ABSTRACT
The colloidal gold quick test card which we developed was used for rapid detection of Alicyclobacillus acidoterrestris (A. acidoterrestris) in apple juice concentrate. Two antibodies against A. acidoterrestris, obtained from Sprague Dawley (SD) rats and Japan White Rabbits, were adopted to construct this quick test card. The results showed that the colloidal gold quick test card had better specificity, and was more rapid and convenient which deserved notice especially (the test could be completed within 5–10 min) and concorded with ELISA method (we previously report) and K medium methods (traditional classical method). This new developed colloidal gold quick test card (we named it TAB quick test card) could be more convenient for apple juice safety testing.
RESUMEN
Se utilizó la tarjeta de test rápido de oro coloidal que desarrollamos para la detección rápida de Alicyclobacillus acidoterrestris (A. acidoterrestris) en concentrado de zumo de manzana (AJC), dos anticuerpos contra A. acidoterrestris que se obtuvieron de ratas Sprague Dawley (SD) y conejos blancos de Japón se utilizaron para realizar esta tarjeta de test rápido. Los resultados mostraron que tenían una mejor especificidad, eran más rápidas y prácticas, además de merecer una especial atención (el test podía ser completado en 5–10 min) y una buena concordancia con el método ELISA (estudiado anteriormente) y los métodos K-medias (método clásico tradicional). Esta nueva tarjeta de test rápido de oro coloidal desarrollada (nombrada tarjeta del test rápido TAB) podría ser más práctica para las pruebas de seguridad del zumo de manzana.
1. Introduction
Alicyclobacillus acidoterrestris is a thermophilic, acidophilic, and spore-forming bacterium (TAB) in many fruit juices (Cerny, Hennlich, & Poralla, Citation1984), such as apple, pear, orange, citrus, and so on. It can survive through pasteurization and cause juice spoilage (Breveglieri, Masiero, Spisani, & De Taddeo, Citation2011; Huang, Yuan, Guo, Gekas, & Yue, Citation2014). The traditional method for the detection of A. acidoterrestris is plate cultivation, which requires 4–5 days to identify the target bacteria (Pinhatti, Variane, Eguchi, & Manfio, Citation1997). In order to monitor and control these spoilage organisms timely and effectively, a rapid detection technology is needed. Numerous studies for rapid detection of A. acidoterrestris have been reported previously. For example, the reverse-transcription polymerase chain reaction (RT-PCR) detection by Yamazaki, Teduka, Inoue, and Shinano (Citation1996), a real-time PCR rapid detection method by Luo, Yousef, and Wang (Citation2004), a quantitatively competitive PCR (QC-PCR) method by Li, Feng, and Qiu (Citation2006), and a Fourier transform infrared (FT-IR) spectroscopy detection by Al-Qadiri, Lin, Cavinato, and Rasco (Citation2006). However, those methods are difficult to use in factory because it requires skilled personnel and relatively expensive equipments (Li, Huang, Xia, & Liu, Citation2014).
We reported a novel method of indirect enzyme-linked immunosorbent assay (ID-ELISA) and staphylococcal protein A enzyme-linked immunosorbent assay (SPA-ELISA) to rapidly detect A. acidoterrestris in apple juice concentrate (AJC) (Li, Wang, & Xia, Citation2011; Li, Xia, & Yu, Citation2013) for the first time. And recently an improved double-antibody sandwich ELISA (DAS-ELISA) (Li et al., Citation2014) has been proved to be convenient and suitable for application.
In this study, a new colloidal gold quick card (TAB quick test card) was developed with the aid of colloidal gold immunochromatographic assay. This test card showed more rapidness and convenience for rapid detection of A. acidoterrestris in apple juice.
