ABSTRACT
Oleoresin of Capsicum sp. is considered a food additive due to its color and pungency, and its active compound, capsaicin, is characterized by antimicrobial and antioxidant properties. However, Chilean variety of Capsicum sp. has not been characterized yet. The purpose of this study was to characterize the oleoresin of Capsicum annuum var. Cacho de cabra (OCc). Solvent extraction of OCc was carried out with hexane and physical properties of the OCc obtained were determined. Antimicrobial and antioxidant properties of OCc were compared with its active compound, capsaicin. Results showed that OCc has good optical properties compared with other commercial oleoresins and better antimicrobial properties than pure capsaicin. Due to the good optical properties obtained for OCc, this oleoresin could have a higher commercial value to be used as a coloring and flavor of various products, giving an added value to this variety of pepper.
RESUMEN
La oleorresina de Capsicum sp. es considerada un aditivo alimentario debido a su color y pungencia, y su compuesto activo capsaicina se caracteriza por poseer propiedades antimicrobianas y antioxidantes. Sin embargo, la oleorresina de la variedad chilena de Capsicum sp. no ha sido caracterizada. El objetivo de este estudio fue caracterizar la oleorresina de Capsicum annuum var. Cacho de cabra (OCc). Se realizó la extracción por solvente de OCc con hexano y se determinaron las propiedades físicas de la OCc obtenida. Las propiedades antimicrobianas y antioxidantes de la OCc fueron comparadas con su compuesto activo capsaicina. Los resultados mostraron que la OCc presenta buenas propiedades ópticas en comparación con otras oleorresinas comerciales y mejores propiedades antimicrobianas que la capsaicina pura. Debido a las buenas propiedades ópticas obtenidas para la OCc, esta podría ser de interés comercial para ser usada como colorante y saborizante de diversos productos, otorgándole un valor a esta variedad de ají.
PALABRAS CLAVES:
Introduction
Capsicum sp. fruits are among the most popular in the world because they have been used as ingredients due to their sensory attributes of color, flavor, and pungency (Giuffrida et al., Citation2013; Perva-Uzunalić, Škerget, Weinreich, & Kneiz, Citation2004; Topuz & Ozdemir, Citation2007). Today, the interest of natural additives such as carotenoids and pigments fruit of the genus Capsicum sp. are increasing due to the restrictive regulations on the use of chemical additives in the food, cosmetic and pharmaceutical industry (Fernández-Ronco et al., Citation2010). Food industry is the major user of the genus Capsicum sp. fruits because they are used as coloring and flavoring in sauces, soups, processed meats, snacks, sweets, soft drinks, and alcoholic beverages (Pino et al., Citation2007).
The genus of Capsicum sp. includes more than 30 species, for example, Capsicum annuum, Capsicum frutescens, Capsicum chinense, Capsicum baccatum, and Capsicum pubescens (Bosland & Votava, Citation2000). The most commonly cultivated species worldwide is the Capsicum annuum, which has subsequently become one of the most important spice commodities as well as an important vegetable crop globally (Perry et al., Citation2007). In Chile, the most cultivated chilli peppers come from the species Capsicum annuum var. ‘Cacho de cabra’, which belongs to Solanaceae family, a local ecotype of the ninth region (38°54′00″S, 72°40′00″O). This traditional culture has been used for generations, especially by Mapuches. They mainly used it for the preparation of merkén, a local condiment that has an important economic value in the country (González, Merino, & Leonelli, Citation2005).
The group of active compounds responsible for the pungency of the fruits Capsicum sp. is capsaicinoids. The latter are made of a mixture of vanillyl-amides of branches fatty acids with 9–11 carbons, which is produced naturally in the placenta and the white ribs of the fruit (Topuz & Ozdemir, Citation2007). The most common capsaicinoid is the capsaicin (8-methyl-N-vanillyl-6-nonenamide), a powerful irritant of receptors participating in circulatory and respiratory reflexes (Perva-Uzunalić et al., Citation2004). Also, the capsaicin is a lipophilic, odorless and colorless, waxy and nonnutrient compound, and the spiciest within capsaicinoids compound with a value of 16.1*106 equivalent Scoville heat units (SHU) (Dong, Citation2000). Furthermore, the capsaicinoids have strong physiological and pharmacological properties, having been reported to also exhibit antioxidant properties (Caris-Veyrat, Citation2008) and antibacterial properties against Listeria monocytogenes, Staphylococcus aureus, Salmonella typhimurium, and Bacillus cereus (Dorantes et al., Citation2000), Vibrio cholerae (Koffi-Nevry, Kouassi, Nanga, Koussémon, & Loukou, Citation2012), Pseudomonas aeruginosa (El Ksibi,Slama, Faidi, Ticha, & M’henni, Citation2015), Klebsiella pneumonia, Bacillus subtilis, and Enterococcus faecali (Nascimento et al., Citation2014).
The fruit of Capsicum sp. is consumed worldwide fresh, as processed natural colorant, and in the form of paste, paprika, or oleoresin (Giuffrida et al., Citation2013). Oleoresin from Capsicum sp. is the liquid extract oil obtained from fresh, ripe, or dried fruits from the genus Capsicum sp. In addition, this viscous oil is bright red color, with a typical pepper aroma, having a complex blend of essential oils, waxes, and various carotenoids with pungent properties and colorants (Restrepo, Citation2007). The most important capsaicinoids reported on oleoresin were dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, homocapsaicin, capsanthin, and capsorubin (Giuffrida et al., Citation2013; Perva-Uzunalić et al., Citation2004).
