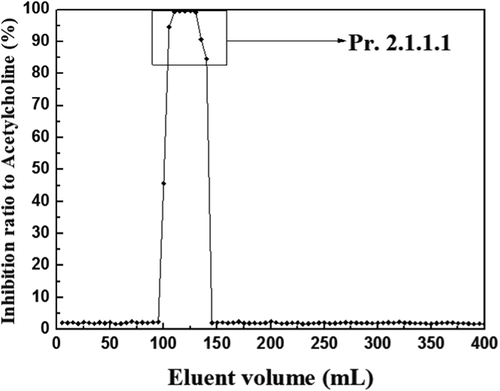
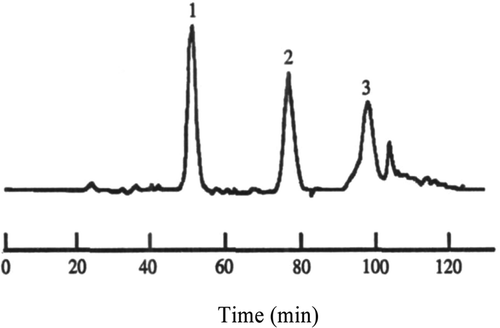
Pr. 2.1.1 was dissolved with 5 mL methanol and then filtrated and separated with Sephadex LH-20 column chromatography; the elution curve was shown in . After reiterative purification and vacuum freeze-drying, Pr. 2.1.1.1 (1.112 g) was obtained and prepared for separation by HSCCC. The HSCCC of Pr. 2.1.1.1 is shown in .
3.6. Structure identification of Compound 1
Compound 1, whose melting point was 297–298°C, was white acicular crystal. Its spectral data is as follows:
EI-MS m/z: 271 [M + H]+, 270 [M+], 269 [M-H]+, 241 [M-H-CO]+, 213 [M-H-CO-CO]+, 153[A1 + H]+, 152 +, 124 [A1-CO]+, 118 [B1]+ .
1 H-NMR (DMSO-d6) δ: 6.21 (1 H, d, J = 2.1 Hz, H-6), 6.37 (1 H, d, J = 2.1 Hz, H-8), 6.81 (2 H, dd, J = 8.7, 2.1 Hz, H-3ʹ, 5ʹ), 7.36(2 H, dd, J = 8.7, 2.1 Hz, H-2ʹ, 6ʹ), 8.31 (1 H, s, H-2), 9158 (1 H, s, OH-4ʹ), 10.87 (1 H, s,OH-7), 12.95 (1 H, s, OH-5).
13 C-NMR (DMSO-d6) δ: 94.0 (C-8), 99.3 (C-6), 104.8 (C-10), 115.4 (C-3ʹ, 5ʹ), 121.5 (C-3), 122.6(C-1ʹ), 130.5(C-2ʹ, 6ʹ), 154.2 (C-2), 157.7 (C-9), 157.9 (C-4ʹ), 162.3 (C-5), 164.6 (C-7), 180.5 (C-4).
The spectral data of Compound 1 was consistent with that of genistein reported by Kinjo et al. (Citation1987). And the melting point of mixture of Compound 1 and genistein was still 297–298°C, so Compound 1 was genistein and showed the highest enzyme inhibition activity against AchE with an IC50 value 17.48 ųg/mL (The IC50 values were calculated from inhibition curves:inhibitior concentration vs. percent of inhibition.)
3.7. Structure identification of Compound 2
Compound 2, whose melting point was 320–321°C, was colorless acicular crystal. Its spectral data is as follows:
EI-MS m/z: 255 [M + H]+, 254 [M]+, 253 [M-H]+, 236 [M-H2O]+, 225 [M-H-CO]+, 197[M-H-CO-CO]+, 137 [A1 + H]+, 118 [B2]+, 108 [A1-CO]+ .