2. Materials and methods
2.1. Bacterial strains and materials
Alicyclobacillus acidoterrestris DSM 3922 was purchased from DSMZ (Brunswick, Germany). Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 6538 and Shigella ATCC 12022 were obtained from the Laboratory of Microbiology, College of LifeScience, Shaanxi Normal University. Japanese White Rabbits and Sprague Dawley (SD) rats were obtained from Laboratory Animal Research Center, College of Medicine, Xi’an Jiaotong University. Juice concentrate (pH 3.2–4.6) was obtained from Shaanxi Hengxing Fruit Juice Co., Ltd. Freund’s complete and incomplete adjuvant was purchased from Sigma Chemical (St. Louis, MO, USA). ELISA Well Strips and Plate Frames were bought from JET BIOFIL (Canada). Colloidal gold and Goat anti-rabbit were purchased from Shanghai Jiening Biotechnology Co., Ltd (Shanghai, CN). Nitrocellulose membranes, sample pads, absorbent pads and conjugate pads were purchased from Millipore Corporation. Polyvinylpyrrolidone (PVP) was purchased from JingBo, Ltd (Shaanxi, CN). All inorganic chemicals and organic solvents used in this study were of reagent grade or chemically pure, which were purchased from DingGuo Biotech (Beijing, CN) or Sigma (USA).
The formula of K medium (pH 4.0): Tween 80(1.0 g), glucose (1.0 g), yeast extract (2.5 g), peptone (5.0 g), agar (15 g) and distilled water (1000 mL).
The formula of 402 medium(pH 4.0): (NH4)2SO4(0.2 g), CaCl2 · 2H2O (0.25 g), MgSO4(0.5 g), yeast extract (2.0 g), glucose (5.0 g),K H2PO4 (5.0 g)and distilled water (1000 mL).
2.2. Antigen preparation
A. acidoterrestris were cultured on K medium at pH 3.7 at43 C for 36 h and then they were harvested with sterile saline (0.9% NaCl), and the cells were washed three times by centrifugation (3500 g, 10 min), and keep resuspension in sterile saline. Bacterial density of the final suspension was 2 × 109 CFU/mL, which was determined by spectrophotometer (OD/600 nm) (Wang, Li, Liu, & Jiang, Citation2009; Xia, Li, & Yu, Citation2012).
2.3. Antibody preparation and purification
The preparation of Japanese White Rabbits and SD rats polyclonal antibodies against A. acidoterrestris was reported by Wang et al. (Citation2009) and Xia et al. (Citation2012). The polyclonal antibody was precipitated by caprylic acid and ammonium sulfate and then purified by Hitrap Protein A chromatography column (Wang et al., Citation2009; Xia et al., Citation2012).
2.4. Preparation of colloidal gold probe
The colloidal gold probe was prepared according to the standard method with modifications (Ju et al., Citation2010). The optimal pH and concentration of capture antibody were added to the colloidal gold solution (10 mL), shaking for 30 min. Then 200 μL 10% BSA (Bull Serum Albumin) were added for 15 min after, the mixtures were washed three times by centrifugation(10 × 103 g,10 min). The precipitate was collected and suspended with 1 mL of the suspension buffer (50 mM pH 8.1Tris-HCl, 0.1% PVP, 0.2% tween-20, 0.2% BSA). The colloidal gold was stored in a dark bottle at 4 C.
2.5. Preparation of the immunochromatographic strips
The immunochromatographic strip (ICS) included four parts (named as TAB quick test card): a sample pad, a conjugate pad, an immobilized nitrocellulose membrane, and an absorbent pad. The sample pads were treated with the treatment liquid (50 mM pH 8.1Tris-HCl, 0.3% tween) for 30 min. The conjugate pads were sprayed with colloidal gold-labeled rabbit antibody against A. acidoterrestris. The T line was coated with rat antibody and the C line was coated with goat anti-rabbit IgG. When the samples were slowly added to the sample pads, it was moved through immobilized nitrocellulose membrane under capillarity field effect. After 5–10 min, the positive effect displayed two red lines, while the negative effect displayed one red line in the control lines. The immunochromatographic strips lost effectiveness if there was nothing in the control lines.