Oleoresin and/or oils extraction of natural compounds is carried out, commonly, by steam distillation, and traditional extraction, by using organic solvents such as acetone, petroleum ether, benzene, hexane, diethyl ether, chloroform, ethanol, and methanol (Park, Kim, & Chu, Citation2007). Indeed, the use of hexane is predominant in the food industry. Less extraction time (about 15 min) is required in comparison with other solvents (Pérez-Gálvez, Jarén-Galán, & Mínguez-Mosquera, Citation2006). The use of hexane prevents the removal of undesirable compounds during use, and it is authorized by the Food and Drugs Administration (FDA) from the U.S.A.
Commercial oleoresin is mainly extracted from Capsicum frutescens. However, the variety grown in Chile (Capsicum annuum var. Cacho de cabra) could be a good alternative to extract oleoresin since it is characterized by a deep red color. Advantageously, this product is a source of antioxidants, antimicrobials, natural colorants, and flavors, giving it an added commercial value.
Capsaicinoids derived from the fruits of Capsicum sp. have been extensively studied in relation to its antimicrobial and antioxidant activity; nevertheless, the obtaining of these pure compounds is very expensive. Therefore, alternative sources of these compounds with the same properties are necessary to find at lower production cost. Thus, the purpose of this study was to obtain and characterize the oleoresin of Capsicum annuum var. Cacho de cabra, in order to find a cheaper alternative to active compounds (capsaicin).
Materials and methods
Materials
Oleoresin of Capsicum annuum var. Cacho de cabra (OCc) were obtained from Capsicum annuum var. Cacho de cabra fruits, which were purchased at a local market (Lider S.A., Chile), capsaicin pure was obtained from Sigma-Aldrich (India), and the others reagents used herein were of analytical grade.
For antimicrobial activity measurements, the microorganisms used were Escherichia coli ATCC 25922 and Listeria innocua ISP65-08 (Instituto de Salud Pública, Chile). While Botrytis cinerea (B. cinerea), Aspergillus sp., and Rhizopus sp. were previously isolated and identified in the laboratory. Also, capsaicin was dissolved in medium chain triglycerides oil (MCT oil, SHS International England), which is an inert oil (no antimicrobial activity for bacteria studied). Capsaicinoids are indeed compounds of hydrophobic nature, soluble in fats, and are not water soluble at room temperature (Zhu et al., Citation2015). Capsaicin concentrations used in dilutions correspond to 5% w/w.
Extraction of OCc
First, a chilli paste is obtained from extraction of the OCc. In order to do that, damaged fruits (with cuts and/or visual deterioration due to microorganisms) were discarded, while the selected fruits were washed and cut into half, eliminating their seeds. The fruits were milled using a blender (Ostersizer 4172, Oster-Mexico) to form a paste which was stored at 4ºC until use (Vega-Gálvez et al., Citation2009), but before the extraction process of the oleoresin, for a maximum period time of 5 days, where no sign of quality loss were observed.
In order to characterize the chilli paste, a proximal analysis was determined by Center for Studies in Science and Food Technology (CECTA) of the University of Santiago of Chile (laboratory accredited by Instituto de Normalización-INN under standard NCh-ISO 17025 LE: 1164-1165-1166), using the following Chilean Norms (NCh): Moisture content was determined according to NCh841 Of78 (AOAC 15Ed-1990); proteins PTR-711.02–173 ISP-2009 (AOAC 19Ed-2012); lipids PTR-701.04–156 ISP-1998 (AOAC 15Ed-1990); ashes NCh842 Of.78 (AOAC 923.03–2005); crude fiber AOAC 962.09–2010; extracts not nitrogen Schmindt-Hebbel, 32–1981 and energy Schmindt-Hebbel, 32–1981.
The extraction was carried out using the methodology of Arjona et al. (Citation2002), wherein the paste (400 g in total) is mixed with hexane (Winkler-Chile) in a relation 1:2 (fruits: solvent) in weight, keeping the mixture at 25°C during 20 h at constant stirring in an incubator (NB-205, N-Biotek-Korea). Then, the mixture is vacuum filtered with Whatman No.1 filter paper to separate the residues of the extract and the solvent was evaporated and recovered by a rotavapor (Heidolph, Germany) at 40ºC (at this temperature the compounds are not degraded and based on other studies (Duarte et al., Citation2004)). Five different extractions (replicates) were performed, where no significant differences (p-value>0.05) on all measurements (also determined by triplicate) were obtained among them. Thus, results were reported as mean value of replicates with their corresponding standard deviation.
Moreover, yield of the total extraction process and yield based on the content of lipids in the chilli paste are determined by Equation (1) (Fernández-Ronco et al., Citation2010).
The calculations of the extraction yield of at least five different extractions were performed using the same batch of chilli peppers.
For antimicrobial measurements, the obtained extract (OCc) was dissolved in the same inert oil (MCT) that capsaicin, to obtain different concentrations (1%, 1.5%, 2%, 2.5%, and 3% extract weigh/inert oil volume).