1H-NMR (DMSO-d6) δ: 6.80 (2 H, dd, J = 8.7, 2.1 Hz, H-3ʹ, 5ʹ), 6.85 (1 H, d, J = 2.1 Hz, H-8), 6.93(1 H, dd, J = 8.7, 2.1 Hz, H-6), 7.38 (2 H, dd, J = 8.7, 2.1 Hz, H-2ʹ, 6ʹ), 7.95 (1 H, d, J = 8.7 Hz, H-5), 8.28 (1 H, s, H-2), 9.52 (1 H, s, OH-4ʹ), 10.78 (1 H, s, OH-7).
13 C-NMR (DMSO-d6) δ: 102.1 (C-8), 114.9 (C-3ʹ, 5ʹ), 115.1 (C-6), 116.6 (C-10), 122.5 (C-3),123.5 (C-1ʹ), 127.3 (C-5), 130.1 (C-2ʹ, 6ʹ), 152.8 (C-2), 157.2 (C-9), 157.4 (C-4ʹ), 162.5 (C-7), 174.7 (C-4).
The spectral data of Compound 2 is consistent with that of daidzein reported by Kinjo et al. (Citation1987). And the melting point of mixture of Compound 2 and daidzein was still 320–321°C, so Compound 2 was daidzein and the inhibition activity against AchE with an IC50 value of 17.29 ųg/mL.
3.8. Structure identification of Compound 3
Compound 3, whose melting point was 269–270°C, was yellow powder. Its spectral data is as follows:
EI-MS m/z: 287 [M + H]+, 286[M+], 285 [M-H]+, 257 [M-CO-H]+
1 H-NMR (DMSO-d6) δ: 6.18 (1 H, d, J = 2.0 Hz, H-6), 6.43 (1 H, d, J = 2.0 Hz, H-8), 6.92 (2 H, dd,J = 2.8, 11.6 Hz, H-3ʹ, 5ʹ), 8.04 (2 H, dd, J = 2.8, 11.6 Hz, H-2ʹ, 6ʹ), 12.48(1 H,s,OH-5).
13C-NMR (DMSO-d6) δ: 93.4 (C-8), 98.2 (C-6), 103.0 (C-10), 115.5 (C-3ʹ, 5ʹ), 121.6 (C-1ʹ), 129.4(C-2ʹ, 6ʹ), 135.6 (C-3), 146.6 (C-2), 156.2 (C-9), 159.1 (C- 4ʹ), 160.7 (C-5), 163.8 (C-7), 175.9 (C-4).
The spectral data of Compound 3 was consistent with that of kaempferol reported by Markham, Ternai, Stanley, Geiger, and Mabry (Citation1978). And the melting point of mixture of Compound 3 and kaempferol was still 269–270°C, so Compound 3 was kaempferol and the inhibition activity against AchE with an IC50 value of 18.14 ųg/mL.
4. Conclusion
In conclusion, inhibitory activities of ethanol extracts from seven fermented soybean products, including douchi, sufu, and stinky tofu, to acetylcholinesterase were studied, and crude extract from Yongchuan douchi, which showed the strongest inhibitory effect, was further extracted systematically. And highly active anticholinesterase ingredients were mainly found in petroleum ether extracts; after multistep column chromatography, three compounds were purified finally with HSCCC. By their spectral data of MS, 1H-NMR, and 13C-NMR, three compounds were identified as genistein, daidzein, and kaempferol, which should be recommended for further research on the relationship between their structure and high anticholinesterase activity.