2.6. Specificity and sensitivity of the TAB quick test card
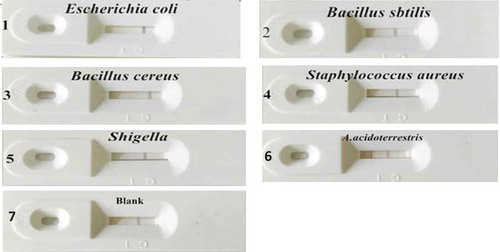
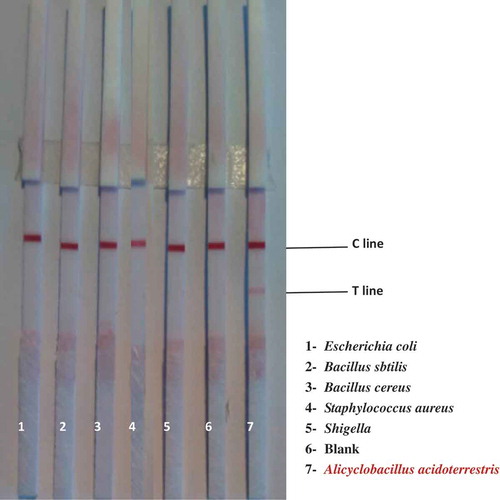
In order to determine the specificity of the TAB quick test card, we used the test card to test Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 6538 and Shigella ATCC 12022.
The five representative food-borne bacteria were cultured on medium at 37 C for 12 h, then they were harvested with sterile saline (0.9% NaCl), and the cells were washed three times by centrifugation (3500 g, 10 min), and kept in resuspension in sterile saline.
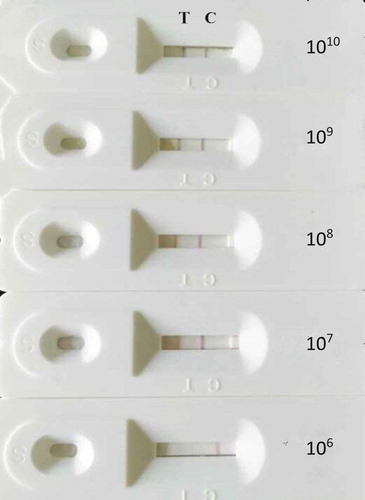
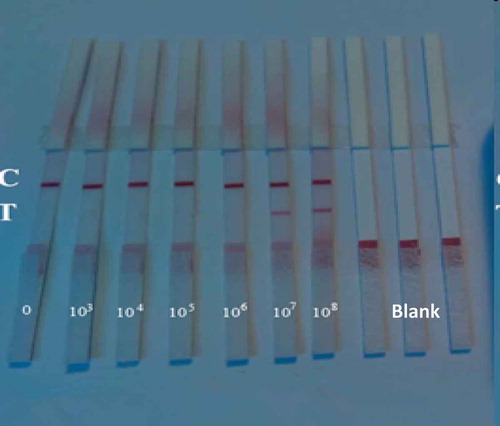
The bacterial suspensions (A. acidoterrestris) were diluted by pH 7.4 PBS buffer (0.2 g KH2PO4, 2.9 g Na2HPO4, 0.2 g KCl, 8.0 g NaCl, 1000 mL distilled water) from concentrations of 1 × 1010 CFU/mL, 1 × 109 CFU/mL, 1 × 108 CFU/mL, 1 × 107 CFU/mL, 1 × 106 CFU/mL to 0 CFU/mL (Y = 0.0844X+0.1126, R2 = 0.9992. Y: absorbance, X: concentrations(billion/mL).
Each suspension with A. acidoterrestris of varying levels was detected by the TAB quick test card. The sensitivity of the TAB quick test card was the positive reaction with the lowest concentration.