Characterization of OCc
Color analysis
Color of OCc was determined through image analysis using a computer vision system (previously calibrated), which consists of a black box with four natural lights D65 (18 W, Phillips) and a digital camera (Canon EOS Rebel XS) at a distance of 22.5 cm from the sample (camera lens angle and lights at 45º) (Matiacevich, Silva, Osorio, & Enrione, Citation2012; Pedreschi, León, Mery, & Moyano, Citation2006). The OCc photographs for the triplicate were taken using white background, so for that purpose, 2 mL of OCc were uniformly distributed in a Petri dish of 5 cm of diameter.
Digital characteristics of color parameters in RGB space were obtained using software Adobe Photoshop 7.0 (Adobe Systems Incorporated, 2007), and then converted to space CIE L*a*b*, where L* indicates lightness, a* the red-green axis and b* the blue-yellow axis.
FTIR spectroscopy
The oleoresin and capsaicin was characterized using Fourier transform infrared spectroscopy (FTIR), in order to find differences and/or similarities between the two compounds. FTIR with attenuated total reflectance unit (ATR) (Diamond Two, Perkin Elmer, England) was used in the range 4000–450 cm−1 at a resolution of 1 cm−1, where 16 scans were done in order to obtain spectra without noise. Spectra were obtained in triplicate.
Antioxidant properties
The scavenging of free radical’s activity of the oleoresin and capsaicin was determined using two, 2-Diphenyl-1-picrylhydrazyl (DPPH) method. A standard curve from a Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid) solution was prepared at initial concentration of the 1 × 10−3 g/mL. Aliquots of 3 mL of different dilutions of Trolox solution or sample reacted with 0.3 mL of DPPH solution at 7.9 × 10−4 g/mL, for 30 min in darkness, and then its absorbance at 517 nm was measured in a spectrophotometer (UVmini-1240, Shimadzu, Japan). Similarly, capsaicin and OCc reacted with DPPH solution and its absorbance at 517 nm was measured after 30 min in the dark. The results were expressed in g Trolox/g sample.
Antimicrobial properties
Kinetics of bacterial growth
The kinetics of bacterial growth in presence of OCc and capsaicin against Escherichia coli (E. coli) and Listeria innocua (L. innocua) were determined, following the methodology described by Celis-Cofré, Azócar, Enrione, Páez, and Matiacevich (Citation2012).
In a microplate (Bottger, Germany) 200 μL of a mixture were added, containing: 200 mL of Mueller Hinton broth (Biokar Diagnostics, France) concentrated (21 g/100 mL), 200 mL of fresh bacteria at a concentration of 1 × 106 UFC/mL and 600 μL of oil solutions with oleoresin or capsaicin at different concentrations (1%, 1.5%, 2%, 2.5%, and 3% w/v). Then, the microplate was incubated at 37ºC for 24 h on a microplate-reader (Multiskan Go, Thermo Fisher, U.S.A), which recorded the absorbance of samples at 625 nm every 1 h.
Antifungal properties
In order to evaluate the antifungal activity of the OCc and its active compound capsaicin, it was first necessary to obtain active growth fungi. For that purpose, an inoculating loop tip of fungi was inoculated in Potato dextrose agar (PDA) acidified with tartaric acid 10% w/w and stored for 7 days at 22°C to B. cinerea and 5 days at 37ºC to Aspergillus sp. and Rhizopus sp.
After obtaining the active fungi, the antifungal activity was evaluated, where 1 mL of oleoresin or capsaicin solution was placed of the medium PDA at different concentrations (1%, 1.5%, 2%, 2.5%, and 3% v/v), and then inoculated with the different fungi. Finally, they were stored at the conditions previously mentioned for each fungi.
Percent inhibition of growth was calculated according to the Equation (2), where the diameter of fungal growth of positive control (without sample) was measured and compared with the growth in the presence of active compounds.
Statistical analysis
All experiments were performed in triplicate and for five different extracts (replicates). The results were reported by the mean and their corresponding standard deviation. Statistical analysis of results was performed for antimicrobial and antioxidant properties, which was evaluated by analysis of variance (ANOVA). Multiple comparison by the Tukey test, with a significance level of 95% using Graph Pad Prism 5.01 software (GraphPad Prism Ink., U.S.A) in order to assess the significance of differences among means.
Results and discussion
Extraction of OCc
Pepper paste used for the extraction of OCc was analyzed by proximate analysis to determine, mainly, moisture content and lipid content. As seen in , the moisture content of pepper paste was about 84 g/100 g sample, being the main component of the paste and thus of the fruit, while the lipid content only reached 0.9 g/100 g sample. Other parameters can be observed in , where a high protein content and crude fiber stand out. Giuffrida et al. (Citation2013) reported similar values for different varieties of Capsicum sp. where the dry matter content was ~14% and the moisture content of ~86%, which is a very similar value to that obtained in this study. In contrast, the nitrogen content was reported in ~1.9%, which is a higher value to that obtained in this study.
Table 1. Proximal analysis of the Capsicum annuum var. ‘Cacho de cabra’ and yields of extraction of OCc. The values corresponding a mean and standard deviation, respectively.