Acknowledgements
This work was funded by the the Science and Technology Commission of Shanghai Municipality with project reference no.13120503300. We also give thanks to all the people who joined and helped in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adewusi, E.A., Moodley, N., & Steenkamp, V. (2011). Antioxidant and acetylcholinesterase inhibitory activity of selected southern African medicinal plants. South African Journal of Botany, 77(3), 638–644. doi:10.1016/j.sajb.2010.12.009
- Aidoo, A.Y., & Ward, K. (2006). Spatio-temporal concentration of acetylcholine in vertebrate synaptic cleft. Mathematical & Computer Modelling, 44(9–10), 952–962. doi:10.1016/j.mcm.2006.03.003
- Aremu, A.O., Amoo, S.O., Ndhlala, A.R., Finnie, J.F., & Staden, J.V. (2011). Antioxidant activity, acetylcholinesterase inhibition, iridoid content and mutagenic evaluation of leucosidea sericea. Food & Chemical Toxicology An International Journal Published for the British Industrial Biological Research Association, 49(5), 1122–1128. doi:10.1016/j.fct.2011.02.003
- Chao, S.-H., Tomii, Y., Watanabe, K., & Tsai, Y.-C. (2008). Diversity of lactic acid bacteria in fermented brines used to make stinky tofu. International Journal of Food Microbiology, 123(1–2), 134–141. doi:10.1016/j.ijfoodmicro.2007.12.010
- Chen, J., Cheng, Y.-Q., Yamaki, K., & Li, L.-T. (2007). Anti-α-glucosidase activity of Chinese traditionally fermented soybean (douchi). Food Chemistry, 103(4), 1091–1096. doi:10.1016/j.foodchem.2006.10.003
- Chen, J., Quan, M.-H., Cheng, Y.-Q., Sun, J., & Li, L.-T. (2012). Acetylcholinesterase inhibitory activity of Chinese sufu (fermented tofu) ethanol-extract. Food Chemistry, 134(3), 1263–1266. doi:10.1016/j.foodchem.2012.02.141
- Cho, Y.-S., Kim, S.-K., Ahn, C.-B., & Je, J.-Y. (2011). Inhibition of acetylcholinesterase by gallic acid- grafted -chitosans. Carbohydrate Polymers, 84(1), 690–693. doi:10.1016/j.carbpol.2010.12.040
- Ellman, G.L., Courtney, K.D., Andres, V., Jr, & Featherstone, R.M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7(2), 88–95. doi:10.1016/0006-2952(61)90145-9
- Garhyan, P., Mahecha-Botero, A., & Elnashaie, S.S.E.H. (2006). Complex bifurcation/chaotic behavior of acetylcholinesterase and cholineacetyltransferase enzymes system. Applied Mathematical Modelling, 30(9), 824–853. doi:10.1016/j.apm.2005.06.009
- Han, B., Cao, C.-F., Rombouts, F.M., & Nout, M.J.R. (2004). Microbial changes during the production of sufu – a Chinese fermented soybean food. Food Control, 15(4), 265–270. doi:10.1016/S0956-7135(03)00066-5
- Kinjo, J.-E., Furusawa, J.-I., Baba, J., Takeshita, T., Yamasaki, M., & Nohara, T. (1987). Studies on the constituents of Pueraria lobata. iii. isoflavonoids and related compounds in the roots and the voluble stems. Chemical & Pharmaceutical Bulletin, 35(12), 4846–4850. doi:10.1248/cpb.35.4846
- Lenigk, R., Lam, E., Lai, A., Wang, H., Han, Y., Carlier, P., & Renneberg, R. (2000). Enzyme biosensor for studying therapeutics of Alzheimer’s disease. Biosensors & Bioelectronics, 15(9–10), 541–547. doi:10.1016/S0956-5663(00)00078-6
- Ma, X., & Gang, D.R. (2008). In vitro production of huperzine a, a promising drug candidate for Alzheimer’s disease. Phytochemistry, 69(10), 2022–2028. doi:10.1016/j.phytochem.2008.04.017
- Markham, K.R., Ternai, B., Stanley, R., Geiger, H., & Mabry, T.J. (1978). Carbon-13 nmr studies of flavonoids—iii: Naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron, 34(9), 1389–1397. doi:10.1016/0040-4020(78)88336-7