2.7. AJC sample preparation for the TAB quick test card assay
AJC sample preparation was performed using procedures based on the methods of Li et al. (Citation2011). About 10 mL AJC samples was heat-treated at 80 C for 10 min and then deionized water was added. The diluted sample was filter sterilized by discmembrane filters (0.45 μm pore size; 50 mm in diameter). After filtering, the membranes were transported into an enrichment broth of 402 medium (DingGuo Biotech, pH 4.0, 200 mL) and incubated at 45 C for 12 h with shaking for pre-enrichment. After incubation, the broth was centrifuged at 10,000 g for 10 min to collect precipitation and then diluted the precipitation with pH 7.4 PBS buffer (0.2 g KH2PO4, 2.9 g Na2HPO4, 0.2 g KCl, 8.0 g NaCl, 1000 mL distilled water) (Li et al., Citation2014) that achieved concentration above the sensitivity of the TAB quick test card.
2.8. Analysis of consistency among quick test card, ELISA kit and K medium method
Adding 150 μL of samples on the TAB quick test cards, after 5–10 min, we observed the phenomena and compared with the results of DAS-ELISA kit (Li et al., Citation2014) and K medium method (The bacteria were cultured on K medium(plate) at pH 3.7 at 43 C for 36 h).
3. Results and discussion
3.1. Polyclonal antibodies
The titers of the Japanese White Rabbits polyclonal antibodies against A. acidoterrestris were reached at 1/25,600 and the titers of the SD rats polyclonal antibodies against A. acidoterrestris were reached at 1/12,800.
3.2. Specificity of the TAB quick test card
Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 6538 and Shigella ATCC 12022 were cultured on beef-protein medium at 37 C for 12 h. A. acidoterrestris was cultured on K medium at pH 3.7 at 43 C for 12 h. Different suspensions were collected to evaluate the specificity of the strip. Only A. acidoterrestris showed positive effect and other samples showed negative effect. As shown in and , the TAB quick test card exhibited better specificity.
3.3. Sensitivity of the TAB quick test card
Different concentrations of A. acidoterrestris were detected by the TAB quick test card. When the concentration was 1 × 106 CFU/mL, just the control line showed red, while the concentration exceeded 1 × 107 CFU/mL(45 C for 12 h with shaking for pre-enrichment), the test line and control line both displayed red. The phenomena are shown in and , and the sensitivity of the TAB quick test cards is 1 × 107 CFU/mL.
3.4. Analysis of consistency among quick test card, ELISA kit and K medium method
Twelve samples of AJC were assayed, respectively, by TAB quick test card, ELISA kit and K medium method. For the quick test card assay, samples with the test line and control line both displayed red, which indicated a positive effect. For the DAS-ELISA assay, samples with OD 450 nm more than 0.038 were considered as a positive effect. In the terms of the K medium, 10 mL samples with more than 1 CFU were considered as a positive effect. The results are shown in . The TAB quick test card assay was well in concordance with ELISA kit and K medium method.
Table 1. Detection of Alicyclobacillus acidoterrestris by quick test card, ELISA kit and K medium.
Tabla 1. Detección de Alicyclobacillus acidoterrestris mediante tarjeta de test rápido, kit ELISA y K-medias.
The traditional method and the main current way for the detection of A. acidoterrestris is plate cultivation (K medium), but it took long times (Chang & Kang, Citation2004). In our previous study, we reported an ID-ELISA, SPA-ELISA and DAS-ELISA method to detect the A. acidoterrestris, which is suitable and time-saving in ordinary laboratory (Li et al., Citation2014, Citation2011, Citation2013). The ICS assay method (TAB quick test card) proved to be more rapid and convenient. It took 5–10 min, excluding sample pretreatment and enrichment time. The TAB quick test card (sealed package) could be stored at room temperature in dry condition for 1 year.
4. Conclusion
A high sensitivity and more convenience TAB quick test card based on colloidal gold-based immunochromatographic assay was developed to detect A. acidoterrestris in juice. The results can be obtained within 5–10 minutes excluding pretreatment and enrichment (see Section 2.7 for the details). Our results showed that the quick test card had no cross-reaction with the five representative food-borne bacteria and showed high sensitivity and excellent agreement with K medium method and DAS-ELISA kit. TAB quick test card showed a great potential in fruit juice detection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Qadiri, H.M., Lin, M.S., Cavinato, A.G., & Rasco, B.A. (2006). Fourier transform infrared spectroscopy, detection and identification of Escherichia coli O157: H7and Alicyclobacillus strains in apple juice. International Journal of Food Microbiology, 111(1), 73–80. doi:10.1016/j.ijfoodmicro.2006.05.004
- Breveglieri, G., Masiero, L., Spisani, S., & De Taddeo, H. (2011). Detection methods for the identification of Alicyclobacillus species involved in food spoilage. Minerva Biotecnologica, 23(1), 23–32.