Tabla 1. Análisis proximal de Capsicum annuum var. ‘Cacho de cabra’ y rendimiento de la extracción de OCc. Los valores corresponden al promedio y su respectiva desviación estándar.
The yields of the extraction process were performed according to Equation (1). The obtained extract was pasty, red, and semisolid at room temperature. The yields can be seen in the , where the overall efficiency of the process (<2%) is significant below average yield values obtained by other researchers, about ~10% (Catchpole et al., Citation2003; Perva-Uzunalić et al., Citation2004). Regardless of the optimization of extraction process, the lowest yield extraction obtained may be due to several factors such as species or cultivar, region, and season in which it was planted and/or harvested the fruit, as mentioned by Fernández-Trujillo (Citation2007). Furthermore, the yield obtained with respect to the lipid content reached ~50% of extraction, which is a good yield, since the active components of the OCc are hydrophobic and it is at that stage where they can be found.
Optical properties
The chromaticity diagram of OCc and their photographs can be seen in . Color parameters obtained for OCc are: L* = 13.1 ± 5.9; a* = 36.5 ± 3.2; b* = 17.9 ± 9.7. The L* parameter has a value close to zero, indicating that the OCc tends to darkness. Furthermore, the color parameter a* is greater than zero, therefore tends to red, while the parameter b* is greater than zero which tends to yellow. Surassmo, Min, Bejrapha, and Choi (Citation2010) reported the color parameters of Capsicum sp. oleoresin commercially obtained from M&S Industry (Korea), which states that it has a strong dark red color. The parameter values obtained by color Surassmo et al. (Citation2010) were 27.0 ± 0.0, 0.3 ± 0.1, and 3.6 ± 0.0 for the L*, a*, and b*, respectively, which differ with those obtained in this research. This difference may be due to the concentration of carotenoid pigments in commercial Capsicum sp. oleoresin, since these vary considerably depending on the kind of fruit from which extraction was performed. However, a visual dark red color is also observed in the OCc obtained.
Figure 1. Chromaticity diagram of OCc and photographs of OCc obtained by extraction solvent.
Figura 1. Diagrama de cromaticidad y fotografías de la OCc obtenidas durante su extracción por solvente.
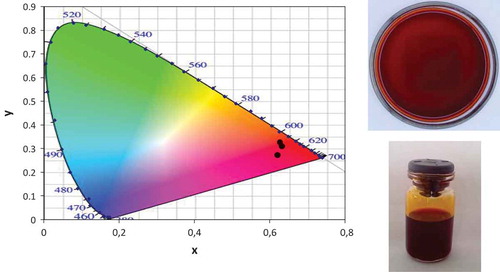
Other color parameters such as Hue (H*) and chrome (C*) were also determined and are: H* = 0.43 ± 0.2; C* = 41.1 ± 7. The angle H* parameter corresponds to the hue of the color of the samples and their values range from 0 to 360, while the C* parameter corresponds to the color saturation of the samples and their values, ranging from 0 to 100. Results reported by Surassmo et al. (Citation2010) for the H* parameter commercial oleoresin, were above those obtained in this research, which shows that OCc obtained by solvent extraction has a lower color tone from that obtained in commercial oleoresin, which might be, as already mentioned, due to the concentration of carotenoid pigments. The value of the parameter C* oleoresin corresponds to an intermediate value of the corresponding scale for this parameter, so no evidence of increased color saturation of the OCc was observed.
FTIR spectroscopy
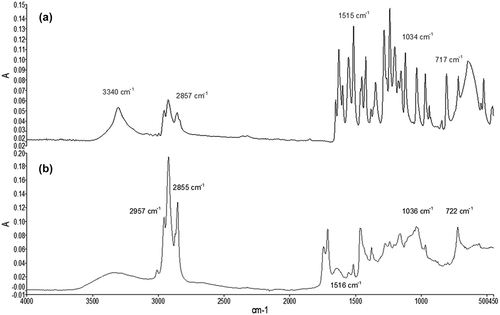
FTIR spectra of capsaicin and OCc are shown in . The peaks observed in the spectra are consistent with those obtained by Wang and Sihao (Citation2010), who reported that the peaks observed by FTIR for capsaicin at 3350, 2900, and 1050 cm−1 correspond to vibrations of bonds OH/NH, CH, and COC, respectively, while Wang, Dong, Chen, and Lou (Citation2013) observed the same peaks in microcapsules of capsaicin. However, Wang and Sihao (Citation2010) also reported peaks at 1600 and 1630 cm−1, which correlates with the vibrations of the links of the amides and peaks groups at 1300 cm−1, corresponding to the vibrations of the C–N groups. While the latter peaks are not observed in the spectra of OCc and capsaicin obtained, similar peaks such as 1378, 1627, and 1650 cm−1 for OCc and 1347 cm−1 for capsaicin were observed. Notably, these types of vibrations correspond to links observed in the molecular structure of capsaicin. In the detail of the peaks obtained (), we can see that peaks are present in both samples (OCc and capsaicin), such as 2857, 1517, and 1036 cm−1. These samples could indicate the presence of capsaicin in the OCc, since they correspond to the active compound oleoresin Capsicum sp. Also, is the capsaicinoid found in greater proportion, about 90% of the capsaicinoids (Fernández-Ronco et al., Citation2010; Giuffrida et al., Citation2013; Hayman & Kam, Citation2008; Surassmo et al., Citation2010).