- Moy, Y.-S., Lu, T.-J., & Chou, C.-C. (2012). Volatile components of the enzyme-ripened sufu, a Chinese traditional fermented product of soy bean. Journal of Bioscience & Bioengineering, 113(2), 196–201. doi:10.1016/j.jbiosc.2011.09.021
- Moyo, M., Ndhlala, A.R., Finnie, J.F., & Staden, J.V. (2010). Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of sclerocarya birrea, and harpephyllum caffrum, (anacardiaceae) extracts. Food Chemistry, 123(1), 69–76. doi:10.1016/j.foodchem.2010.03.130
- Orhan, I., Kartal, M., Naz, Q., Ejaz, A., Yilmaz, G., Kan, Y., … Iqbal Choudhary, M. (2007). Antioxidant and anticholinesterase evaluation of selected Turkish salvia, species. Food Chemistry, 103(4), 1247–1254. doi:10.1016/j.foodchem.2006.10.030
- Orhan, I., & Üstün, O. (2011). Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. Journal of Food Composition & Analysis, 24(3), 386–390. doi:10.1016/j.jfca.2010.11.005
- Racchi, M., Mazzucchelli, M., Porrello, E., Lanni, C., & Govoni, S. (2004). Acetylcholinesterase inhibitors: Novel activities of old molecules. Pharmacological Research, 50(4), 441–451. doi:10.1016/j.phrs.2003.12.027
- Rahim, F., Ullah, H., Taha, M., Wadood, A., Javed, M.T., Rehman, W., … Khan, K.M. (2016). Synthesis and in vitro, acetylcholinesterase and butyrylcholinesterase inhibitory potential of hydrazide based schiff bases. Bioorganic Chemistry, 68, 30–40. doi:10.1016/j.bioorg.2016.07.005
- Sacan, O., & Yanardag, R. (2010). Antioxidant and antiacetylcholinesterase activities of chard (beta vulgaris, l. var. cicla). Food & Chemical Toxicology An International Journal Published for the British Industrial Biological Research Association, 48(5), 1275–1280. doi:10.1016/j.fct.2010.02.022
- Sancheti, S., Sancheti, S., Um, B.-H., & Seo, S.-Y. (2010). 1,2,3,4,6-penta-o-galloyl-β-d-glucose: A cholinesterase inhibitor from Terminalia chebula. South African Journal of Botany, 76(2), 285–288. doi:10.1016/j.sajb.2009.11.006
- Stasiuk, M., Bartosiewicz, D., & Kozubek, A. (2008). Inhibitory effect of some natural and semisynthetic phenolic lipids upon acetylcholinesterase activity. Food Chemistry, 108(3), 996–1001. doi:10.1016/j.foodchem.2007.12.011
- Tsai, Y.-H., Kung, H.-F., Chang, S.-C., Lee, T.-M., & Wei, C.-I. (2007). Histamine formation by histamine-forming bacteria in douchi, a Chinese traditional fermented soybean product. Food Chemistry, 103(4), 1305–1311. doi:10.1016/j.foodchem.2006.10.036
- Wang, D., Wang, L.-J., Zhu, F.-X., Zhu, J.-Y., Chen, X.D., Zou, L., … Li, L.-T. (2008). In vitro, and in vivo, studies on the antioxidant activities of the aqueous extracts of douchi (a traditional Chinese salt-fermented soybean food). Food Chemistry, 107(4), 1421–1428. doi:10.1016/j.foodchem.2007.09.072
- Wang, L.-J., Yin, L.-J., Li, D., Zou, L., Saito, M., Tatsumi, E., & Li, L.-T. (2007). Influences of processing and Nacl supplementation on isoflavone contents and composition during douchi manufacturing. Food Chemistry, 101(3), 1247–1253. doi:10.1016/j.foodchem.2006.03.029
- Yoon, N. Y., Ngo, D. N., & Kim, S. K. (2009). Acetylcholinesterase inhibitory activity of novel chitooligosaccharide derivatives. Carbohydrate Polymers, 78(4), 869–872. doi:10.1016/j.carbpol.2009.07.004
- Zhang, J.-H., Tatsumi, E., Ding, C.-H., & Li, L.-T. (2006). Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product. Food Chemistry, 98(3), 551–557. doi:10.1016/j.foodchem.2005.06.024
- Zhang, P., Chen, L., Gu, W., Xu, Z., Gao, Y., & Li, Y. (2007). In vitro and in vivo evaluation of donepezil-sustained release microparticles for the treatment of Alzheimer’s disease. Biomaterials, 28(10), 1882–1888. doi:10.1016/j.biomaterials.2006.12.016