- Cerny, G., Hennlich, W., & Poralla, K. (1984). Spoilage of fruit juice by bacilli: Isolation and characterization of the spoiling microorganisms. Zeitschrift Fur Lebensmittel-Untersuchung Und -Forschung, 179(3), 224–227. doi:10.1007/BF01041898
- Chang, S.S., & Kang, D.H. (2004). Alicyclobacillus spp. in the fruit juice industry: History, characteristics, and current isolation/detection procedures. Critical Reviews in Microbiology, 30(2), 55–74. doi:10.1080/10408410490435089
- Huang, X.C., Yuan, Y.H., Guo, C.F., Gekas, V., & Yue, T.L. (2014). Alicylobacillus in the fruit juice industry: Spoilage,detection, and prevention/control. Food Reviews International, 31(13), 91–124.
- Ju, Y., Hao, H.J., Xiong, G.H., Geng, H.R., Zheng, Y.L., Wang, J. … Jiang, Y.Q. (2010). Development of colloidal gold-based immunochromatographic assay for rapid detection of Streptococcus suis serotype 2. Veterinary Immunology and Immunopathology, 133(2–4), 207–211. doi:10.1016/j.vetimm.2009.08.010
- Li, J.K., Feng, Z.P., & Qiu, N.X. (2006). Optimization and establishment of quantitatively competitive PCR system for the detection of. Alicyclobacillus Acidoterrestris. Scientia Agricultura Sinica, 39(2), 375–380.
- Li, J.K., Huang, R.R., Xia, K., & Liu, L. (2014). Double antibodies sandwich enzyme-linked immunosorbent assay for the detection of Alicyclobacillus acidoterrestris in apple juice concentrate. Food Control, 40, 172–176. doi:10.1016/j.foodcont.2013.11.037
- Li, J.K., Wang, F., & Xia, K. (2011). An indirect and accurate ELISA for detection of Alicyclobacillus acidoterrestris in apple juice concentrate. Scientia Agricultura Sinica, 44(22), 4669–4677.
- Li, J.K., Xia, K., & Yu, C.Z. (2013). Detection of Alicyclobacillus acidoterrestris in apple juice concentrate by enzyme-linked immunosorbent assay. Food Control, 30(1), 251–254. doi:10.1016/j.foodcont.2012.07.009
- Luo, H., Yousef, A.E., & Wang, H.H. (2004). A real-time polymerase chain reaction-based method for rapid and specific detection of spoilage Alicyclobacillus spp. in apple juice. Letters in Applied Microbiology, 39(4), 376–382. doi:10.1111/lam.2004.39.issue-4
- Pinhatti, M.E.M.C., Variane, S., Eguchi, S.Y., & Manfio, G.P. (1997). Detection of acidothermic bacilli in industrialized fruit juices. Fruit Process, 7, 350–353.
- Wang, F., Li, J.K., Liu, H.X., & Jiang, K. (2009). Preparation and purification of polyclonal antibody against Alicyclobacillus Acidoterrestrisfrom apple juice concentrate. Food and Fermentation Industries, 5(4), 33–37.
- Xia, K., Li, J.K., & Yu, Z. (2012). Preparation and purification of rat polyclonal antibody against. Aliyclobacillua Acidoterrestris. Science and Technology of Food Industry, 33(15), 162–168.
- Yamazaki, K., Teduka, H., Inoue, N., & Shinano, H. (1996). Specific primers for detection of Alicyclobacillus acidoterrestris by RT-PCR. Letters in Applied Microbiology, 23(5), 350–354. doi:10.1111/j.1472-765X.1996.tb00206.x