Antioxidant properties
Due to the nature of the molecular structure of the capsaicinoids, these compounds present antioxidant activity, which is conferred to the presence of a phenol group (a methoxy group in ortho position to OH) (Giuffrida et al., Citation2013; Maksimova, Koleva, Ruskovska, Cvetabovska, & Gulaboski, Citation2014). Apart from capsaicinoids present on OCc (where capsaicin is the main component), different phenolic compounds and flavonoids with antioxidant activity were reported (Henderson, Slickman, & Henderson, Citation1999; Kittisakulnam, Saetae, & Suntornsuk, Citation2016; Maksimova et al., Citation2014). Considering that free radical scavenging capacity by DPPH method is used for compounds of hydrophobic nature, and was reported for capsaicin (Amarowicz, Citation2014; Gang, Park, Rhim, & Kwon, Citation2008; Okada, Tanaka, Sato, & Okajima, Citation2010), and other oil extracts from Capsicum sp. (Kittisakulnam et al., Citation2016; Vega-Gálvez et al., Citation2009). It is therefore, in order to compare with literature, decided to study the antioxidant property using the radical scavenger of DPPH activity for OCc and capsaicin.
For the DPPH assay, the curve pattern of standard was calculated using six data points in the range of 1 × 10−3–1 × 10−6 g Trolox/mL, and these curves were fitted showing a linear adjustment (R2 = 0.989). While both samples (OCc and capsaicin) showed antioxidant activity, it is important to note that capsaicin showed greater activity (1.8 × 10−6 ± 0.1 × 10−6 g Trolox/g sample) than OCc (3.7 × 10−7 ± 0.3 × 10−7 g Trolox/g sample). Gang et al. (Citation2008) reported similar results, where the capsaicin had greater DPPH radical scavenging activity than hot peppers extracts. Also importantly, it is difficult to compare the values obtained by DPPH method with others reported results, mainly because of the different methods used, and the different ways of expressing the results (Carvalho et al., Citation2015).
Oleoresins present compounds with antioxidant properties due to their redox properties that allow them to act as reducing agents, such as hydrogen donors ion, deactivators oxygen ion, and metal chelators (Deepa, Kaura, Geroge, Singh, & Kapoor, Citation2007). For example, β-carotene, capsanthin, capsorubin, cryptoxanthin, (Márkus, Daood, Kapitány, & Biacs, Citation1999; Zúñiga, Jiménez, & Gordillo, Citation2005), and protocatechuic, chlorogenic, coumaric, ferulic, cinnamic, caffeic (Estrada, Bernal, Díaz, Pomar, & Merino, Citation2000). Maksimova et al. (Citation2014) reported that the antioxidant activity of ethanolic oleoresins from hot peppers cultivars (genotypes Vezena, Feferona, and Bombona) is dependent on the concentration of capsaicin, deducing that capsaicin is mainly responsible for antioxidant capacity of oleoresins, in accordance to what is reported by Zimmer et al. (Citation2012) in Capsicum baccatum var. pendulum for oleoresin obtained by ethanolic extract. According to those authors, and considering that although OCc is composed of various constituents of phenolic nature, it is expected that at the same concentration of oil, the oleoresin (OCc) presents lower antioxidant capacity due to lower concentration of capsaicin than the capsaicin solutions used.
Antibacterial properties
The antimicrobial activity of OCc and capsaicin against E. coli and L. innocua can be observed in . As expected, the OCc presented a bacteriostatic effect on E. coli at all concentrations studied (1–3% w/w), since a significant (p-value<0.05) lower growth was observed in comparison with bacterial growth (positive control). Kittisakulnam et al. (Citation2016) indicated bacteriostatic effect on E. coli at a minimum concentration of 0.27% w/w for ethanolic extracts from ripe bird chilli. However, OCc presented bacteriostatic effect on L. innocua only at the maximum concentration studied (3% w/w) (p-value<0.05).
Figure 3. Antimicrobial properties of OCc. against (a) Escherichia coli and (b) Listeria innocua, and capsaicin against (c) Escherichia coli and (d) Listeria innocua.
Figura 3. Propiedad antimicrobiana de la OCc contra (A) Escherichia coli y (b) Listeria innocua, y de la capsaicina contra (c) Escherichia coli y (d) Listeria innocua.
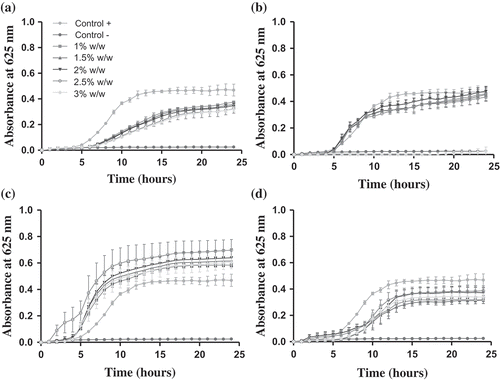
On the contrary, capsaicin did not show antimicrobial activity against E. coli and L. innocua at the concentrations studied, since no significant differences (p-value>0.05) were observed between the positive control of both bacteria and the different concentrations of capsaicin. Considering that Molina-Torres, García-Chávez, and Ramírez-Chávez (Citation1999) reported that capsaicin isolated from Capsicum sp. fruits only retarded the growth of E. coli at higher concentrations (200–300 μg/mL) than those used in this study (50–150 μg/mL); it could be possible to obtain antibacterial effect at higher concentrations of capsaicin. On the other hand, no inhibitory effect against Listeria monocytogenes were observed by Dorantes et al. (Citation2000) using capsaicin and capsaicinoids from Capsicum annuum extracts; besides, these authors reported that the antibacterial activity is associated to the presence of m-coumeric acid and cinnamic acid but no capsaicin in those extracts.
Antifungal properties
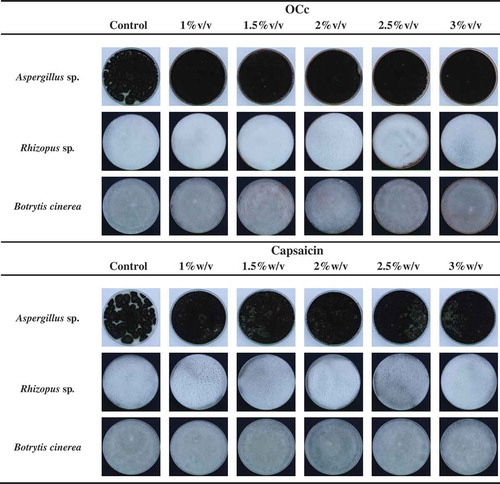
The antifungal activity of OCc and capsaicin is shown in , where regardless of the type of active compound and its concentration, there is no inhibition of growth of the fungi studied. These results differ from those obtained by Soumya and Nair (Citation2012), where extracts obtained from Capsicum frutescens L. showed inhibition growth (over 60%) of Aspergillus flavus, Aspergillus niger, and Rhizopus sp. Also, results obtained by Singh, Fairs, and Syarhabil (Citation2011) reported inhibition of growth of Aspergillus niger using extracts of Capsicum annuum. It is important to note that the differences observed are related to the concentrations used in this study, which are lower than those used by those authors. Accordingly, if the concentration of OCc or capsaicin was increased, antifungal effects were observed. However, at higher concentrations, the pungency of these active compounds appears, which is uncomfortable during handling, and it is unavailable for use in food industry.
Conclusions
The oleoresin of Capsicum annuum var. Cacho de cabra was characterized showing better antibacterial properties than the pure compound, capsaicin. Consequently, a bacteriostatic effect against E. coli and L. innocua was observed, while capsaicin did not show antibacterial activity for these bacteria. In the case of antifungal properties, both the oleoresin and capsaicin did not present activity against B. cinerea, Aspergillus sp., and Rhizopus sp. at the concentration studied. The oleoresin obtained by solvent extraction showed a lower antioxidant activity than capsaicin.
Due to the good optical properties obtained for this oleoresin, it could have a high commercial value in order to be used as a coloring and flavor of various products. Furthermore, considering the antimicrobial properties of OCc observed at lower concentrations, this variety of pepper acquires then active properties and added value. However, it is necessary to focus the application on specific foods because the addition of this oleoresin could modify the organoleptic characteristic, namely color and pungency.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amarowicz, R. (2014). Antioxidant activity of peppers. European Journal of Lipid Science and Technology, 116, 237–239. doi:10.1002/ejlt.v116.3
- Arjona, M., Amaya, S., Iriarte, A., García, V., Carabajal, D., & Sosa, B. (2002). Efecto del sistema de secado en el color y rendimiento de la oleorresina de pimentón en la variedad Capsicum annuum Trompa de elefante. Congreso Regional de Ciencia y Tecnología NOA. Catamarca: Universidad Nacional de Catamarca.
- Bosland, P.W., & Votava, E.J. (2000). Pepper: Vegetable and spice capsicum. London: CAB Publishing.
- Caris-Veyrat, C. (2008). Antioxidant and prooxidant actions and stabilities of carotenoids in vitro and in vivo and carotenoid oxidation products. In C. Socaciu (Ed.), Food colorants, chemical and functional properties. New York, NY: CRC Press.
- Carvalho, A.V., de Andrade-Mattietto, R., Oliveira-Rios, A., de Almeida, R., Moresco, K., & de Souza- Oliveira, T. (2015). Bioactive compounds and antioxidant activity of pepper (Capsicum sp.) genotypes. Journal of Food Science and Technology, 52, 7457–7464. doi:10.1007/s13197-015-1833-0
- Catchpole, O.J., Grey, J.B., Perry, N.B., Burgess, E.J., Redmond, W.A., & Porter, N.G. (2003). Extraction of chili, black pepper, and ginger with near-critical CO2, propane, and dimethyl ether: analysis of the extracts by quantitative nuclear magnetic resonance. Journal of Agricultural and Food Chemistry, 51, 4853–4860. doi:10.1021/jf0301246
- Celis-Cofré, D., Azócar, M.I., Enrione, J., Páez, M., & Matiacevich, S. (2012). Influence of glassy or rubbery state on the antimicrobial activity of chitosan-gelatin films. Journal of Food Research, 1, 184–193. doi:10.5539/jfr.v1n4p184
- Deepa, N., Kaura, C., George, B., Singh, B., & Kapoor, H. (2007). Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT - Food Science and Technology, 40, 121–129. doi:10.1016/j.lwt.2005.09.016
- Dong, M.W. (2000). How hot is that pepper? Quantifying capsaicinoids with chromatography. Today’s Chemist at Work, 9, 17–20.
- Dorantes, L., Colmenero, R., Hernandez, H., Mota, L., Jaramillo, M.E., Fernandez, E., & Solano, C. (2000). Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. International Journal of Food Microbiology, 57, 125–128. doi:10.1016/S0168-1605(00)00216-6
- Duarte, C., Moldão-Martins, M., Gouveia, A.F., Beirão Da Costa, S., Leitão, A.E., & Bernardo-Gil, M.G. (2004). Supercritical fluid extraction of red pepper (Capsicum frutescens L.). The Journal of Supercritical Fluids, 30, 155–161. doi:10.1016/j.supflu.2003.07.001
- El Ksibi, I., Slama, R.B., Faidi, K., Ticha, M.B., & M’henni, M.F. (2015). Mixture approach for optimizing the recovery of colored phenolics from red pepper (Capsicum annum L.) by products as potential source of natural dye and assessment of its antimicrobial activity. Industrial Crops and Products, 70, 34–40. doi:10.1016/j.indcrop.2015.03.017
- Estrada, B., Bernal, M.A., Díaz, J., Pomar, F., & Merino, F. (2000). Fruit development in Capsicum annuum: Changes in capsaicin, lignin, free phenolics, and peroxidase patterns. Journal of Agricultural and Food Chemistry, 48, 6234–6239. doi:10.1021/jf000190x
- Fernández-Ronco, M.P., Ortega-Noblejas, C., Gracia, I., De Lucas, A., García, M.T., & Rodríguez, J.F. (2010). Supercritical fluid fractionation of liquid oleoresin Capsicum: Statistical analysis and solubility parameters. The Journal of Supercritical Fluids, 54, 22–29. doi:10.1016/j.supflu.2010.03.011
- Fernández-Trujillo, J.P. (2007). Extracción convencional de oleorresina de pimentón dulce y picante I. Generalidades, composición, proceso e innovaciones y aplicaciones. Grasas y Aceites, 58, 252–253.
- Gang, H.-M., Park, H.-S., Rhim, T.-J., & Kwon, K.-R. (2008). A study on the comparison of antioxidant effects between hot pepper extract and capsaicin. Journal of Pharmacopuncture, 11, 109–118. doi:10.3831/KPI.2008.11.1.109
- Giuffrida, D., Dugo, P., Torre, G., Bignardi, C., Cavazza, A., Corradini, C., & Dugo, G. (2013). Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chemistry, 140, 794–802. doi:10.1016/j.foodchem.2012.09.060
- González, C., Merino, D., & Leonelli, G. (2005). Caracterización y evaluación de la producción de semilla orgánica de ají (Capsicum annuum L.) y determinación de la calidad organoléptica del merkén, en comunidades mapuche de la IX Región, Chile. Simiente, 75, 3–4.
- Hayman, M., & Kam, P. (2008). Capsaicin: A review of its pharmacology and clinical applications. Current Anaesthesia & Critical Care, 19, 338–343. doi:10.1016/j.cacc.2008.07.003
- Henderson, D.E., Slickman, A.M., & Henderson, S.K. (1999). Quantitative HPLC determination of the antioxidant activity of capsaicin on the formation of lipid hydroperoxides of linoleic acid: A comparative study against BHT and melatonin. Journal of Agricultural and Food Chemistry, 47, 2563–2570. doi:10.1021/jf980949t
- Kittisakulnam, S., Saetae, D., & Suntornsuk, W. (2016). Antioxidant and antibacterial activities of spices traditionally used in fermented meat products. Journal of Food Processing and Preservation. doi:10.1111/jfpp.13004
- Koffi-Nevry, R., Kouassi, K., Nanga, Z., Koussémon, M., & Loukou, G. (2012). Antibacterial activity of two bell pepper extracts: capsicum annuum L. and Capsicum frutescens. International Journal of Food Properties, 15, 961–971. doi:10.1080/10942912.2010.509896
- Maksimova, V., Koleva, L., Ruskovska, T., Cvetanovska, A., & Gulaboski, R. (2014). Antioxidative effect of capsicum oleoresins compared with pure capsaicin. Journal of Pharmacy, 4, 44–48.
- Márkus, F., Daood, H.G., Kapitány, J., & Biacs, P.A. (1999). Change in the carotenoid and antioxidant content of spice red pepper (Paprika) as a function of ripening and some technological factors. Journal of Agricultural and Food Chemistry, 47, 100–107. doi:10.1021/jf980485z
- Matiacevich, S., Silva, P., Osorio, F., & Enrione, J. (2012). Evaluation of blueberry color during storage using image analysis. In J.L. Caivano & M.P. Buera (Eds.), Color in food: Technological and psychophysical aspects. Buenos Aires: CRC Press. doi:10.1201/b11878-26
- Molina-Torres, J., Garcı́a-Chávez, A., & Ramı́rez-Chávez, E. (1999). Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. Journal of Ethnopharmacology, 64, 241–248. doi:10.1016/S0378-8741(98)00134-2
- Nascimento, P.L., Nascimento, T.C., Ramos, N.S., Silva, G.R., Gomes, J.E.G., Falcão, R.E., … Silva, T. (2014). Quantification, antioxidant and antimicrobial activity of phenolics isolated from different extracts of Capsicum frutescens (Pimenta Malagueta). Molecules, 19, 5434–5447. doi:10.3390/molecules19045434
- Okada, Y., Tanaka, K., Sato, E., & Okajima, H. (2010). Kinetics and antioxidative sites of capsaicin in homogeneous solution. Journal of the American Oil Chemists’ Society, 87, 1397–1405. doi:10.1007/s11746-010-1628-4
- Park, P., Kim, E., & Chu, K. (2007). Chemical disruption of yeast cells for the isolation of carotenoid pigments. Separation and Purification Technology, 53, 148–152. doi:10.1016/j.seppur.2006.06.026
- Pedreschi, F., León, J., Mery, D., & Moyano, P. (2006). Development of a computer vision system to measure the color of potato chips. Food Research International, 39, 1092–1098. doi:10.1016/j.foodres.2006.03.009
- Pérez-Gálvez, A., Jarén-Galán, M., & Mínguez-Mosquera, I. (2006). Processing of red pepper fruits (Capsicum annuum L.) for production of paprika and paprika oleoresin. Handbook of Fruits and Fruits Processing, 30, 565–579.
- Perry, L., Dickau, R., Zarrillo, S., Holst, I., Pearsall, D.M., Piperno, D.R., … Zeidler, J.A. (2007). Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science, 315, 986–988. doi:10.1126/science.1136914
- Perva-Uzunalić, A., Škerget, M., Weinreich, B., & Knez, Ž. (2004). Extraction of chilli pepper (var. Byedige) with supercritical CO2: Effect of pressure and temperature on capsaicinoid and color extraction efficiency. Food Chemistry, 87, 51–58. doi:10.1016/j.foodchem.2003.10.016
- Pino, J., Gonzalez, M., Ceballos, L., Centurionyah, A.R., Trujillo-Aguirre, J., Latournerie-Moreno, L., & Sauri-Duch, E. (2007). Characterization of total capsaicinoids, color and volatile compounds of Habanero chilli pepper (Capsicum chinense Jack.) cultivars grown in Yucatan. Food Chemistry, 104, 1682–1686. doi:10.1016/j.foodchem.2006.12.067
- Restrepo, M. (2007). Oleorresinas de Capsicum en la industria alimentaria. Revista Lasallista de Investigación, 3, 43–47.
- Singh, H., Fairs, G., & Syarhabil, M. (2011, June 4–5). Antifungal activity of Capsicum frutescens and Zingiber officinale against key post-harvest pathogens in citrus. In International Conference on Biomedical Engineering and Technology IPCBEE vol. 11, Kuala Lumpur. Singapore: IACSIT Press.
- Soumya, S.L., & Nair, B. (2012). Antifungal efficacy of Capsicum frutescens L. extract against some prevalent fungal strains associated with groundnut storage. Journal of Agricultural Technology, 8, 739–750.
- Surassmo, S., Min, S.-G., Bejrapha, P., & Choi, M.-J. (2010). Effects of surfactants on the physical properties of capsicum oleoresin-loaded nanocapsules formulated through the emulsion-diffusion method. Food Research International, 43, 8–17. doi:10.1016/j.foodres.2009.07.008
- Topuz, A., & Ozdemir, F. (2007). Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. Journal of Food Composition and Analysis, 20, 596–602. doi:10.1016/j.jfca.2007.03.007
- Vega-Gálvez, A., Di Scala, K., Rodríguez, K., Lemus-Mondaca, R., Miranda, M., López, J., & Perez-Won, M. (2009). Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, color and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chemistry, 117, 647–653. doi:10.1016/j.foodchem.2009.04.066
- Wang, J., Dong, X., Chen, S., & Lou, J. (2013). Microencapsulation of capsaicin by solvent evaporation method and thermal stability study of microcapsules. Colloid Journal, 75, 26–33. doi:10.1134/S1061933X13010134
- Wang, J., & Sihao, C. (2010). Preparation and characterization of microcapsules containing capsaicin. Journal of Applied Polymer Science, 116, 2234–2241.
- Zhu, Y., Zhang, J., Zheng, Q., Wang, M., Deng, W., Li, Q. … Yu, J. (2015). In vitro and in vivo evaluation of capsaicin-loaded microemulsion for enhanced oral bioavailability. Journal of the Science of Food and Agriculture, 95, 2678–2685. doi:10.1002/jsfa.2015.95.issue-13
- Zimmer, A.R., Leonardi, B., Miron, D., Schapoval, E., Rodrigues de Oliveira, J., & Gosmann, G. (2012). Antioxidant and anti-inflammatory properties of Capsicum baccatum: From traditional use to scientific approach. Journal of Ethnopharmacology, 139, 228–233. doi:10.1016/j.jep.2011.11.005
- Zúñiga, O.C., Jiménez, F.G., & Gordillo, R.M. (2005). Comparative study of carotenoid composition in three Mexican varieties of Capsicum annuum L. Food Chemistry, 90, 109–114. doi:10.1016/j.foodchem.2004.03.032